Abstract
Asthma is a chronic inflammatory disease with no definite treatment and more research is needed to overcome this condition. The aim of this study was to investigate the effect of the extract of Zataria multiflora (Z. multiflora) as a medicinal plant on cytokine genes expression in an experimental mouse model of asthma. Adult mice were randomly divided into the following groups: control (C), untreated asthma (A), asthmatic groups treated with dexamethasone (D) and Z. multiflora extract (200, 400, and 800 μg/mL; Z1, Z2, and Z3, respectively), (for groups C, A, and D n = 5 and for groups Z1, Z2, and Z3 n = 6). For induction of the mouse model of asthma, animals were sensitized with intraperitoneal injection and inhalation of ovalbumin (OVA). The number of T helper (Th) subtype cells (using flow cytometry) and the levels of IFN-γ, FOXP3, IL-4, TGF-β, IL-17 gene expression (by real time PCR) were assessed in mice splenocytes. The observed changes in spleen cells of group A compared to group C were increased number of Th2 and Th17 cells, enhancement of gene expression of IL-4, IL-17, and TGF-β (p < 0.001 for all cases), reduction of Th1 cells and Th1/Th2 ratio (p < 0.001 for both cases) and decrease in gene expression of IFN-γ, FOXP3 and IFN-γ/IL-4 ratio (p < 0.01 for IFN-γ and p < 0.001 for other cases). The observed changes in spleen cells of treated compared to untreated A group were enhancement of Treg cells and Th1/Th2 ratio (p < 0.001 for both cases), increase in IFN-γ (p < 0.05) and FOXP3 (p < 0.001) gene expression and IFN-γ/IL-4 ratio (p < 0.01) as well as reduction of Th2 and Th17 cells (p < 0.01 to p < 0.001), decrease gene expression of IL-4, IL-17, and TGF-β (p < 0.05 to p < 0.001). The findings showed that the extract of Z. multiflora decreased pro-inflammatory cytokines in asthma (IL-4 and IL-17 and TGF-β) but increased anti-inflammatory cytokines (IFN-γ) gene expression and the number of Treg (FOXP3) in splenocytes of asthmatic mice which may indicate the specific therapeutic effect of the plant extract in allergy, autoimmunity, and infectious diseases via potentiating Th1 and suppressing Th2 and Th17 cells.
Keywords: animal model of asthma, flow cytometry, mice, real time PCR, Zataria multiflora
Introduction
There are a growing use of herbal medicines and natural products, especially in recent years. Currently, 25% of existing drugs are made from herbal sources and 10% of medications are produced from microbial sources (Demain and Sanchez, 2009; Gurnani et al., 2014). Zataria multiflora (Z. multiflora) is native to Southwestern Asia such as Iran, Afghanistan, Pakistan, and Kashmir (Manikandan et al., 2012). This plant contains compounds such as thymol and carvacrol which are likely to be responsible for its pharmacological effects. The amount of thymol in fresh plant was reported as 73.21% while in dried plant the amount of carvacrol is 62.87% (Saleem et al., 2004). Various pharmacological and therapeutic effects were reported for Z. multiflora including effect on gastrointestinal disorders such as reduction of colon inflammation, and duodenal ulcer in inflammatory bowel disease (Minaiyan et al., 2005; Nakhai et al., 2007), dose-dependent anti-nociceptive activity (Hosseinzadeh et al., 2000), anti-microbial mainly on streptococci and E. coli (Owlia et al., 2004; Rahmani et al., 2012a,b), anti-fungal activity against Saprolegnia parasitica (Khosravi et al., 2012; Mahammadi purfard and Kavoosi, 2012), anti-oxidant (Babaie et al., 2007; Karimian et al., 2012; Boskabady and Mahtaj, 2015), and anti-inflammatory properties against acute and chronic inflammation, and lung inflammation (Hosseinzadeh et al., 2000; Jaffary et al., 2000; Boskabady et al., 2013, 2014b). The immuno-modulatory effects of the plant such as increased IFN-γ and IFN-γ/IL-4 ratio (Th1/Th2 balance) as well as decreased IL-4 were also demonstrated (Boskabady et al., 2013). In addition, several pharmacological effects of Z. multiflora were shown on respiratory system including stimulation of β2 adrenoceptors, inhibition of muscarinic (Boskabady et al., 2010, 2012b,a; Jafari et al., 2011) and histamine (H1) receptors (Boskabady et al., 2012b). Reduction of tracheal responsiveness (Gholami Mahtaj et al., 2015) and inflammatory mediators (Boskabady et al., 2013; Boskabady and Mahtaj, 2015) as well as improvement of lung pathological changes (Gholami Mahtaj et al., 2015) in sensitized guinea pigs due to treatment with the plant extract were also demonstrated. In addition, the preventive effects of Z. multiflora on emphysema and pathological changes of the lung and systemic inflammation in animal models of COPD (Gholami Mahtaj et al., 2015) were documented. The effects of the extract of Z. multiflora on coughs due to colds, bronchitis, disorders of the oral cavity (Mozaffarian, 1996), respiratory disorders of chemical war victims (Mostafavi and Shasavari, 2005) and its antitussive effect (Afzali et al., 2003) were also shown. The plant also traditionally used as an antibacterial agent for oral hygiene in Iran (Avicenna, 1985).
Asthma is mainly characterized by: (1) Intermittent and reversible obstruction of the airways which leads to recurrent attacks of wheezing, shortness of breath and cough (2) Bronchial hyper-responsiveness (BHR), which is the narrowing of the airways as a result of small amounts of broncho constrictor agents such as histamine or cholinergic agonists and (3) Airway inflammation. Cytokines play a key role in organizing the chronic inflammation of asthma. Increased number of CD4+ Th cells and subsequent increased secretion of IL-4, IL-5, IL-9, and IL-13 from Th2 cells, were shown in the airways of asthmatic patients (Barnes et al., 1998). Therefore, Th2 cells can induce allergic inflammatory diseases (Salmon et al., 1999; Babayigit et al., 2009). The above-mentioned cytokines stimulate B cells to induce immunoglobulin E (IgE) secretion, eosinophilic inflammation and mast cell proliferation. IFN-γ is the predominant cytokine produced by Th1 and its level is reduced in individuals with asthma (Renauld, 2001; Mehta and Mahajan, 2006; Barnes, 2008). Th1 can inhibit Th2 activity, and enhancement of Th1 activity could be a possible asthma therapy (Boskabady et al., 2013). Therefore, changes in IFN-γ/IL-4 cytokines ratio (Th1/Th2 balance) toward IL-4 (Th2) may cause allergic and atopic diseases such as atopic dermatitis, anaphylactic shock, allergic rhinitis, and asthma.
The regulatory T cells (Treg) activity is regulated by a specific transcription factor namely forkhead/winged helix (FOXP3) transcription factor. Treg cells normally suppress other Th cells by releasing TGF-β and IL-10 and the function of Treg may be impaired in asthma (Fontenot et al., 2003; Hori et al., 2003; Khattri et al., 2003; Barnes, 2008). Th17 cells can cause neutrophilic inflammation, airway remodeling and irreversible airway obstruction via secretion of IL-17. IL-17 also inhibits FOXP3 expression (Bullens et al., 2006) and has an important role in the progress of autoimmune disorders (Annunziato et al., 2008).
In this study, the preventive effect of Z. multiflora administered during the sensitization period was examined on subsets of T cells determination by flow cytometry and their cytokine gene expression in splenocytes of experimentally-induced asthma in BALB/c.
Materials and methods
Animals and groups
BALB/c mice weighted 20–25 g and age of 6–8 weeks were used in this study. The animals were housed in cages under standard temperature (20 ± 2°C), humidity (55 ± 5%) and light (12 h: 12 h light: dark) conditions with food and water were available ad libitum, in the animal house of School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.
Animals were randomly divided into the following groups:
(1) Non-asthmatic control animals (group C).
(2) Untreated asthmatic animals (group A).
(3-5) Asthmatic animals treated with Z. multiflora extract (200, 400, and 800 μg/mL) added to animals' drinking water (groups Z1, Z2, and Z3, respectively) during sensitization period. Each mouse drank an average of 4 ml drinking water per day and there was no significant difference in the amount of used drinking water among groups.
(6) Asthmatic group treated with dexamethasone (1 mg/kg/day; Sigma Chemicals, LTD.) from day 34 to 40 of sensitization period (group D). For groups C, A, and D, there were 5 animals in each group (n = 5) while in groups Z1, Z2, and Z3, there were 6 animals in each groups (n = 6).
Induction of the mouse model of asthma and extraction of splenocytes
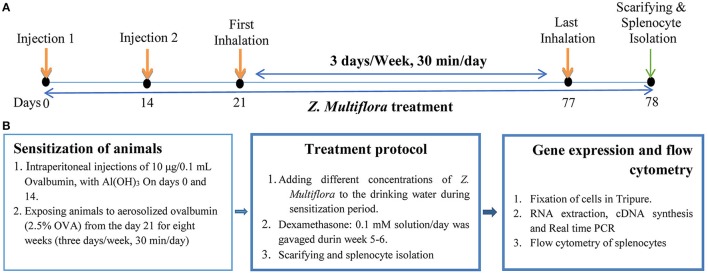
On days 0 and 14 of the experiment, mice were sensitized by intraperitoneal (i.p) injections of 10 μg/0.1 mL chicken egg albumin (Ovalbumin or OVA, grade V, 98% pure; Sigma, St. Louis, MO, USA) together with Al(OH)3. From the day 21 of the study, animals were exposed to aerosolized ovalbumin (2.5% OVA) for 8 weeks (30 min/day, 3 days/week; Temelkovski et al., 1998; Babayigit et al., 2009; Figures 1A,B). During experimental period, non-sensitized animals (control group) received injected and aerosolized saline instead of OVA. To evaluate the sensitivity of the animal, DTH (delayed-type hypersensitivity) test was carried out. For this purpose, 0.1 mg OVA (up to 10-fold diluted) was injected subcutaneously. Increased skin thickness, as well as inflamed and redness of the skin 1 day after injection indicated animal sensitization.
Figure 1.
The schematic time-course of inducing animal model of asthma, treatment, spleocyte isolation, flow cytometry and gene expression. (A) Induction of experimental animal model of asthma (sensitization) in mice. In control animals saline was administrated instead of OVA. (B) Treatment of animals with dexamethasone and three concentrations of Z. multiflora. In control and sensitized groups: animals were given drinking water alone. In group D: dexamethasone (0.1 mmol) was gavaged from the beginning of week 5 for 1 week. In groups Z1, Z2, and Z3: animals were given drinking water containing extract of Z. multiflora at three concentrations of 200, 400, and 800 μg/mL.
One day after the last inhalation (day 78) animals were sacrificed, spleen was isolated and its lymphocytes were extracted and cultured. The study protocol was approved by the Ethics Committee, Mashhad University of Medical Sciences and all experiments were conducted in accordance with the National Institute of Health guide for the care and use of laboratory animals (NIH Publications No. 80-23).
Plant and extracts
Z. multiflora was collected from mountains between Tabas and Yazd, in central Iran. The plant was identified by botanists and kept in the herbarium of Ferdowsi University of Mashhad with herbarium number: 35314. For preparation of hydro-ethanolic extract, 100 g of dried plant was soaked with 875 mL of 50% ethanol for 72 h at room temperature. The extract was then passed through the filter paper, the solvent was removed under reduced pressure and the dried extract was kept in refrigerator. The yield extract was 33.2 g. Three concentrations (200, 400, and 800 μg/mL) of the extract were then used for treatment groups.
HPLC finger print of Z. multiflora extract
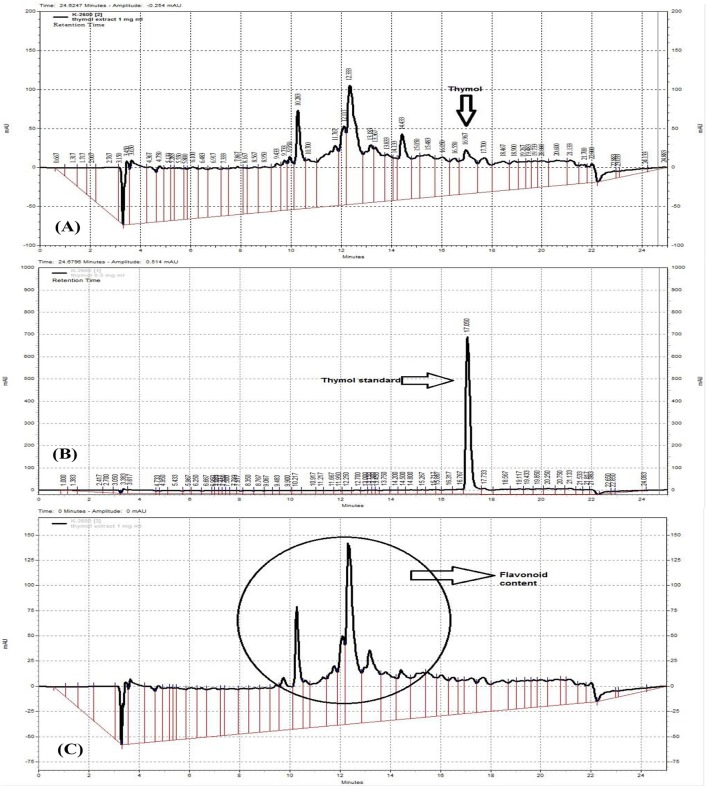
The hydro-ethanolic extract of Z. multiflora was characterized using a fingerprint profile prepared by a reversed-phase high performance chromatography (RP-HPLC). RP-HPLC was carried out by a linear gradient mobile phase included 0–100% methanol with a 1 ml/min flow rate at RT. Sample was prepared by filtering through 0.45 μm pore size ultra-membrane filter followed by sonication for about 45 min. It was loaded on a C18 column (5 μm particle size, 250 × 4.6 mm) from Capital (Broxburn, UK) with 20 μl injection volume. HPLC-UV (multi-wavelengths) measurements were performed using a Knauer HPLC system (Berlin, Germany) equipped with UV detector K-2600 and K-1001 HPLC Pump. The HPLC system was supplied with Chromgate HPLC software, version 3.3 and analyses were monitored at 240, 280, and 320 nm (Figure 2).
Figure 2.
RP-HPLC fingerprinting analysis of Z. multiflora extract (A,C) at 280 and 320 nm (C), thymol standard curve (B). The presence of flavonoids was confirmed at the wavelength 320 nm which is specific for flavonoids and on the basis of previous works.
Flow cytometry
To determine different subsets of regulatory T cells, single-cell suspension prepared from mice spleen (1 × 106 of cells) was poured in flow tube and surface stained with CD4 and CD25 and anti-mouse FoxP3+ according to eBioscience mouse regulatory T cell staining kit's guidelines (BD, eBioscience, USA). Spleen cells were suspended in the presence of PMA (Phorbol 12-myristate 13-acetate, Sigma), ionomycin (Sigma) and Brefeldyn for 4 h to evaluate Th1, Th2, and Th17 cells. According to kit protocol, for the entry of IFN-γ, IL-4, and IL-17 antibodies (Anti-Mouse IFN-γ, IL-4, and IL-17, PE eBioscience, USA) cells membrane was permeabilized by permeabilization buffer and fixed by fixation buffer. Then, all samples were analyzed using a BD FACSCalibur flow cytometer and the results were analyzed using Flowjo Software.
Real-time PCR analysis
The RNA extracted from splenocytes (5 × 106 cells) was mixed with 1 ml of Tripure Isolation Reagents (Roche Applied Science, Germany), and cDNA was prepared with cDNA Synthesis Kit (Fermentas, Germany) according to instructions, for quantitative real-time analysis.
Primers and quantitation probes for β2- Microglobulin (β2M), Interferon gamma (IFN-γ), IL-4, IL-17, FOXP3 and Transforming growth factor beta (TGF-β), were designed using the Beacon Designer software Version 7.9. The primers and probe are mentioned in Table 1.
Table 1.
Sequences of primers used for real-time PCR.
| Gene name | Sence/antisence primers-probs |
|---|---|
| 1. β2-Microglobin | 5-GCCGAACATACTGAACTGCTAC-3 (forward) |
| 5-CTTGCTGAAGGACATATCTGACATC-3 (reverse) | |
| 5-AACACAGTTCCACCCGCCTCACATTGA-3 (probe) | |
| 2. IFN-γ | 5-GTATTGCCAAGTTTGAGGTCAAC-3 (forward) |
| 5-GCTTCCTGAGGCTGGATTC-3 (reverse) | |
| 5-CCACAGGTCCAGCGCCAAGCATTCAA-3 (probe) | |
| 3. IL-4 | 5-TCCTCACAGCAACGAAGAAC-3 (forward) |
| 5-CAAGCATGGAGTTTTCCCATG-3 (reverse) | |
| 5-AGCACCTTGGAAGCCCTACAGACGAGC-3 (probe) | |
| 4. IL-17 | 5-CAGACTACCTCAACCGTTCC-3 (forward) |
| 5-TTCCCTCCGCATTGACAC-3 (reverse) | |
| 5-ACTCTCCACCGCAATGAAGACCCTGA-3 (probe) | |
| 5. TGF-β | 5-CCTGGATACCAACTATTGCTTCAG-3 (forward) |
| 5-CAGACAGAAGTTGGCATGGTAG-3 (reverse) | |
| 5-TCCACTTCCAACCCAGGTCCTTCCT-3 (probe) | |
| 6. FOXP3 | 5-GGTACACCCAGGAAAGACAG-3 (forward) |
| 5-GCTTGGCAGTGCTTGAGA-3 (reverse) | |
| 5-TGGCTCCTCGAAGACCTTCTCACAACC-3 (probe) |
Real-time PCR was performed using TaqMan PCR Mastermix and pre-formulated primers for mentioned genes and β2M by Rotor Gene Q Real-Time PCR machine (Corbett Research, Australia). The results were analyzed by the comparative threshold cycle method and normalized by β2M as an internal control with Rotor Gene 6000 software (Corbett Research, Australia). Using the below equation, the fold change in expression of gene of interest for each group was calculated: mean of gene of interest normalized. Index of test group/mean of gene of interest normalized index of healthy controls.
Statistical analysis
Data were expressed as mean ± SEM. Comparison between groups was performed using one way ANOVA with Tukey Kramer post-hoc tests. A P-value less than 0.05 was considered as statistically significant. For data analysis, SPSS software (version 16) was used.
Results
Characteristics of Z. multiflora extract
The HPLC fingerprint shows 5 major peaks in retention times between 10 and 18 min. According to thymol standard curve (Figure 2B), the peak appeared at 16.967 in Figure 2A is thymol. The quantification of thymol in the total extract of Z. multiflora was also carried out using HPLC. The total content of thymol was determined to be 1.6% w/w of the total extract (Figure 2A). According to previous studies, peaks of HPLC chromatogram appeared at 320 nm are ascribed to flavonoids (Figure 2C). These compounds include apigenin, luteolin and 6-hydroxyluteolin (Ali et al., 2000).
Delayed-type hypersensitivity (DTH) results
All animals in the OVA-sensitized groups showed positive DTH test which confirm animal sensitization (induction of mice animal model of asthma).
Flow cytometry results
Subsets of Th lymphocytes and Th1/Th2 balance in the spleen of asthmatic mice
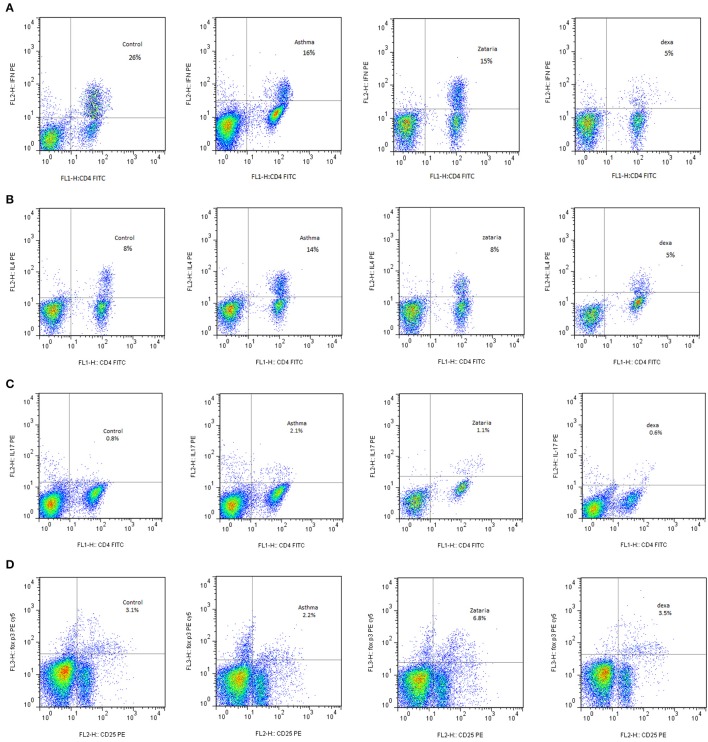
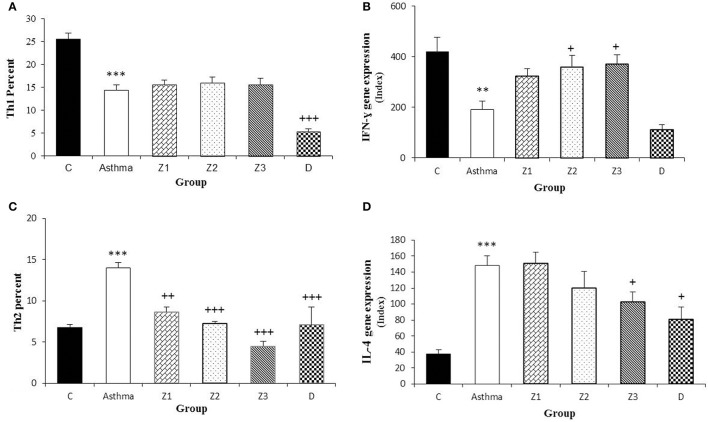
In asthma group, Th2 and Th17 cells were significantly increased but Th1 cells was significantly decreased (p < 0.001 for all cases), while Treg cells did not show significant change compared to the control group (Figures 3A,D, 4A,C, 5A). In addition, the ratio of Th1/Th2 in asthmatic group was significantly decreased compared to the control group (p < 0.001; Figure 6C).
Figure 3.
Flow cytometry of various T Cells sub types including; (A) In plot FSC and SSC, Th1 lymphocyte population between CD4 markers and IFN-γ were selected. The Th1 in the group treated with extracts in comparison with untreated group increased. (B) In plot FSC and SSC, Th2 lymphocyte population between the CD4 and IL-4 markers were selected. Th2 levels in treated groups with extract and dexamethasone compared with untreated group decreased. (C) In the plot FSC and SSC, Th17 lymphocyte population between the CD4 and IL-17 markers were selected. The Th17 in the treated groups with extract and dexamethasone compared with untreated group decreased. (D) Treg cells on the lymphocyte population were selected between Sec and CD4. Then this population on CD25 and FOXP3 was placed for determine the amount of these cells. Treg levels in groups treated with extracts and dexamethasone compared with untreated group increased.
Figure 4.
Percent of Th1 and Th2 cells (A,C) and values of IFN-γ and IL-4 gene expression (B,D) in splenocyte from control group (C, n = 5), Asthma group (A, n = 5) and A treated groups with the extract of Z. multiflora at concentrations 200, 400, and 800 μg/mL (groups Z1, Z2, and Z3, respectively, n = 6) and dexamethasone (D, n = 5). Data were presented as mean ± SEM. **p < 0.01, ***p < 0.001, vs. control group. +p < 0.05, ++p < 0.01, +++p < 0.001, vs. asthma group. The statistical comparisons were made using ANOVA with Tukey–Kramer multiple post-hoc test.
Figure 5.
Percent of Treg cells (A), values of FOXP3 (B), and TGF-β (C) gene expression in splenocyte from control group (C, n = 5), Asthma group (A, n = 5), and A treated groups with the extract of Z. multiflora at concentrations 200, 400, and 800 μg/mL (groups Z1, Z2, and Z3, respectively, n = 6) and dexamethasone (D, n = 5). Data were presented as mean ± SEM. ***p < 0.001, vs. control group. +++p < 0.001, vs. asthma group. The statistical comparisons were made using ANOVA with Tukey–Kramer multiple post-hoc test.
Figure 6.
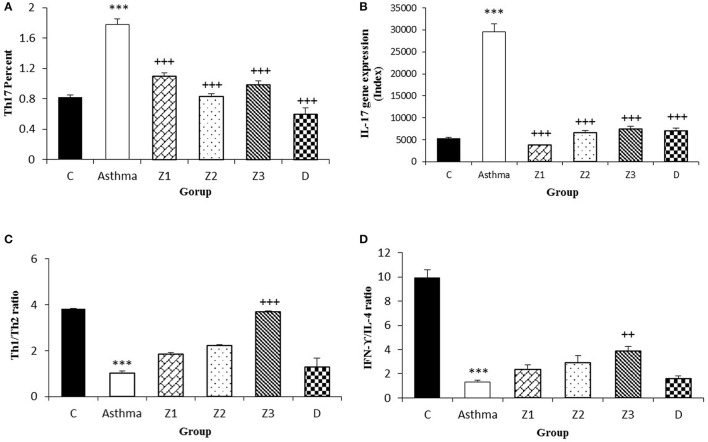
Percent of Th17 cells (A), values of IL-17 gene expression (B), Th1/Th2 ratio (C), and values of IFN-γ/IL-4 ratio (D) in splenocyte from control group (C, n = 5), Asthma group (A, n = 5) and A treated groups with the extract of Z. multiflora at concentrations 200, 400, and 800 μg/mL (groups Z1, Z2, and Z3, respectively, n = 6) and dexamethasone (D, n = 5). Data were presented as mean ± SEM. ***p < 0.001, vs. control group. ++p < 0.01, +++p < 0.001, vs. asthma group. The statistical comparisons were made using ANOVA with Tukey–Kramer multiple post-hoc test.
Subsets of lymphocytes and Th1/Th2 balance in asthmatic animals treated with Z. multiflora extract and dexamethasone
Compared to untreated asthmatic group, Th2 and Th17 cells in asthmatic animals treated with all extract concentrations were significantly reduced (p < 0.01 for Th2 in Z1, and p < 0.001 for other cases; Figures 3B,D, 4C, 6A). Th1/Th2 ratio in asthmatic animals treated with high concentration of the extract (800 μg/ml) was significantly increased compared to untreated asthmatic group (p < 0.001; Figure 6C).
There was no significant difference in Th1 and Th17 among the asthmatic groups treated with different extract concentrations. The effects of treatment with high concentration of the extract (800 μg/ml) on Th2 were significantly higher than its low concentration (p < 0.05). The effect of high concentration of the extract on Th1/Th2 ratio was also significantly higher than its low concentration (p < 0.01; Table 2).
Table 2.
The percent of T cells and values of IFN-γ, IL-4, TGF-β, FOXP3, and IL-17 gene expressions as well as IFN-γ/IL-4 ratio of splenocyte in asthmatic groups treated with the extract of Z. multiflora at concentrations 200, 400, and 800 μg/ml (groups Z1, Z2, and Z3, respectively) and dexamethasone (D, 0.1 mM).
| Variable | Z1 | Z2 | Z3 | D |
|---|---|---|---|---|
| Th1 | 15.66 ± 0.88*** | 16 ± 1.29*** | 15.66 ± 1.38*** | 5.4 ± 0.54 |
| Th2 | 8.66 ± 0.55 | 7.25 ± 0.25 | 4.5 ± 0.56# | 7.1 ± 2.12 |
| Th17 | 1.1 ± 0.04** | 0.83 ± 0.03 | 0.98 ± 0.05* | 0.6 ± 0.08 |
| Treg | 5.45 ± 0.14** | 7.66 ± 0.25***,### | 5.63 ± 0.22**,††† | 4.08 ± 0.45 |
| Th1/Th2 | 1.85 ± 0.19 | 2.22 ± 0.19 | 3.68 ± 0.49***,##,† | 1.27 ± 0.39 |
| IFN-γ | 323 ± 29.29** | 359.9 ± 45.68*** | 371.5 ± 37.33*** | 112.4 ± 18.71 |
| FOXP3 | 2,196 ± 254.1 | 4,599 ± 228.6***,### | 1,565 ± 262.4††† | 1,580 ± 223.2 |
| IL-4 | 150.7 ± 13.97* | 119.8 ± 20.95 | 102.6 ± 12.18 | 80.62 ± 15.47 |
| TGF-β | 2,667 ± 81.38*** | 2,020 ± 134.6**,# | 1,259 ± 256###,†† | 1,168 ± 8.36 |
| IL-17 | 3,787 ± 89.68 | 6,687 ± 466.7 | 7,496 ± 645.5 | 7,015 ± 618.9 |
| IFN-γ/IL-4 ratio | 2.327 ± 0.42 | 2.91 ± 0.56 | 3.89 ± 0.36** | 1.57 ± 0.25 |
Results are presented as mean ± SEM.
p < 0.05,
p < 0.01,
p < 0.001 vs. dexamethasone (D) group.
p < 0.05,
p < 0.01,
p < 0.001 vs. Z1 group.
p < 0.05,
p < 0.01,
p < 0.001 vs. Z2 group.
Statistical comparisons were made using ANOVA with Tukey–Kramer multiple post-hoc test.
In asthmatic animals treated with dexamethasone, all T cells subset (Th1, Th2, and Th17) were significantly decreased while Treg was increased compared to untreated asthma group (p < 0.001 for all cases; Figures 3A–D, 4A,C, 6A). Th1/Th2 ratio in the asthmatic group treated with dexamethasone was not significantly different with that of untreated group (Figure 6C).
The effects of treatment with all extract concentrations on Th1 and Treg were significantly higher than the effect of dexamethasone treatment (p < 0.01 to p < 0.001, Table 2). However, the effects of low and high extract concentrations on Th17, were lower than the effect of dexamethasone treatment (p < 0.01 and p < 0.05 for low and high extract concentrations, respectively; Table 2). In addition, the effect of high concentration of the extract on Th1/Th2 ratio was also significantly higher than that of dexamethasone (p < 0.001; Table 2).
Cytokines gene expression
Cytokine gene expression in the spleen cells extracted from asthmatic animals
The gene expression of IFN-γ, FOXP3, and IFN-γ/IL-4 ratio were significantly reduced, while IL-4, TGF-β, and IL-17 gene expression were increased in asthmatic animals compared to the control group (p < 0.01 for IFN-γ and p < 0.001 for other cases, Figures 4B,D, 5B,C, 6B,D).
Cytokine gene expression in the spleen cells of treated groups with Z. multiflora extract and dexamethasone
Gene expression of IFN-γ in spleen cells of asthma groups treated with medium and high concentrations of the extracts (400 and 800 μg/ml; p < 0.05 for both cases) and that of FOXP3 in the group treated with medium extract concentration (p < 0.001) were significantly increased compared to untreated asthma group (Figures 4B, 5B).
Gene expression of IL-4 in the group treated with high concentration of the extract (p < 0.05), IL-17 in asthma groups treated with all extract concentrations (p < 0.001 for all cases) and TGF-β in the groups treated with the medium and high extract concentrations (p < 0.001 for both cases) were significantly decreased compared with untreated asthma group (Figures 4D, 5B, 6B).
The ration of IFN-γ/IL-4 in the group treated with high concentration of the extract was significantly increased compared with the untreated asthma group (p < 0.01; Figure 6D).
There was no significant difference in genes expression of IFN-γ, IL-4, IL-17, and IFN-γ/IL-4 ratio among the asthma groups treated with different concentrations of extract. The effect of the treatment with medium concentration of the extract (400 μg/ml) on FOXP3 gene expression was significantly higher than the other two concentrations (p < 0.001 for both cases). The effect of treatment with medium and high concentrations of the extract on TGF-β gene expression was significantly higher than treatment with its low concentration (p < 0.05 and p < 0.001 for the medium and low concentration, respectively). In addition, the effect of high concentration of the extract was higher than its medium concentration on TGF-β gene expression (p < 0.01, Table 2).
In animals treated with dexamethasone, gene expression of IL-4 (p < 0.05), IL-17 and TGF-β (p < 0.001 for both cases) were significantly reduced compared with untreated asthma group (Figures 4D, 5B, 6C). However, there were no significant changes in IFN-γ, FOXP3, and IFN-γ/IL-4 ratio in asthma group treated with dexamethasone compared with untreated asthma group (Figures 4B, 5B, 6D).
The effects of all extract concentrations on genes expression of IFN-γ and its medium concentration (400 μg/ml) on FOXP3 (p < 0.01 to p < 0.001) were significantly greater than the effect of dexamethasone. However, the effects of treatment with low concentration of the extract (400 μg/ml) on IL-4 gene expression and its two lower concentrations (200 and 400 μg/ml) on TGF-β gene, were significantly lower than the effect of dexamethasone (p < 0.05 to p < 0.001; Table 2).
Discussion
The most important characteristic feature of asthma is chronic airway inflammation which is associated with increased number of Th2lymphocytes and their cytokines (IL-4, IL-5, IL-9, and IL-13) while Th1 lymphocytes and their cytokines (IL-2, IFN-γ, and IL-12) are reduced. There are also increased activated eosinophils and mast cells counts in asthma (Ray and Cohn, 1999; Larché et al., 2003). Th2 cells can induce B cell response to produce IgE and consequently activate mastocytes and eosinophils and regulate pro-inflammatory response, whereas Th1 cells directly or indirectly, inhibit inflammatory activities by preventing the Th2 response and thus, regulate the inflammatory responses of mast cells and eosinophils which are the main player cells in allergic asthma. Stimulation of Th1 may inhibit Th2 cells and allergic inflammation by secreting IL-2 and IFN-γ. IFN-γ not only suppresses Th2 responses but also stimulates B cells to secret IgG. Therefore, inducing an increase in Th1 activity could be considered as a treatment strategy for asthma (Lindemann and Racké, 2003). FOXP3 is essential for the differentiation of primary T cells to Treg phenotype. Treg cells control not only pathogenic Th2 cells, but also its effect on Th1 cells and the reduction of FOXP3 had been demonstrated in patients with stable asthma (Provoost et al., 2009). IL-17 may play an important role in the development of autoimmune disorders and chronic inflammation by secretion of other cytokines and growth factors from stromal cells (Molet et al., 2001; Annunziato et al., 2008). In addition, the effect of TGF-β during the late phase of lung inflammation and its role in airway remodeling in asthma has been reported (Magnan et al., 1996; Duvernelle et al., 2003). Asthma is an inflammatory disease and there is greater emphasis on its prevention using anti-inflammatory drugs in the treatment of this disease. Therefore, in this study, the preventive effect of Z. multiflora on Th cell sub types and the gene expression of several pro-inflammatory and anti-inflammatory cytokines in splenocytes of asthmatic BALB/c mice were evaluated.
In asthmatic animals, flow cytometry results showed significant increase in Th2 and Th17 but significant decrease in Th1 compared to control group. In addition, the ratio of Th1/Th2 in asthmatic animals was significantly decreased compared to control group.
IFN-γ and FOXP3 gene expression showed a significant decrease, while a significant increase was observed in IL-4, TGF-β, and IL-17 gene expression in spleen cells of asthmatic animals compared to control group. The ratio of IFN-γ/IL-4 in asthmatic animals compared to control group was also significantly reduced. These results confirmed the induction of experimental model of asthma in mice (sensitization of the animals) as previously shown using similar method of sensitization (Larché et al., 2003).
Th2 and Th17 cells in treated animals with three concentrations of the extract were significantly reduced but Treg cells was significantly increased compared to untreated asthma group. In addition, treatment with high concentration of the extract significantly increased Th1/Th2 ratio. The findings also showed that the effect of high concentration of the extract on Th2 cells and Th1/Th2 ratio was significantly higher compared to its low concentration. These results suggest a preventive effect for the plant on subset of T lymphocyte and gene expression of their cytokines in an animal model of asthma (sensitized animals) when the extract was administered during the sensitization period.
Although, the percentage of Th17 cells in Z1 treated group was higher than Z2 and Z3 treated groups, the differences between three concentrations of the extract on Th17 cells were not statistically significant. IL-17 gene expression also was not significantly different among treated groups with three different concentrations of the extract. In addition, IL-17 gene expression was significantly reduced due to treatment with all three concentrations of the extract which was consistent with the results of the extract on Th17 cells.
In previous studies, the relaxant effect of Z. multiflora and carvacrol as its main constituent on tracheal smooth muscle had been shown which may indicate the bronchodilator properties of the plant and carvacrol (Boskabady and Jalali, 2013; Boskabady et al., 2014a). The effect of Z. multiflora on cytokines of human lymphocytes and lung lavage of guinea pigs model of asthma showed reduced IL-4 and enhanced IFN-γ gene expression as well as increased IFN-γ/IL-4 ratio (Boskabady et al., 2013) which support the findings of the present study. In addition, Z. multiflora improved IL-8 level, total WBC, eosinophil counts, and MDA level in an experimental animal model of COPD (Boskabady et al., 2014b). In animal model of COPD, Z. multiflora extract and its constituent, carvacrol showed preventive effect on tracheal responsiveness and pathological changes of the lung that may be due to its anti-inflammatory properties (Boskabady and Mahtaj, 2015; Gholami Mahtaj et al., 2015). The effects of the plant on animal model of COPD also suggest the preventive effect of the plant on inflammatory lung disorders and support the findings of the present study.
Treatment of sensitized animals with dexamethasone caused a significant reduction in Th1, Th2, and Th17 cells and a significant increase in Treg cells compared to untreated asthmatic animals. The results of previous studies also showed that dexamethasone treatment lead to reduction of both IFN-γ and IL-4 gene expression in human lymphocyte and sensitized guinea pigs (Boskabady et al., 2013). The results of this study also showed that treatment of asthmatic mice with dexamethasone reduced Th1 cells and did not affect Th1/Th2 ratio, IFN-γ gene expression and IFN-γ/IL-4 ratio which was in line with the previous findings (Boskabady et al., 2013). The effect of treatment with the extract on Th1 and Treg cells was significantly higher and more selective than that of dexamethasone. The ratio of Th1/Th2 in asthmatic animals treated with the extract was also significantly higher than in treated with dexamethasone. The results of the present study showed that treatment of asthmatic animals with the extracts of Z. multiflora reduced Th17 and Th2 cells as well as IL-17, IL-4, and TGF-β gene expression but increased Treg cells as well as FOXP3 and IFN-γ gene expression. These results indicate the effect of the extract on both measured pro-inflammatory and anti-inflammatory cytokines. However, dexamethasone only affected Th2, Th17, and Treg subtypes and gene expression of only pro-inflammatory cytokines. Therefore, the findings of the present study suggested a more specific preventive effect for the plant in inflammatory disorders such as asthma compared to dexamethasone.
The extract also showed a dose dependently inhibitory effect on TGF-β gene expression. Treatment of animals with high and medium concentrations of the extract showed overexpression of anti-inflammatory cytokine (IFN-γ) in asthma and increased number of Treg (FOXP3) compared to untreated asthmatic animals. These findings may indicate more pronounced preventive effect of high and medium concentrations of extract on anti-inflammatory Th subsets.
Reduced IL-4, increased IFN-γ and enhanced IFN-γ/IL-4 ratio due to Z. multiflora extract were shown in OVA-sensitized guinea pigs as well as in PHA-stimulated human lymphocytes, using ELISA assay, previously (Boskabady et al., 2013) which support the results of the present study. However, it would be also helpful to examine the effect of the plant on the levels of cytokine using Western Blot and immunohistochemistry (IHC) in sensitized animals in future studies.
Taken together, these results may predicate the therapeutic effect of Z. multiflora extract on inflammatory diseases and immunologic disorders of Type I hypersensitivities (IgE-mediated) such as hay fever, urticaria, and asthma. The prophylactic effect of the plant extract on tracheal response, inflammatory mediators (Boskabady et al., 2014a) and pathological changes (Boskabady et al., 2014b) in sensitized guinea pigs.
In the present study the cellular and molecular mechanisms of the preventive effect of Z. multiflora on asthma was studied by flow cytometry measurements of Th subtype cells and also gene expression of their major cytokines which showed promising cellular and molecular effect of the plant on asthma. The results of the present study are in line and supported with the findings of previous studies of the effect of Z. multiflora on animal model of asthma and COPD (described in the rest of discussion previously). However, as it is clear all aspects of cellular and molecular bases of the effect of the plant could not be conducted in a single study and further studies needed to be performed in this regard in the future.
In conclusion, the results of the present study indicated that the extract of Z. multiflora decreased pro-inflammatory cytokines in asthma (IL-4, IL-17, and TGF-β) whereas increased anti-inflammatory cytokine (IFN-γ) gene expression in splenocytes of experimentally-induced asthma in mice. These results may indicate a more specific preventive effect for the plant extract compared to dexamethasone in allergy, autoimmunity and infectious diseases by both potentiating the production of Th1 cytokines and suppressing the inflammatory cytokines from Th2 and Th17. In future studies, Western Blot and immunohistochemistry should be conducted to ensure the effect of Z. multiflora on Th1/Th2 and Th17/T regulatory in a mouse model of allergic asthma.
Author contributions
MB, MK, and AR designed the work, and analyzed and interpreted of data. MK, DH, and RN helped in execution of research. AA and MB wrote the manuscript and all the other authors read, improved and approved the manuscript.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was financially supported by a grant from Research Council of Mashhad University of Medical Sciences. The results of this paper are a part of a Ph.D. thesis of MK.
Glossary
Abbreviations
- Z. multiflora
Zataria multiflora
- BHR
bronchial hyper-responsiveness
- OVA
ovalbumin
- i.p
intraperitoneal
- DTH
delayed-type hypersensitivity
- Treg
regulatory T cells
- β2M
β2-Microglobin
- IFN-γ
interferon gamma
- IL-4
interleukin 4
- IL-17
interleukin 17
- FOXP3
forkhead box P3
- TGF-β
transforming growth factor beta
- IgE
immunoglobulin E.
References
- Afzali H., Darabi M., Kashanian M. (2003). The effect of Zataria multiflora and Foeniculum vulgare in acute cough, in Proceeding of the 16th Iranian Congresss of Physiology and Pharmacology (Tehran: ), 192. [Google Scholar]
- Ali M. S., Saleem M., Ali Z., Ahmad V. U. (2000). Chemistry of Zataria multiflora (Lamiaceae). Phytochemistry 55, 933–936. 10.1016/S0031-9422(00)00249-1 [DOI] [PubMed] [Google Scholar]
- Annunziato F., Cosmi L., Liotta F., Maggi E., Romagnani S. (2008). The phenotype of human Th17 cells and their precursors, the cytokines that mediate their differentiation and the role of Th17 cells in inflammation. Int. Immunol. 20, 1361–1368. 10.1093/intimm/dxn106 [DOI] [PubMed] [Google Scholar]
- Avicenna (1985). Al-Qanun fi al Tibb, (The Canon of Medicine), Persian Edition by Sharaf-Kandi. Tehran: Soroush. [Google Scholar]
- Babaie M., Yasa N., Mohammadirad A., Khorasani R., Abdollahi M. (2007). On the anti oxidative stress potential of Zataria multiflora Boiss (Avishan shirazi) in rats. Int. J. Pharmacol. 3, 510–514. 10.3923/ijp.2007.510.514 [DOI] [Google Scholar]
- Babayigit A., Olmez D., Karaman O., Ozogul C., Yilmaz O., Kivcak B., et al. (2009). Effects of Ginkgo biloba on airway histology in a mouse model of chronic asthma. Allergy Asthma Proc. 30, 186–191. 10.2500/aap.2009.30.3187 [DOI] [PubMed] [Google Scholar]
- Barnes P. J. (2008). The cytokine network in asthma and chronic obstructive pulmonary disease. J. Clin. Invest. 118, 3546–3556. 10.1172/JCI36130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes P. J., Chung K. F., Page C. P. (1998). Inflammatory mediators of asthma: an update. Pharmacol. Rev. 50, 515–596. [PubMed] [Google Scholar]
- Boskabady M. H., Jafari Z., Pouraboli I., Babazade B., Rahbardar M. G. (2012a). Anti-cholinergic effect of Zataria multiflora Boiss on guinea pig tracheal chains. Nat. Prod. Res. 26, 1523–1528. 10.1080/14786419.2011.565007 [DOI] [PubMed] [Google Scholar]
- Boskabady M. H., Jalali S. (2013). Effect of carvacrol on tracheal responsiveness, inflammatory mediators, total and differential WBC count in blood of sensitized guinea pigs. Exp. Biol. Med. 238, 200–208. 10.1177/1535370212474604 [DOI] [PubMed] [Google Scholar]
- Boskabady M. H., Jalali S., Farkhondeh T., Byrami G. (2014a). The extract of Zataria multiflora affect tracheal responsiveness, serum levels of NO, nitrite, PLA2, TP and histamine in sensitized Guinea pigs. J. Ethnopharmacol. 156, 301–308. 10.1016/j.jep.2014.08.024 [DOI] [PubMed] [Google Scholar]
- Boskabady M. H., Kaveh M., Eftekhar N., Nemati A. (2010). Zataria multiflora Boiss and carvacrol affect β2-adrenoceptors of guinea pig trachea. J. Evid. Based Complementary Altern. Med. 2011:857124 10.1155/2011/857124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boskabady M. H., Mahtaj L. G. (2015). Lung inflammation changes and oxidative stress induced by cigarette smoke exposure in guinea pigs affected by Zataria multiflora and its constituent, carvacrol. BMC Complement. Altern. Med. 15:39. 10.1186/s12906-015-0574-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boskabady M. H., Mehrjardi S. S., Rezaee A., Rafatpanah H., Jalali S. (2013). The impact of Zataria multiflora Boiss extract on in vitro and in vivo Th1/Th2 cytokine (IFN-gamma/IL4) balance. J. Ethnopharmacol. 150, 1024–1031. 10.1016/j.jep.2013.10.003 [DOI] [PubMed] [Google Scholar]
- Boskabady M. H., Tabanfar H., Gholamnezhad Z., Sadeghnia H. R. (2012b). Inhibitory effect of Zataria multiflora Boiss and carvacrol on histamine (H1) receptors of guinea-pig tracheal chains. Fundam. Clin. Pharmacol. 26, 609–620. 10.1111/j.1472-8206.2011.00971.x [DOI] [PubMed] [Google Scholar]
- Boskabady M. H., Tabatabaee A., Jalali S. (2014b). Potential effect of the extract of Zataria multiflora and its constituent, carvacrol, on lung pathology, total and differential WBC, IgE and eosinophil peroxidase levels in sensitized guinea pigs. J. Funct. Foods 11, 49–61. 10.1016/j.jff.2014.08.021 [DOI] [Google Scholar]
- Bullens D., Truyen E., Coteur L., Dilissen E., Hellings P. W., Dupont L. J., et al. (2006). IL-17 mRNA in sputum of asthmatic patients: linking T cell driven inflammation and granulocytic influx. Respir. Res. 7, 135–144. 10.1186/1465-9921-7-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demain A. L., Sanchez S. (2009). Microbial drug discovery: 80 years of progress. J. Antibiot. 62, 5–16. 10.1038/ja.2008.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvernelle C., Freund V., Frossard N. (2003). Transforming growth factor-b and its role in asthma. Pulm. Pharmacol. Ther. 16, 181–196. 10.1016/S1094-5539(03)00051-8 [DOI] [PubMed] [Google Scholar]
- Fontenot J. D., Gavin M. A., Rudensky A. Y. (2003). Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 4, 330–336. 10.1038/ni904 [DOI] [PubMed] [Google Scholar]
- Gholami Mahtaj L., Boskabady M., Mohamadian Roshan N. (2015). The effect of Zataria multiflora and its constituent, carvacrol, on tracheal responsiveness and lung pathology in guinea pig model of COPD. Phytother. Res. 29, 730–736. 10.1002/ptr.5309 [DOI] [PubMed] [Google Scholar]
- Gurnani N., Mehta D., Gupta M., Mehta B. (2014). Natural Products: source of potential drugs. Afr. J. Basic. Appl. Sci. 6, 171–186. 10.5829/idosi.ajbas.2014.6.6.21983 [DOI] [Google Scholar]
- Hori S., Nomura T., Sakaguchi S. (2003). Control of regulatory T cell development by the transcription factor Foxp3. Science 299, 1057–1061. 10.1126/science.1079490 [DOI] [PubMed] [Google Scholar]
- Hosseinzadeh H., Ramezani M., Salmani G. (2000). Antinociceptive, anti-inflammatory and acute toxicity effects of Zataria multiflora Boiss extracts in mice and rats. J. Ethnopharmacol. 73, 379–385. 10.1016/S0378-8741(00)00238-5 [DOI] [PubMed] [Google Scholar]
- Jafari Z., Boskabady M. H., Pouraboli I., Babazade B. (2011). Zataria multiflora Boiss inhibits muscarinic receptors of incubated tracheal smooth muscle with propranolol. Avicenna J. Phytomed. 1, 7–13. 10.22038/ajp.2011.115 [DOI] [Google Scholar]
- Jaffary F., Ghannadi A., Poush A. S. (2000). Antiinflammatory activity of Zataria multiflora Boiss. J. Res. Med. Sci. 5, 6–9. [Google Scholar]
- Karimian P., Kavoosi G., Saharkhiz M. J. (2012). Antioxidant, nitric oxide scavenging and malondialdehyde scavenging activities of essential oils from different chemotypes of Zataria multiflora. Nat. Prod. Res. 26, 2144–2147. 10.1080/14786419.2011.631136 [DOI] [PubMed] [Google Scholar]
- Khattri R., Cox T., Yasayko S. A., Ramsdell F. (2003). An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat. Immunol. 4, 337–342. 10.1038/ni909 [DOI] [PubMed] [Google Scholar]
- Khosravi A. R., Shokri H., Sharifrohani M., Mousavi H. E., Moosavi Z. (2012). Evaluation of the antifungal activity of Zataria multiflora, Geranium herbarium, and Eucalyptus camaldolensis essential oils on Saprolegnia parasitica–infected rainbow trout (Oncorhynchus mykiss) eggs. Foodborne Pathog. Dis. 9, 674–679. 10.1089/fpd.2011.1086 [DOI] [PubMed] [Google Scholar]
- Larché M., Robinson D. S., Kay A. B. (2003). The role of T lymphocytes in the pathogenesis of asthma. J. Allergy Clin. Immunol. 111, 450–463. 10.1067/mai.2003.169 [DOI] [PubMed] [Google Scholar]
- Lindemann D., Racké K. (2003). Glucocorticoid inhibition of interleukin-4 (IL-4) and interleukin-13 (IL-13) induced up-regulation of arginase in rat airway fibroblasts. Naunyn. Schmiedebergs. Arch. Pharmacol. 368, 546–550. 10.1007/s00210-003-0839-8 [DOI] [PubMed] [Google Scholar]
- Magnan A., Mege J. L., Escallier J. C., Brisse J., Capo C., Reynaud M., et al. (1996). Balance between alveolar macrophage IL-6 and TGF-beta in lung-transplant recipients. marseille and montreal lung transplantation group. Am. J. Respir. Crit. Care Med. 153, 1431–1436. 10.1164/ajrccm.153.4.8616577 [DOI] [PubMed] [Google Scholar]
- Mahammadi purfard A., Kavoosi G. (2012). Chemical composition, radical scavenging, antibacterial and antifungal activities of Zataria multiflora bioss essential oil and aqueous extract. J. Food Saf. 32, 326–332. 10.1111/j.1745-4565.2012.00384.x [DOI] [Google Scholar]
- Manikandan R., Chandrasekar K., Srivastava S. K. (2012). Life form analysis of the family Lamiaceae in Jammu & Kashmir, India. Phytotaxonomy 12, 7–19. [Google Scholar]
- Mehta A. A., Mahajan S. (2006). Role of cytokines in pathophysiology of asthma. Iran. J. Pharmacol. Ther. 5, 1–14. [Google Scholar]
- Minaiyan M., Ghannadi A., Salehi E. (2005). Antiulcerogenic effect of Zataria multiflora Boiss. on cysteamine induced duodenal ulcer in rats. Iran. J. Pharm. Res. 1, 223–229. [Google Scholar]
- Molet S., Hamid Q., Davoineb F., Nutku E., Tahaa R., Pagé N., et al. (2001). IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J. Allergy Clin. Immunol. 108, 430–438. 10.1067/mai.2001.117929 [DOI] [PubMed] [Google Scholar]
- Mostafavi B., Shasavari S. (2005). The effect of Zataria on respiratory disorders of chemical war victims. Behbood J. 7, 293–297. [Google Scholar]
- Mozaffarian V. (1996). A Dictionary of Iranian Plant Names: Latin, English, Persian. Tehran: Farhang Mo'aser. [Google Scholar]
- Nakhai L. A., Mohammadirad A., Yasa N., Minaie B., Nikfar S., Ghazanfari G., et al. (2007). Benefits of Zataria multiflora Boiss in experimental model of mouse inflammatory bowel disease. J. Evid. Based Complementary Altern. Med. 4, 43–50. 10.1093/ecam/nel051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owlia P., Pirveicy H., Saderi H., Rezvani M., Mansouri S. (2004). Evaluation of the antimicrobial effects of extract of Zataria multiflora against oral Streptococci. Iran. J. Pharm. Res. 3, 74–75. [Google Scholar]
- Provoost S., Maes T., Van Durme Y., Gevaert P., Bachert C., Schmidt-Weber C., et al. (2009). Decreased FOXP3 protein expression in patients with asthma. Allergy 64, 1539–1546. 10.1111/j.1398-9995.2009.02056.x [DOI] [PubMed] [Google Scholar]
- Rahmani M., Afshari H., Dahesht A. E., Vaskas A. T., Nasiri D. (2012a). Evaluating the antimicrobial effect of Zataria multiflora essential oil on E. coli 0157: H7 in MDM (mechanical deboned meat) on different days of storage in refrigerator. J. Pure Appl. Microbiol. 6, 653–658. [Google Scholar]
- Rahmani M., Afshari H., Dahesht A. E., Vaskas A. T., Nasiri D. (2012b). Evaluating the antimicrobial effects of Zataria multiflora essential oils on bacterial growth of Listeria monocytogenes in roast-chicken fillets. J. Pure Appl. Microbiol. 6, 577–582. [Google Scholar]
- Ray A., Cohn L. (1999). Th2 cells and GATA-3 in asthma: new insights into the regulation of airway inflammation. J. Clin. Investig. 104, 985–993. 10.1172/JCI8204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renauld J. C. (2001). New insights into the role of cytokines in asthma. J. Clin. Pathol. 54, 577–589. 10.1136/jcp.54.8.577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem M., Nazli R., Afza N., Sami A., Shaiq Ali M. (2004). Biological significance of essential oil of Zataria multiflora Boiss. Nat. Prod. Res. 18, 493–497. 10.1080/14786410310001608064 [DOI] [PubMed] [Google Scholar]
- Salmon M., Walsh D. A., Huang T. J., Barnes P. J., Leonard T. B., et al. (1999). Involvement of cysteinyl leukotrienes in airway smooth muscle cell DNA synthesis after repeated allergen exposure in sensitized Brown Norway rats. Br. J. Pharmacol. 127, 1151–1158. 10.1038/sj.bjp.0702669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temelkovski J., Hogan S. P., Shepherd D. P., Foster P. S., Kumar R. K. (1998). An improved murine model of asthma: selective airway inflammation, epithelial lesions and increased methacholine responsiveness following chronic exposure to aerosolised allergen. Thorax 53, 849–856. 10.1136/thx.53.10.849 [DOI] [PMC free article] [PubMed] [Google Scholar]