Abstract
Among the endodermal tissues of adult mammals, the gastrointestinal (GI) epithelium exhibits the highest turnover rate. As the ingested food moves along the GI tract, gastric acid, digestive enzymes, and gut resident microbes aid digestion as well as nutrient and mineral absorption. Due to the harsh luminal environment, replenishment of new epithelial cells is essential to maintain organ structure and function during routine turnover and injury repair. Tissue-specific adult stem cells in the GI tract serve as a continuous source for this immense regenerative activity. Tissue homeostasis is achieved by a delicate balance between gain and loss of cells. In homeostasis, temporal tissue damage is rapidly restored by well-balanced tissue regeneration, whereas prolonged imbalance may result in diverse pathologies of homeostasis and injury repair. Starting with a summary of the current knowledge of GI tract homeostasis, we continue with providing models of acute injury and chronic diseases. Finally, we will discuss how primary organoid cultures allow new insights into the mechanisms of homeostasis, injury repair, and disease, and how this novel 3D culture system has the potential to translate into the clinic.
Homeostasis
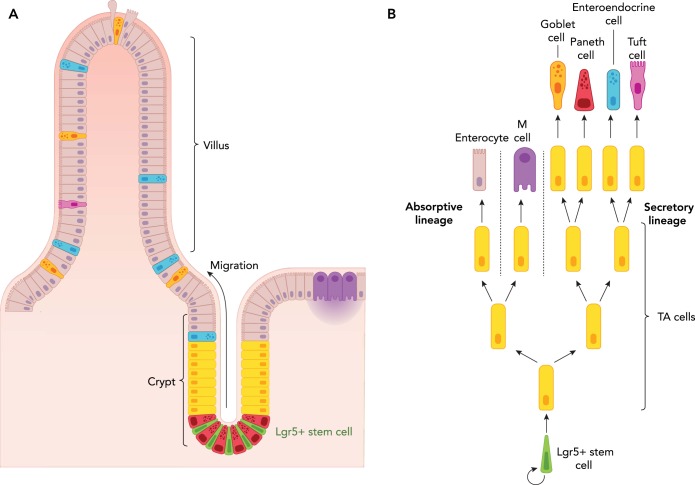
Homeostasis was first described in 1854 as “the fixity of the milieu supposes a perfection of the organism such that the external variations are at each instant compensated for and equilibrated” (8). Today, in the field of adult stem cell biology, homeostasis is defined as the fine balance between self-renewal and cell loss caused by differentiation, apoptosis, as well as environmental tissue damage. Adult stem cell homeostasis, plasticity, and regeneration have been extensively studied in the small intestinal epithelium, since it represents an ideal model system due to its robust regeneration and simple, repetitive architecture. The small intestine is a tube-shaped structure, of which the inner wall is lined by a single layer of epithelium folded into distinct units. Each unit can be subdivided into a villus, protruding into the gut lumen and containing differentiated cells responsible for digestion and absorption, and crypt of Lieberkühn, harboring intestinal stem cells (ISCs), progenitors, and terminally differentiated Paneth cells (14). At the base of each crypt, ~15 stem cells (71) divide daily to renew the epithelial tissue. As a result of the limited niche space in the crypt base, only about half of the newly generated daughter stem cells can remain as ISCs. The other half is pushed upward from the crypt bottom and differentiates into transit-amplifying (TA) progenitors, which undergo a few rounds of rapid cell division before differentiating into functional cell types while migrating upward along the crypt-villus axis. Finally at the villus tip, the terminally differentiated cells are shed into the lumen (46) (FIGURE 1). This conveyor belt-like migration was first described by Leblonde (12). ISC self-renewal and differentiation is governed by a complex signaling network encompassing Wnt, Notch, EGF, and BMP signaling to ensure the production of an adequate number of cells during various conditions, such as homeostasis and injury repair.
FIGURE 1.
Architecture of the small intestine
A: intestinal Lgr5+ stem cells (green) are located at the bottom of the intestinal crypt in between Paneth (niche) cells (magenta). Following stem cell division, the progenitors migrate upward in a conveyor belt-like manner to undergo a few rounds of rapid division before differentiating into functional cell types. On reaching the tip of the villi, the terminally differentiated cells are shed into the lumen. B: schematic drawing illustrating how division of Lgr5+ stem cells give rise to progenitor transit amplifying (TA) cells, which then differentiate into either the secretory or absorptive lineage.
A number of studies proved Wnt signaling to be essential for crypt homeostasis (37, 39, 61), and the search for Wnt target genes expressed in colon cancer and crypts led to the identification of Lgr5 (Leucin-rich repeat-containing G-protein-coupled receptor 5) as a marker specific for ISCs, the crypt base columnar (CBC) cells (76). A genetic approach was taken to prove the stem cell identity of these cells: a genetic tracing experiment for lineage identification (lineage tracing) performed in a mouse with Lgr5EGFP-ires-CreERT2 and R26R-lacZ Cre reporter alleles (5) revealed that the CBCs display both stem cell characteristics: long-term self-renewal and multipotency. A follow-up study combining Lgr5EGFP-ires-CreERT2 with a multicolor reporter (R26R-Confetti) showed that Lgr5-positive (Lgr5+) stem cells divide symmetrically, with the fate of each daughter cell being unpredictable, since all stem cell clones within a single crypt have an equal chance to outcompete the others and generate a monoclonal crypt (71). This somewhat surprising outcome was independently confirmed by the Winton group (43). Although this phenomenon known as “Neutral drift” holds true at a population level, intravital imaging of the Lgr5eGFP-Ires-CreERT2, R26R-Confetti mice revealed that, at the cellular level, Lgr5+ stem cells close to the bottom and center of the crypt have a higher chance to remain in the stem cell zone compared with those at the border (83). These findings suggest the “proximity” of ISCs to their niche at the crypt base to be an important factor in regulating stem cell maintenance and differentiation.
The intestinal stem cell niche is located at and around the crypt bottom, and consists of Paneth cells (epithelial niche) and underlying stromal cells (stromal niche). Both epithelial and stromal niches supply ISCs with EGF and Wnt ligands and show apparent redundancy in genetic studies (20, 53). Interestingly, the Notch ligand Dll4 is exclusively provided by the epithelial niche, the Paneth cells (20). Notch ligands are juxtaposed factors that work via cell-to-cell interaction. Notch signaling thus represents another key factor promoting stemness since physical contact with a Paneth cell is essential for the maintenance of ISCs. Moreover, the genetic depletion of Notch ligands produced by Paneth cells (Dll4) and secretory progenitor cells (Dll1) (60, 72), as well as Mib1, an E3 ligase necessary for Notch ligand activation (36), causes massive differentiation of ISCs and TA cells into secretory goblet cells (18, 50). Thus Notch signaling plays a crucial role in stem cell maintenance and lineage specification by balancing the generation of the absorptive vs. the secretory lineage. This balance provides insights into why ablation of Paneth cells does not result in long-term perturbation of homeostasis (7, 23, 24, 55, 70). Paneth cell depletion leaves ISCs in an environment without Notch signaling activation but in the presence of high levels of Wnt supplied from the underlying stroma. This condition promotes ISC differentiation toward the Paneth cell lineage (3, 20). Once new Paneth cells are differentiated from ISCs, they can provide Notch ligands to “reinstate” the stem cells and regain crypt homeostasis. Conversely, Paneth cell overproduction leads to an increase in available Paneth cell surface, carrying high-density Notch ligands. Thus, by touching Paneth cells, ISCs receive increased Notch signaling, attenuating the secretory cell production, i.e., Paneth cells (22, 79). This example displays a remarkable stability of the “signaling network” in regulating crypt homeostasis (FIGURE 2A).
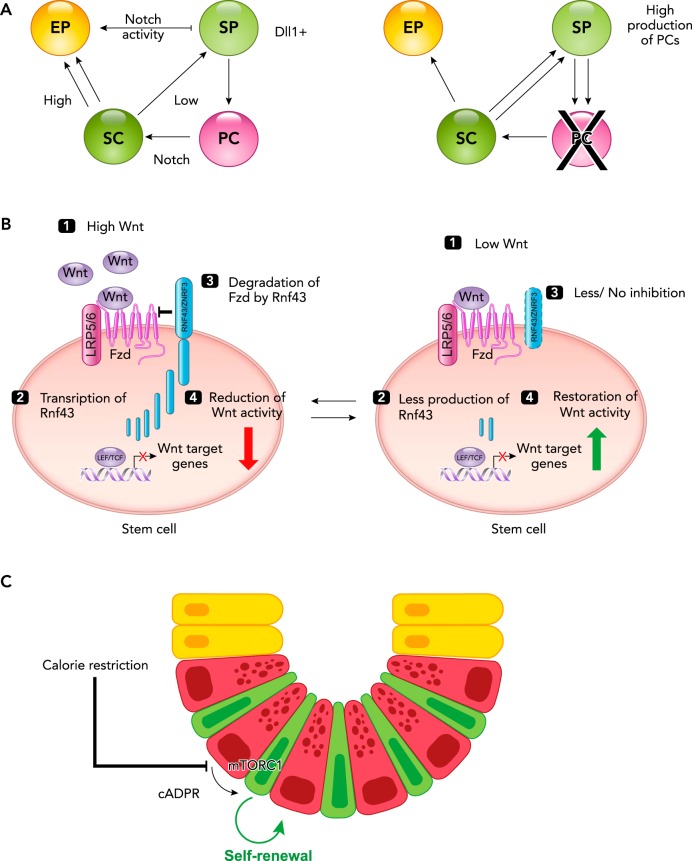
FIGURE 2.
Signaling in intestinal stem cells
A: Notch signaling in homeostasis (left) and in the absence of Paneth cells (right). Notch signaling plays a crucial role in stem cell (SC) maintenance and is supplied by the Paneth (niche) cells (PC). In the presence of Paneth cells (left), high levels of Notch signaling promote the daughter cell to adopt an absorptive progenitor fate (EP; enterocyte progenitor), whereas low levels of Notch signaling facilitate the formation of secretory progenitors (SP), some of which will differentiate into Paneth or enteroendocrine cells. In the absence of Paneth cells (right), the lack of Notch signaling promotes intestinal SC differentiation toward the secretory lineage, thus facilitating the generation of new Paneth cells. B: high (left) and low (right) levels of Wnt signaling. Left: high levels of Wnt signaling (1) leads to transcription of Rnf43 (a Wnt target gene; 2), which degrades the Wnt receptor Frizzled (3) and subsequently reduces the level of Wnt activity (4). Right: during low levels of Wnt signaling (1), the production of Rnf43 is reduced (2), and consequently there is less to no degradation of Frizzled (3), and the Wnt activity is enhanced. C: calorie restriction promotes intestinal stem cell renewal. Calorie restriction inhibits mTOR complex 1 (mTORC1) signaling in Paneth cells, resulting in the production of cyclic ADP ribose (cADPR). cADPR acts as a paracrine factor and promotes stem cell division.
The interaction between ISCs and Paneth cells include other master regulatory mechanisms for the crypt homeostasis, without which it can cause uncontrolled stem cell expansion or depletion. For example, RNF43 and its paralogue ZNRF3 are key regulatory molecules expressed by ISCs. RNF43 and ZNRF3 (RZ) are Wnt downstream target genes that act in a negative feedback loop by removing the Wnt receptor complex (Frizzled and LRP5/6) from the cell surface to the endo-lysosomal pathway for degradation (25, 35). Consequently, low levels of Wnt activity in ISCs result in downregulation of RZ, which leads to accumulation of Wnt receptors at the ISC surface, thus restoring the Wnt activity in ISCs. On the contrary, high Wnt activity induces RNF43 expression and thereby a downregulation of the Wnt receptor-mediated signaling cascade (FIGURE 2B). Simultaneous genetic ablation of these Wnt regulators (RZ) in the gut epithelium causes uncontrolled hyper-Wnt activity, resulting in stem cell hyperplasia (25, 35). Additionally, Paneth cells regulate the speed of ISC self-renewal under calorie restriction as attenuated mTOR complex 1 (mTORC1) activity triggers Paneth cells to produce cADPR to promote ISC self-renewal (78) (FIGURE 2C). Thus, besides its well-known role in gut immunity, Paneth cell plays another important master regulatory role in ISC homeostasis. Together, these examples demonstrate the importance of ISC regulation and how this is achieved via a signaling network acting on multiple levels with Paneth cells providing key regulatory molecules and spatial restriction to ISCs.
Acute Injury
Despite the presence of a robust signaling network governing intestinal crypt homeostasis, gut homeostasis can be challenged upon severe acute damage. In nature, this could be the loss of a large surface area of epithelium following a severe bacterial or viral infection. Compared with the other terminally differentiated cells, the ISCs and Paneth cells reside deep within the crypt base and are thereby less exposed to external damage. Both ISCs and Paneth cells work together to reinstate homeostasis by replenishing lost cells and secreting anti-bacterial peptides, respectively. As far as functional ISCs remain, the gut epithelium is able to restore its function eventually (FIGURE 3A). However, what happens if all Lgr5+ ISCs are affected?
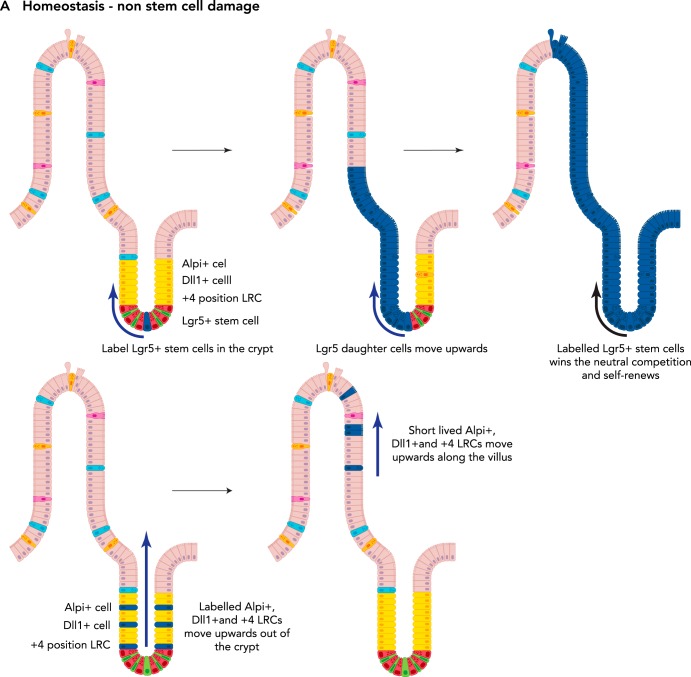
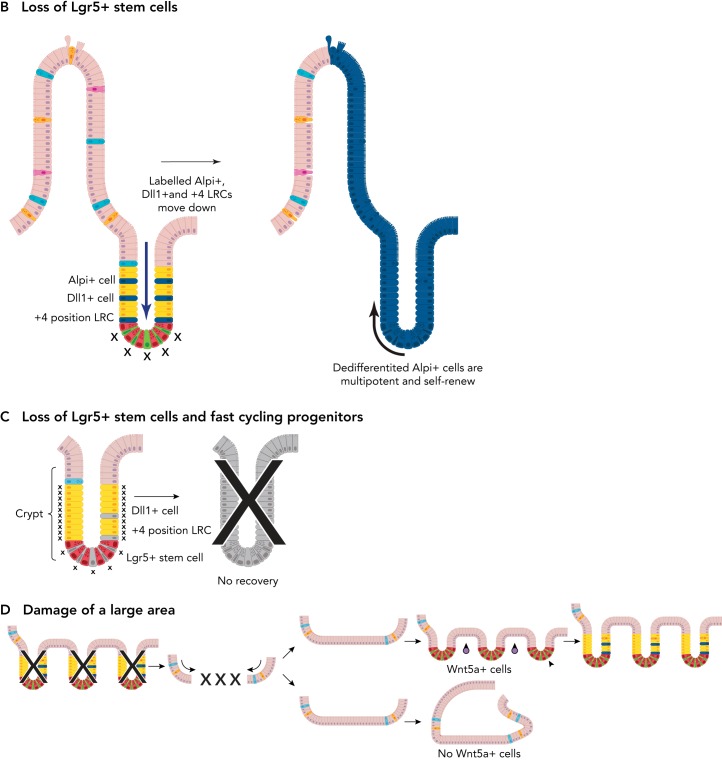
FIGURE 3.
Plasticity of the intestinal epithelium upon damage
A: during homeostasis, the Lgr5+ stem cells (top) self-renew and give rise to all different cell types of the intestinal epithelium. Thus, at a later time point following labeling of an Lgr5+ stem cell, the whole crypt and villus will consist of its progeny (as long as the labeled Lgr5+ stem cell wins the “neutral competition” against the other unlabelled stem cell clones). Label-retaining cells (LRCs), Dll1+ progenitors, and Alpi+ progenitors migrate upward along the crypt villus axis under homeostatic conditions (bottom). They differentiate and are eventually lost; hence no tracing can be observed from these labeled cells. B: upon loss of Lgr5+ stem cells, LRCs, Dll1+ progenitors, and Alpi+ progenitors can dedifferentiate into a stem cell-like state and replace the lost Lgr5+ stem cells. C: upon loss of Lgr5+ stem and progenitor cells as well as rapidly dividing Lgr5- progenitors, the intestinal epithelium cannot recover. D: following loss of multiple crypts in the colonic epithelium, cells originating from the adjacent tissue migrate to quickly seal the wound. Then, the presence of Wnt5a-positive cells (purple circles) in the underlying mesenchyme stimulate crypt regeneration. In the absence of Wnt5a+ cells, no new crypts are formed.
The complete loss of Lgr5+ stem cells can be induced by a sublethal dose of radiation (19) or by the use of a transgenic mouse model where the diphtheria toxin receptor (DTR) is specifically expressed under the Lgr5 promoter (75). Since murine cells do not express DTR, diphtheria toxin (DT) administration specifically ablates Lgr5+ stem cells expressing DTR. Surprisingly, ablation of Lgr5+ stem cells did not show long-term effects on crypt homeostasis (75). For a long time, the predominant hypothesis was that a postulated quiescent stem cell population located at the +4 position in the crypt acted as a reserve stem cell (4, 13) population. The “+4 cells” were first identified by Potten et al. by their label-retaining ability, and several +4 cell-specific markers were proposed (57, 62, 63). However, subsequent studies revealed that these markers are co-expressed by Lgr5+ stem cells, and some were found to be scattered along the crypt-villus axis (57). This led to the question whether +4 cells constitute a separate stem cell population or are mere descendants of the Lgr5+ CBC cells.
To address these seemingly contradictory hypotheses, Buczacki et al. used an elegant, unbiased, marker-free approach, showing that these label-retaining (LR) +4 cells represent secretory progenitors able to revert back (dedifferentiate) to a stem cell-like state upon the loss of Lgr5+ CBC cells (11) (FIGURE 3B). Additional observations have shown that Dll1high secretory progenitors as well as primed enterocyte-lineage progenitors share this ability (19, 74) (FIGURE 3B). This dedifferentiation capability of otherwise committed progenitors reverting into a stem cell state is termed “plasticity.” Again, Paneth cells and the underlying stromal niche play important roles in this remarkable regenerative activity of intestinal epithelium. Under homeostatic conditions, committed progenitors lose their contact to stem cell niches as they migrate upward along the crypt-villus axis. Thus, even if committed early progenitors retain plasticity, they eventually commit to full differentiation as they migrate out from their niche environments. Following ISC loss, committed progenitors can “fall back” into the crypt base and once within the niche environment become ISCs again (FIGURE 3B). The dedifferentiation process involves transit of the progenitor cells in the opposite direction of the normal flow of cells, such that they settle back into the crypt base, where they can keep in direct contact with Paneth cells. Paneth cells provide important niche factors such as Notch ligand, EGF, and Wnt3. The Paneth cell-secreted Wnt3 has been identified as a crucial factor for Dll1high secretory progenitors to regain stemness using in vitro organoid experiments (19). The importance of the environment is further strengthened by the data from Kim et al., showing that the epigenetic status remains almost unchanged upon ISC differentiation. The permissive chromatin state likely contributes to the high level of plasticity of the committed progenitors, whose identity strongly depends on the environment rather than cell-intrinsic features (34).
Metcalfe et al. have shown that applying both radiation or DT-mediated Lgr5+ depletion led to complete loss of regeneration in the intestinal epithelium (48). Only in this artificial condition, the regenerative capacity of the intestinal epithelium can be suppressed by absence of Lgr5 stem cells. Upon radiation-induced loss of proliferative Lgr5+ and TA cells, slow-cycling secretory progenitors regain stemness to become Lgr5+ stem cells. Simultaneous and subsequent DT treatments are thought to negatively affect these plastic progenitors while regaining Lgr5 expression (FIGURE 3C). This result demonstrates the importance of proliferative ISCs as well as plastic slow-cycling progenitors to achieve robust regeneration of intestinal epithelium.
The repair mechanism of larger areas of the intestinal epithelium is not well understood. Damage, involving the loss of whole epithelial tissue including both crypts and villi, requires either de novo crypt formation or crypt fission to reinstate tissue homeostasis. Seno et al. established an endoscopic method for generating oval-shaped epithelial wounds (51) by the excision of ∼1-mm2 areas from the inner lining of mouse colons. The first response of the epithelia to this type of injury is to rapidly cover the wounded area with a transient type of flattened, non-proliferative epithelium [wound-associated epithelial (WAE) cells] to quickly reestablish the epithelial barrier. The absence of WAE results in inefficient repair of the epithelia, showing that they play an important role in the regeneration process. Formation of the WAE cells is mediated by prostaglandin E2 (PGE2) signaling (52), which is produced by mesenchymal cells (10, 44, 45) as a result of injury-induced signaling, potentially by TLR/Myd88 (10, 45). Following secretion, PGE2 binds to its receptor Ptger4 on stem and progenitor cells, thereby promoting their differentiation into WAEs (52). Also at this stage of repair, the relationship between the gut microbiota and ISCs seems to play a protective role. The soluble metabolite butyrate has the ability to suppress ISC proliferation via Foxo3-mediated cell-cycle regulation and is under homeostatic conditions metabolized by colonocytes to prevent it from coming into contact with ISCs. However, upon mucosal injury, the contact of ISCs with butyrate protects them by reducing their chance of acquiring cancerous mutations, which may otherwise occur during division in genotoxic environments (33). Mutations in PTGER4 and single nucleotide polymorphisms (SNPs) in Foxo3 are frequently occurring in IBD (33, 52). Following this initial phase of repair, adjacent crypts elongate and form extensions toward the center of the wound bed. These wound-responsive crypts finally undergo fission events to replace the lost crypts in the wounded area (FIGURE 3D). Interestingly, noncanonical Wnt, Wnt5a, was found to be important for proper crypt fission during this wound-healing process (51). Thus both canonical (Wnt3 secreted by Paneth cells) and noncanonical Wnt (Wnt5a produced by mesenchymal cells) play a role during epithelial injury repair.
This new observation provides clues to how new crypts are formed to repair damaged epithelia. However, many questions remain. For example, it is still not known whether Lgr5+ cells in the newly generated crypts are directly derived from Lgr5+ cells of adjacent crypts or whether they originate from primed progenitors having undergone dedifferentiation to regain Lgr5+ stem cell state in the new crypts. Moreover, we do not know what signals instruct the stem cells to initiate the repair program and how Wnt5a+ mesenchymal cells are recruited from the serosa to the wound site. Also, the proposed mesothelial origin of these Wnt5a+ cells is another interesting point for further studies.
Chronic Damage
Given the critical role for intestinal epithelial cell function in health, it is perhaps not surprising that conditions disrupting homeostasis have been linked to the development of several intestinal conditions. Among them are chronic inflammatory bowel diseases (IBD) such as Crohn’s disease and ulcerative colitis (UC). Both conditions are defined by chronic relapsing inflammation, which can affect any part of the digestive tract in CD and, in the case of UC, is generally restricted to the large bowel. Although disease pathogenesis remains only partly understood, a degree of genetic predisposition, environmental triggers, the intestinal microbiome, and an altered immune response are among the most widely accepted key factors contributing to the development of IBD. As a multi-factorial, complex disease, it is highly likely that alterations in the function of more than one cell type leads to the wide variety of phenotypes currently summarized as IBD. However, substantial evidence points toward a critical role for the intestinal epithelium to play an important part.
For example, large-scale, genome-wide association studies (GWAS) have identified over 200 single nucleotide polymorphisms (SNPs), which predispose to the development of IBD (31, 41). Among them are several that implicate the involvement of intestinal epithelium. Examples include SNPs located in the region of nucleotide-binding oligomerization domain containing 2 (NOD2) gene. NOD2 is known to play a critical role as pattern recognition receptor in sensing bacterial muramyl dipeptide (MDP) followed by activation of innate defense response. Although our knowledge of the exact mechanistic effects of NOD2 mutations in patients with IBD remains incomplete, impaired function of Paneth cells, specifically in terms of the production of alpha-defensins, has been proposed (40, 58, 64, 82). In fact, IBD-predisposing genetic variants in the transcription factor XBP1 gene have provided further evidence for altered Paneth cells and intestinal epithelial function by demonstrating an effect on ER stress both in Paneth cells as well as in the intestinal epithelium (68).
Another important cellular mechanism, which has been highlighted by IBD GWAS and known to be important for the intestinal epithelium is autophagy. Several studies have linked variants in the autophagy-related 16-like 1 (ATG1L1) gene to the development of CD. Moreover, functional studies in both mice and humans have provided evidence that ATG1L1 mutations can lead to altered Paneth cell function and may even be involved in the initiation of the inflammatory cascade (1, 16). As mentioned above, given the important role of Paneth cells in the maintenance and homeostasis of the intestinal epithelial stem cell compartment, it seems plausible to speculate that altered Paneth cell function may impact on stem cell behavior and/or vice versa contribute to chronic inflammation.
There are other loci found in IBD GWAS studies strongly implied in epithelial barrier function, such as CDH1 (E-cadherin) and HNF4A. As described above, the intestinal epithelium maintains a large number of stem cells for rapid regeneration of the tissue, ensuring the integrity of the single-layer epithelium, acting as a physical barrier separating the microbiota and foreign antigens in the luminal area from immune cells in the lamina propria. The tight connection between epithelial cells is mediated through adherens junctions (AJs), tight junctions (TJs), and desmosomes, and is essential for barrier formation. E-caderine is an AJ component. Intestine-specific deletion of E-cadherin results in increased permeability (56), and intestinal-specific expression of a dominant negative form of N-cadherin causes inflammatory responses similar to those of CD (27). HNF4α expression is significantly downregulated in CD and UC patients. Hnf4α mutant mice exhibit increased intestinal permeability and higher vulnerability in the DSS-induced colitis model (2, 6). Mice carrying an activated form of the inflammatory cytokine IL-6 receptor display severe Paneth (defensin production) and goblet cell (mucous production) deficiency, but the concomitant increase in proliferation and generation of enterocyte progenitors is enough to maintain the epithelial barrier, thereby showing the importance of barrier integrity to prevent microbial translocation (73).
Epigenetic mechanisms known to be operating at the interface between the human genome and the environment are increasingly being considered as playing a vital role in the development of multi-factorial diseases, including IBD (30). Interestingly, DNA methylation has been shown to play a role in regulating cellular function in the intestinal epithelium (32, 69). Moreover, recent studies have demonstrated the important role of postnatal bacterial colonization in the functional maturation of the intestinal epithelium, which seems to be at least in part mediated through changes in DNA methylation (80). These findings suggest that intestinal epithelial cell-microbial cross talk is likely to impact on cellular function by modulating the epigenetic signature. Hence, it seems plausible to speculate that alterations in this process resulting in changes to the epigenetic program may lead to the development of IBD. Indeed, altered DNA methylation patterns have been observed in purified primary intestinal epithelium obtained from children newly diagnosed with IBD compared with cells derived from healthy, non-IBD controls (38).
Despite the rapidly increasing evidence pointing toward a critical role of the intestinal epithelium in IBD, until recently the field has been suffering from the lack of a suitable human-based experimental model. The current development of primary intestinal organoid culture merits our attention as a novel disease-modeling platform, where the direct effect on the intestinal epithelium can be investigated without other direct and indirect effects from the microbiota or immune cells. Moreover, this new platform provides an ideal reductionist approach since it allows modular incorporation of other components (e.g., bacterial components, cytokines, growth factors, and immune cells) of disease pathogenesis in a controlled manner. An excellent example of this approach was recently reported by the group of James Wells, where coculture of developing human pluripotent cell-derived organoids and neural crest cells resulted in intestinal tissue with a functional enteric nervous system (ENS) (77). Next, we will discuss the uses and applications of intestinal and colonic organoids.
The Use of Organoids to Enhance Tissue Injury Repair and Relieve Chronic Disease Symptoms
Due to their long-term self-renewal and multipotency, adult stem cells (ASCs) represent a potential source of transplantable cells for regenerative medicine and gene therapy. However, this requires the ability to culture these ASCs ex vivo, an idea that for a long time was regarded as nonfeasible (26) for any organ except the skin (29, 54). In 2009, two studies proved this to be possible. Single adult small intestinal stem cells were isolated from murine crypts and, in combination with an extracellular matrix and a cocktail of growth factors mimicking the in vivo niche, were cultured continuously for more than a year (59, 66). These cultured ISCs not only retained their proliferative potential but gave rise to all major epithelial cell types and self-organized into “mini-guts” showing budding crypt-like structures (66). Here, we will focus on ASC-derived intestinal organoids of mouse and human (65, 66), and on their current and potential applications (FIGURE 4).
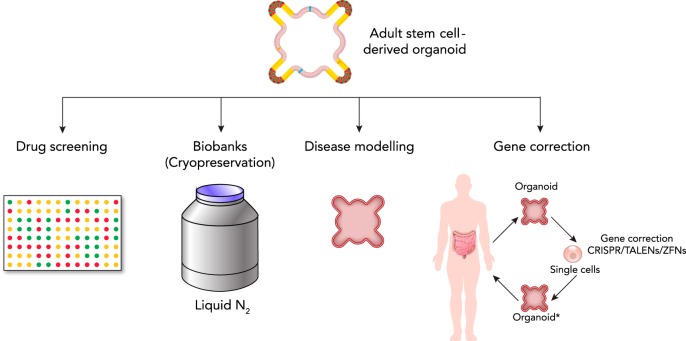
FIGURE 4.
Uses and applications of adult stem cell-derived organoids
The potential uses and applications of adult stem cell-derived organoids are many. For example, they can be used for drug screening of new and already existing drugs, and be cryopreserved to form a biobank that can be used for the purpose of basic science and translational medicine. Moreover, they can be used for disease modeling, either by gene editing or by derivation from human patients. In the future, they might have the potential to be used in personalized gene therapy, e.g., correction of the genetic defect in patient-derived organoids, and then transplantation back into the patient. *Genetic defect has been corrected.
The adult intestinal stem cell drives homeostatic tissue turnover and injury repair. The entire epithelium renews every 5 days. Thus, in chronic diseases like IBD or cystic fibrosis (CF), the persisting failure of regeneration is likely to involve stem cell failure or dysfunction, although the pathogenesis of these diseases are complicated and multifactorial. If epithelial dysfunction plays a crucial role as proposed above, it is probable to assume that the dysfunctional stem cells have acquired genetic mutations and/or altered epigenetic traits. It has been technically difficult to isolate pure epithelia from tissue biopsies. Thus exact molecular changes in the affected epithelium have not been thoroughly investigated.
Here, human intestinal and colonic organoid culture technology provides an attractive epithelial culture model for studying GI tract diseases. Using this new technology, epithelium of isolated biopsies can be cultured in vitro as a pure epithelium. In a sterile condition, ISCs in the cultured epithelium drives the expansion of the epithelial tissue, whereas stromal and immune cells fail to propagate. After several rounds of passages, a pure epithelial culture can be established in a few weeks that allows subsequent molecular analysis. This approach has been applied to several genetic diseases (9, 67) as well as colon cancer (65). As the organoid culture method is confirmed to maintain a high level of genetic stability, the original genetic alterations are well retained in established organoids derived from patient material. Thus organoid culture enables rapid investigation of genetic alteration as well as long-term cryopreservation for future studies (FIGURE 4). It is also known that organoids from different gut segments retain their original regional identity and expression profile (49). This implies that the ISCs of organoid retain their genetic and epigenetic identity ex vivo. Therefore, aberrant epigenetic marks caused by severe inflammation and/or infection can also be retained in vitro, allowing further molecular studies of the pertinent diseases.
By collecting and cryopreserving a large number of patient and healthy donor material, a live biobank of organoids can be established (FIGURE 4). Such biobanking will facilitate biomedical and clinical studies by serving as a platform in which new hypotheses can be tested. For example, CF patient-derived organoids have been used in a functional swelling assay with forskolin treatment. Organoids lacking Phenylalanine 508 (F508) in the CFTR gene failed to swell on forskolin treatment due to the absence of CFTR channel activity on the cell surface (15). Using CRISPR/Cas genome engineering technology, the deletion of F508 in the CFTR coding sequence was corrected in CF patient-derived organoids, enabling them to respond to forskolin treatment (67). This precise genetic confirmation proves that the lack of forskolin responsiveness is solely caused by the deletion of a single amino acid in the CFTR gene. Another example of the application of organoid culture is disease modeling, where colon cancer organoids were generated using a minimum set of genetic alterations. Again using CRISPR/Cas genome engineering, two groups showed that mutations in APC, KRAS, SMAD4, and TP53 are sufficient to transform a normal organoid to exhibit adenocarcinoma characteristics (17, 47). Since the CRISPR/Cas technology can modify genetic information as well as epigenetic marks, organoids can be used to test the consequence of diverse genetic and epigenetic alterations in the gut epithelium (28, 42) (FIGURE 4).
For detrimental epithelial damage caused by a genetic disease or by infection/inflammation like IBD, autologous cell therapy would be preferred over surgical dissection of a large portion of the intestine. Replacement of the severely affected epithelium with healthy tissue while suppressing the hyper-immune activity may significantly alleviate the disease symptom and might even represent a curative approach. For this purpose, the intestinal and colonic organoid culture again provides a promising opportunity. Both types of human organoids can be expanded three- to fivefold every week for more than 6 months, resulting in enormous expansion of the primary cell source for transplantation. A proof-of-concept where murine colonic organoids were introduced into mice with DSS-induced colitis showed that not only did the organoids localize to the injured areas but they engrafted and remained as a transplant with proper differentiation (81). Importantly, no tumor formation was observed as the organoids preserved their tissue identity. Moreover, based on the faster recovery of body weight in organoid-transplanted mice after DSS-induced colitis, the therapeutic potential of organoids seems significant. Additionally, fetal-derived organoids transplanted into mice with DS-induced colonic injury successfully engrafted, and a subset of cells differentiated into goblet cells (21). For autologous cell transplantation, organoids can be isolated from the unaffected area of the epithelium or may be genetically engineered to remove disease-causing elements (FIGURE 4).
The past decade has brought major advances in the gastrointestinal field, largely driven by the discovery of Lgr5+ intestinal stem cells. Although much knowledge has already been gained, new and old unanswered questions remain. Organoid culture is a rapidly evolving technology that potentially helps to solve many of our current challenges and questions. In this review, we have described the regulatory signaling network of stem cells and plasticity of committed progenitors that together ensure epithelial tissue homeostasis during everyday loss and acute injury. The repair process of larger areas of epithelium and the potential importance of epithelium in chronic gut inflammatory diseases have also been highlighted. Last, a number of potential applications of intestinal and colonic human organoids were discussed with several milestone examples. We hope that the technical developments of 3D organoid culture systems and genome engineering, as well as the accumulating knowledge, will benefit basic science and translational research.
Footnotes
We are grateful to Dr. Christopher J. Hindley and Lawrence Bates for critical reading of the manuscript.
H.C. is supported by the Cancer Genomics Center (CGC) via The Netherlands Genomics Initiative/Netherlands Organisation for Scientific Research and the European Research Council (ERC). A.A-R. is supported by the Medical Research Council. B-K.K. is supported by supported by a Sir Henry Dale Fellowship from the Wellcome Trust and the Royal Society (101241/Z/13/Z), and the ERC starting grant, and receives a core support grant from the Wellcome Trust and MRC to the WT-MRC Cambridge Stem Cell Institute. M.Z. is supported by the Evelyn Trust, Crohn's in Childhood Research Association (CICRA), and Crohn's and Colitis in Childhood (3Cs) charity.
No conflicts of interest, financial or otherwise, are declared by the author(s).
Author contributions: A.A.-R. prepared figures; A.A.-R., M.Z., and B.-K.K. drafted manuscript; A.A.-R., M.Z., B.-K.K., and H.C. edited and revised manuscript; A.A.-R., M.Z., B.-K.K., and H.C. approved final version of manuscript.
References
- 1.Adolph TE, Tomczak MF, Niederreiter L, Ko H-J, Böck J, Martinez-Naves E, Glickman JN, Tschurtschenthaler M, Hartwig J, Hosomi S, Flak MB, Cusick JL, Kohno K, Iwawaki T, Billmann-Born S, Raine T, Bharti R, Lucius R, Kweon M-N, Marciniak SJ, Choi A, Hagen SJ, Schreiber S, Rosenstiel P, Kaser A, Blumberg RS. Paneth cells as a site of origin for intestinal inflammation. Nature 503: 272–276, 2013. doi: 10.1038/nature12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahn S-H, Shah YM, Inoue J, Morimura K, Kim I, Yim S, Lambert G, Kurotani R, Nagashima K, Gonzalez FJ, Inoue Y. Hepatocyte nuclear factor 4alpha in the intestinal epithelial cells protects against inflammatory bowel disease. Inflamm Bowel Dis 14: 908–920, 2008. doi: 10.1002/ibd.20413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andreu P, Peignon G, Slomianny C, Taketo MM, Colnot S, Robine S, Lamarque D, Laurent-Puig P, Perret C, Romagnolo B. A genetic study of the role of the Wnt/beta-catenin signalling in Paneth cell differentiation. Dev Biol 324: 288–296, 2008. doi: 10.1016/j.ydbio.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 4.Bach SP, Renehan AG, Potten CS. Stem cells: the intestinal stem cell as a paradigm. Carcinogenesis 21: 469–476, 2000. doi: 10.1093/carcin/21.3.469. [DOI] [PubMed] [Google Scholar]
- 5.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449: 1003–1007, 2007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 6.Barrett JC, Lee JC, Lees CW, Prescott NJ, Anderson CA, Phillips A, Wesley E, Parnell K, Zhang H, Drummond H, Nimmo ER, Massey D, Blaszczyk K, Elliott T, Cotterill L, Dallal H, Lobo AJ, Mowat C, Sanderson JD, Jewell DP, Newman WG, Edwards C, Ahmad T, Mansfield JC, Satsangi J, Parkes M, Mathew CG, Donnelly P, Peltonen L, Blackwell JM, Bramon E, Brown MA, Casas JP, Corvin A, Craddock N, Deloukas P, Duncanson A, Jankowski J, Markus HS, Mathew CG, McCarthy MI, Palmer CNA, Plomin R, Rautanen A, Sawcer SJ, Samani N, Trembath RC, Viswanathan AC, Wood N, Spencer CCA, Barrett JC, Bellenguez C, Davison D, Freeman C, Strange A, Donnelly P, Langford C, Hunt SE, Edkins S, Gwilliam R, Blackburn H, Bumpstead SJ, Dronov S, Gillman M, Gray E, Hammond N, Jayakumar A, McCann OT, Liddle J, Perez ML, Potter SC, Ravindrarajah R, Ricketts M, Waller M, Weston P, Widaa S, Whittaker P, Deloukas P, Peltonen L, Mathew CG, Blackwell JM, Brown MA, Corvin A, McCarthy MI, Spencer CCA, Attwood AP, Stephens J, Sambrook J, Ouwehand WH, McArdle WL, Ring SM, Strachan DP, Ring SM, Strachan DP; UK IBD Genetics Consortium; Wellcome Trust Case Control Consortium 2 . Genome-wide association study of ulcerative colitis identifies three new susceptibility loci, including the HNF4A region. Nat Genet 41: 1330–1334, 2009. doi: 10.1038/ng.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bastide P, Darido C, Pannequin J, Kist R, Robine S, Marty-Double C, Bibeau F, Scherer G, Joubert D, Hollande F, Blache P, Jay P. Sox9 regulates cell proliferation and is required for Paneth cell differentiation in the intestinal epithelium. J Cell Biol 178: 635–648, 2007. doi: 10.1083/jcb.200704152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernard C. Lectures on the Phenomena of Life Common to Animals and Plants. Springfield, IL: Charles C. Thomas, 1974. [Google Scholar]
- 9.Bigorgne AE, Farin HF, Lemoine R, Mahlaoui N, Lambert N, Gil M, Schulz A, Philippet P, Schlesser P, Abrahamsen TG, Oymar K, Davies EG, Ellingsen CL, Leteurtre E, Moreau-Massart B, Berrebi D, Bole-Feysot C, Nischke P, Brousse N, Fischer A, Clevers H, de Saint Basile G. TTC7A mutations disrupt intestinal epithelial apicobasal polarity. J Clin Invest 124: 328–337, 2014. doi: 10.1172/JCI71471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown SL, Riehl TE, Walker MR, Geske MJ, Doherty JM, Stenson WF, Stappenbeck TS. Myd88-dependent positioning of Ptgs2-expressing stromal cells maintains colonic epithelial proliferation during injury. J Clin Invest 117: 258–269, 2007. doi: 10.1172/JCI29159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buczacki SJ, Zecchini HI, Nicholson AM, Russell R, Vermeulen L, Kemp R, Winton DJ. Intestinal label-retaining cells are secretory precursors expressing Lgr5. Nature 495: 65–69, 2013. doi: 10.1038/nature11965. [DOI] [PubMed] [Google Scholar]
- 12.Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. Am J Anat 141: 537–561, 1974. doi: 10.1002/aja.1001410407. [DOI] [PubMed] [Google Scholar]
- 13.Chwalinski S, Potten CS, Evans G. Double labelling with bromodeoxyuridine and [3H]-thymidine of proliferative cells in small intestinal epithelium in steady state and after irradiation. Cell Tissue Kinet 21: 317–329, 1988. [DOI] [PubMed] [Google Scholar]
- 14.Clevers H. The intestinal crypt, a prototype stem cell compartment. Cell 154: 274–284, 2013. doi: 10.1016/j.cell.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Dekkers JF, Wiegerinck CL, de Jonge HR, de Jong NWM, Bijvelds MJC, Nieuwenhuis EES, van den Brink S, Clevers H, van der Ent CK, Middendorp S, Beekman JM. A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat Med 19: 939–945, 2013. doi: 10.1016/S1569-1993(12)60101-5. [DOI] [PubMed] [Google Scholar]
- 16.Deuring JJ, Fuhler GM, Konstantinov SR, Peppelenbosch MP, Kuipers EJ, de Haar C, van der Woude CJ. Genomic ATG16L1 risk allele-restricted Paneth cell ER stress in quiescent Crohn’s disease. Gut 63: 1081–1091, 2014. doi: 10.1136/gutjnl-2012-303527. [DOI] [PubMed] [Google Scholar]
- 17.Drost J, van Jaarsveld RH, Ponsioen B, Zimberlin C, van Boxtel R, Buijs A, Sachs N, Overmeer RM, Offerhaus GJ, Begthel H, Korving J, van de Wetering M, Schwank G, Logtenberg M, Cuppen E, Snippert HJ, Medema JP, Kops GJPL, Clevers H. Sequential cancer mutations in cultured human intestinal stem cells. Nature 521: 43–47, 2015. doi: 10.1038/nature14415. [DOI] [PubMed] [Google Scholar]
- 18.van Es JH, van Gijn ME, Riccio O, van den Born M, Vooijs M, Begthel H, Cozijnsen M, Robine S, Winton DJ, Radtke F, Clevers H. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature 435: 959–963, 2005. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- 19.van Es JH, Sato T, van de Wetering M, Lyubimova A, Nee AN, Gregorieff A, Sasaki N, Zeinstra L, van den Born M, Korving J, Martens ACM, Barker N, van Oudenaarden A, Clevers H. Dll1+ secretory progenitor cells revert to stem cells upon crypt damage. Nat Cell Biol 14: 1099–1104, 2012. doi: 10.1038/ncb2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farin HF, Van Es JH, Clevers H. Redundant sources of Wnt regulate intestinal stem cells and promote formation of Paneth cells. Gastroenterology 143: 1518–1529.e7, 2012. doi: 10.1053/j.gastro.2012.08.031. [DOI] [PubMed] [Google Scholar]
- 21.Fordham RP, Yui S, Hannan NRF, Soendergaard C, Madgwick A, Schweiger PJ, Nielsen OH, Vallier L, Pedersen RA, Nakamura T, Watanabe M, Jensen KB. Transplantation of expanded fetal intestinal progenitors contributes to colon regeneration after injury. Cell Stem Cell 13: 734–744, 2013. doi: 10.1016/j.stem.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fre S, Huyghe M, Mourikis P, Robine S, Louvard D, Artavanis-Tsakonas S. Notch signals control the fate of immature progenitor cells in the intestine. Nature 435: 964–968, 2005. doi: 10.1038/nature03589. [DOI] [PubMed] [Google Scholar]
- 23.Garabedian EM, Roberts LJJ, Mcnevin MS, Gordon JI. Examining the role of Paneth cells in the small intestine by lineage ablation in transgenic mice. J Biol Chem 272: 23729–23740, 1997. [DOI] [PubMed] [Google Scholar]
- 24.Geiser J, Venken KJT, De Lisle RC, Andrews GK. A mouse model of acrodermatitis enteropathica: loss of intestine zinc transporter ZIP4 (Slc39a4) disrupts the stem cell niche and intestine integrity. PLoS Genet 8: e1002766, 2012. doi: 10.1371/journal.pgen.1002766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hao HX, Xie Y, Zhang Y, Charlat O, Oster E, Avello M, Lei H, Mickanin C, Liu D, Ruffner H, Mao X, Ma Q, Zamponi R, Bouwmeester T, Finan PM, Kirschner MW, Porter JA, Serluca FC, Cong F. ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive manner. Nature 485: 195–200, 2012. doi: 10.1038/nature11019. [DOI] [PubMed] [Google Scholar]
- 26.Hayflick L. The limited in vitro lifetime of human diploid cell strains Exp Cell Res 37: 614–636, 1965. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- 27.Hermiston ML, Gordon JI. In vivo analysis of cadherin function in the mouse intestinal epithelium: essential roles in adhesion, maintenance of differentiation, and regulation of programmed cell death. J Cell Biol 129: 489–506, 1995. doi: 10.1083/jcb.129.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hilton IB, D’Ippolito AM, Vockley CM, Thakore PI, Crawford GE, Reddy TE, Gersbach CA. Epigenome editing by a CRISPR-Cas9-based acetyltransferase activates genes from promoters and enhancers. Nat Biotechnol 33: 510–517, 2015. doi: 10.1038/nbt.3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rheinwald JG, Green H; JG; H . Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell 6: 331–343, 1975. [DOI] [PubMed] [Google Scholar]
- 30.Jenke AC, Zilbauer M. Epigenetics in inflammatory bowel disease. Curr Opin Gastroenterol 28: 577–584, 2012. doi: 10.1097/MOG.0b013e328357336b. [DOI] [PubMed] [Google Scholar]
- 31.Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, Lee JC, Schumm LP, Sharma Y, Anderson CA, Essers J, Mitrovic M, Ning K, Cleynen I, Theatre E, Spain SL, Raychaudhuri S, Goyette P, Wei Z, Abraham C, Achkar J-P, Ahmad T, Amininejad L, Ananthakrishnan AN, Andersen V, Andrews JM, Baidoo L, Balschun T, Bampton PA, Bitton A, Boucher G, Brand S, Büning C, Cohain A, Cichon S, D’Amato M, De Jong D, Devaney KL, Dubinsky M, Edwards C, Ellinghaus D, Ferguson LR, Franchimont D, Fransen K, Gearry R, Georges M, Gieger C, Glas J, Haritunians T, Hart A, Hawkey C, Hedl M, Hu X, Karlsen TH, Kupcinskas L, Kugathasan S, Latiano A, Laukens D, Lawrance IC, Lees CW, Louis E, Mahy G, Mansfield J, Morgan AR, Mowat C, Newman W, Palmieri O, Ponsioen CY, Potocnik U, Prescott NJ, Regueiro M, Rotter JI, Russell RK, Sanderson JD, Sans M, Satsangi J, Schreiber S, Simms LA, Sventoraityte J, Targan SR, Taylor KD, Tremelling M, Verspaget HW, De Vos M, Wijmenga C, Wilson DC, Winkelmann J, Xavier RJ, Zeissig S, Zhang B, Zhang CK, Zhao H, Silverberg MS, Annese V, Hakonarson H, Brant SR, Radford-Smith G, Mathew CG, Rioux JD, Schadt EE, Daly MJ, Franke A, Parkes M, Vermeire S, Barrett JC, Cho JH; International IBD Genetics Consortium (IIBDGC) . Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491: 119–124, 2012. doi: 10.1038/nature11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaaij LTJ, van de Wetering M, Fang F, Decato B, Molaro A, van de Werken HJG, van Es JH, Schuijers J, de Wit E, de Laat W, Hannon GJ, Clevers HC, Smith AD, Ketting RF. DNA methylation dynamics during intestinal stem cell differentiation reveals enhancers driving gene expression in the villus. Genome Biol 14: R50, 2013. doi: 10.1186/gb-2013-14-5-r50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaiko GE, Ryu SH, Koues OI, Collins PL, Solnica-Krezel L, Pearce EJ, Pearce EL, Oltz EM, Stappenbeck TS. The colonic crypt protects stem cells from microbiota-derived metabolites. Cell 165: 1708–1720, 2016. doi: 10.1016/j.cell.2016.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim T-H, Li F, Ferreiro-Neira I, Ho L-L, Luyten A, Nalapareddy K, Long H, Verzi M, Shivdasani RA. Broadly permissive intestinal chromatin underlies lateral inhibition and cell plasticity. Nature 506: 511–515, 2014. doi: 10.1038/nature12903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koo B-K, Spit M, Jordens I, Low TY, Stange DE, van de Wetering M, van Es JH, Mohammed S, Heck AJR, Maurice MM, Clevers H. Tumour suppressor RNF43 is a stem-cell E3 ligase that induces endocytosis of Wnt receptors. Nature 488: 665–669, 2012. doi: 10.1038/nature11308. [DOI] [PubMed] [Google Scholar]
- 36.Koo BK, Lim HS, Song R, Yoon MJ, Yoon KJ, Moon JS, Kim YW, Kwon MC, Yoo KW, Kong MP, Lee J, Chitnis AB, Kim CH, Kong YY. Mind bomb 1 is essential for generating functional Notch ligands to activate Notch. Development 132: 3459–3470, 2005. doi: 10.1242/dev.01922. [DOI] [PubMed] [Google Scholar]
- 37.Korinek V, Barker N, Willert K, Molenaar M, Roose J, Wagenaar G, Markman M, Lamers W, Destree O, Clevers H. Two members of the Tcf family implicated in Wnt/beta-catenin signaling during embryogenesis in the mouse. Mol Cell Biol 18: 1248–1256, 1998. doi: 10.1128/MCB.18.3.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kraiczy J, Nayak K. Ross A, Raine T, Mak TN, Gasparetto M, Cario E, Rakyan V, Heuschkel R, Zilbauer M. Assessing DNA methylation in the developing human intestinal epithelium: potential link to inflammatory bowel disease. Mucosal Immunol 9: 1–12, 2015. doi: 10.1038/mi.2015.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuhnert F, Davis CR, Wang H-T, Chu P, Lee M, Yuan J, Nusse R, Kuo CJ. Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc Natl Acad Sci USA 101: 266–271, 2004. doi: 10.1073/pnas.2536800100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lala S, Ogura Y, Osborne C, Hor SY, Bromfield A, Davies S, Ogunbiyi O, Nuñez G, Keshav S. Crohn’s disease and the NOD2 gene: a role for paneth cells. Gastroenterology 125: 47–57, 2003. doi: 10.1016/S0016-5085(03)00661-9. [DOI] [PubMed] [Google Scholar]
- 41.Liu JZ, van Sommeren S, Huang H, Ng SC, Alberts R, Takahashi A, Ripke S, Lee JC, Jostins L, Shah T, Abedian S, Cheon JH, Cho J, Daryani NE, Franke L, Fuyuno Y, Hart A, Juyal RC, Juyal G, Kim WH, Morris AP, Poustchi H, Newman WG, Midha V, Orchard TR, Vahedi H, Sood A, Sung JJY, Malekzadeh R, Westra H-J, Yamazaki K, Yang S-K, Barrett JC, Franke A, Alizadeh BZ, Parkes M, B K T, Daly MJ, Kubo M, Anderson CA, Weersma RK; International Multiple Sclerosis Genetics Consortium; International IBD Genetics Consortium . Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet 47: 979–986, 2015. doi: 10.1038/ng.3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu XS, Wu H, Ji X, Stelzer Y, Wu X, Czauderna S, Shu J, Dadon D, Young RA, Jaenisch R. Editing DNA methylation in the mammalian genome. Cell 167: 233–247.e17, 2016. doi: 10.1016/j.cell.2016.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lopez-Garcia C, Klein AM, Simons BD, Winton DJ. Intestinal stem cell replacement follows a pattern of neutral drift. Science 330: 822–825, 2010. doi: 10.1126/science.1196236. [DOI] [PubMed] [Google Scholar]
- 44.Malvin NP, Seno H, Stappenbeck TS. Colonic epithelial response to injury requires Myd88 signaling in myeloid cells. Mucosal Immunol 5: 194–206, 2012. doi: 10.1038/mi.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Manieri NA, Drylewicz MR, Miyoshi H, Stappenbeck TS. Igf2bp1 is required for full induction of Ptgs2 mRNA in colonic mesenchymal stem cells in mice. Gastroenterology 143: 110–21.e10, 2012. doi: 10.1053/j.gastro.2012.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marshman E, Booth C, Potten CS. The intestinal epithelial stem cell. BioEssays 24: 91–98, 2002. doi: 10.1002/bies.10028. [DOI] [PubMed] [Google Scholar]
- 47.Matano M, Date S, Shimokawa M, Takano A, Fujii M, Ohta Y, Watanabe T, Kanai T, Sato T. Modeling colorectal cancer using CRISPR-Cas9-mediated engineering of human intestinal organoids. Nat Med 21: 256–262, 2015. doi: 10.1038/nm.3802. [DOI] [PubMed] [Google Scholar]
- 48.Metcalfe C, Kljavin NM, Ybarra R, de Sauvage FJ. Lgr5+ stem cells are indispensable for radiation-induced intestinal regeneration. Cell Stem Cell 14: 149–159, 2014. doi: 10.1016/j.stem.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 49.Middendorp S, Schneeberger K, Wiegerinck CL, Mokry M, Akkerman RDL, van Wijngaarden S, Clevers H, Nieuwenhuis EES. Adult stem cells in the small intestine are intrinsically programmed with their location-specific function. Stem Cells 32: 1083–1091, 2014. doi: 10.1002/stem.1655. [DOI] [PubMed] [Google Scholar]
- 50.Milano J, McKay J, Dagenais C, Foster-Brown L, Pognan F, Gadient R, Jacobs RT, Zacco A, Greenberg B, Ciaccio PJ. Modulation of notch processing by gamma-secretase inhibitors causes intestinal goblet cell metaplasia and induction of genes known to specify gut secretory lineage differentiation. Toxicol Sci 82: 341–358, 2004. doi: 10.1093/toxsci/kfh254. [DOI] [PubMed] [Google Scholar]
- 51.Miyoshi H, Ajima R, Luo CT, Yamaguchi TP, Stappenbeck TS. Wnt5a potentiates TGF-β signaling to promote colonic crypt regeneration after tissue injury. Science 338: 108–113, 2012. doi: 10.1126/science.1223821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miyoshi H, VanDussen KL, Malvin NP, Ryu SH, Wang Y, Sonnek NM, Lai CW, Stappenbeck TS. Prostaglandin E2 promotes intestinal repair through an adaptive cellular response of the epithelium. EMBO J 36: 5–24, 2017. doi: 10.15252/embj.201694660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Valenta T, Degirmenci B, Moor AE, Herr P, Zimmerli D, Moor MB, Hausmann G, Cantù C, Aguet M, Basler K. Wnt ligands secreted by subepithelial mesenchymal cells are essential for the survival of intestinal stem cells and gut homeostasis. Cell Reports 15: 911–918, 2016. doi: 10.1016/j.celrep.2016.03.088. [DOI] [PubMed] [Google Scholar]
- 54.Morgan JR, Barrandon Y, Green H, Mulligan RC. Expression of an exogenous growth hormone gene by transplantable human epidermal cells Science 237: 1476–1479, 1987. doi: 10.1126/science.3629250. [DOI] [PubMed] [Google Scholar]
- 55.Mori-Akiyama Y, van den Born M, van Es JH, Hamilton SR, Adams HP, Zhang J, Clevers H, de Crombrugghe B. SOX9 is required for the differentiation of paneth cells in the intestinal epithelium. Gastroenterology 133: 539–546, 2007. doi: 10.1053/j.gastro.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 56.Muise AM, Walters TD, Glowacka WK, Griffiths AM, Ngan B-Y, Lan H, Xu W, Silverberg MS, Rotin D. Polymorphisms in E-cadherin (CDH1) result in a mis-localised cytoplasmic protein that is associated with Crohn’s disease. Gut 58: 1121–1127, 2009. doi: 10.1136/gut.2008.175117. [DOI] [PubMed] [Google Scholar]
- 57.Muñoz J, Stange DE, Schepers AG, van de Wetering M, Koo B-K, Itzkovitz S, Volckmann R, Kung KS, Koster J, Radulescu S, Myant K, Versteeg R, Sansom OJ, van Es JH, Barker N, van Oudenaarden A, Mohammed S, Heck AJR, Clevers H. The Lgr5 intestinal stem cell signature: robust expression of proposed quiescent '+4′ cell markers. EMBO J 31: 3079–3091, 2012. doi: 10.1038/emboj.2012.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ogura Y, Lala S, Xin W, Smith E, Dowds TA, Chen FF, Zimmermann E, Tretiakova M, Cho JH, Hart J, Greenson JK, Keshav S, Nuñez G. Expression of NOD2 in Paneth cells: a possible link to Crohn’s ileitis. Gut 52: 1591–1597, 2003. doi: 10.1136/gut.52.11.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ootani A, Li X, Sangiorgi E, Ho QT, Ueno H, Toda S, Sugihara H, Fujimoto K, Weissman IL, Capecchi MR, Kuo CJ. Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat Med 15: 701–706, 2009. doi: 10.1038/nm.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pellegrinet L, Rodilla V, Liu Z, Chen S, Koch U, Espinosa L, Kaestner KH, Kopan R, Lewis J, Radtke F. Dll1- and dll4-mediated notch signaling are required for homeostasis of intestinal stem cells. Gastroenterology 140: 1230–1240.e1, 2011. doi: 10.1053/j.gastro.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pinto D, Gregorieff A, Begthel H, Clevers H. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev 17: 1709–1713, 2003. doi: 10.1101/gad.267103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Potten CS, Hume WJ, Reid P, Cairns J. The segregation of DNA in epithelial stem cells. Cell 15: 899–906, 1978. doi: 10.1016/0092-8674(78)90274-X. [DOI] [PubMed] [Google Scholar]
- 63.Potten CS, Owen G, Booth D. Intestinal stem cells protect their genome by selective segregation of template DNA strands. J Cell Sci 115: 2381–2388, 2002. [DOI] [PubMed] [Google Scholar]
- 64.Rocha JD, Schlossmacher MG, Philpott DJ. LRRK2 and Nod2 promote lysozyme sorting in Paneth cells. Nat Immunol 16: 898–900, 2015. doi: 10.1038/ni.3255. [DOI] [PubMed] [Google Scholar]
- 65.Sato T, Stange DE, Ferrante M, Vries RGJ, Van Es JH, Van den Brink S, Van Houdt WJ, Pronk A, Van Gorp J, Siersema PD, Clevers H. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 141: 1762–1772, 2011. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 66.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459: 262–265, 2009. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 67.Schwank G, Koo B-K, Sasselli V, Dekkers JF, Heo I, Demircan T, Sasaki N, Boymans S, Cuppen E, van der Ent CK, Nieuwenhuis EES, Beekman JM, Clevers H. Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell 13: 653–658, 2013. doi: 10.1016/j.stem.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 68.Schwitalla S, Fingerle AA, Cammareri P, Nebelsiek T, Göktuna SI, Ziegler PK, Canli O, Heijmans J, Huels DJ, Moreaux G, Rupec RA, Gerhard M, Schmid R, Barker N, Clevers H, Lang R, Neumann J, Kirchner T, Taketo MM, van den Brink GR, Sansom OJ, Arkan MC, Greten FR. Intestinal tumorigenesis initiated by dedifferentiation and acquisition of stem-cell-like properties. Cell 152: 25–38, 2013. doi: 10.1016/j.cell.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 69.Sheaffer KL, Kim R, Aoki R, Elliott EN, Schug J, Burger L, Schübeler D, Kaestner KH. DNA methylation is required for the control of stem cell differentiation in the small intestine. Genes Dev 28: 652–664, 2014. doi: 10.1101/gad.230318.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shroyer NF, Wallis D, Venken KJT, Bellen HJ, Zoghbi HY. Gfi1 functions downstream of Math1 to control intestinal secretory cell subtype allocation and differentiation. Genes Dev 19: 2412–2417, 2005. doi: 10.1101/gad.1353905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Snippert HJ, van der Flier LG, Sato T, van Es JH, van den Born M, Kroon-Veenboer C, Barker N, Klein AM, van Rheenen J, Simons BD, Clevers H. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell 143: 134–144, 2010. doi: 10.1016/j.cell.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 72.Stamataki D, Holder M, Hodgetts C, Jeffery R, Nye E, Spencer-Dene B, Winton DJ, Lewis J. Delta1 expression, cell cycle exit, and commitment to a specific secretory fate coincide within a few hours in the mouse intestinal stem cell system. PLoS One 6: e24484, 2011. doi: 10.1371/journal.pone.0024484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Taniguchi K, Wu L-W, Grivennikov SI, de Jong PR, Lian I, Yu F-X, Wang K, Ho SB, Boland BS, Chang JT, Sandborn WJ, Hardiman G, Raz E, Maehara Y, Yoshimura A, Zucman-Rossi J, Guan K-L, Karin M. A gp130-Src-YAP module links inflammation to epithelial regeneration. Nature 519: 57–62, 2015. doi: 10.1038/nature14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tetteh PW, Farin HF, Clevers H. Plasticity within stem cell hierarchies in mammalian epithelia. Trends Cell Biol 25: 100–108, 2015. doi: 10.1016/j.tcb.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 75.Tian H, Biehs B, Warming S, Leong KG, Rangell L, Klein OD, de Sauvage FJ. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature 478: 255–259, 2011. doi: 10.1038/nature10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.van de Wetering M, Sancho E, Verweij C, de Lau W, Oving I, Hurlstone A, van der Horn K, Batlle E, Coudreuse D, Haramis AP, Tjon-Pon-Fong M, Moerer P, van den Born M, Soete G, Pals S, Eilers M, Medema R, Clevers H. The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells Cell 111: 241–250, 2002. doi: 10.1016/S0092-8674(02)01014-0. [DOI] [PubMed] [Google Scholar]
- 77.Workman MJ, Mahe MM, Trisno S, Poling HM, Watson CL, Sundaram N, Chang C-F, Schiesser J, Aubert P, Stanley EG, Elefanty AG, Miyaoka Y, Mandegar MA, Conklin BR, Neunlist M, Brugmann SA, Helmrath MA, Wells JM. Engineered human pluripotent-stem-cell-derived intestinal tissues with a functional enteric nervous system. Nat Med 23: 49–59, 2017. doi: 10.1038/nm.4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yilmaz ÖH, Katajisto P, Lamming DW, Gültekin Y, Bauer-Rowe KE, Sengupta S, Birsoy K, Dursun A, Yilmaz VO, Selig M, Nielsen GP, Mino-Kenudson M, Zukerberg LR, Bhan AK, Deshpande V, Sabatini DM. mTORC1 in the Paneth cell niche couples intestinal stem-cell function to calorie intake. Nature 486: 490–495, 2012. doi: 10.1038/nature11163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yin X, Farin HF, van Es JH, Clevers H, Langer R, Karp JM. Niche-independent high-purity cultures of Lgr5+ intestinal stem cells and their progeny. Nat Methods 11: 106–112, 2014. doi: 10.1038/nmeth.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yu D-H, Gadkari M, Zhou Q, Yu S, Gao N, Guan Y, Schady D, Roshan TN, Chen M-H, Laritsky E, Ge Z, Wang H, Chen R, Westwater C, Bry L, Waterland RA, Moriarty C, Hwang C, Swennes AG, Moore SR, Shen L. Postnatal epigenetic regulation of intestinal stem cells requires DNA methylation and is guided by the microbiome. Genome Biol 16: 211, 2015. doi: 10.1186/s13059-015-0763-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yui S, Nakamura T, Sato T, Nemoto Y, Mizutani T, Zheng X, Ichinose S, Nagaishi T, Okamoto R, Tsuchiya K, Clevers H, Watanabe M. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5+ stem cell. Nat Med 18: 618–623, 2012. doi: 10.1038/nm.2695. [DOI] [PubMed] [Google Scholar]
- 82.Zhang Q, Pan Y, Yan R, Zeng B, Wang H, Zhang X, Li W, Wei H, Liu Z. Commensal bacteria direct selective cargo sorting to promote symbiosis. Nat Immunol 16: 918–926, 2015. doi: 10.1038/ni.3233. [DOI] [PubMed] [Google Scholar]
- 83.Zomer A, Ellenbroek SIJ, Ritsma L, Beerling E, Vrisekoop N, Van Rheenen J. Intravital imaging of cancer stem cell plasticity in mammary tumors. Stem Cells 31: 602–606, 2013. doi: 10.1002/stem.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]