Abstract
Cell culture has become an indispensable tool to help uncover fundamental biophysical and biomolecular mechanisms by which cells assemble into tissues and organs, how these tissues function, and how that function becomes disrupted in disease. Cell culture is now widely used in biomedical research, tissue engineering, regenerative medicine, and industrial practices. Although flat, two-dimensional (2D) cell culture has predominated, recent research has shifted toward culture using three-dimensional (3D) structures, and more realistic biochemical and biomechanical microenvironments. Nevertheless, in 3D cell culture, many challenges remain, including the tissue-tissue interface, the mechanical microenvironment, and the spatiotemporal distributions of oxygen, nutrients, and metabolic wastes. Here, we review 2D and 3D cell culture methods, discuss advantages and limitations of these techniques in modeling physiologically and pathologically relevant processes, and suggest directions for future research.
Cell cultures in vitro are frequently used to advance understanding of the mechanisms that underlie cell behavior in vivo. These behaviors include cell differentiation, migration, growth, and mechanics, all of which are impacted by their biochemical and biomechanical microenvironment (57). Deciphering the mechanisms behind these behaviors is vital to understanding in vivo processes that result in formation and function of tissues and organs. Ideally, laboratory experiments could be performed with a user-defined three-dimensional (3D) model that closely mimics the cellular microenvironment. However, creating such a model faces challenges that include construction of the tissue-tissue interface, control of the spatiotemporal distibutions of oxygen and carbon dioxide, nutrients, and waste, and the customization of other microenvironmental factors that are known to regulate activities in vivo (57).
For over a century, two-dimensional (2D) cell cultures have been used as in vitro models to study cellular responses to stimulations from biophysical and biochemical cues. Although these approaches are well-accepted and have significantly advanced our understanding of cell behavior, growing evidence now shows that, under some circumstances, the 2D systems can result in cell bioactivities that deviate appreciably the in vivo response. For instance, some important characteristics of cancer cells cannot be appropriately modeled in 2D cultures (22). To overcome this limitation, novel 3D cell culture platforms are being created to better mimic in vivo conditions and are sometimes called spheroid or organoid culture, as described below (37, 61, 84, 98, 117, 118). In many cases, these new platforms have proven more capable of inducing in vivo-like cell fates for the specific processes under study. Results from 3D studies demonstrate that increasing the dimensionality of extracellular matrix (ECM) around cells from 2D to 3D can significantly impact cell proliferation, differentiation, mechano-responses, and cell survival (2, 10, 41). Although these discoveries might suggest that 3D systems should be applied whenever possible, the platform of choice is often dictated by the specific process of interest, and a universal 3D platform does not currently exist; additionally, 2D cell culture approaches can still recapitulate in vivo behavior for many bioactivities, while new advances in substrate design continue to offer new capabilities for this platform. Overall, 3D platforms are likely to provide an increasingly attractive alternative for 2D cell culture as the technology develops to enable a wider range of processes.
Here, we provide an overview of traditional culture methods in 2D and 3D, and discuss the current techniques, immediate challenges, and the differences in results in 2D and 3D, as well as their implications. Topics included are microtopographies in 2D cultures (18, 72, 81, 109, 113), biopolymers for scaffold creation in 3D cultures (5, 23, 26, 27, 60, 63, 68, 84, 88), and the effect of the extracellular matrix on culturing techniques (78, 127, 129). We aim to provide a reasonably comprehensive review of the benefits and downfalls of both 2D and 3D cultures in this rapidly evolving and expanding field.
Current 2D Cell Culture Methods
Considerations
Conventional 2D cell culture relies on adherence to a flat surface, typically a petri dish of glass or polystyrene, to provide mechanical support for the cells. Cell growth in 2D monolayers allows for access to a similar amount of nutrients and growth factors present in the medium, which results in homogenous growth and proliferation (31). This characteristic makes 2D platforms attractive to biologists and clinical users due to simplicity and efficiency. However, most of these 2D methods do not provide control of cell shape, which determines biophysical cues affecting cell bioactivities in vivo. To control cell shape in 2D cell culture, micro-patterned substrates, such as cell-adhesive islands (30), microwells (121), and micropillars (40), have been created to customize the 2D shape of cells and help study the effects of cell shape on bioactivities. Nonetheless, these pseudo-3D models induce an apical-basal polarity, which is unnatural in vivo for some cell types, for example, mesenchymal cells. This induced polarity may alter the functions of native cells with regard to spreading, migrating, and sensing soluble factors and other microenvironmental cues (61). The effect of cell polarization in 2D cell culture can be mitigated using a sandwich culture method that adds a layer of ECM atop the cells (9, 29, 34, 65, 73) to eliminate apical-basal polarity and provide a mimic of 3D ECM.
Techniques
Sandwich culture.
Modeling physiologically relevant events can be challenging for many cell lines. Indeed, alternative methods to traditional 2D cell culture were developed for hepatocytes because they do not survive well under traditional 2D culture methods. In 2D cultures, there are decreases in transcriptional genes and rapid changes in morphology (29, 34). The sandwich culture method, e.g., placing cells between two layers comprised of ECM, polyacrylamide, or collagen, has long been demonstrated to produce hepatocyte cell cultures, where morphology and function more accurately reproduce in vivo behavior (9, 29, 34, 65, 73). This is particularly relevant in the area of drug discovery, where researchers are interested in understanding pharmacokinetics in relation to the liver (9, 65).
Hepatocytes in the liver are surrounded by a complex ECM, and several researchers have shown that conventional 2D culture methods, flasks with or without ECM protein coatings, do not establish well enough the complex microenvironment that hepatocytes need to function (29, 34, 73). Dunn et al. (29) compared the behavior of hepatocytes cultured on a one-gel system to a sandwich culture comprised of two collagen gels. Their results showed that the sandwich culture could maintain a proper albumin production rate for at least 6 wk, whereas the one-gel system already had rates that were tenfold lower than normal by the end of the first week (29). LeCluyse et al. (73) found that the polarity of hepatocytes was also affected when cultured on only single-layer gels. When a sandwich culture was used, a normal distribution of the apical membrane markers was found at the canalicular membrane, and bile canaliculi were also well established (73). The development of bile canaliculi is important in the study of pharmacokinetics, since understanding drug uptake is critical when modeling pathologically relevant events (9).
Sandwich cultures not only have allowed researches to study the uptake and efflux transport of hepatocytes but also have helped them to understand the conditions for hippocampal neuron culture (12). Hippocampal neurons do not culture well at low densities under traditional 2D methods. Brewer et al. (12) desired low-density cultures to image individual neurites contacting each other and for drug testing. They determined that low levels of oxygen (9%) are ideal for low density (<20,000 cells/cm2) hippocampal neuron cultures, since higher levels become toxic. The sandwich method was shown to reduce the diffusion of oxygen sufficiently so that an 80% survival rate was found over 5 days compared with a 32% survival rate over 2 days in a traditional mixed-cell population culture (12). The sandwich culture has allowed many researchers to examine the effects of pharmacokinetics, which is important to consider when modeling physiological and pathological events.
Micro-pattering.
Engineered 2D surface patterning and modification creates a 2D microenvironment with unique biochemical factors, topography, stiffness, and mechanical load for cell culture (72, 81). In a typical 2D cell culture, cells are exposed to a homogenous surface devoid of any defects that could influence their development (109). Several researchers have begun to test the effects of varying microtopographies on cell differentiation and proliferation (18, 106, 113).
Chaubey et al. (18) studied the effect of adipocyte differentiation based on cell culture surface microtopography. D1 cells, multipotent mouse bone marrow stromal precursors, were used to study the effect that patterned and non-patterned poly-L-lactide (PLLA) surfaces had on cell differentiation and lipid production. The patterned surfaces contained horizontal, 3-μm-wide micro-grooves that were separated by 100 μm. The patterned PLLA surfaces were shown to have a faster rate of differentiation than non-patterned PLLA. However, cells cultured on patterned and non-patterned PLLA surfaces showed a lower rate of differentiation compared with cells on tissue culture-treated polystyrene (PS), a control for the experiment. As for lipid production, it was found that, at later time points, patterned PLLA surfaces produced the highest amount of lipids, followed by PS, and then non-patterned PLLA. The results indicated that the micro-patterning and type of surface both affected the rate of differentiation of cells (18).
Wan et al. (113) also studied the effects of microtopography using PLLA and polystyrene (PS) with hemispherical pitting and islands to understand mechanical interactions of OCT-1 osteoblast-like cells within micro-niches. They demonstrated that cells preferred to round up on smooth-surfaced PLLA substrates and stretch on rough surfaces. The efficiency of the surface attachment was also shown to be higher on rounded surfaces compared with smooth surfaces. In response to variations in microtopography, cells tend to exhibit an increase in filopodia and microspikes, which play important roles in cell migration and neurite outgrowth (106). The effect of microtopography on cell migration was further supported by Wan et al. (113), whose experiments also revealed that pseudopods extending from cells could encroach into pits in culture surfaces. The less rigid pseudopods could act as anchors for the cell and influence its migration patterns, thereby providing a contact guidance (113).
Altering substrate stiffness.
The microenvironment of cells is not only affected by microtopography but also by the stiffness of the substrate that cells adhere to, which affects bioactivities ranging from migration to differentiation (28). For example, Engler et al. (32) demonstrated that the differentiation of mesenchymal stem cells (MSCs), which possess the ability to develop into neurons, myoblasts, and osteoblasts, can be regulated by substrate stiffness. MSCs were cultured on collagen I-coated, inert polyacrylamide gels, with the elasticity of the matrix determined by the degree to which the gels were cross-linked. Cultures on the softest gels with Young’s modulus (E) similar to the brain (0.1–1 kPa) exhibited the highest expression of neuron-specific markers. Cells on a substrate with a moderate stiffness similar to muscle tissue (E = 8–17 kPa) expressed sixfold higher levels of myogenic factor messages. Last but not least, cells cultured on the stiffest matrix (E = 25–40 kPa) expressed fourfold higher osteogenic factor messages.
3D Cell Culture Models and Applications
Considerations
Cells in our body perform bioactivities in response to the stimulation from a highly complex, 3D microenvironment (15, 98, 112). The makeup and distribution of cell-ECM and cell-cell interactions influence fundamental cellular behaviors and are tied to the functions of whole organs. For 3D culture systems looking at cell clusters, the aggregates formed in 3D create a gradient of nutrient access and waste buildup. Surfaces of the aggregated spheroids, for example, have the highest levels of proliferation, whereas the interior of the 3D cell bodies possesses the highest number of quiescent or necrotic cells (31). The introduction of 3D cell culture approaches aimed at modeling the in vivo interactions of tissues and organs has opened new possibilities in studying the underlying biochemical and biomechanical signals (46, 57). A well-designed microenvironment in tissue and cell engineering can be used to promote proliferation, migration, matrix production, and stem cell differentiation. The following subsection will discuss various 3D cell culture techniques that have been established to try and create in vivo conditions for cellular development.
Techniques
Spheroid culture.
There are multiple methods for spheroid cultures, including microfluidics (35), microchips (110), embryoid bodies (EBs) (91), collagen gels (GELs) (91), and hanging-drop culture (35, 55, 110, 111). Torisawa et al. (110) created a novel chip system utilizing microfluidics that allows spheroids to grow in a controlled environment for long-term culture. PDMS wells with a triangular pyramid shape opening promoted the growth of spheroids, with some control over the size achievable. This device was able to maintain spheroid cultures for up to 2 wk (110). Additionally, microwells made out of a non-adhesion promoting material, such as agarose, can be used to grow several arrays of spheroids at once (86). Micropatterning can also be used to pattern areas or to create “microwells” for the controlled growth of spheroids (i.e., agarose) (35).
Pineda et al. (91) conducted experiments on the proliferation and differentiation of embryonic stem cells (ESCs) in two different 3D culture methods. Cells were cultured as embryoid bodies (EBs) in either a collagen type I gel (GEL) or by growing them in non-tissue culture-treated dishes to prevent adherence. GEL and unattached EB cultures produced similar cluster morphology, with defined boundaries and the occasional cavities. A key difference in the morphology of GEL cultures was the presence of elongated masses along the edges of the gel form. Free EBs had more dynamic changes in the genotypic expression levels of cytoskeletal genes over the same 12-day span compared with the GEL cultures. By day 12, unattached EB cultures had higher expression levels of the intermediate filaments lamin, keratin 8, and vimentin (91). These results indicate that, even with similar morphologies, the gene expression of each 3D culture is specific in adaptation to its microenvironment. Another culture method involves spheroids forming within hanging droplets of media (35, 55, 110, 111). Conventional hanging-drop method does not allow for extended cell culture due to difficulty in changing media. To overcome this, one group, Hsiao et al. (55), designed a plate using micro-ring structures to stabilize the drops, allowing for 14-day culture of prostate cancer cells. They continued on to use these plates as a high throughput method for testing anticancer drug sensitivities on H9 human epithelial carcinoma cells. Their results from 2D and 3D spheroid culture indicated spheroids were more resistant to one anti-cancer drug, 5-fluorouracil, which inhibits proliferation, whereas 2D cultures were more resistant to a different anti-cancer drug, tirapazamine, which is cytotoxic in hypoxic conditions (111).
Biopolymer scaffolds.
Significant progress has been achieved during the last decade on the techniques to encapsulate cells in 3D using tissue-engineering scaffolds with customized biochemical and biophysical components. These 3D scaffolds and associated cell-encapsulation techniques provide the valuable tools for helping us decipher how the ECM affects the fate of cells (5, 13, 14, 25–27, 68, 77, 80, 87, 88, 92, 108). Biopolymers derived from animal tissues are particularly popular, since they contain the similar biochemical components that cells experience in their native tissue and may promote tissue regeneration. Among the most commonly used natural polymers are hyaluronic acid, gelatin, collagen, and chondroitin sulfate (14, 108). Biopolymers from non-mammal tissues, such as alginate and chitosan, are also used for making 3D scaffolds (23, 63, 68, 84). Despite the progress in developing biopolymers that promote the desirable cell fates for regenerating cartilages (50, 53), bones (126), skin (107, 128), and arteries (56, 90), creating a 3D scaffold for tissue repair remains highly challenging. Among the leading issues is the difficulty to independently control the key elements crucial for controlling cell bioactivities, including biochemical property, matrix elasticity, and macroporosity (51, 116).
Prefabricated scaffolds.
Prefabricated scaffolds provide a customizable biochemical composition, matrix elasticity, and micro-architectures. These scaffolds can be fabricated by 3D printing (36), stereo-lithography (38, 41, 89), polymer phase separation, lyophilizing (22, 119), gas foaming (96), and porogen leaching using soluble templates to form pores or channels (20, 54, 60, 83, 99). However, current methods to create prefabricated scaffolds often involve conditions that are too harsh for cells to survive, such as extreme pressure, non-physiological salt concentration, and the use of organic solvents. Therefore, the delivery of cells into the scaffolds is mainly achieved by cell diffusion (66, 79, 101, 117, 122, 124), and this method is often subjected to low cell penetration rate and poor scaffold cellularization. A relatively new approach to vascularization of fabricated scaffolds has been explored by Zhang et al. (127). Their experiments involved the use of silk fibroin, a protein polymer shown to be biocompatible and degradable. The silk fibroin scaffolding was processed to contain porous, hollow channels that were pre-vascularized with human umbilical vein endothelial cells. They observed that the host tissue grew into the scaffold while the channels aided in the development of in vitro tube formations resulting from the human umbilical vein endothelial cells.
Building blocks-based scaffold formation.
To overcome challenges with cellularization, building blocks-based scaffold formation has emerged as a new trend of technology for 3D scaffold construction (48, 49, 51). This technology aims to provide independent control of porosity, mechanics, and ECM properties by building designer base units which are then controllably crosslinked to ensure the desired porosity is achieved. Their results demonstrated that microribbon-based scaffolds facilitated cell engraftment, cell proliferation, and ECM production. Stem cells encapsulated within a designer scaffold at a low initial density (2 to ~5 million/ml) were shown to proliferate up to 30-fold within 3 wk. This process has been utilized in bone regeneration scaffolds, where implanting building block-based scaffolds with adipose-derived stromal cells (ADSC) accelerated bone regeneration in a mouse cranial defect. Endogenic bone formation was promoted by the prolonged survival of ADSC and associated stem cells’ paracrine signaling (49). Both ribbon-shaped and micro-bead-like building blocks were used to create 3D scaffolds that support nutrient diffusion, cell proliferation, ECM production, and wound healing (44).
Hydrogels.
In contrast to prefabricated scaffolds, hydrogels made of different types of biopolymers have been widely used as scaffolds due to their ease of cell encapsulation (27, 88, 103). Hydrogels also provide tissue-like water content and easily tunable biochemical and mechanical properties. However, most hydrogels consist of micron/nanometer-sized mesh that is often too small to allow postfabrication cellularization and lack the micro-topography needed for controlling cell shape and supporting cell mobility, cell proliferation, and matrix production. Attempts have been made to overcome the above limitations by using hydrolytically and enzymatically degradable biopolymers (6, 50). These hydrogels have components that gradually dissolve and produce internal space to facilitate matrix production and other cell bioactivities. The main downside of hydrogels, in terms of tissue regeneration, is that matrix degradation simultaneously changes the biochemical elements and the matrix elasticity, both of which need delicate control. Furthermore, it is extremely difficult to match the speed of hydrogel degradation with the pace of tissue formation (74), which is important in maintaining the shape and mechanical integrity of tissue-engineering constructs.
Cell sheets.
Another approach to engineering organs and tissues without the use of constructed scaffolds is to make multiple layers of cell sheets. Shimizu et al. successfully replicated the pulsatile function of cardiomyocytes in a 3D construct by layering multiple cell sheets (FIGURE 1) (52, 100). This approach demonstrated that the use of a synthesized, biodegradable scaffold was not necessary to develop potentially properly functioning tissue. The tissue constructs were transplanted into rats and were shown to have a survival rate of up to 3 mo, and vascularization of the cells sheets by the host tissue was observed. Shimizu et al. (100) went on to compare their research to other studies that used biodegradable 3D scaffolds to regenerate cardiac tissue. The biodegradable 3D scaffolds had poor influx of migratory cells and caused inflammatory reactions as opposed to the cell sheets that provided a more optimal recovery.
FIGURE 1.
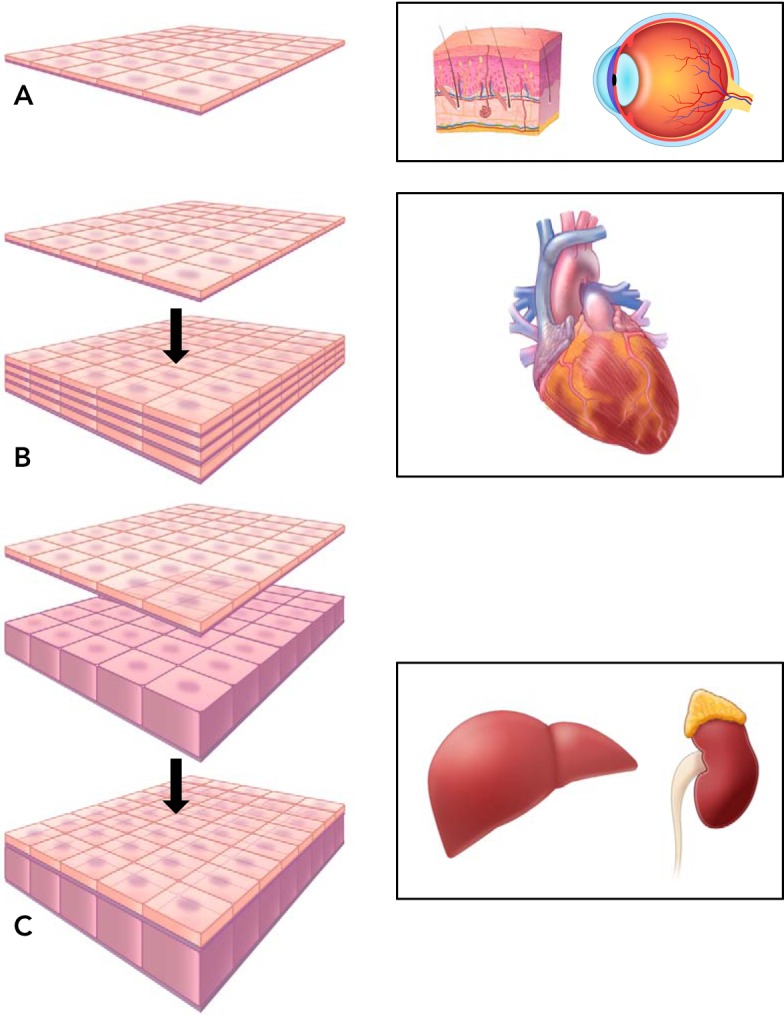
Three different applications and variations of cell sheet engineering
A: a cornea transplant using a single cell sheet. B: layered identical cell sheets for myocardial reconstruction. C: layers of various cell sheet types for liver or kidney applications. Reprinted from Ref. 100, with permission from Biomaterials.
Bioreactors with perfusion, dynamic loading, and microfluidic actuation.
Bioreactors are created to understand cell behaviors in construction of micro-tissues or organs and produce more cells for clinical application or laboratory research. Large-scale bioreactors include simple systems such as spinner flasks and rotating wall bioreactors, which allow for semi-adherent cell growth, as well as more complicated systems. Beyond traditional uses, bioreactors can be used to assist newer variants of 3D cell culture. For example, in addition to relying on natural diffusion, transportation of gas, nutrients, and wastes through a 3D cellular scaffold can be accelerated by using a bioreactor with perfusion pumps and dynamic stress-loading actuators (123). Using bioreactors with cell-sized conduits, the effects of fluid transport in a cellular scale have been explored. Recently, micro-bioreactors such as micro-fluid bioreactors, bioreactors-on-chip, and micro-bioreactors with microchannels have been established as effective models for studying the bioactivities of various cells, including MSCs, embryonic stem cells (ESCs) and tissue (liver and heart)-specific cells (15, 24, 97, 104). Micro-bioreactors also showed promise in screening therapeutic drugs and in controling cell microenvironment (1, 24, 37, 94).
Microfluidics.
Beyond traditional large-scale bioreactors, the advancement of micro- and nano-scale fabrication has led to the development of various devices for cell studies, of which microfluidics is at the forefront. The advancement of microelectromechanical systems (MEMs) led researchers to consider ways that similar devices could be applied in biology, and thus microfluidics were developed (118). Microfluidic devices are often made using polymers, with poly(dimethylsiloxane) (PDMS) being the most predominant (118). PDMS does not corrode or become affected by aqueous solutions used in cell culture, pressure, or temperature, and cells are able to survive or grow as a result (107). Microfluidic devices have been used for cancer detection (43), as a diagnostic tool in developing countries (95), for understanding the mechanics of embryonic development (118), and for many more applications. Many of these devices remain 2D in nature, but recently some microfluidic devices have evolved into more 3D-like cultures to mimic organ functions (8, 57).
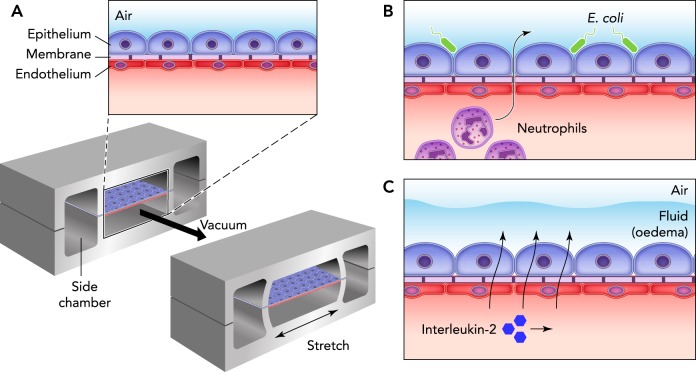
Organs-on-chip refers to microfluidic devices whose structures allow them to mimic the functions of organs (8). This ability to simulate organs in vivo allows for new drug tests to be performed before animal or clinical studies. One example is the lung-on-chip (FIGURE 2), which provides a novel method for testing the efficacy of pharmaceuticals rapidly and easily (33, 58, 59). In addition, other organs-on-chip have been developed (58), and there is potential for the systems to be connected together much in the same way as in vivo, which could allow for a better understanding of the interdisciplinary mechanisms of organs in diseased and healthy states. It is important to note that these devices mimic both the physical and the biochemical environments in vivo; this suggests that chemical signaling and biological pathways stay the same and can therefore be investigated using these devices (8, 58).
FIGURE 2.
A lung-on-a-chip created by culturing human alveolar epithelial cells and pulmonary microvascular endothelial cells
A: a lung-on-a-chip created by culturing human alveolar epithelial cells and pulmonary microvascular endothelial cells on opposing sides of a porous membrane. B: the system could recreate organ-level functions, such as an inflammatory response bacteria. C: the system was also used as a model for lung diseases like pulmonary edema. Reprinted from Ref. 33, with permission from Nature Reviews Drug Discovery.
Comparison of Cell Development in 2D and 3D Environments
3D cell cultures differ greatly from standard 2D cultures in cell-cell interaction, cellular mechanics, and nutrient access (31). The prevalence of 3D culture systems is increasing due to the ability to mimic, with acceptable accuracy, the in vivo environment. However, many epithelial systems are reasonably approximated in 2D, even compared with the in vivo systems. For example, lung airway epithelia will develop normally in air-liquid interface culture on 2D surfaces (67, 85). With that said, 2D monolayer cell culture systems sometimes fail to exhibit the cell development process seen in the physiological environment in vivo due to the simplicity of the systems. The lack of a complex and biological information-rich environment in these 2D systems could be the cause of this disparity. Both 2D and 3D cell culture models have different influences on in vivo-like cell development behaviors, such as cell proliferation, differentiation, apoptosis, and cell motion, compared with 2D planar cell cultures (2, 10, 39). These developmental behaviors will be discussed in the following sections.
Proliferation, Differentiation, and Gene Expression
Several researchers have examined the effects of 2D and 3D culture methods on the proliferation, differentiation, and gene expression levels of cells (21, 81, 91). Pineda et al. (91) demonstrated that OCT4 in mouse ESCs decreased in both 2D and 3D cultures, showing a loss of pluripotency, whereas NES (ectoderm) and BRACHY-T (mesoderm) markers were shown to increase. However, the ESCs cultured on glass slides (2D) coated with collagen type I had a faster rate of differentiation than in 3D cultures (EBs and GELs). It was also shown that the duration of cell culture and organization of cells affected their differentiation. Based on assessment of GATA4, a potential myocardial transcription factor, it was shown that EB cultures possessed the greatest ability, among these culture types, to support cardiovascular differentiation. Furthermore, neural differentiation was only supported in EB cultures that had been sustained over time (91).
Chitcholtan et al. (21) also showed that some characteristics of tumor cells are not properly modeled in 2D. In all the tumor cells lines that were observed, there were higher proliferation rates in 2D monolayers than in 3D reconstituted basement membrane (rBM) cultures. Although 3D cultures exhibited a reduction in proliferation, there was an increase in β4 and β1 integrins that serve as markers for cell polarization and differentiation. Previous experimentations have shown that 2D cell culture of endometrial cancer cells led to a loss in tissue-specific function and organization. The complexity of studying 3D cultures is highlighted by how different cell lines uptake a ubiquitous energy source, such as glucose. A glucose analog, 2-NBDG, was introduced to both 2D and 3D cultures of KLE, Ish-ikawa, and EN-1078D cells. KLE and Ish-ikawa cells in 3D conformations had a lower influx of 2-NBDG than their counterparts in a 2D conformation. This contrasts with the EN-1078D cell line, which showed higher uptake of 2-NBDG in 3D models compared with 2D. KLE cancer cells had the highest overall rate of 2-NBDG uptake in both 2D and 3D cultures but expressed the lowest levels of cellular proliferation. These findings imply that glucose uptake levels may not impact cell proliferation rates regardless of the culture method (21). Findings such as these demonstrate the challenge in assessing whether 2D or 3D cultures are preferable for cellular proliferation and differentiation, since many of the differences are cell line specific.
Phenotypic expression is also altered depending on the culture method used. Microarray analysis of valvular interstitial cells (VIC), the primary cell type in heart valves, revealed that substrate stiffness can affect the gene expression of cell lines. Cells cultured on stiff, 2D tissue culture polystyrene presented with more gene expression changes than 2D or 3D cultures conducted in less stiff materials such as hydrogels. The Young’s modulus of the material that VIC cells were cultured on impacted the expression of cytoskeletal, contractility, and matrix remodeling genes (81). Additionally, Pineda et al. showed that cells grown in a monolayer expressed higher levels of cytoskeletal elements and extracellular matrix proteins than those grown in 3D cultures (91). The combinations of these factors influences cellular proliferation, differentiation, and gene expression, and makes both 2D and 3D cultures valuable to scientific experimentation.
Apoptosis
A recent study has indicated that the formation of dense spheroids can prevent apoptosis in some breast cancer cell lines when exposed to cancer drugs such as paclitaxel (62). In 2D cultures, access to nutrients is not affected by a gradient of cells, such as in 3D cultures, since necrotic cells detach into the medium, leaving only viable cells exposed on the culture surface (31). For instance, in aggregated spheroids, the highest levels of proliferation are at the surface, whereas the interior of the 3D cell bodies possesses the highest number of quiescent or necrotic cells (31). These quiescent cells are less susceptible to drug treatment, yet can provide the seed of new tumor growth (69). This suggests that, beyond simple diffusion limitations, the changes in cell behavior due to geometry can have far-reaching impacts in terms of understanding physiological response.
Motion and Migration
Cell migration differs between dimensionalities (11, 42, 45), potentially due to the more complex cell interactions within a 3D substrate. Cells in 3D are surrounded and attached on all sides, creating obstacles for migration and subsequently causing alterations in cell motility and the mechanisms cells use (45, 125). These differences are significant, since migration is a key aspect of cellular mechanics and plays a key role in the metastasis of cancer, as well as other diseases and disorders. For example, Hakkinen et al. (47) found that fibroblasts migrate at least 1.3 times faster in 3D culture compared with their corresponding 2D culture in collagen, fibrin, and cell-derived matrix, whereas, on the basement membrane extract, the reverse was true. Additionally, it has been suggested that 3D scaffolds provide a more realistic environment for the study of cell migration and may prove crucial in the advancement of the understanding and prevention of cancer metastasis or other diseases affected by cell migration (71). Varying signaling cascades were found between 2D and 3D cultures, with more signaling mechanisms becoming evident in 3D (46). For instance, coupled interactions between β1-integrin and epidermal growth factor receptor signaling were identified in 3D culture but not found in 2D (114).
Matrix topography, including porosity and microarchitectures, is required for regulating cell mobility and retaining ECM components produced upon cell differentiation (50, 53). More recently, cell shape formation in response to ECM properties has been established as a key mechanochemical regulator for stem cell differentiation (4, 30, 82).
Extracellular Matrix
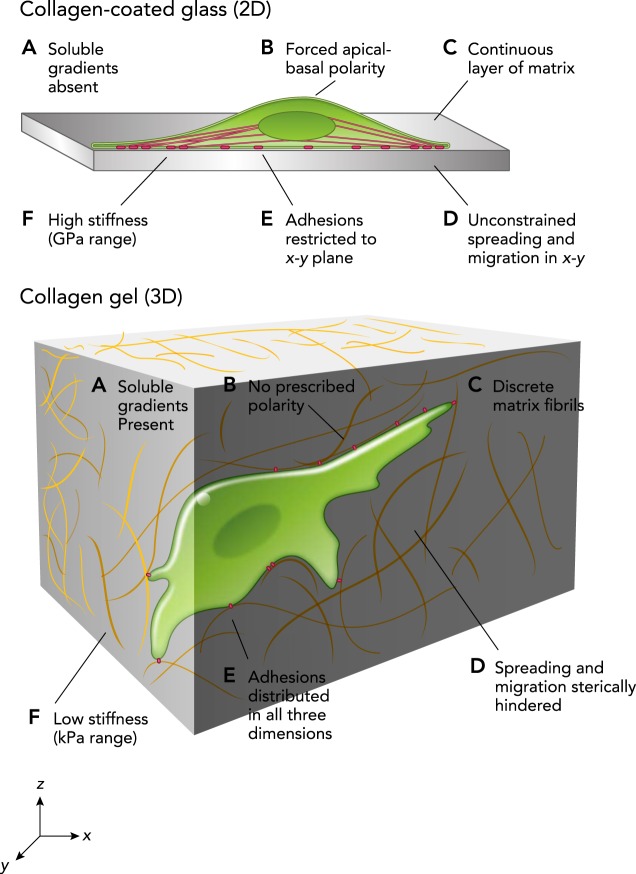
The ECM produces multifactorial signals to trigger bioactivities and regulate cell fates; these signals are determined by the ECM’s mechanical, chemical, and topographical properties (15, 98, 112). Mimicking the in vivo ECM seen by cells in 2D typically involves the use of collagen or fibronectin, which allows the cells to bind to substrates (18, 58). Usually, a cross-linker is used that allows collagen to bind to the surface of polyacrylamide or other gels; cells are then able to attach to the collagen fibers coating the surface of the gel. Collagen is also found in 3D studies, but to reduce the effect of degradation and allow for greater control over singular properties, several 3D studies use materials other than collagen. The chemical and mechanical microenvironment in 2D vs. 3D cultures is different, as illustrated in FIGURE 3.
FIGURE 3.
Cells in 2D and 3D microenvironments interact differently with their surroundings due to differences in the cues, mechanical and chemical, that they experience
Reprinted from Ref. 2, with permission from Journal of Cell Science.
These 3D culture systems allow for studying the effect of ECM stiffness on different cancer cells, as well as how other properties affect cancer tumors (46). By combining polyethylene glycol with a photodegradable compound, Kloxin et al. (70) were able to alter the physical properties of hydrogels, thereby altering the microenvironment cell experience. This alteration enables investigations into mechanical and chemical sensing between cells and their environment, which can in turn be used to control cell behavior for a variety of applications. Other studies have reported alternate recipes for hydrogels and investigated the effect of tuning characteristics of these hydrogels. For example, Chaudhuri et al. (19) used alginate/collagen hydrogels as synthetic ECM to examine the effect of stress relaxation of the hydrogel on the cell spreading, proliferation, and differentiation of mesenchymal stem cells. They were able to tune hydrogels to have different stress relaxation rates and illustrated the significance of this characteristic in cell physiology.
Mechanical Responses
Assessing the mechanical response of cells is significantly easier in 2D than in 3D (71). For example, cells in 2D must only move along a planar surface through generating enough traction to overcome surface inhibition. Cells in 3D arrangements not only have inhibition from potential surface contact but also more contact inhibition from other cells as well as the ECM. One technique for assessing 2D cell traction forces measures the displacement of beads embedded into gel substrates that cells are cultured on (71). Most research into 3D traction forces have been centered on substrates that have a linear force-displacement response, but this is not as physiologically relevant, since the connective tissue in most organs is not linearly elastic.
Obtaining accurate measurements of forces exerted on the ECM are necessary to understand physiological processes such as cancer or immune cell migration. Steinwechs et al. (105) quantified, for the first time, the forces resulting from a breast carcinoma cell that migrates within a 3D collagen fibril matrix. They found that similar forces are generated even as the stiffness of the matrix is changed. This is in contrast to 2D matrices whereby physical forces increase as the matrix stiffness is increased (115). However, they did not determine that matrix stiffness was the only cause of the observed similarity in forces. Other factors such as ligand density and pore size may have simply inhibited cell spreading, which in turn could have caused a reduction in force (105, 115).
Conclusions and Discussion
We have provided an overview of various methods for culturing cells in both 2D and 3D, analyzing the benefits and disadvantages, and discussing current challenges. Classically, 2D studies have been favored, but 3D studies are becoming more necessary to replicate the behaviors of some in vivo conditions. Currently, there are several 3D methods that have become popular and well developed, such as hydrogels and bioreactors. These methods allow for a more physiologically relevant environment to investigate cell and tissue behavior.
New culture methods are continuously appearing, and older methods are constantly evolving to address the current challenges. One major problem in the field is the lack of a standard approach in 3D cultures, whereas 2D cell culture practice is standard and roughly comparable. However, a standard system for 3D cultures will be difficult to implement since different cell types will most likely be better matched for certain 3D systems. The fluidity of this field is what allows advances to continuously be made.
In 3D cell culture, multicellular spheroids are closer to living tissues in vivo in terms of biostructural and biofunctional properties compared with conventional 2D planar cell cultures. Cells in spheroids often initiate different characteristics than monolayer cells, such as the deposition of ECM, secretion of growth factors, and gene expression profiles. Spheroid cell culture is of interest to researchers due to its simplicity, reproducibility, and similarity to physiological tissues. This approach has been developed in the research of cancer (7, 76, 102), stem cells (17, 120), and many organs (such as liver, kidney, heart, etc.) (3, 16, 64, 75). The difficulty in growing physiologically similar 3D tissue or organ cultures stems from finding viable cell types, biocompatible scaffold materials, and a scaffold design that supports the development of an ECM and vascularization. Even though 3D spheroid cultures are more similar to in vivo physiological conditions, more guidance is needed for culture conditions to replicate tissue conditions more accurately.
In conclusion, the situation and questions at hand will determine whether a 2D or 3D culture method is best suited. In some cases, 2D studies are more feasible, and the results can be meaningful. However, in other cases, 3D studies will yield more accurate results and can be done in combination with 2D studies.
Footnotes
Z.C. acknowledges the Dartmouth startup fund and the support by the Society in Science-Branco Weiss Fellowship, administered by ETH Zürich. This work is in part supported by the National Cancer Institute of the National Institutes of Health under Award No. U01 CA-202123.
No conflicts of interest, financial or otherwise, are declared by the author(s).
Author contributions: K.D., H.G., and A.F.P. prepared figures; K.D., H.G., L.H., Y.M., and Z.C. drafted manuscript; K.D., H.G., L.H., A.F.P., J.F., and Z.C. edited and revised manuscript; A.F.P., J.F., and Z.C. approved final version of manuscript.
References
- 1.Amanullah A, Otero JM, Mikola M, Hsu A, Zhang J, Aunins J, Schreyer HB, Hope JA, Russo AP. Novel micro-bioreactor high throughput technology for cell culture process development: Reproducibility and scalability assessment of fed-batch CHO cultures. Biotechnol Bioeng 106: 57–67, 2010. doi: 10.1002/bit.22664. [DOI] [PubMed] [Google Scholar]
- 2.Baker BM, Chen CS. Deconstructing the third dimension: how 3D culture microenvironments alter cellular cues. J Cell Sci 125: 3015–3024, 2012. doi: 10.1242/jcs.079509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beauchamp P, Moritz W, Kelm JM, Ullrich ND, Agarkova I, Anson BD, Suter TM, Zuppinger C. Development and characterization of a scaffold-free 3D spheroid model of induced pluripotent stem cell-derived human cardiomyocytes. Tissue Eng Part C Methods 21: 852–861, 2015. doi: 10.1089/ten.tec.2014.0376. [DOI] [PubMed] [Google Scholar]
- 4.Bellas E, Chen CS. Forms, forces, and stem cell fate. Curr Opin Cell Biol 31: 92–97, 2014. doi: 10.1016/j.ceb.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benoit DS, Schwartz MP, Durney AR, Anseth KS. Small functional groups for controlled differentiation of hydrogel-encapsulated human mesenchymal stem cells. Nat Mater 7: 816–823, 2008. doi: 10.1038/nmat2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benton JA, Fairbanks BD, Anseth KS. Characterization of valvular interstitial cell function in three dimensional matrix metalloproteinase degradable PEG hydrogels. Biomaterials 30: 6593–6603, 2009. doi: 10.1016/j.biomaterials.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berens EB, Holy JM, Riegel AT, Wellstein A. A cancer cell spheroid assay to assess invasion in a 3D setting. J Vis Exp 105: 53409, 2015. doi: 10.3791/53409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhatia SN, Ingber DE. Microfluidic organs-on-chips. Nat Biotechnol 32: 760–772, 2014. doi: 10.1038/nbt.2989. [DOI] [PubMed] [Google Scholar]
- 9.Bi YA, Kazolias D, Duignan DB. Use of cryopreserved human hepatocytes in sandwich culture to measure hepatobiliary transport. Drug Metab Dispos 34: 1658–1665, 2006. doi: 10.1124/dmd.105.009118. [DOI] [PubMed] [Google Scholar]
- 10.Bonnier F, Keating ME, Wróbel TP, Majzner K, Baranska M, Garcia-Munoz A, Blanco A, Byrne HJ. Cell viability assessment using the Alamar blue assay: a comparison of 2D and 3D cell culture models. Toxicol In Vitro 29: 124–131, 2015. doi: 10.1016/j.tiv.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 11.Bott K, Upton Z, Schrobback K, Ehrbar M, Hubbell JA, Lutolf MP, Rizzi SC. The effect of matrix characteristics on fibroblast proliferation in 3D gels. Biomaterials 31: 8454–8464, 2010. doi: 10.1016/j.biomaterials.2010.07.046. [DOI] [PubMed] [Google Scholar]
- 12.Brewer GJ, Cotman CW. Survival and growth of hippocampal neurons in defined medium at low density: advantages of a sandwich culture technique or low oxygen. Brain Res 494: 65–74, 1989. doi: 10.1016/0006-8993(89)90144-3. [DOI] [PubMed] [Google Scholar]
- 13.Van Den Bulcke AI, Bogdanov B, De Rooze N, Schacht EH, Cornelissen M, Berghmans H. Structural and rheological properties of methacrylamide modified gelatin hydrogels. Biomacromolecules 1: 31–38, 2000. doi: 10.1021/bm990017d. [DOI] [PubMed] [Google Scholar]
- 14.Burdick JA, Chung C, Jia X, Randolph MA, Langer R. Controlled degradation and mechanical behavior of photopolymerized hyaluronic acid networks. Biomacromolecules 6: 386–391, 2005. doi: 10.1021/bm049508a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burdick JA, Vunjak-Novakovic G. Engineered microenvironments for controlled stem cell differentiation. Tissue Eng Part A 15: 205–219, 2009. doi: 10.1089/ten.tea.2008.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buzhor E, Harari-Steinberg O, Omer D, Metsuyanim S, Jacob-Hirsch J, Noiman T, Dotan Z, Goldstein RS, Dekel B. Kidney spheroids recapitulate tubular organoids leading to enhanced tubulogenic potency of human kidney-derived cells. Tissue Eng Part A 17: 2305–2319, 2011. doi: 10.1089/ten.tea.2010.0595. [DOI] [PubMed] [Google Scholar]
- 17.Cesarz Z, Tamama K. Spheroid Culture of Mesenchymal Stem Cells. Stem Cells Int 2016: 9176357, 2016. doi: 10.1155/2016/9176357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaubey A, Ross KJ, Leadbetter RM, Burg KJL. Surface patterning: tool to modulate stem cell differentiation in an adipose system. J Biomed Mater Res B Appl Biomater 84: 70–78, 2008. doi: 10.1002/jbm.b.30846. [DOI] [PubMed] [Google Scholar]
- 19.Chaudhuri O, Gu L, Klumpers D, Darnell M, Bencherif SA, Weaver JC, Huebsch N, Lee HP, Lippens E, Duda GN, Mooney DJ. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat Mater 15: 326–334, 2016. doi: 10.1038/nmat4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen VJ, Ma PX. Nano-fibrous poly(L-lactic acid) scaffolds with interconnected spherical macropores. Biomaterials 25: 2065–2073, 2004. doi: 10.1016/j.biomaterials.2003.08.058. [DOI] [PubMed] [Google Scholar]
- 21.Chitcholtan K, Asselin E, Parent S, Sykes PH, Evans JJ. Differences in growth properties of endometrial cancer in three dimensional (3D) culture and 2D cell monolayer. Exp Cell Res 319: 75–87, 2013. doi: 10.1016/j.yexcr.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 22.Choi SW, Yeh YC, Zhang Y, Sung HW, Xia Y. Uniform beads with controllable pore sizes for biomedical applications. Small 6: 1492–1498, 2010. doi: 10.1002/smll.201000544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chueh BH, Zheng Y, Torisawa YS, Hsiao AY, Ge C, Hsiong S, Huebsch N, Franceschi R, Mooney DJ, Takayama S. Patterning alginate hydrogels using light-directed release of caged calcium in a microfluidic device. Biomed Microdevices 12: 145–151, 2010. doi: 10.1007/s10544-009-9369-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cimetta E, Figallo E, Cannizzaro C, Elvassore N, Vunjak-Novakovic G. Micro-bioreactor arrays for controlling cellular environments: design principles for human embryonic stem cell applications. Methods 47: 81–89, 2009. doi: 10.1016/j.ymeth.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science 294: 1708–1712, 2001. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- 26.DeForest CA, Polizzotti BD, Anseth KS. Sequential click reactions for synthesizing and patterning three-dimensional cell microenvironments. Nat Mater 8: 659–664, 2009. doi: 10.1038/nmat2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeForest CA, Sims EA, Anseth KS. Peptide-functionalized click hydrogels with independently tunable mechanics and chemical functionality for 3D cell culture. Chem Mater 22: 4783–4790, 2010. doi: 10.1021/cm101391y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science 310: 1139–1143, 2005. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 29.Dunn JCY, Tompkins RG, Yarmush ML. Hepatocytes in collagen sandwich: evidence for transcriptional and translational regulation. J Cell Biol 116: 1043–1053, 1992. doi: 10.1083/jcb.116.4.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, Elvassore N, Piccolo S. Role of YAP/TAZ in mechanotransduction. Nature 474: 179–183, 2011. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 31.Edmondson R, Broglie JJ, Adcock AF, Yang L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev Technol 12: 207–218, 2014. doi: 10.1089/adt.2014.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell 126: 677–689, 2006. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 33.Esch EW, Bahinski A, Huh D. Organs-on-chips at the frontiers of drug discovery. Nat Rev Drug Discov 14: 248–260, 2015. doi: 10.1038/nrd4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ezzell RM, Toner M, Hendricks K, Dunn JC, Tompkins RG, Yarmush ML. Effect of collagen gel configuration on the cytoskeleton in cultured rat hepatocytes. Exp Cell Res 208: 442–452, 1993. doi: 10.1006/excr.1993.1266. [DOI] [PubMed] [Google Scholar]
- 35.Fennema E, Rivron N, Rouwkema J, van Blitterswijk C, de Boer J. Spheroid culture as a tool for creating 3D complex tissues. Trends Biotechnol 31: 108–115, 2013. doi: 10.1016/j.tibtech.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 36.Fierz FC, Beckmann F, Huser M, Irsen SH, Leukers B, Witte F, Degistirici O, Andronache A, Thie M, Müller B. The morphology of anisotropic 3D-printed hydroxyapatite scaffolds. Biomaterials 29: 3799–3806, 2008. doi: 10.1016/j.biomaterials.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 37.Figallo E, Cannizzaro C, Gerecht S, Burdick JA, Langer R, Elvassore N, Vunjak-Novakovic G. Micro-bioreactor array for controlling cellular microenvironments. Lab Chip 7: 710–719, 2007. doi: 10.1039/b700063d. [DOI] [PubMed] [Google Scholar]
- 38.Fozdar DY, Soman P, Lee JW, Han LH, Chen S. Three-Dimensional Polymer Constructs Exhibiting a Tunable Negative Poisson’s Ratio. Adv Funct Mater 21: 2712–2720, 2011. doi: 10.1002/adfm.201002022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Friedl P, Sahai E, Weiss S, Yamada KM. New dimensions in cell migration. Nat Rev Mol Cell Biol 13: 743–747, 2012. doi: 10.1038/nrm3459. [DOI] [PubMed] [Google Scholar]
- 40.Fu J, Wang YK, Yang MT, Desai RA, Yu X, Liu Z, Chen CS. Mechanical regulation of cell function with geometrically modulated elastomeric substrates. Nat Methods 7: 733–736, 2010. doi: 10.1038/nmeth.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gauvin R, Chen YC, Lee JW, Soman P, Zorlutuna P, Nichol JW, Bae H, Chen S, Khademhosseini A. Microfabrication of complex porous tissue engineering scaffolds using 3D projection stereolithography. Biomaterials 33: 3824–3834, 2012. doi: 10.1016/j.biomaterials.2012.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gjorevski N, Piotrowski AS, Varner VD, Nelson CM. Dynamic tensile forces drive collective cell migration through three-dimensional extracellular matrices. Sci Rep 5: 11458, 2015. doi: 10.1038/srep11458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gogoi P, Sepehri S, Zhou Y, Gorin MA, Paolillo C, Capoluongo E, Gleason K, Payne A, Boniface B, Cristofanilli M, Morgan TM, Fortina P, Pienta KJ, Handique K, Wang Y. Development of an automated and sensitive microfluidic device for capturing and characterizing circulating tumor cells (CTCs) from clinical blood samples. PLoS One 11: e0147400, 2016. doi: 10.1371/journal.pone.0147400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Griffin DR, Weaver WM, Scumpia PO, Di Carlo D, Segura T. Accelerated wound healing by injectable microporous gel scaffolds assembled from annealed building blocks. Nat Mater 14: 737–744, 2015. doi: 10.1038/nmat4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grinnell F. Fibroblast biology in three-dimensional collagen matrices. Trends Cell Biol 13: 264–269, 2003. doi: 10.1016/S0962-8924(03)00057-6. [DOI] [PubMed] [Google Scholar]
- 46.Gu L, Mooney DJ. Biomaterials and emerging anticancer therapeutics: engineering the microenvironment. Nat Rev Cancer 16: 56–66, 2016. doi: 10.1038/nrc.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hakkinen KM, Harunaga JS, Doyle AD, Yamada KM. Direct comparisons of the morphology, migration, cell adhesions, and actin cytoskeleton of fibroblasts in four different three-dimensional extracellular matrices. Tissue Eng Part A 17: 713–724, 2011. doi: 10.1089/ten.tea.2010.0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Han L-H, Yu S, Wang T, Behn AW, Yang F. Microribbon-like elastomers for fabricating macroporous and highly flexible scaffolds that support cell proliferation in 3D. Adv Funct Mater 23: 346–358, 2013. doi: 10.1002/adfm.201201212. [DOI] [Google Scholar]
- 49.Han LH, Conrad B, Chung MT, Deveza L, Jiang X, Wang A, Butte MJ, Longaker MT, Wan D, Yang F. Winner of the Young Investigator Award of the Society for Biomaterials at the 10th World Biomaterials Congress, May 17-22, 2016, Montreal QC, Canada: Microribbon-based hydrogels accelerate stem cell-based bone regeneration in a mouse critical-size cranial defect model. J Biomed Mater Res A 104: 1321–1331, 2016. doi: 10.1002/jbm.a.35715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Han LH, Lai JH, Yu S, Yang F. Dynamic tissue engineering scaffolds with stimuli-responsive macroporosity formation. Biomaterials 34: 4251–4258, 2013. doi: 10.1016/j.biomaterials.2013.02.051. [DOI] [PubMed] [Google Scholar]
- 51.Han LH, Tong X, Yang F. Photo-crosslinkable PEG-based microribbons for forming 3D macroporous scaffolds with decoupled niche properties. Adv Mater 26: 1757–1762, 2014. doi: 10.1002/adma.201304805. [DOI] [PubMed] [Google Scholar]
- 52.Haraguchi Y, Shimizu T, Sasagawa T, Sekine H, Sakaguchi K, Kikuchi T, Sekine W, Sekiya S, Yamato M, Umezu M, Okano T. Fabrication of functional three-dimensional tissues by stacking cell sheets in vitro. Nat Protoc 7: 850–858, 2012. doi: 10.1038/nprot.2012.027. [DOI] [PubMed] [Google Scholar]
- 53.Hollister SJ. Porous scaffold design for tissue engineering. Nat Mater 4: 518–524, 2005. doi: 10.1038/nmat1421. [DOI] [PubMed] [Google Scholar]
- 54.Hong Y, Guan J, Fujimoto KL, Hashizume R, Pelinescu AL, Wagner WR. Tailoring the degradation kinetics of poly(ester carbonate urethane)urea thermoplastic elastomers for tissue engineering scaffolds. Biomaterials 31: 4249–4258, 2010. doi: 10.1016/j.biomaterials.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hsiao AY, Tung Y-C, Kuo C-H, Mosadegh B, Bedenis R, Pienta KJ, Takayama S. Micro-ring structures stabilize microdroplets to enable long term spheroid culture in 384 hanging drop array plates. Biomed Microdevices 14: 313–323, 2012. doi: 10.1007/s10544-011-9608-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang AH, Niklason LE. Engineering of arteries in vitro. Cell Mol Life Sci 71: 2103–2118, 2014. doi: 10.1007/s00018-013-1546-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huh D, Hamilton GA, Ingber DE. From 3D cell culture to organs-on-chips. Trends Cell Biol 21: 745–754, 2011. doi: 10.1016/j.tcb.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huh D, Kim HJ, Fraser JP, Shea DE, Khan M, Bahinski A, Hamilton GA, Ingber DE. Microfabrication of human organs-on-chips. Nat Protoc 8: 2135–2157, 2013. doi: 10.1038/nprot.2013.137. [DOI] [PubMed] [Google Scholar]
- 59.Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE. Reconstituting organ-level lung functions on a chip. Science 328: 1662–1668, 2010. doi: 10.1126/science.1188302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hwang CM, Sant S, Masaeli M, Kachouie NN, Zamanian B, Lee SH, Khademhosseini A. Fabrication of three-dimensional porous cell-laden hydrogel for tissue engineering. Biofabrication 2: 035003, 2010. doi: 10.1088/1758-5082/2/3/035003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ihalainen TO, Aires L, Herzog FA, Schwartlander R, Moeller J, Vogel V. Differential basal-to-apical accessibility of lamin A/C epitopes in the nuclear lamina regulated by changes in cytoskeletal tension. Nat Mater 14: 1252–1261, 2015. doi: 10.1038/nmat4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Imamura Y, Mukohara T, Shimono Y, Funakoshi Y, Chayahara N, Toyoda M, Kiyota N, Takao S, Kono S, Nakatsura T, Minami H. Comparison of 2D- and 3D-culture models as drug-testing platforms in breast cancer. Oncol Rep 33: 1837–1843, 2015. doi: 10.3892/or.2015.3767. [DOI] [PubMed] [Google Scholar]
- 63.Ji C, Khademhosseini A, Dehghani F. Enhancing cell penetration and proliferation in chitosan hydrogels for tissue engineering applications. Biomaterials 32: 9719–9729, 2011. doi: 10.1016/j.biomaterials.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 64.Jiang H-L, Kim Y-K, Cho K-H, Jang Y-C, Choi Y-J, Chung J-H, Cho C-S. Roles of spheroid formation of hepatocytes in liver tissue engineering. Int J Stem Cells 3: 69–73, 2010. doi: 10.15283/ijsc.2010.3.2.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jones HM, Barton HA, Lai Y, Bi YA, Kimoto E, Kempshall S, Tate SC, El-Kattan A, Houston JB, Galetin A, Fenner KS. Mechanistic pharmacokinetic modeling for the prediction of transporter-mediated disposition in humans from sandwich culture human hepatocyte data. Drug Metab Dispos 40: 1007–1017, 2012. doi: 10.1124/dmd.111.042994. [DOI] [PubMed] [Google Scholar]
- 66.Kawazoe N, Inoue C, Tateishi T, Chen G. A cell leakproof PLGA-collagen hybrid scaffold for cartilage tissue engineering. Biotechnol Prog 26: 819–826, 2010. doi: 10.1002/btpr.375. [DOI] [PubMed] [Google Scholar]
- 67.Kesimer M, Kirkham S, Pickles RJ, Henderson AG, Alexis NE, Demaria G, Knight D, Thornton DJ, Sheehan JK. Tracheobronchial air-liquid interface cell culture: a model for innate mucosal defense of the upper airways? Am J Physiol Lung Cell Mol Physiol 296: L92–L100, 2009. doi: 10.1152/ajplung.90388.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Khalil S, Sun W. Bioprinting endothelial cells with alginate for 3D tissue constructs. J Biomech Eng 131: 111002, 2009. doi: 10.1115/1.3128729. [DOI] [PubMed] [Google Scholar]
- 69.Kimlin LC, Casagrande G, Virador VM. In vitro three-dimensional (3D) models in cancer research: an update. Mol Carcinog 52: 167–182, 2013. doi: 10.1002/mc.21844. [DOI] [PubMed] [Google Scholar]
- 70.Kloxin AM, Kasko AM, Salinas CN, Anseth KS. Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science 324: 59–63, 2009. doi: 10.1126/science.1169494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Koch TM, Münster S, Bonakdar N, Butler JP, Fabry B. 3D Traction forces in cancer cell invasion. PLoS One 7: e33476, 2012. doi: 10.1371/journal.pone.0033476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kolind K, Leong KW, Besenbacher F, Foss M. Guidance of stem cell fate on 2D patterned surfaces. Biomaterials 33: 6626–6633, 2012. doi: 10.1016/j.biomaterials.2012.05.070. [DOI] [PubMed] [Google Scholar]
- 73.LeCluyse EL, Audus KL, Hochman JH. Formation of extensive canalicular networks by rat hepatocytes cultured in collagen-sandwich configuration. Am J Physiol 266: C1764–C1774, 1994. [DOI] [PubMed] [Google Scholar]
- 74.Lee K, Hubbell JA. Tissue, cell and engineering. Curr Opin Biotechnol 24: 827–829, 2013. doi: 10.1016/j.copbio.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 75.Lee S-A, No Y, Kang E, Ju J, Kim D-S, Lee S-H. Spheroid-based three-dimensional liver-on-a-chip to investigate hepatocyte-hepatic stellate cell interactions and flow effects. Lab Chip 13: 3529–3537, 2013. doi: 10.1039/c3lc50197c. [DOI] [PubMed] [Google Scholar]
- 76.Lemmo S, Atefi E, Luker GD, Tavana H. Optimization of Aqueous Biphasic Tumor Spheroid Microtechnology for Anti-Cancer Drug Testing in 3D Culture. Cell Mol Bioeng 7: 344–354, 2014. doi: 10.1007/s12195-014-0349-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li Q, Williams CG, Sun DD, Wang J, Leong K, Elisseeff JH. Photocrosslinkable polysaccharides based on chondroitin sulfate. J Biomed Mater Res A 68: 28–33, 2004. doi: 10.1002/jbm.a.20007. [DOI] [PubMed] [Google Scholar]
- 78.Liao S, Chan CK, Ramakrishna S. Stem cells and biomimetic materials strategies for tissue engineering. Mater Sci Eng C 28: 1189–1202, 2008. doi: 10.1016/j.msec.2008.08.015. [DOI] [Google Scholar]
- 79.Lu H, Ko YG, Kawazoe N, Chen G. Cartilage tissue engineering using funnel-like collagen sponges prepared with embossing ice particulate templates. Biomaterials 31: 5825–5835, 2010. doi: 10.1016/j.biomaterials.2010.04.019. [DOI] [PubMed] [Google Scholar]
- 80.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol 23: 47–55, 2005. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 81.Mabry KM, Payne SZ, Anseth KS. Microarray analyses to quantify advantages of 2D and 3D hydrogel culture systems in maintaining the native valvular interstitial cell phenotype. Biomaterials 74: 31–41, 2016. doi: 10.1016/j.biomaterials.2015.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell 6: 483–495, 2004. doi: 10.1016/S1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 83.Mondrinos MJ, Dembzynski R, Lu L, Byrapogu VK, Wootton DM, Lelkes PI, Zhou J. Porogen-based solid freeform fabrication of polycaprolactone-calcium phosphate scaffolds for tissue engineering. Biomaterials 27: 4399–4408, 2006. doi: 10.1016/j.biomaterials.2006.03.049. [DOI] [PubMed] [Google Scholar]
- 84.Muzzarelli RA, Mattioli-Belmonte M, Tietz C, Biagini R, Ferioli G, Brunelli MA, Fini M, Giardino R, Ilari P, Biagini G. Stimulatory effect on bone formation exerted by a modified chitosan. Biomaterials 15: 1075–1081, 1994. doi: 10.1016/0142-9612(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 85.Nalayanda DD, Puleo C, Fulton WB, Sharpe LM, Wang TH, Abdullah F. An open-access microfluidic model for lung-specific functional studies at an air-liquid interface. Biomed Microdevices 11: 1081–1089, 2009. doi: 10.1007/s10544-009-9325-5. [DOI] [PubMed] [Google Scholar]
- 86.Napolitano AP, Chai P, Dean DM, Morgan JR. Dynamics of the self-assembly of complex cellular aggregates on micromolded nonadhesive hydrogels. Tissue Eng 13: 2087–2094, 2007. doi: 10.1089/ten.2006.0190. [DOI] [PubMed] [Google Scholar]
- 87.Nichol JW, Koshy ST, Bae H, Hwang CM, Yamanlar S, Khademhosseini A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 31: 5536–5544, 2010. doi: 10.1016/j.biomaterials.2010.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nii M, Lai JH, Keeney M, Han LH, Behn A, Imanbayev G, Yang F. The effects of interactive mechanical and biochemical niche signaling on osteogenic differentiation of adipose-derived stem cells using combinatorial hydrogels. Acta Biomater 9: 5475–5483, 2013. doi: 10.1016/j.actbio.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 89.Ovsianikov A, Schlie S, Ngezahayo A, Haverich A, Chichkov BN. Two-photon polymerization technique for microfabrication of CAD-designed 3D scaffolds from commercially available photosensitive materials. J Tissue Eng Regen Med 1: 443–449, 2007. doi: 10.1002/term.57. [DOI] [PubMed] [Google Scholar]
- 90.Pashneh-Tala S, MacNeil S, Claeyssens F. The tissue-engineered vascular graft—past, present, and future. Tissue Eng Part B Rev. In press. doi: 10.1089/ten.teb.2015.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pineda ET, Nerem RM, Ahsan T. Differentiation patterns of embryonic stem cells in two- versus three-dimensional culture. Cells Tissues Organs 197: 399–410, 2013. doi: 10.1159/000346166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Place ES, Evans ND, Stevens MM. Complexity in biomaterials for tissue engineering. Nat Mater 8: 457–470, 2009. doi: 10.1038/nmat2441. [DOI] [PubMed] [Google Scholar]
- 94.Reis N, Gonçalves CN, Vicente AA, Teixeira JA. Proof-of-concept of a novel micro-bioreactor for fast development of industrial bioprocesses. Biotechnol Bioeng 95: 744–753, 2006. doi: 10.1002/bit.21035. [DOI] [PubMed] [Google Scholar]
- 95.Sackmann EK, Fulton AL, Beebe DJ. The present and future role of microfluidics in biomedical research. Nature 507: 181–189, 2014. doi: 10.1038/nature13118. [DOI] [PubMed] [Google Scholar]
- 96.Salerno A, Guarnieri D, Iannone M, Zeppetelli S, Netti PA. Effect of micro- and macroporosity of bone tissue three-dimensional-poly(epsilon-caprolactone) scaffold on human mesenchymal stem cells invasion, proliferation, and differentiation in vitro. Tissue Eng Part A 16: 2661–2673, 2010. doi: 10.1089/ten.tea.2009.0494. [DOI] [PubMed] [Google Scholar]
- 97.Sarvi F, Jain K, Arbatan T, Verma PJ, Hourigan K, Thompson MC, Shen W, Chan PPY. Cardiogenesis of embryonic stem cells with liquid marble micro-bioreactor. Adv Healthc Mater 4: 77–86, 2015. doi: 10.1002/adhm.201400138. [DOI] [PubMed] [Google Scholar]
- 98.Scadden DT. The stem-cell niche as an entity of action. Nature 441: 1075–1079, 2006. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- 99.Scott EA, Nichols MD, Kuntz-Willits R, Elbert DL. Modular scaffolds assembled around living cells using poly(ethylene glycol) microspheres with macroporation via a non-cytotoxic porogen. Acta Biomater 6: 29–38, 2010. doi: 10.1016/j.actbio.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shimizu T, Yamato M, Kikuchi A, Okano T. Cell sheet engineering for myocardial tissue reconstruction. Biomaterials 24: 2309–2316, 2003. doi: 10.1016/S0142-9612(03)00110-8. [DOI] [PubMed] [Google Scholar]
- 101.Silva MMCG, Cyster LA, Barry JJA, Yang XB, Oreffo ROC, Grant DM, Scotchford CA, Howdle SM, Shakesheff KM, Rose FRAJ. The effect of anisotropic architecture on cell and tissue infiltration into tissue engineering scaffolds. Biomaterials 27: 5909–5917, 2006. doi: 10.1016/j.biomaterials.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 102.Sirenko O, Mitlo T, Hesley J, Luke S, Owens W, Cromwell EF. High-content assays for characterizing the viability and morphology of 3D cancer spheroid cultures. Assay Drug Dev Technol 13: 402–414, 2015. doi: 10.1089/adt.2015.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Slaughter BV, Khurshid SS, Fisher OZ, Khademhosseini A, Peppas NA. Hydrogels in regenerative medicine. Adv Mater 21: 3307–3329, 2009. doi: 10.1002/adma.200802106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Soldatow VY, Lecluyse EL, Griffith LG, Rusyn I. In vitro models for liver toxicity testing. Toxicol Res (Camb) 2: 23–39, 2013. doi: 10.1039/C2TX20051A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Steinwachs J, Metzner C, Skodzek K, Lang N, Thievessen I, Mark C, Münster S, Aifantis KE, Fabry B. Three-dimensional force microscopy of cells in biopolymer networks. Nat Methods 13: 171–176, 2016. doi: 10.1038/nmeth.3685. [DOI] [PubMed] [Google Scholar]
- 106.Stevens MM, George JH. Exploring and engineering the cell surface interface. Science 310: 1135–1138, 2005. doi: 10.1126/science.1106587. [DOI] [PubMed] [Google Scholar]
- 107.Sun BK, Siprashvili Z, Khavari PA. Advances in skin grafting and treatment of cutaneous wounds. Science 346: 941–945, 2014. doi: 10.1126/science.1253836. [DOI] [PubMed] [Google Scholar]
- 108.Suri S, Schmidt CE. Cell-laden hydrogel constructs of hyaluronic acid, collagen, and laminin for neural tissue engineering. Tissue Eng Part A 16: 1703–1716, 2010. doi: 10.1089/ten.tea.2009.0381. [DOI] [PubMed] [Google Scholar]
- 109.Théry M. Micropatterning as a tool to decipher cell morphogenesis and functions. J Cell Sci 123: 4201–4213, 2010. doi: 10.1242/jcs.075150. [DOI] [PubMed] [Google Scholar]
- 110.Torisawa YS, Takagi A, Nashimoto Y, Yasukawa T, Shiku H, Matsue T. A multicellular spheroid array to realize spheroid formation, culture, and viability assay on a chip. Biomaterials 28: 559–566, 2007. doi: 10.1016/j.biomaterials.2006.08.054. [DOI] [PubMed] [Google Scholar]
- 111.Tung Y-C, Hsiao AY, Allen SG, Torisawa YS, Ho M, Takayama S. High-throughput 3D spheroid culture and drug testing using a 384 hanging drop array. Analyst (Lond) 136: 473–478, 2011. doi: 10.1039/C0AN00609B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Underhill GH, Bhatia SN. High-throughput analysis of signals regulating stem cell fate and function. Curr Opin Chem Biol 11: 357–366, 2007. doi: 10.1016/j.cbpa.2007.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wan Y, Wang Y, Liu Z, Qu X, Han B, Bei J, Wang S. Adhesion and proliferation of OCT-1 osteoblast-like cells on micro- and nano-scale topography structured poly(L-lactide). Biomaterials 26: 4453–4459, 2005. doi: 10.1016/j.biomaterials.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 114.Wang F, Weaver VM, Petersen OW, Larabell CA, Dedhar S, Briand P, Lupu R, Bissell MJ. Reciprocal interactions between beta1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: a different perspective in epithelial biology. Proc Natl Acad Sci USA 95: 14821–14826, 1998. doi: 10.1073/pnas.95.25.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang K, Cai L-H, Lan B, Fredberg JJ. Hidden in the mist no more: physical force in cell biology. Nat Methods 13: 124–125, 2016. doi: 10.1038/nmeth.3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang T, Lai JH, Han L-H, Tong X, Yang F. Chondrogenic differentiation of adipose-derived stromal cells in combinatorial hydrogels containing cartilage matrix proteins with decoupled mechanical stiffness. Tissue Eng Part A 20: 2131–2139, 2014. doi: 10.1089/ten.tea.2013.0531. [DOI] [PubMed] [Google Scholar]
- 117.Wang Y, Bella E, Lee CSD, Migliaresi C, Pelcastre L, Schwartz Z, Boyan BD, Motta A. The synergistic effects of 3-D porous silk fibroin matrix scaffold properties and hydrodynamic environment in cartilage tissue regeneration. Biomaterials 31: 4672–4681, 2010. doi: 10.1016/j.biomaterials.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 118.Whitesides GM. The origins and the future of microfluidics. Nature 442: 368–373, 2006. doi: 10.1038/nature05058. [DOI] [PubMed] [Google Scholar]
- 119.Xiao J, Duan H, Liu Z, Wu Z, Lan Y, Zhang W, Li C, Chen F, Zhou Q, Wang X, Huang J, Wang Z. Construction of the recellularized corneal stroma using porous acellular corneal scaffold. Biomaterials 32: 6962–6971, 2011. doi: 10.1016/j.biomaterials.2011.05.084. [DOI] [PubMed] [Google Scholar]
- 120.Xie L, Mao M, Zhou L, Jiang B. Spheroid mesenchymal stem cells and mesenchymal stem cell-derived microvesicles: two potential therapeutic strategies. Stem Cells Dev 25: 203–213, 2016. doi: 10.1089/scd.2015.0278. [DOI] [PubMed] [Google Scholar]
- 121.Xu X, Wang W, Kratz K, Fang L, Li Z, Kurtz A, Ma N, Lendlein A. Controlling major cellular processes of human mesenchymal stem cells using microwell structures. Adv Healthc Mater 3: 1991–2003, 2014. doi: 10.1002/adhm.201400415. [DOI] [PubMed] [Google Scholar]
- 122.Yang B, Yin Z, Cao J, Shi Z, Zhang Z, Song H, Liu F, Caterson B. In vitro cartilage tissue engineering using cancellous bone matrix gelatin as a biodegradable scaffold. Biomed Mater 5: 045003, 2010. doi: 10.1088/1748-6041/5/4/045003. [DOI] [PubMed] [Google Scholar]
- 123.Yeatts AB, Choquette DT, Fisher JP. Bioreactors to influence stem cell fate: augmentation of mesenchymal stem cell signaling pathways via dynamic culture systems. Biochim Biophys Acta 1830: 2470–2480, 2013. doi: 10.1016/j.bbagen.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yin N, Stilwell MD, Santos TMA, Wang H, Weibel DB. Agarose particle-templated porous bacterial cellulose and its application in cartilage growth in vitro. Acta Biomater 12: 129–138, 2015. doi: 10.1016/j.actbio.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yoshii Y, Waki A, Yoshida K, Kakezuka A, Kobayashi M, Namiki H, Kuroda Y, Kiyono Y, Yoshii H, Furukawa T, Asai T, Okazawa H, Gelovani JG, Fujibayashi Y. The use of nanoimprinted scaffolds as 3D culture models to facilitate spontaneous tumor cell migration and well-regulated spheroid formation. Biomaterials 32: 6052–6058, 2011. doi: 10.1016/j.biomaterials.2011.04.076. [DOI] [PubMed] [Google Scholar]
- 126.Zanetti AS, Sabliov C, Gimble JM, Hayes DJ. Human adipose-derived stem cells and three-dimensional scaffold constructs: a review of the biomaterials and models currently used for bone regeneration. J Biomed Mater Res B Appl Biomater 101: 187–199, 2013. doi: 10.1002/jbm.b.32817. [DOI] [PubMed] [Google Scholar]
- 127.Zhang W, Wray LS, Rnjak-Kovacina J, Xu L, Zou D, Wang S, Zhang M, Dong J, Li G, Kaplan DL, Jiang X. Vascularization of hollow channel-modified porous silk scaffolds with endothelial cells for tissue regeneration. Biomaterials 56: 68–77, 2015. doi: 10.1016/j.biomaterials.2015.03.053. [DOI] [PubMed] [Google Scholar]
- 128.Zöller N, Valesky E, Butting M, Hofmann M, Kippenberger S, Bereiter-Hahn J, Bernd A, Kaufmann R. Clinical application of a tissue-cultured skin autograft: an alternative for the treatment of non-healing or slowly healing wounds? Dermatology 229: 190–198, 2014. doi: 10.1159/000362927. [DOI] [PubMed] [Google Scholar]
- 129.Zuppinger C. 3D culture for cardiac cells. Biochim Biophys Acta 1863: 1873–1881, 2016. doi: 10.1016/j.bbamcr.2015.11.036. [DOI] [PubMed] [Google Scholar]