Abstract
Background
Cessation of long-term aspirin treatment before noncardiac surgery can cause adverse cardiac events in patients at risk, particularly in those with previous percutaneous coronary interventions (PCI) with stent implantation. The factors influencing the clinical decision to stop aspirin treatment are currently unknown.
Methods
In a single-center, cross-sectional study (retrospective registration: NCT03049566) carried out from February to December 2014, we took a survey among patients scheduled for noncardiac surgery who were under long-term aspirin treatment, and among their treating anesthesiologists using standardized questionnaires on preoperative aspirin use, comorbidities, and risk-benefit assessments. The main objective was to identify factors associated with the decision to stop aspirin treatment. The results of multivariable logistic regressions and intraclass correlations are presented.
Results
805 patients were included in the study, and 636 questionnaires were returned (203 of which concerned patients with coronary stents). 46.8% of the patients stopped their long-term aspirin treatment before surgery; 38.7% of these patients stopped it too early (>10 days before surgery) or too late (= 3 days before surgery). A prior PCI with stent implantation lowered the probability of aspirin cessation (odds ratio [OR] = 0.47 [0.31; 0.72]; p <0.001). On the other hand, patients were more likely to stop their long-term aspirin treatment if it had already been discontinued once before (OR = 4.58 [3.06; 6.84]; p <0.001), if there was a risk of bleeding into a closed space (OR = 4.54 [2.02; 10.22]; p <0.001), if they did not know why they were supposed to take aspirin (OR = 2.12 [1.05; 4.28]; p = 0.036), or if the preoperative consultation with the anesthesiologist occurred <2 days before surgery (OR = 1.60 [1.08; 2.37]; p = 0.018). Patients often assessed the risks related to aspirin cessation lower than their physicians did.
Conclusion
This study reveals discordance between guideline recommendations and everyday clinical practice in patients with coronary stents. The early integration of cardiologists and anesthesiologists and a more widespread use of stent implant cards could promote adherence to the guidelines.
Aspirin (acetyl salicylic acid) has been shown to be effective for secondary prevention of cardiovascular disease (CVD) (1). In addition, aspirin is used by a large proportion of the population for primary prevention (2, 3). In Germany, more than 1 in 10 adults aged between 45 and 75 are taking aspirin as an antiplatelet agent (prevalence 11.5%, in men: 13.2%, in women: 9.8%; source: unpublished data from the DEGS1 study 2008–2011 [4]).
In patients with CVD, the lifelong use of aspirin for secondary prevention is recommended. Discontinuation of antiplatelet therapy may trigger a prothrombotic rebound phenomenon (5). When aspirin therapy for secondary CVD prevention is stopped, the risk of myocardial infarction may increase by more than 60% (6). A meta-analysis found that aspirin discontinuation conferred a three-fold increase in the risk of major adverse cardiac events in patients at risk for, or with confirmed coronary artery disease (CAD) (7).
In Germany, the lifetime prevalence of CAD during the period from 2008 to 2011 was 9.3% (8). In 2008, the number of percutaneous coronary interventions (PCIs) performed in Germany exceeded 300 000 (9). The risk of stent thrombosis and myocardial infarction is markedly increased when antiplatelet therapy is discontinued soon after PCI (=6 months with drug-eluting stents and =1 month with bare-metal stents) (10). During surgical procedures, patients develop a proinflammatory, hypercoagulable state which promotes thromboembolic events (11).
This is a clinically relevant problem because approximately 20% of patients undergo noncardiac surgery during the first 2 years after PCI (12– 17). In these patients, a considerably increased incidence of major cardiac events has to be expected (12). Perioperative cessation of aspirin therapy is considered a key risk factor in patients with coronary stents (18– 21).
Clinical decision making with regard to perioperative cessation of aspirin therapy is challenging as two opposed risks need to be balanced: the risk of cardiovascular thromboembolic complications in case of therapy cessation and the risk of hemorrhagic surgical complication in case of therapy continuation (22).
Thus, the European (10) and US (23) guidelines recommend to perform an individualized risk-benefit analysis prior to elective surgery: If the perioperative risk of hemorrhage clearly exceeds the potential cardiovascular benefits, aspirin therapy should be stopped. In patients with previous PCI and stent implantation, long-term aspirin therapy should be continued perioperatively, if possible. However, a high risk of hemorrhage in a closed space (intracranial, intramedullary or posterior chamber of the eye) is regarded as an important exception (24, 25). In patients treated with dual antiplatelet therapy after recent PCI, elective surgical procedures should be postponed.
It has been recommended in guidelines to stop aspirin therapy, if indicated, 7 to 10 days (10, 26) before surgery. However, studies involving preoperative platelet function tests reported faster recovery of platelet function (27, 28). It is an expert opinion of some authors to stop aspirin, if indicated, 5 days before surgery (29). Unfortunately, larger comparative randomized studies evaluating optimal timing of aspirin cessation prior to noncardiac surgery with regard to thromboembolic and hemorrhagic complications are lacking (26).
While the association between perioperative aspirin therapy and cardiovascular complications has been investigated in many studies, it has not yet been studied systematically which factors contribute to the individual decision to preoperatively stop aspirin therapy in everyday clinical practice.
The primary aim of this study was to identify factors associated with the decision to preoperatively stop aspirin therapy and in particular to determine whether coronary stents represent an independent factor influencing decision-making. The secondary aim of this study was to explore in which way the decision to stop aspirin therapy is associated with individual risk-benefit assessments of patients and treating physicians.
Methods
This single-center cross-sectional study received the approval of the Ethics Committee of the Medical Association of Hamburg (reference number WF03013) and was retrospectively registered at ClinicalTrials.gov (NCT03049566). Selective sampling was used: Between 10 February 2014 and 30 December 2014, adult patients
presenting at the Anesthesiology Pre-assessment Clinic of the University Medical Center Hamburg-Eppendorf prior to elective noncardiac surgery,
stating to regularly receive antiplatelet therapy with low-dose aspirin (even if this treatment was discontinued within 30 days of study inclusion) and
giving their informed consent to participate
were included in this study.
The study population included a large subgroup of patients with previous PCI.
Using a two-part standardized questionnaire (part 1: patient; part 2: treating anesthesiologist), detailed information about aspirin medication, perioperative aspirin use and factors which may influence the decision to discontinue aspirin therapy was collected in an anonymized fashion. In addition, patients and the corresponding physicians were asked to make risk-benefit assessments using two numerical rating scales (evaluation of the benefits of long-term aspirin therapy and the risk associated with perioperative aspirin cessation: 1 = very low, 10 = very high). Detailed information is provided in the eMethods section.
Methods of statistical analysis
“Cessation of aspirin therapy” was the primary outcome measure of this study and its primary aim was to identify factors associated with the decision to preoperatively stop an existing long-term aspirin therapy. To answer the primary question, logistic regression analysis was used to identify factors potentially influencing the primary outcome. Missing data were not imputed. In multivariable regression analyses, cases were deleted listwise. We chose a two-step procedure for variable selection: In the context of our research question, potential parameters were initially selected based on clinical aspects and then identified using univariate regression analysis. Redundant independent variables with subsequent multicollinearity were excluded. In the second step, a multivariable logistic regression analysis with backward elimination was performed. The results are reported as odds ratios (ORs) with 95% confidence intervals (95% CIs).
The four ratings resulting from the risk-benefit assessment were tested in a multivariable model with regard to the primary outcome measure, using an explorative approach. As a quantitative measure of the agreement between physician and patient ratings, the respective intraclass correlation coefficients (ICCs) were calculated. An ICC <0.4 is indicative of low interrater agreement (30).
Other questions were answered in a descriptive manner. Categorical variables are reported as relative frequencies in relation to the sum of the valid answers. Time variables are reported as medians (range). Continuous variables are reported as means and, after verification of normal distribution, were compared using Student‘s t-test.
We used a significance level of 0.05 (two-sided) for the primary question. This significance level was not adjusted for multiple testing. For statistical analysis, we used SPSS for Windows Version 22.0 (IBM SPSS Inc, Chicago, IL, USA) as well as the statistical analysis software solution R 3.2.3 (31).
Results
Altogether, 9481 patients were screened and 836 met the inclusion criteria; of these, 31 did not consent to participe in this study. Questionnaires were handed out to the 805 included participants and 636 evaluable questionnaires were returned (response rate of 79.0%).
Our study population comprised a broad range of noncardiac surgery patients, but no patients scheduled for ophthalmologic surgery. 46.8% of the patients participating in the study stopped their long-term aspirin therapy preoperatively. 31.9% of the participants had coronary stents. In 7.8% of the participants, a risk of hemorrhage in a closed space was found (table 1).
Table 1. Patient characteristics of the study cohort (n = 636).
| Patient characteristic |
Missing values |
Number of patients (n) |
% |
|
Pre-exiting conditions and medications Previous percutaneous coronary intervention (PCI) with stent implantation History of cardiac disease Coronary artery disease Previous coronary artery bypass graft surgery (CABG) Cardiac arrhythmia History of stroke or transient ischemic attack Peripheral arterial occlusive disease Oral anticoagulants Dual antiplatelet therapy |
0 0 0 1 1 1 1 14 23 |
203 343 300 72 56 137 92 22 32 |
31.9 53.9 47.2 11.3 8.8 21.6 14.5 3.5 5.2 |
|
Aspirin therapy cessation in patients with previous PCI and stent implantation in patients without coronary stents |
27 | 285 67 218 |
46.8 11.0 35.8 |
|
Surgical discipline Gynecology and urology ENT or oral and maxillofacial surgery General and hepatobiliary surgery Traumatology and orthopedic surgery Neurosurgery Spinal surgery Other operations/interventions |
17 |
223 145 112 37 33 24 45 |
36.0 23.4 18.1 6.0 5.3 3.9 7.3 |
|
Surgical risk High-risk procedure („Revised Cardiac Risk Index“ definition) High-risk procedure („ESC/ESA“ definition) Moderate-risk procedure („ESC/ESA“ definition) Bleeding risk in a closed space |
19 19 20 4 |
101 50 400 49 |
16.4 8.1 64.9 7.8 |
|
American Society of Anesthesiology classification [scale 1–5] Class 1 or 2 Class 3 Class 4 |
30 |
181 389 36 |
29.9 64.2 5.9 |
|
Revised Cardiac Risk Index [scale 0–6] Score 0 Score 1 Score 2 Score 3 Score ≥ 4 |
198* |
130 187 96 20 5 |
29.7 42.7 21.9 4.6 1.1 |
|
Duration of aspirin therapy in patients after PCI (n = 203) <1 month 1–3 months 4–6 months 7–12 months 13–24 months more than 2 years |
20 |
5 2 6 7 17 146 |
2.7 1.1 3.3 3.8 9.3 78.8 |
Data are presented as absolute frequency (n) and relative frequency (%) in relation to valid responses for individual characteristics;the Revised Cardiac Risk Index (RCRI) is a prospective validated 6-point scoring system: The risk of perioperative cardiac complications increases with the number of risk factors (32);
* In case of missing information about one point in the RCRI (e.g. missing creatinine level to verify renal failure), the entire score was evaluated as „missing value“. Aspirin, acetylsalicylic acid; ESA, European Society of Anaesthesiology; ESC, European Society of Cardiology; ENT, ear, nose and throat; PCI, percutaneous ?coronary intervention
Which factors influence the decision to discontinue aspirin therapy?
The factors “previous PCI with stent implantation” (OR = 0.47 [0.31; 0.72]; p <0.001), “history of cardiac disease” and „aspirin therapy for secondary prevention“ reduce the chance of preoperative cessation of a long-term aspirin therapy (table 2).
Table 2. Uni- and multivariable analysis (n = 636), logistic regression model with the outcome variable “cessation of aspirin therapy”.
| Patient characteristic |
Missing values |
Number of patients (n) |
Effect estimates univariate analysis OR [95% CI] |
p-value univariate analysis |
Adjusted effect estimates multivariable analysis OR [95% CI] |
p-value multivariable analysis |
| Previous PCI with stent implantation | 0 | 203 | 0.49 [0.34; 0.70] | <0.001 | 0.47 [0.31; 0.72] | <0.001 |
| History of cardiac disease | 0 | 343 | 0.47 [0.34; 0.64] | <0.001 | –*3 | – |
| Aspirin for secondary prevention | 9 | 455 | 0.34 [0.24; 0.49) | <0.001 | –*3 | – |
| Aspirin therapy already discontinued once before in the past | 57 | 227 | 4.35 [3.03; 6.25] | <0.001 | 4.58 [3.06; 6.84] | <0.001 |
| Patient does not know the indication for which aspirin was prescribed | 34 | 53 | 2.07 [1.14; 3.75] | 0.016 | 2.12 [1.05; 4.28] | 0.036 |
| Preanesthetic assessment less than 2 days before surgery | 47 | 274 | 1.71 [1.23; 2.39] | 0.002 | 1.60 [1.08; 2.37] | 0.018 |
| History of stroke or transient ‧ischemic attack | 1 | 137 | 0.95 [0.64; 1.41] | 0.803 | – | – |
| Cardiac arrhythmias | 1 | 56 | 1.49 [0.84; 2.64] | 0.173 | – | – |
| Neurosurgical operations | 17 | 33 | 12.49 [3.77; 41.40] | <0.001 | –*2 | – |
| Bleeding risk in a closed space*1 | 4 | 49 | 4.84 [2.36; 9.91] | <0.001 | 4.54 [2.02; 10.22] | <0.001 |
| Expected transfusion risk (as stated by physicians) Risk <5% (reference) Risk 5–10 % Risk >10 % |
23 |
478 108 27 |
1.00 0.84 [0.54; 1.29] 1.20 [0.54; 2.68] |
0.636 0.424 0.662 |
– | – |
Univariate logistic regression was used to identify potentially eligible factors; for categorical variables, the first category was defined as reference; OR: Odds ratio for the primary outcome
“cessation of aspirin therapy”;
*1 The category „Bleeding risk in a closed space“ comprises neurosurgical and neuroradiological intracranial and intramedullary procedures.
The adjusted OR originates from a multivariable model which includes 5 variables; because of:
*2 redundance and subsequent multicollinearity with „bleeding risk in a closed space“ and
*3 redundance and subsequent multicollinearity with“previous PCI with stent implantation” variables were not included in the multivariable model;
Aspirin, acetylsalicylic acid; OR, odds ratio; PCI, percutaneous coronary intervention; [95% CI], 95% confidence interval; the level of statistical significance was set at 0.05.
By contrast, the chance is markedly increased in patients scheduled for neurosurgery or at risk of hemorrhage in a closed space (OR = 4.54 [2.02; 10.22]; p<0.001). In addition, the chance of therapy cessation is increased more than fourfold if long-term aspirin therapy has already been stopped once before (OR = 4.58 [3,06; 6.84]; p <0.001); more than doubled if the patient does not know the indication for which aspirin was prescribed (OR = 2.12 [1,05; 4.28]; p = 0.036); and increased by 60% if the preanesthetic assessment is conducted less than 2 days prior to surgery (OR = 1.60 [1.08; 2.37]; p = 0.018).
However, our data showed no association between the decision to preoperatively discontinue aspirin therapy and the expected perioperative transfusion risk or risk factors for cerebrovascular thromboembolism („cardiac arrhythmia“ or „history of stroke or transient ischemic attack“).
Patients with previous PCI and stent implantation?
34.9% of patients with implanted coronary stents stopped long-term aspirin therapy preoperatively. Our study population included a subgroup of 140 patients with coronary stents who had neither a relevant transfusion risk >5% nor a risk of hemorrhage in a closed space, i.e. no reproducible reason for cessation of their aspirin therapy. Despite these facts, 35.0% of the patients within this subgroup stopped their long-term aspirin therapy. Of the patients with coronary stents who stopped taking aspirin, 60.9% had already done so once before and 31.3% stopped aspirin therapy without seeking medical advice. In 45.5% of patients with coronary stents (vs 47.0% of patients without coronary stents), the preanesthetic assessment was conducted less than 2 days prior to surgery. In only 28.1% of patients with coronary stents, the stent type could be clearly classified during anesthesiological pre-assessment (for example, „bare metal stents“ or „drug-eluting stents“). Since only patients scheduled for elective surgery were included in our study, the number of patients with recently implanted coronary stents receiving dual antiplatelet therapy was, as expected, low (table 1).
What about the risk-benefit assessment?
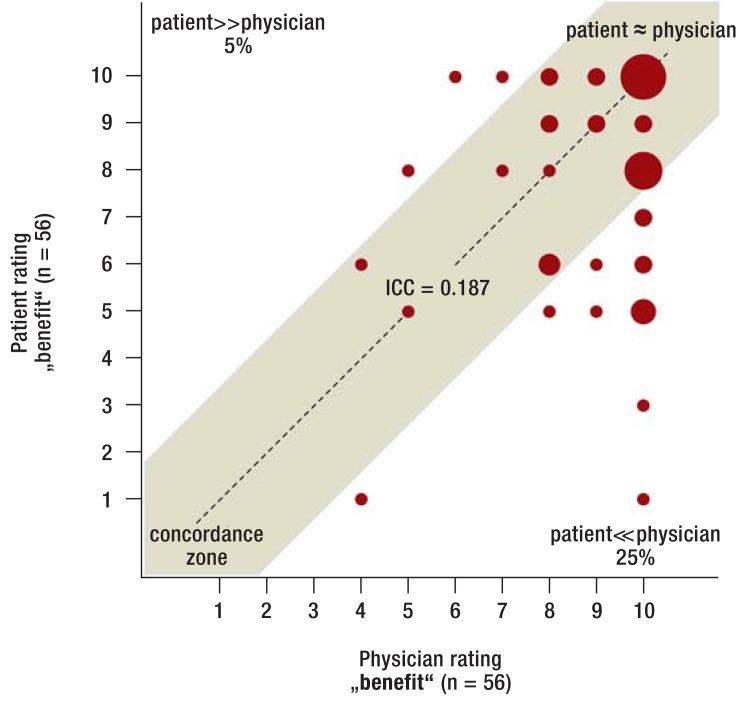
Benefit of aspirin therapy—Even though the overall “benefit” of aspirin therapy was rated as high among patients who stopped taking aspirin, there was considerable discrepancy between the ratings of physicians and patients (eFigure 1: ICC 0.348, eFigure 2: Subgroup of patients with coronary stents: ICC 0.187). The logistic regression analysis found no noticeable association between the rating of the „benefit“ and the actual clinical decision to stop aspirin therapy (patient: OR = 0.98, 95% CI: [0.90; 1.07]; p = 0.63; physician: OR = 0.99 [0.87; 1.12]; p = 0.84).
eFigure 1.
Benefit rating among patients who stopped aspirin (total sample)
Rating of the benefit of aspirin therapy (numeric rating scale from 1–10): ratings by patients (y-axis) and by treating physicians (x-axis). Valid benefit ratings among patients who stopped aspirin therapy preoperatively are depicted. The area of the diagram points represents the relative frequency of a data pair. In the center, a concordance zone is shown (grey area in which the physician and patient ratings differ = 2 points from each other). In the areas outside of the concordance zone, patient ratings are considerably above (percentage top left) or below (percentage bottom right) the physician ratings (difference of at least 3 points). Intraclass correlation (ICC) is used to quantify the concordance between patient and physician ratings (value at the center of the concordance zone). An ICC <0.4 indicates low agreement between the respective ratings (30).
Aspirin, acetylsalicylic acid
eFigure 2.
Benefit rating among patients who stopped aspirin (subgroup with coronary stents) Rating of the benefit of aspirin therapy (numeric rating scale from 1–10) among patients with coronary stents who stopped aspirin therapy preoperatively.
Aspirin, acetylsalicylic acid
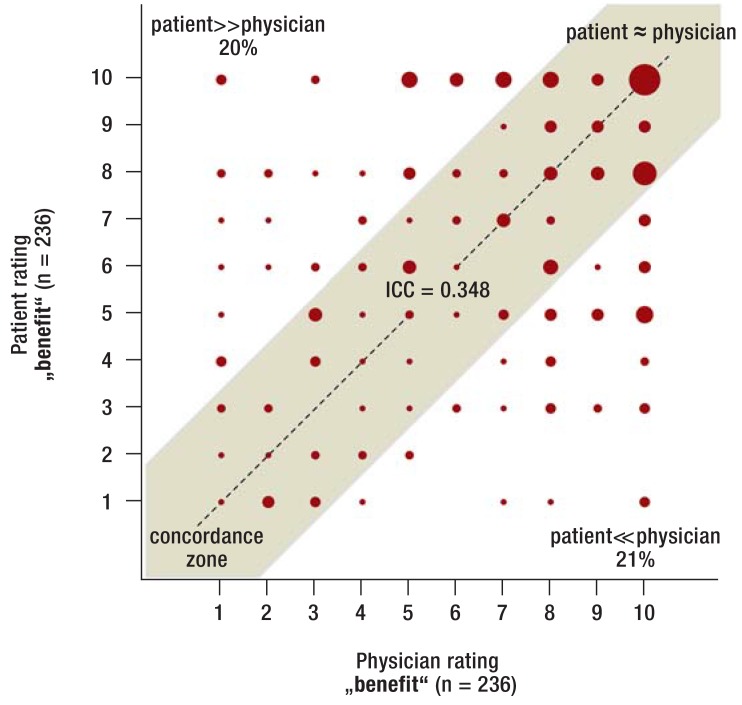
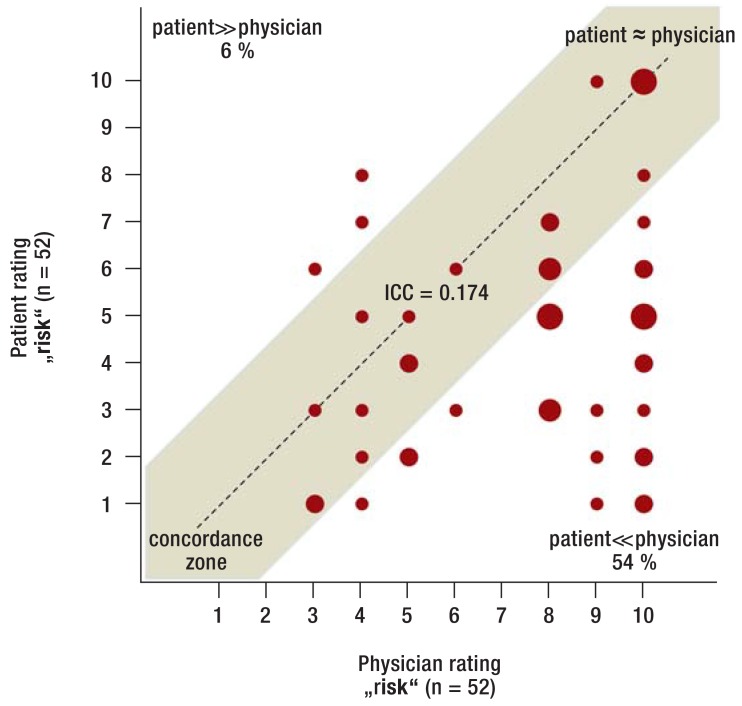
Risk associated with aspirin cessation—Among patients who stopped taking aspirin, the rating of the physicians and patients differed relevantly (figure 1: ICC 0.349), especially in patients with coronary stents (figure 2: ICC 0.174). The patients‘ risk ratings were frequently considerably lower compared with those of the physicians (54% of patients with coronary stents in figure 2). On average, patients rated the „risk“ as significantly lower compared with physicians (4.9 [4.6; 5.1] versus 6.4 [6.1; 6.6] points; p<0.001), especially in the subgroup with coronary stents (5.8 [5.3; 6.3] versus 8.0 [7.7; 8.3] points; p<0.001). This is relevant as based on the logistic regression analysis it can be statistically estimated that with every point added by the patient on the risk rating scale the chance of aspirin therapy cessation is reduced by 19% (OR = 0.81 [0.74; 0.87]; p<0.001) and with every point added by physicians it is reduced by 14% (OR = 0.86 [0.77; 0.96]; p = 0.007).
Figure 1.
Risk rating among patients who stopped aspirin (total sample)
Rating of the risk associated with aspirin therapy cessation (numeric rating scale from 1–10): ratings by patients (y-axis) and by treating physicians (x-axis). Valid risk ratings among patients who stopped aspirin therapy preoperatively are depicted. The area of the diagram points represents the relative frequency of a data pair. In the center, a concordance zone is shown (grey area in which the physician and patient ratings differ = 2 points from each other). In the areas outside of the concordance zone, patient ratings are considerably above (percentage top left) or below (percentage bottom right) the physician ratings (difference at least 3 points). Intraclass correlation (ICC) is used to quantify the concordance between patient and physician ratings (value at the center of the concordance zone). An ICC <0.4 indicates low agreement between the respective ratings (30). Aspirin, acetylsalicylic acid
Figure 2.
Risk rating among patients who stopped aspirin (subgroup with coronary stents)
Rating of the risk associated with aspirin therapy cessation (numeric rating scale from 1–10) among patients with coronary stents who preoperativley stopped aspirin therapy.
Aspirin, acetylsalicylic acid; ICC, intraclass correlation
Who decides to stop aspirin therapy?
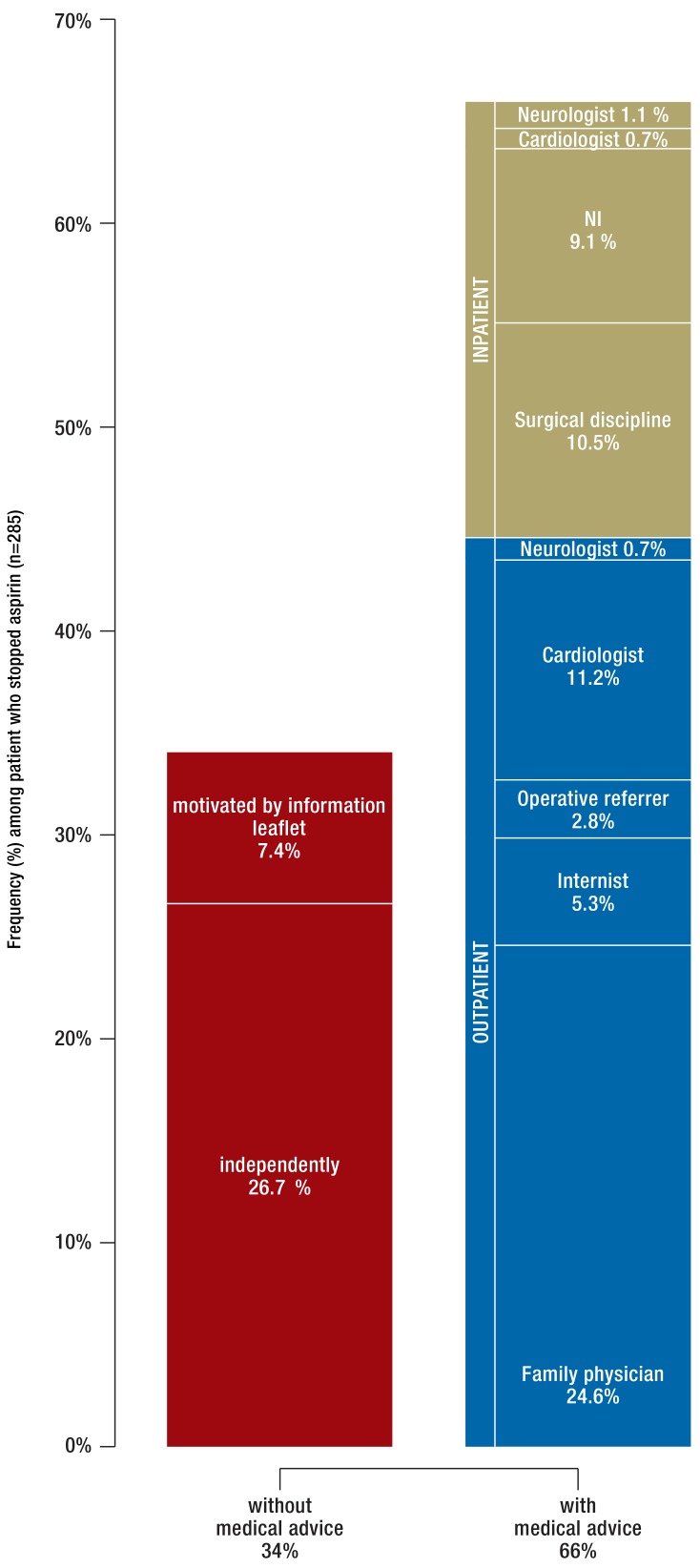
The decision to stop aspirin was frequently made on an outpatient basis (in 44.6% of patients who stopped taking aspirin) and less often on an inpatient basis (21.4%). However, within the subgroup of patients who discontinued aspirin therapy about one third (34.0%) stopped the intake without first consulting a physician (efigure 3).
eFigure 3.
Decision-making regarding preoperative cessation of aspirin therapy. Analysis for the subgroup of patients who stopped aspirin therapy preoperatively (n = 285)
Aspirin, acetylsalicylic acid; NI; no information
When was aspirin stopped?
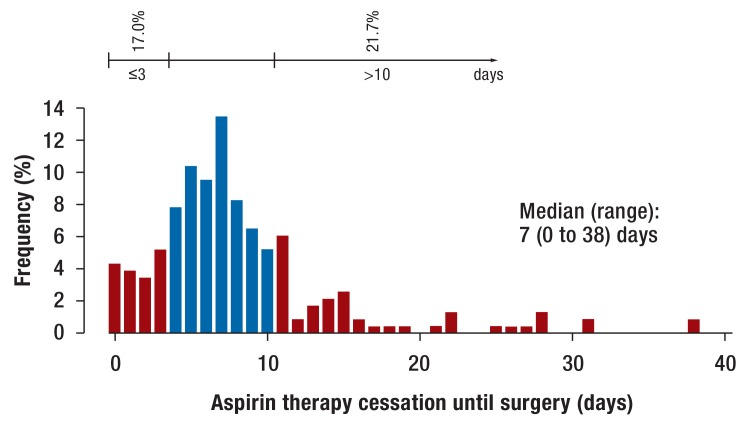
The median interval of preoperative aspirin cessation was 7 days (0–38) (figure 3). However, in 38.7% of these patients the timing was incorrect: 17.0% stopped the therapy too late (= 3 days preoperatively) and 21.7% too early (>10 days preoperatively). Many patients stopped taking aspirin already prior to the day of the anesthesiological pre-assessment (81.6%) (efigure 4). Thus at the date of preanesthetic assessment it was often no longer possible to influence the continuation or timely cessation of aspirin therapy (figure 4).
Figure 3.
When was aspirin stopped? Interval of preoperative aspirin cessation (days) among patients who stopped aspirin therapy. Shown is the relative frequency (in %) in relation to the sum of valid responses. Red bars: Too late (= 3 days preoperatively) or too early (>10 days preoperatively) therapy cessation.
Aspirin, acetylsalicylic acid
eFigure 4.
Interval between preanesthetic assessment and cessation of aspirin therapy.
Shown is the relative frequency (in %) in relation to the sum of valid responses.
Aspirin, acetylsalicylic acid
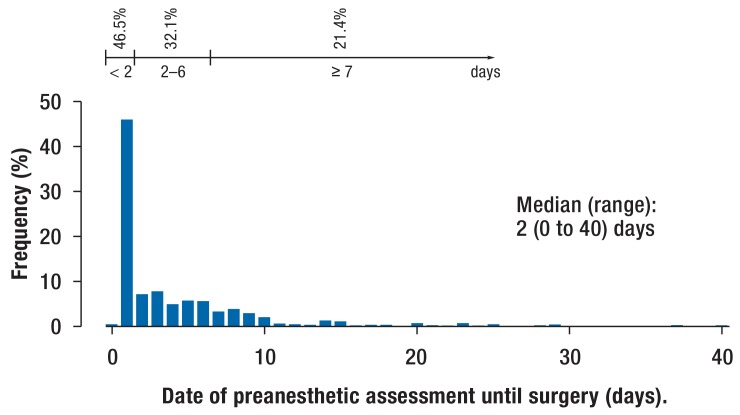
Figure 4.
Interval between preanesthetic assessment and surgery (days). Shown is the relative frequency (in %) in relation to the sum of valid responses.
Discussion
This single-center cross-sectional study evaluating 636 noncardiac surgery patients with long-term aspirin therapy in a German metropolis showed high variability in the perioperative management of aspirin medication and revealed discrepancies between guideline recommendations and routine clinical practice in patients with coronary stents.
Which factors influence the decision to discontinue aspirin therapy?
Guidelines recommend to individually balance the risk of thromboembolism against the risk of hemorrhage (10, 23, 33, 34). However, our study found no association between the decision to stop aspirin and the expected transfusion risk or anamnestic risk factors for cerebrovascular thromboembolism.
Risk-benefit assessment?
Our data indicate that patients rate the risk associated with aspirin cessation significantly lower than the treating physicians; however, patients‘ risk ratings are very strongly associated with the actual clinical decision to stop aspirin therapy.
Who decides to stop aspirin therapy?
Almost half of the patients stopped long-term aspirin therapy—frequently without medical recommendation to do so. Cardiologists were rarely consulted. At the date of preanesthetic assessment, the continuation or timely cessation of aspirin medication could frequently not be influenced any longer.
When was aspirin stopped?
It is recommended to stop aspirin therapy, if indicated, 5–10 days preoperatively (10, 24– 26, 29, 35). In 39% of cases, however, the timing of aspirin therapy cessation was incorrect (=3 days preoperatively: unnecessary bleeding risk or, if >10 days, unnecessary thromboembolic risk).
Management of patients with coronary stents
Patients with previous PCI and coronary stenting should continue aspirin therapy whenever possible (10, 23). However, 35% of patients with coronary stents in our study population stopped their long-term aspirin intake—in the majority of cases without reproducible reason. During the preanesthetic assessment, the stent type was unknown in more than 70% of patients, making cardiac risk stratification more difficult.
The POISE-2 study, published in 2014, showed no significant reduction of the composite outcome of death and nonfatal myocardial infarction, but a significantly increased risk of major bleeding in patients who received 200 mg aspirin perioperatively (22). Since patients who recently underwent PCI with stent implantation were excluded, these results should not be extrapolated to this patient population. Due to multiple methodology-related limitations, it has been recommended to interpret the findings of that study with caution. Nearly two-thirds of patients in the aspirin group may not have met primary or secondary prevention criteria for aspirin therapy (24, 36). Smaller studies did not demonstrate a significant increase in bleeding risk in patients who received aspirin perioperatively (21, 37, 38). A large meta-analysis (35) found a 50% increase in bleeding complications, but not a higher level of the severity of bleeding complications.
Perioperative therapy adherence
The World Health Organization (WHO) has defined five dimensions of treatment adherence (39); in our context, the focus is on the complexity of the medical regimen, the level of information provided to the patient (participative decision-making) and the anxieties about possible adverse effects (surgical bleeding complications) (40). The inhomogeneous, partially contradictory evidence from studies with regard to the risk-benefit profile of perioperative aspirin therapy in patients without coronary stents may be a cause for nonadherence. There are grounds for concern that these conflicting data might have negative implications on a patient group in which perioperative aspirin therapy is particularly relevant: patients with coronary stents.
Do we have to reconsider our principles? To date, scientific debates have focused on the question which patient– and operation-specific risk profile requires perioperative continuation of long-term aspirin therapy. By contrast, our data raise the issue of how we can improve the provision of available scientific insights to our patients in order to affect their participation and treatment adherence. New generations of coronary stents as well as pharmacological innovations make the perioperative management of cardiovascular high-risk patients increasingly complex. In order to realize an optimal perioperative treatment, cardiovascular high-risk patients (e.g. with previous PCI and stent implantation) under long-term aspirin therapy should schedule for preanesthetic assessment in time (typically at least 7 days preoperatively), unless they have already been evaluated by a specialist for internal medicine or cardiology. In these patients, perioperative management with regard to aspirin therapy should be decided in agreement with the surgeon based on an individual risk-benefit analysis. Corresponding clinical pathways should be on file in a standardized and written form. Suitable media in this respect include standard operating procedures (SOPs), guideline-based recommendations of professional societies, preoperative patient information brochures, and stent implant cards.
Stent implant cards
Since 1 October 2015, implant cards („stent cards“) are legally required (see example on the website of the German Cardiac Society (DGK): www.dgk.org/daten/Stent-Pass_2015.pdf). Stent cards bundle essential information, including stent type, indication for implantation and required anti-platelet regimen, thus providing valuable guidance for the individual preoperative risk-benefit analysis. In addition, stent cards offer relevant instructions and clear warnings for patients. Thus, stent cards have the potential to improve adherence to perioperative anti-platelet therapy in patients with previous PCI and stent implantation.
Limitations
Methodological limitations of this study should be taken into consideration when interpreting its results. There is the selected and monocentric nature of the patient population. Due to the study design, it cannot be ruled out that the results of the study are distorted by selection bias and information bias. Consequently, we make no claim that our data are representative nationwide—the generalization of our results is only possible to a limited extent. Since at the time of the survey the anesthesiologists had already preliminary procedural information available, it is possible that their ratings were biased. In order to ensure anonymity, relevant sociodemographic information was not collected; thus, these parameters are missing as potential factors in the multivariable model used. Finally, cross-sectional studies only allow to claim association, not causation.
Key Messages.
In this study, almost half of the patients stopped long-term aspirin therapy preoperatively, of these one third without seeking medical advice.
Of the patients who discontinued aspirin therapy preoperatively, almost 40% stopped taking aspirin either too early (>10 days preoperatively) or too late (=3 days preoperatively).
In the study population, 35% of patients with coronary stents stopped long-term aspirin therapy preoperatively, in most cases without reproducible reason (low transfusion risk and no bleeding risk in a closed space).
Cardiovascular high-risk patients (e.g. with previous PCI and stent implantation) under long-term aspirin therapy should schedule for preanesthetic assessment in time (typically at least 7 days preoperatively), unless they have already been evaluated by a specialist for internal medicine or cardiology.
Patients frequently rated the risk associated with aspirin cessation lower than their treating physicians. Stent implant cards may promote adherence to perioperative aspirin therapy in patients with coronary stents.
eBOX.
Study design: single-center cross-sectional study using standardized questionnaires
Selective sampling was used. Patients presenting at the Anesthesiology Pre-assessment Clinic of the University Medical Center Hamburg-Eppendorf for preanesthetic assessment and meeting the inclusion criteria were included in this study.
Inclusion criteria
Age = 18 years
Elective noncardiac surgery
Regular antiplatelet therapy with low-dose aspirin (defined as a daily aspirin dose =100 mg, even when aspirin therapy was discontinued within 30 days of study inclusion)
Exclusion criteria
No informed consent provided by patient
Age <18 years
Patient allocation: Adult patients with long-term aspirin therapy who presented prior to elective noncardiac surgery at the Anesthesiology Pre-assessment Clinic of the University Medical Center Hamburg-Eppendorf were screened. At the time of registering at the Anesthesiology Pre-assessment Clinic, patients were interviewed by medical staff regarding the inclusion criterion „regular daily intake of low-dose aspirin, even if this therapy was discontinued within 30 days of potential study inclusion“ (screening question). Patients who gave an affirmative response were invited to participate in the study.
Questionnaire-based data collection: Data were immediately collected on site at the Anesthesiology Pre-assessment Clinic. After study inclusion, both the patient (questionnaire part 1) and the treating anesthesiologist (questionnaire part 2) were provided with the respective part of the questionnaire which they then completed independently.
Anonymization: At the end of the preanesthetic assessment, both parts of the questionnaire were stapled together and handed to the patient. Since both parts of the questionnaire did not contain patient-identifying information, such as name, sex and date of birth, it was later impossible to identify a particular patient. Upon leaving the Anesthesiology Pre-assessment Clinic, the patient could then drop the questionnaire into a centrally located box. In this way, the voluntary nature of study participation as well as the anonymity of the patient were ensured, while the direct case-related link between physician and patient responses remained intact. After this point in time, the questionnaire was only available in anonymized form and the data it contained could subsequently only be used and analyzed in a fully anonymized fashion. Prior to the start of the study, this approach was coordinated with the data protection officer of the University Medical Center Hamburg-Eppendorf and with the Ethics Committee of the Medical Association of Hamburg. With this approach, a response rate of 79.0% was achieved. Because of the anonymized data collection method, it was not possible to perform a non-response analysis.
Questionnaires: Patients were asked since when they had been taking aspirin, why aspirin had been prescribed, whether they had stopped taking aspirin, who had advised them to do so, whether they had already paused taking aspirin occasionally in the past prior to surgical procedures. The treating physicians were asked about primary or secondary prevention, stent type, comorbidities, Revised Cardiac Risk Index, American Society of Anesthesiology classification, timing of aspirin therapy cessation, type of surgery, bleeding risk in closed spaces, and expected transfusion risk (<5%, 5–10%, >10%). The rating of the physicians relied on information from the medical history, specific clinical data from the patient file and preoperative examination findings.
In addition, patients and corresponding physicians could each perform a risk-benefit assessment using two numerical rating scales at the respective parts of the questionnaire:
Rating of the benefit of long-term aspirin therapy on a numeric rating scale (1 = very little, 10 = very large)
Rating of the risk associated with the perioperative cessation of aspirin therapy on a numeric rating scale (1 = very low, 10 = very high)
A priori sample size calculation: A priori sample size calculation was based on the null hypothesis that the chance of aspirin cessation would not differ between patients with and without coronary stents. It found that 438 evaluable cases were required to detect a significant difference between the two groups at a significance level of 5% with a power of 90%. Assuming a response rate of 60%, a sample size of 730 participants was calculated.
Aspirin, acetylsalicylic acid
Acknowledgments
The authors would like to thank Dr. Hildtraud Knopf and Daniel Grams of the Robert Koch Institute, Department of Epidemiology and Health Reporting, for calculating the prevalence of the administration of aspirin as an anti-platelet agent from data of the German Health Interview and Examination Survey for Adults (DEGS1) and for granting permission to publish these results in Deutsches Ärzteblatt.
Footnotes
Conflict of interest
The authors declare that no conflict of interest exists.
Translated from the original German by Ralf Thoene, MD
References
- 1.Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849–1860. doi: 10.1016/S0140-6736(09)60503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bibbins-Domingo K. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: US. Preventive Services Task Force Recommendation statement. Ann Intern Med. 2016;164:836–845. doi: 10.7326/M16-0577. [DOI] [PubMed] [Google Scholar]
- 3.Williams CD, Chan AT, Elman MR, et al. Aspirin use among adults in the US.: results of a national survey. Am J Prev Med. 2015;48:501–508. doi: 10.1016/j.amepre.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Knopf H, Grams D. Arzneimittelanwendung von Erwachsenen in Deutschland Ergebnisse der Studie zur Gesundheit Erwachsener in Deutschland (DEGS1) Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2013;56:868–877. doi: 10.1007/s00103-013-1667-8. [DOI] [PubMed] [Google Scholar]
- 5.Alcock RF, Reddel CJ, Pennings GJ, Hillis GS, Curnow JL, Brieger DB. The rebound phenomenon after aspirin cessation: the biochemical evidence. Int J Cardiol. 2014;174:376–378. doi: 10.1016/j.ijcard.2014.03.192. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez LA, Cea-Soriano L, Martin-Merino E, Johansson S. Discontinuation of low dose aspirin and risk of myocardial infarction: case-control study in UK primary care. BMJ. 2011;343 doi: 10.1136/bmj.d4094. d4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biondi-Zoccai GG, Lotrionte M, Agostoni P, et al. A systematic review and meta-analysis on the hazards of discontinuing or not adhering to aspirin among 50, 279 patients at risk for coronary artery disease. Eur Heart J. 2006;27:2667–2674. doi: 10.1093/eurheartj/ehl334. [DOI] [PubMed] [Google Scholar]
- 8.Gosswald A, Schienkiewitz A, Nowossadeck E, Busch MA. Prävalenz von Herzinfarkt und koronarer Herzkrankheit bei Erwachsenen im Alter von 40 bis 79 Jahren in Deutschland - Ergebnisse der Studie zur Gesundheit Erwachsener in Deutschland (DEGS1) Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2013;56:650–655. doi: 10.1007/s00103-013-1666-9. [DOI] [PubMed] [Google Scholar]
- 9.van Buuren F. Bericht über die Leistungszahlen der Herzkatheterlabore in der Bundesrepublik Deutschland. Kardiologe. 2010;4:502–508. [Google Scholar]
- 10.Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: The Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA) Eur Heart J. 2014;35:2383–2431. doi: 10.1093/eurheartj/ehu282. [DOI] [PubMed] [Google Scholar]
- 11.Devereaux PJ, Goldman L, Cook DJ, Gilbert K, Leslie K, Guyatt GH. Perioperative cardiac events in patients undergoing noncardiac surgery: a review of the magnitude of the problem, the pathophysiology of the events and methods to estimate and communicate risk. CMAJ. 2005;173:627–634. doi: 10.1503/cmaj.050011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berger PB, Kleiman NS, Pencina MJ, et al. Frequency of major noncardiac surgery and subsequent adverse events in the year after drug-eluting stent placement results from the EVENT (Evaluation of Drug-Eluting Stents and Ischemic Events) Registry. JACC Cardiovasc Interv. 2010;3:920–927. doi: 10.1016/j.jcin.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 13.Cruden NL, Harding SA, Flapan AD, et al. Previous coronary stent implantation and cardiac events in patients undergoing noncardiac surgery. Circ Cardiovasc Interv. 2010;3:236–242. doi: 10.1161/CIRCINTERVENTIONS.109.934703. [DOI] [PubMed] [Google Scholar]
- 14.Gandhi NK, Abdel-Karim AR, Banerjee S, Brilakis ES. Frequency and risk of noncardiac surgery after drug-eluting stent implantation. Catheter Cardiovasc Interv. 2011;77:972–976. doi: 10.1002/ccd.22744. [DOI] [PubMed] [Google Scholar]
- 15.Hawn MT, Graham LA, Richman JR, et al. The incidence and timing of noncardiac surgery after cardiac stent implantation. J Am Coll Surg. 2012;214:658–666. doi: 10.1016/j.jamcollsurg.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 16.Hawn MT, Graham LA, Richman JS, Itani KM, Henderson WG, Maddox TM. Risk of major adverse cardiac events following noncardiac surgery in patients with coronary stents. JAMA. 2013;310:1462–1472. doi: 10.1001/jama.2013.278787. [DOI] [PubMed] [Google Scholar]
- 17.Wijeysundera DN, Wijeysundera HC, Yun L, et al. Risk of elective major noncardiac surgery after coronary stent insertion: a population-based study. Circulation. 2012;126:1355–1362. doi: 10.1161/CIRCULATIONAHA.112.102715. [DOI] [PubMed] [Google Scholar]
- 18.Albaladejo P, Marret E, Samama CM, et al. Non-cardiac surgery in patients with coronary stents: the RECO study. Heart. 2011;97:1566–1572. doi: 10.1136/hrt.2011.224519. [DOI] [PubMed] [Google Scholar]
- 19.Ferrari E, Benhamou M, Cerboni P, Marcel B. Coronary syndromes following aspirin withdrawal: a special risk for late stent thrombosis. J Am Coll Cardiol. 2005;45:456–459. doi: 10.1016/j.jacc.2004.11.041. [DOI] [PubMed] [Google Scholar]
- 20.Rossini R, Capodanno D, Lettieri C, et al. Prevalence, predictors, and long-term prognosis of premature discontinuation of oral antiplatelet therapy after drug eluting stent implantation. Am J Cardiol. 2011;107:186–194. doi: 10.1016/j.amjcard.2010.08.067. [DOI] [PubMed] [Google Scholar]
- 21.Rossini R, Musumeci G, Capodanno D, et al. Perioperative management of oral antiplatelet therapy and clinical outcomes in coronary stent patients undergoing surgery Results of a multicentre registry. Thromb Haemost. 2015;113:272–282. doi: 10.1160/TH14-05-0436. [DOI] [PubMed] [Google Scholar]
- 22.Devereaux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med. 2014;370:1494–1503. doi: 10.1056/NEJMoa1401105. [DOI] [PubMed] [Google Scholar]
- 23.Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130:e278–e333. doi: 10.1161/CIR.0000000000000106. [DOI] [PubMed] [Google Scholar]
- 24.Gerstein NS, Carey MC, Cigarroa JE, Schulman PM. Perioperative aspirin management after POISE-2: some answers, but questions remain. Anesth Analg. 2015;120:570–575. doi: 10.1213/ANE.0000000000000589. [DOI] [PubMed] [Google Scholar]
- 25.Schlitt A, Jambor C, Spannagl M, Gogarten W, Schilling T, Zwissler B. The perioperative management of treatment with anticoagulants and platelet aggregation inhibitors. Dtsch Arztebl Int. 2013;110:525–532. doi: 10.3238/arztebl.2013.0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e326S–e350S. doi: 10.1378/chest.11-2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jambor C, von Pape KW, Spannagl M, Dietrich W, Giebl A, Weisser H. Multiple electrode whole blood aggregometry, PFA-100, and in vivo bleeding time for the point-of-care assessment of aspirin-induced platelet dysfunction in the preoperative setting. Anesth Analg. 2011;113:31–39. doi: 10.1213/ANE.0b013e31821acddc. [DOI] [PubMed] [Google Scholar]
- 28.Zisman E, Erport A, Kohanovsky E, et al. Platelet function recovery after cessation of aspirin: preliminary study of volunteers and surgical patients. Eur J Anaesthesiol. 2010;27:617–623. doi: 10.1097/EJA.0b013e328335b354. [DOI] [PubMed] [Google Scholar]
- 29.Koenig-Oberhuber V, Filipovic M. New antiplatelet drugs and new oral anticoagulants. Br J Anaesth. 2016;117(2):ii74–ii84. doi: 10.1093/bja/aew214. [DOI] [PubMed] [Google Scholar]
- 30.Hallgren KA. Computing inter-rater reliability for observational data: an overview and tutorial. Tutor Quant Methods Psychol. 2012;8:23–34. doi: 10.20982/tqmp.08.1.p023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.R Core Team. www.r-project.org (last accessed on on 8 May 2017) Vienna: Austria; R: a language and environment for statistical computing. R Foundation for Statistical Computing, [Google Scholar]
- 32.Petzoldt M, Kahler J, Goetz AE, Friederich P. Perioperative medikamentöse Kardioprotektion Systematische Literaturübersicht als rationale Grundlage zur Prozessoptimierung. Anaesthesist. 2008;57:655–669. doi: 10.1007/s00101-008-1396-9. [DOI] [PubMed] [Google Scholar]
- 33.Duceppe E, Mrkobrada M, Thomas S, Devereaux PJ. Role of aspirin for prevention and treatment of perioperative cardiovascular events. J Thromb Haemost. 2015;13(1):S297–S303. doi: 10.1111/jth.12975. [DOI] [PubMed] [Google Scholar]
- 34.Kiberd MB, Hall RI. Aspirin in the perioperative period: a review of the recent literature. Curr Opin Anaesthesiol. 2015;28:349–355. doi: 10.1097/ACO.0000000000000200. [DOI] [PubMed] [Google Scholar]
- 35.Burger W, Chemnitius JM, Kneissl GD, Rucker G. Low-dose aspirin for secondary cardiovascular prevention—cardiovascular risks after its perioperative withdrawal versus bleeding risks with its continuation-review and meta-analysis. J Intern Med. 2005;257:399–414. doi: 10.1111/j.1365-2796.2005.01477.x. [DOI] [PubMed] [Google Scholar]
- 36.Gerstein NS, Charlton GA. Questions linger over POISE-2 and perioperative aspirin management. Evid Based Med. 2014;19:224–225. doi: 10.1136/ebmed-2014-110035. [DOI] [PubMed] [Google Scholar]
- 37.Mantz J, Samama CM, Tubach F, et al. Impact of preoperative maintenance or interruption of aspirin on thrombotic and bleeding events after elective non-cardiac surgery: the multicentre, randomized, blinded, placebo-controlled, STRATAGEM trial. Br J Anaesth. 2011;107:899–910. doi: 10.1093/bja/aer274. [DOI] [PubMed] [Google Scholar]
- 38.Oscarsson A, Gupta A, Fredrikson M, et al. To continue or discontinue aspirin in the perioperative period: a randomized, controlled clinical trial. Br J Anaesth. 2010;104:305–312. doi: 10.1093/bja/aeq003. [DOI] [PubMed] [Google Scholar]
- 39.Sabaté E. Adherence to long-term therapies: evidence for action Geneva, Switzerland: World Health Organization. www.who.int/chp/knowledge/publications/adherence_report/en/ (last accessed on 8 May 2017) [Google Scholar]
- 40.Matthes J, Albus C. Improving adherence with medication—a selective literature review based on the example of hypertension treatment. Dtsch Arztebl Int. 2014;111:41–47. doi: 10.3238/arztebl.2014.0041. [DOI] [PMC free article] [PubMed] [Google Scholar]