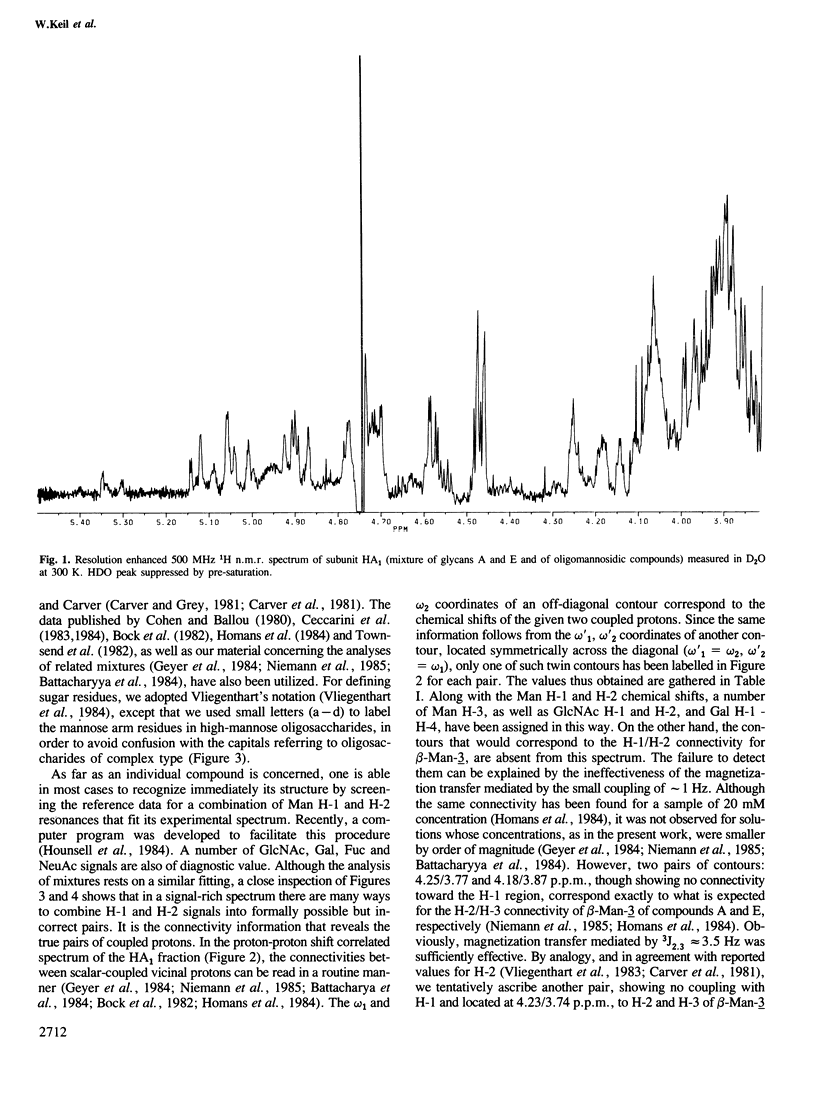
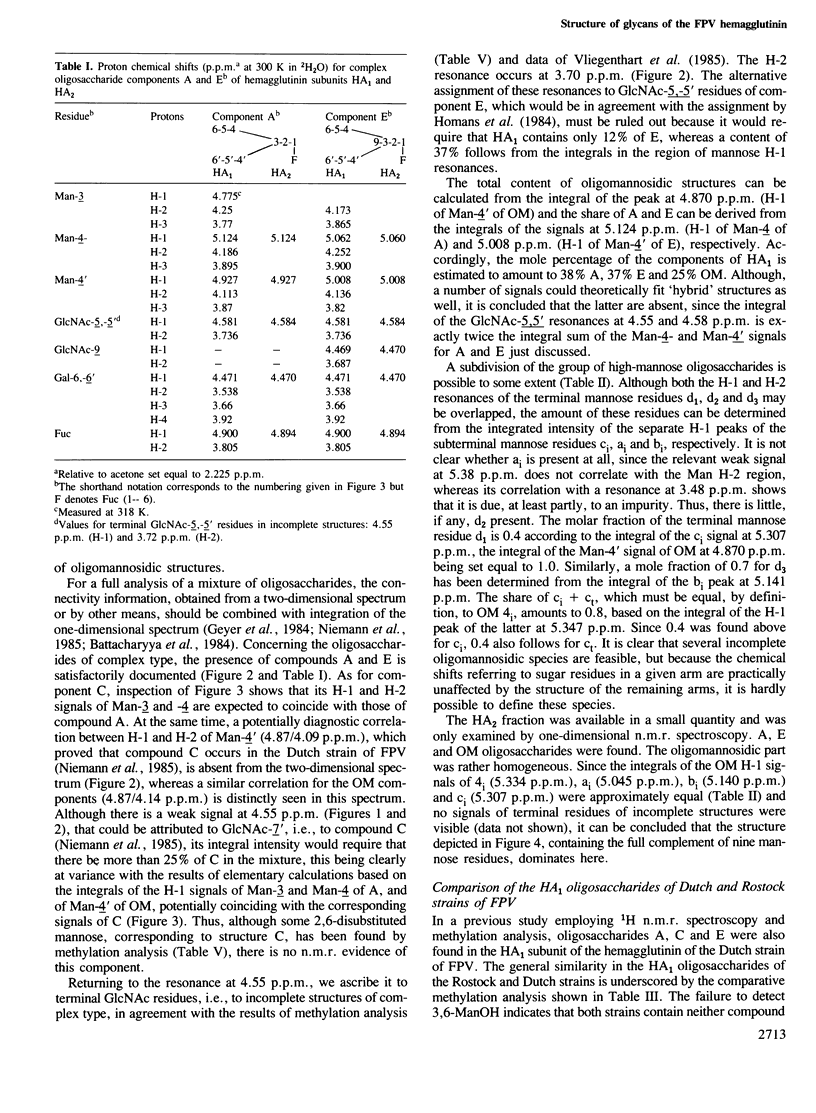
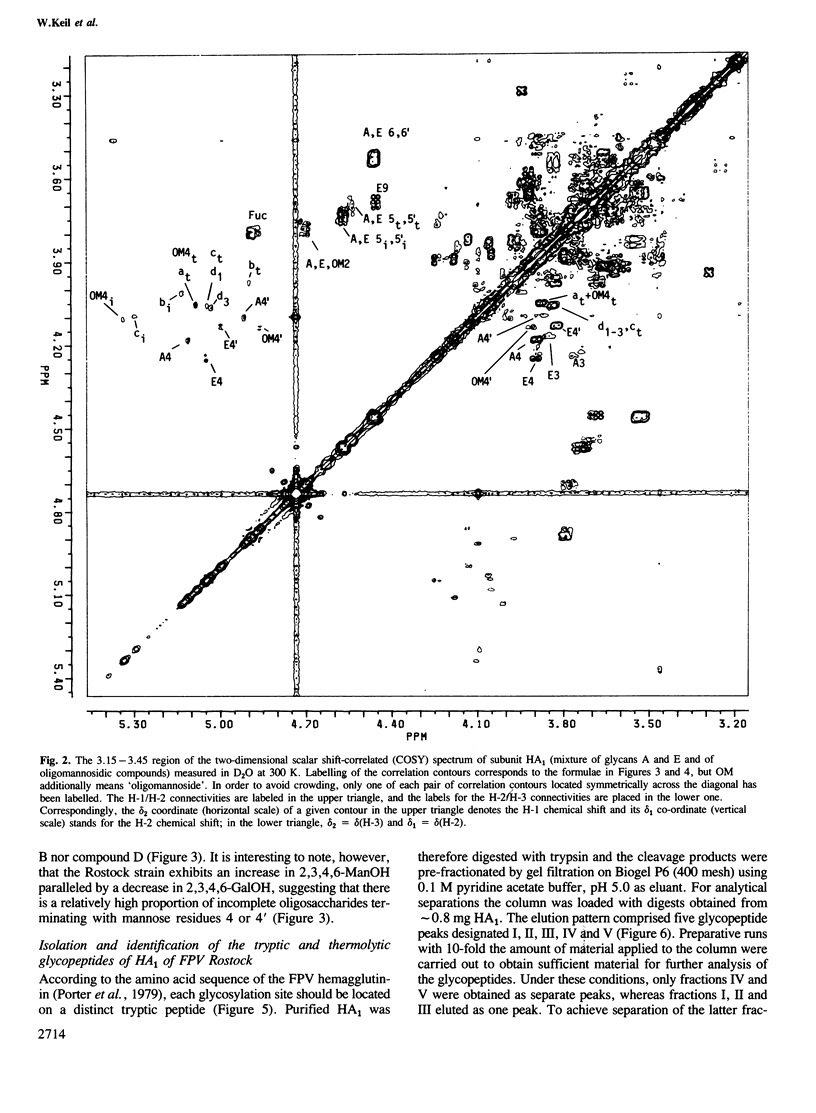
Abstract
The structures of the oligosaccharides of the hemagglutinin of fowl plague virus [influenza A/FPV/Rostock/34 (H7N1)] have been elucidated by one- and two-dimensional 1H n.m.r. spectroscopy at 500 MHz and by microscale methylation analysis. N-Glycosidic oligosaccharides of the oligomannosidic (OM) and of the N-acetyllactosaminic type have been found, the latter type comprising biantennary structures, without (A) or with (E) bisecting N-acetylglucosamine, and triantennary (C) structures. Analysis of the tryptic and thermolytic glycopeptides of the hemagglutinin allowed the allocation of these oligosaccharides to the individual glycosylation sites. Each attachment site contained a unique set of oligosaccharides. Asn12 contains predominantly structures C and E which are highly fucosylated. Asn28 contains OM and A structures that lack fucose and sulfate. Asn123 shows A that has incomplete antennae but is highly fucosylated and sulfated. Asn149 has fucosylated A and E. Asn231 shows fucosylated A and E with incomplete antennae. Asn406 has OM oligosaccharides. Asn478 has A and E with little fucose. Localization of the oligosaccharides on the three-dimensional structure of the hemagglutinin revealed that the oligomannosidic glycans are attached to glycosylation sites at which the enzymes responsible for carbohydrate processing do not have proper access. These observations demonstrate that an important structural determinant for the oligosaccharide side chains is the structure of the glycoprotein itself. In addition, evidence was obtained that the rate of glycoprotein synthesis also has an influence on carbohydrate structure.
Full text
PDF

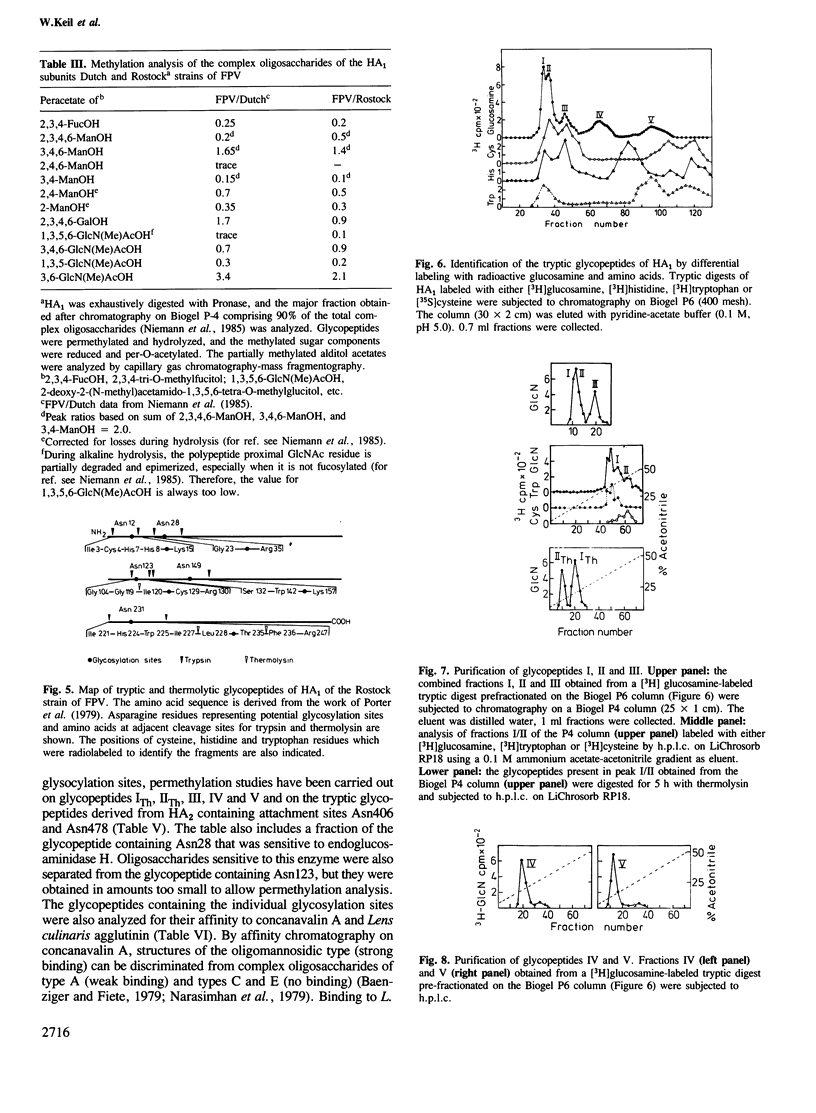
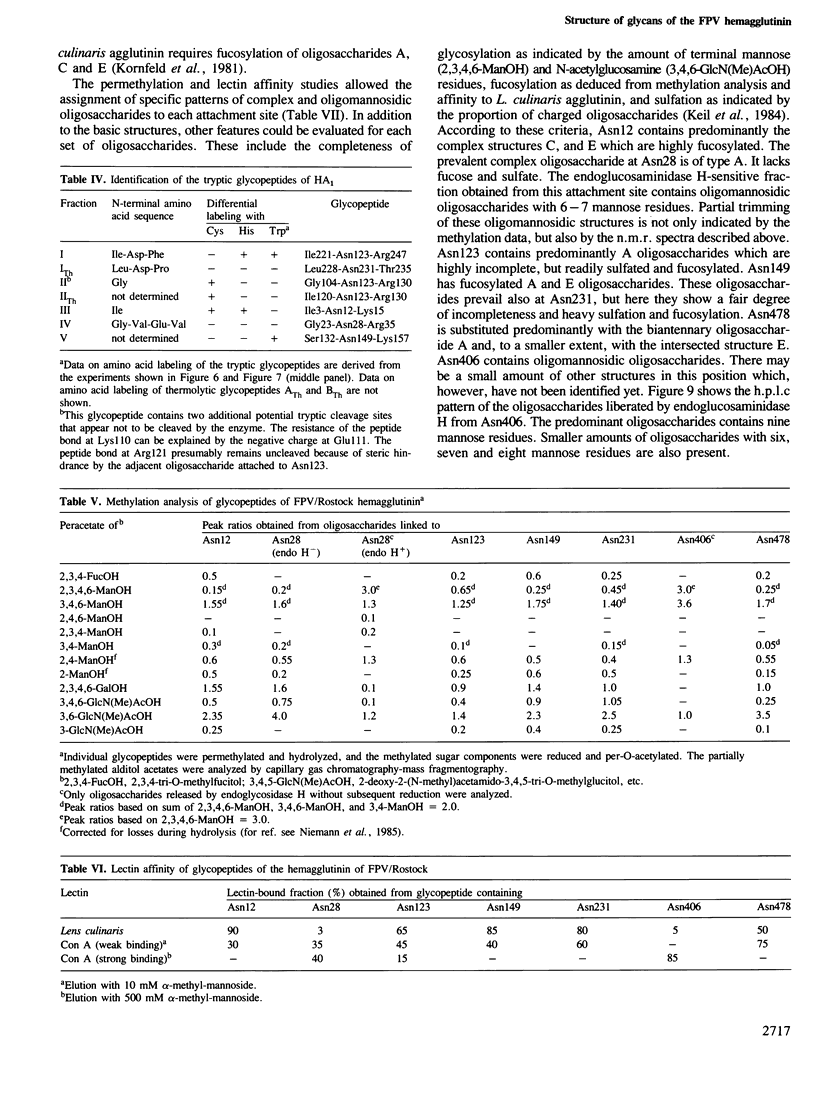
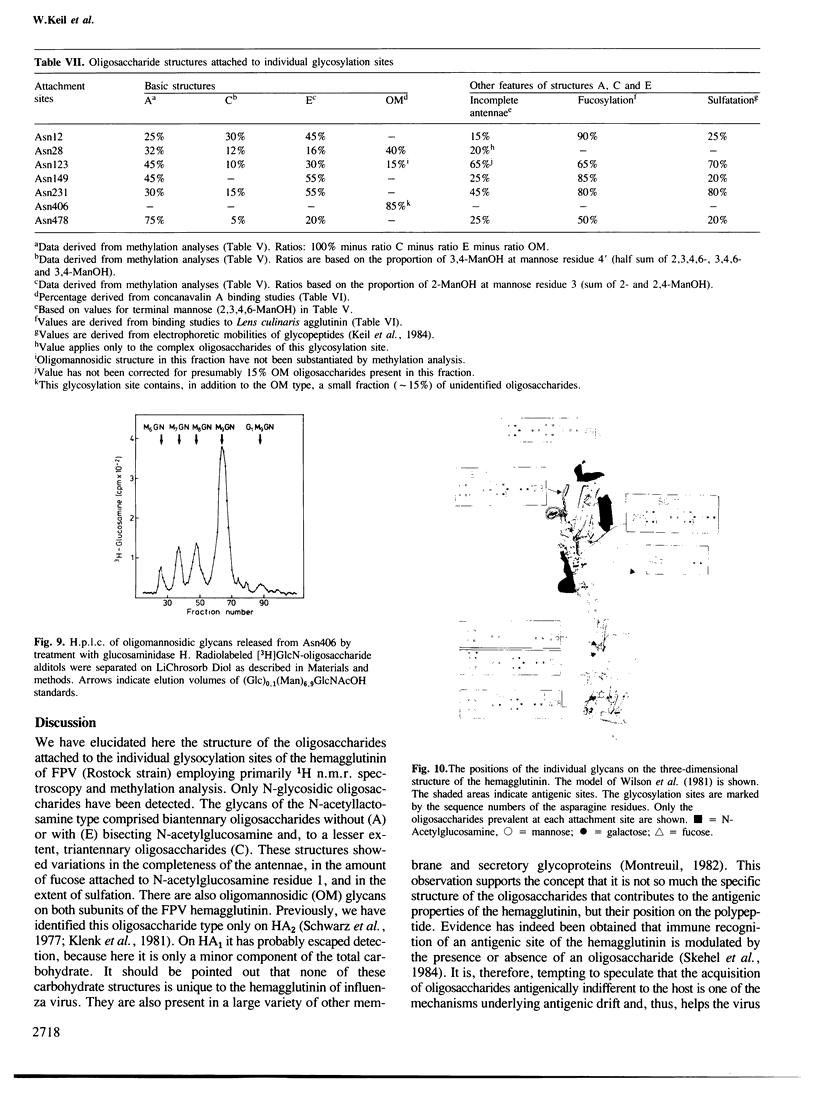


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baenziger J. U., Fiete D. Structural determinants of concanavalin A specificity for oligosaccharides. J Biol Chem. 1979 Apr 10;254(7):2400–2407. [PubMed] [Google Scholar]
- Bhattacharyya S. N., Lynn W. S., Dabrowski J., Trauner K., Hull W. E. Structure elucidation by one- and two-dimensional 360- and 500-MHz 1H NMR of the oligosaccharide units of two glycoproteins isolated from alveoli of patients with alveolar proteinosis. Arch Biochem Biophys. 1984 May 15;231(1):72–85. doi: 10.1016/0003-9861(84)90364-3. [DOI] [PubMed] [Google Scholar]
- Bock K., Arnarp J., Lönngren J. The preferred conformation of oligosaccharides derived from the complex-type carbohydrate portions of glycoproteins. Eur J Biochem. 1982 Dec;129(1):171–178. doi: 10.1111/j.1432-1033.1982.tb07036.x. [DOI] [PubMed] [Google Scholar]
- Carver J. P., Grey A. A. Determination of glycopeptide primary structure by 360-MHz proton magnetic resonance spectroscopy. Biochemistry. 1981 Nov 10;20(23):6607–6616. doi: 10.1021/bi00526a014. [DOI] [PubMed] [Google Scholar]
- Carver J. P., Grey A. A., Winnik F. M., Hakimi J., Ceccarini C., Atkinson P. H. Determination of the Structure of four glycopeptides from hen ovalbumin using 360-MHz proton magnetic resonance spectroscopy. Biochemistry. 1981 Nov 10;20(23):6600–6606. doi: 10.1021/bi00526a013. [DOI] [PubMed] [Google Scholar]
- Ceccarini C., Lorenzoni P., Atkinson P. H. Determination of the structure of ovalbumin glycopeptide AC-B by 1H nuclear magnetic resonance spectroscopy at 500 MHz. J Mol Biol. 1984 Jun 15;176(1):161–167. doi: 10.1016/0022-2836(84)90387-5. [DOI] [PubMed] [Google Scholar]
- Ceccarini C., Lorenzoni P., Atkinson P. H. Fractionation of ovalbumin glycopeptide AC-C by high-pressure liquid chromatography. Determination of structure by 1H-NMR spectroscopy. Biochim Biophys Acta. 1983 Sep 13;759(3):214–221. doi: 10.1016/0304-4165(83)90315-x. [DOI] [PubMed] [Google Scholar]
- Cohen R. E., Ballou C. E. Linkage and sequence analysis of mannose-rich glycoprotein core oligosaccharides by proton nuclear magnetic resonance spectroscopy. Biochemistry. 1980 Sep 2;19(18):4345–4358. doi: 10.1021/bi00559a031. [DOI] [PubMed] [Google Scholar]
- Collins J. K., Knight C. A. Purification of the influenza hemagglutinin glycoprotein and characterization of its carbohydrate components. J Virol. 1978 May;26(2):457–467. doi: 10.1128/jvi.26.2.457-467.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer R., Geyer H., Kühnhardt S., Mink W., Stirm S. Capillary gas chromatography of methylhexitol acetates obtained upon methylation of N-glycosidically linked glycoprotein oligosaccharides. Anal Biochem. 1982 Apr;121(2):263–274. doi: 10.1016/0003-2697(82)90478-x. [DOI] [PubMed] [Google Scholar]
- Geyer R., Geyer H., Kühnhardt S., Mink W., Stirm S. Methylation analysis of complex carbohydrates in small amounts: capillary gas chromatography-mass fragmentography of methylalditol acetates obtained from N-glycosidically linked glycoprotein oligosaccharides. Anal Biochem. 1983 Aug;133(1):197–207. doi: 10.1016/0003-2697(83)90243-9. [DOI] [PubMed] [Google Scholar]
- Geyer R., Geyer H., Stirm S., Hunsmann G., Schneider J., Dabrowski U., Dabrowski J. Major oligosaccharides in the glycoprotein of Friend murine leukemia virus: structure elucidation by one- and two-dimensional proton nuclear magnetic resonance and methylation analysis. Biochemistry. 1984 Nov 6;23(23):5628–5637. doi: 10.1021/bi00318a038. [DOI] [PubMed] [Google Scholar]
- HAKOMORI S. A RAPID PERMETHYLATION OF GLYCOLIPID, AND POLYSACCHARIDE CATALYZED BY METHYLSULFINYL CARBANION IN DIMETHYL SULFOXIDE. J Biochem. 1964 Feb;55:205–208. [PubMed] [Google Scholar]
- Homans S. W., Dwek R. A., Fernandes D. L., Rademacher T. W. The analysis of coupling networks in a complex oligosaccharide mixture derived from the Fc region of rabbit immunoglobulin G using 1H-1H correlated NMR spectroscopy combined with double quantum NMR spectroscopy. Biochim Biophys Acta. 1984 Mar 22;798(1):78–83. doi: 10.1016/0304-4165(84)90012-6. [DOI] [PubMed] [Google Scholar]
- Hounsell E. F., Wright D. J., Donald A. S., Feeney J. A computerized approach to the analysis of oligosaccharide structure by high-resolution proton n.m.r. Biochem J. 1984 Oct 1;223(1):129–143. doi: 10.1042/bj2230129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh P., Rosner M. R., Robbins P. W. Host-dependent variation of asparagine-linked oligosaccharides at individual glycosylation sites of Sindbis virus glycoproteins. J Biol Chem. 1983 Feb 25;258(4):2548–2554. [PubMed] [Google Scholar]
- Hubbard S. C., Ivatt R. J. Synthesis and processing of asparagine-linked oligosaccharides. Annu Rev Biochem. 1981;50:555–583. doi: 10.1146/annurev.bi.50.070181.003011. [DOI] [PubMed] [Google Scholar]
- Keil W., Klenk H. D., Schwarz R. T. Carbohydrates of influenza virus. III. Nature of oligosaccharide-protein linkage in viral glycoproteins. J Virol. 1979 Jul;31(1):253–256. doi: 10.1128/jvi.31.1.253-256.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil W., Niemann H., Schwarz R. T., Klenk H. D. Carbohydrates of influenza virus. V. Oligosaccharides attached to individual glycosylation sites of the hemagglutinin of fowl plague virus. Virology. 1984 Feb;133(1):77–91. doi: 10.1016/0042-6822(84)90427-6. [DOI] [PubMed] [Google Scholar]
- Klenk H. D., Rott R. Cotranslational and posttranslational processing of viral glycoproteins. Curr Top Microbiol Immunol. 1980;90:19–48. doi: 10.1007/978-3-642-67717-5_2. [DOI] [PubMed] [Google Scholar]
- Kornfeld K., Reitman M. L., Kornfeld R. The carbohydrate-binding specificity of pea and lentil lectins. Fucose is an important determinant. J Biol Chem. 1981 Jul 10;256(13):6633–6640. [PubMed] [Google Scholar]
- Matsumoto A., Yoshima H., Kobata A. Carbohydrates of influenza virus hemagglutinin: structures of the whole neutral sugar chains. Biochemistry. 1983 Jan 4;22(1):188–196. doi: 10.1021/bi00270a028. [DOI] [PubMed] [Google Scholar]
- Mellis S. J., Baenziger J. U. Structures of the oligosaccharides present at the three asparagine-linked glycosylation sites of human IgD. J Biol Chem. 1983 Oct 10;258(19):11546–11556. [PubMed] [Google Scholar]
- Nagai Y., Klenk H. D. Activation of precursors to both glycoporteins of Newcastle disease virus by proteolytic cleavage. Virology. 1977 Mar;77(1):125–134. doi: 10.1016/0042-6822(77)90412-3. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Bhown A. S., Compans R. W. Glycosylation sites of influenza viral glycoproteins. Tryptic glycopeptides from the A/WSN (H0N1) hemagglutinin glycoprotein. Virology. 1980 Nov;107(1):208–221. doi: 10.1016/0042-6822(80)90286-x. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Compans R. W. Effects of glucosamine, 2-deoxyglucose, and tunicamycin on glycosylation, sulfation, and assembly of influenza viral proteins. Virology. 1978 Feb;84(2):303–319. doi: 10.1016/0042-6822(78)90250-7. [DOI] [PubMed] [Google Scholar]
- Narasimhan S., Wilson J. R., Martin E., Schachter H. A structural basis for four distinct elution profiles on concanavalin A--Sepharose affinity chromatography of glycopeptides. Can J Biochem. 1979 Jan;57(1):83–96. doi: 10.1139/o79-011. [DOI] [PubMed] [Google Scholar]
- Niemann H., Dabrowski J., Dabrowski U., Geyer R., Keil W., Klenk H. D., Stirm S. The major oligosaccharides in the large subunit of the hemagglutinin from fowl plague virus, strain Dutch. Structure elucidation by one-dimensional and two-dimensional 1H nuclear magnetic resonance and by methylation analysis. Eur J Biochem. 1985 Feb 1;146(3):523–532. doi: 10.1111/j.1432-1033.1985.tb08683.x. [DOI] [PubMed] [Google Scholar]
- Porter A. G., Barber C., Carey N. H., Hallewell R. A., Threlfall G., Emtage J. S. Complete nucleotide sequence of an influenza virus haemagglutinin gene from cloned DNA. Nature. 1979 Nov 29;282(5738):471–477. doi: 10.1038/282471a0. [DOI] [PubMed] [Google Scholar]
- Schlüter M., Linder D., Geyer R., Hunsmann G., Schneider J., Stirm S. The glycoprotein 71 of ecotropic Friend murine leukemia virus. Structure of the oligosaccharides linked to asparagine-12. FEBS Lett. 1984 Apr 24;169(2):194–198. doi: 10.1016/0014-5793(84)80317-8. [DOI] [PubMed] [Google Scholar]
- Schuy W., Garten W., Linder D., Klenk H. D. The carboxyterminus of the hemagglutinin-neuraminidase of Newcastle disease virus is exposed at the surface of the viral envelope. Virus Res. 1984;1(5):415–426. doi: 10.1016/0168-1702(84)90027-3. [DOI] [PubMed] [Google Scholar]
- Schwarz R. T., Schmidt M. F., Anwer U., Klenk H. D. Carbohydrates of influenza virus. I. Glycopeptides derived from viral glycoproteins after labeling with radioactive sugars. J Virol. 1977 Aug;23(2):217–226. doi: 10.1128/jvi.23.2.217-226.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skehel J. J., Stevens D. J., Daniels R. S., Douglas A. R., Knossow M., Wilson I. A., Wiley D. C. A carbohydrate side chain on hemagglutinins of Hong Kong influenza viruses inhibits recognition by a monoclonal antibody. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1779–1783. doi: 10.1073/pnas.81.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiedler S. J., Hart G. W., Tarentino A. L., Plummer T. H., Jr, Freed J. H. Stable oligosaccharide microheterogeneity at individual glycosylation sites of a murine major histocompatibility antigen derived from a B-cell lymphoma. J Biol Chem. 1983 Oct 10;258(19):11515–11523. [PubMed] [Google Scholar]
- Townsend R. R., Hilliker E., Li Y. T., Laine R. A., Bell W. R., Lee Y. C. Carbohydrate structure of human fibrinogen. Use of 300-MHz 1H-NMR to characterize glycosidase-treated glycopeptides. J Biol Chem. 1982 Aug 25;257(16):9704–9710. [PubMed] [Google Scholar]
- Ward C. W. Structure of the influenza virus hemagglutinin. Curr Top Microbiol Immunol. 1981;94-95:1–74. doi: 10.1007/978-3-642-68120-2_1. [DOI] [PubMed] [Google Scholar]
- Wilson I. A., Skehel J. J., Wiley D. C. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 A resolution. Nature. 1981 Jan 29;289(5796):366–373. doi: 10.1038/289366a0. [DOI] [PubMed] [Google Scholar]