Abstract
Autism spectrum disorders (ASDs) have been associated with brain inflammation as indicated by microglia activation, as well as brain expression and increased plasma levels of interleukin-6 (IL-6) and tumor necrosis factor (TNF). Here we report that serum levels of IL-6 and TNF were elevated (61.95±94.76 pg ml−1 and 313.8±444.3 pg ml−1, respectively) in the same cohort of patients with elevated serum levels of corticotropin-releasing hormone (CRH) and neurotensin (NT), while IL-9, IL-31 and IL-33 were not different from controls. The elevated CRH and NT levels did not change after treatment with a luteolin-containing dietary formulation. However, the mean serum IL-6 and TNF levels decreased significantly (P=0.036 and P=0.015, respectively) at the end of the treatment period (26 weeks) as compared with levels at the beginning; these decreases were strongly associated with children whose behavior improved the most after luteolin formulation treatment. Our results indicate that there are distinct subgroups of children within the ASDs that may be identifiable through serum levels of IL-6 and TNF and that these cytokines may constitute distinct prognostic markers for at least the beneficial effect of luteolin formulation.
Introduction
Autism spectrum disorders (ASDs) are neurodevelopmental disorders characterized by impaired social interactions and communication, as well as stereotypic behaviors.1, 2, 3, 4 The prevalence of ASDs is estimated to be 1 in 68 children.5 As many as 50% of children with ASDs regress at 2–3 years old implying the involvement of some epigenetic triggers, such as high fever, infection,6, 7 trauma,8 environmental toxins9, 10, 11 or stress.12 In spite of the identification of a number of mutations in children with ASDs,13 its pathogenesis remains unknown. Moreover there are no objective biomarkers for either diagnosis or prognosis making effective drug development difficult.
There appear to be distinct subgroups within the ASDs, including gastrointestinal problems,14 mitochondrial dysfunction15 and ‘allergic’ symptoms,16 especially food intolerance and eczema.17 However, no group has been identified by objective biomarkers. Increasing evidence indicates that brain inflammation is important in the pathogenesis of neuropsychiatric disorders13, 18, 19 including ASDs. A recent paper reported microglia activation as a common finding in the brain of patients with ASDs.20 Microglia can be activated by mast cells (MC),21 which have been implicated in ASDs.22 In fact, the risk of ASDs appears to be 10 times higher in children with mastocytosis,23 a condition characterized by an increased number of activated MCs.24
We reported increased serum levels of the peptide neurotensin (NT) in children with ASDs.25 NT is a vasoactive peptide isolated from the brain26 and is implicated in immunity.27 We recently reported that serum levels of corticotropin-releasing hormone (CRH), secreted under stress, were also elevated together with NT in children with ASDs.28 CRH increased vascular permeability22 through a synergistic action with NT.29 Interactions among CRH, NT, microglia and MCs could contribute to brain inflammation.30, 31
Many children with ASDs have been reported to have ‘allergic-like’ symptoms32 implicating MC activation.33 Natural flavonoids, like luteolin and quercetin, exhibit potent anti-oxidant and anti-inflammatory activities34 and inhibit the release of inflammatory mediators from human MCs.35 Luteolin and its structurally related quercetin inhibit the release of histamine, leukotrienes, interleukin-8 (IL-8), IL-6 and tumor necrosis factor-alpha (TNF-α) from human cultured MCs36, 37, 38 and allergic inflammation.39 Moreover, luteolin inhibited IL-6 release from activated microglia40 and reduced maternal IL-6-induced autism-like behavioral deficits related to social interactions in mice.41 Luteolin also inhibits MC-dependent stimulation of activated T cells,42 and is neuroprotective.43 It also inhibits stimulation of astrocytes,44 as well as microglial activation and proliferation,45, 46, 47 protects against thimerosal-induced inflammatory mediator release from MCs 48 and methylmercury-induced mouse brain mitochondrial damage.49 One open-label clinical study showed that a luteolin-containing dietary formulation significantly improved sociability in children with ASDs.50
Here we report that serum IL-6 and TNF levels that were elevated in the children with ASDs in that study before treatment were significantly reduced at the end of the treatment period; moreover, this reduction strongly correlated with those children who improved by this luteolin dietary supplement.
Materials and Methods
Fasting blood was obtained from Caucasian children (34 male and 6 female, 4–10 years of age) on the entire ASDs who participated in an open-label clinical trial conducted at the Attikon General Hospital, Athens Medical School, Athens, Greece (registered at ClinicalTrials.gov, NCT 01847521).50 Children were diagnosed with ASDs based on clinical assessment and corroborated by meeting the cutoff scores on both the DSM-IV-TR symptom list and the autism diagnostic observation schedule (ADOS) algorithm. They were medication free prior to blood draw for at least 2 weeks for all psychotropic medications and 4 weeks for fluoxetine or depot neuroleptics. The exclusion criteria were: (1) Any genetic condition linked to ASDs (for example, Rett syndrome, Fragile X syndrome, tuberous sclerosis or focal epilepsy); (2) Any genetic syndrome involving the central nervous system, even if the link with ASDs was uncertain; (3) Any neurologic disorder involving pathology above the brain stem, other than uncomplicated non-focal epilepsy; (4) Contemporaneous evidence, or unequivocal retrospective evidence, of probable neonatal brain damage; (5) Clinically significant visual or auditory impairment, even after correction; (6) Any severe nutritional or psychological deprivation; (7) Systemic or mastocytosis (including urticaria pigmentosa); (8) History of upper airway diseases; (9) History of inflammatory diseases (for example, juvenile rheumatoid arthritis, inflammatory bowel disease); (10) History of allergies. Informed consent was obtained from all subjects. This protocol was approved by the Ethics Committee of Attikon General Hospital, Athens Medical School, Athens, Greece.
Children were administered the dietary formulation (NeuroProtek, GMPCertified, Tishcon, Salisbury, MD, USA) containing the liposomal flavonoids (mg per capsule): luteolin (100), quercetin (70) and the quercetin glucoside rutin (30) in olive fruit extract formulated by a good manufacturing practices–certified facility (Tishcon Laboratories, Westbury, NY, USA) under contract from Algonot (Sarasota, FL, USA; www.algonot.com). Quercetin and rutin were added to the formulation as ‘decoys’ to keep the intestinal and liver enzymes occupied to allow luteolin to escape metabolism and reach the brain.
Serum was also collected from normally developing, healthy children, unrelated to the ASDs subjects, who were seen for routine health visits at the Pediatric Department of the Social Security Administration (IKA) polyclinic. Serum samples were labeled only with a code number, the age and sex of the subjects. All ASDs and control blood samples were prepared immediately and serum was stored in −80 °C. Samples were then transported on dry ice to Boston for analysis.
Assessment of serum cytokine levels
IL-6, IL-9, IL-31, IL-33 and TNF levels were determined with commercially available enzyme-immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s protocol.
Statistical analysis
Prior to analysis, the data were validated and inspected for outliers. The results are presented as scattergrams with symbols representing individual data points and the horizontal lines representing the mean for each group. Normality of distribution was checked with the Shapiro–Wilk’s test. Comparison between the healthy control and the ASDs groups was performed using Mann–Whitney U-tests. Comparison of the ASDs group at baseline and at endpoint was performed using Wilcoxon matched pair test. The effect of Vineland Adaptive Behavior Scale (VABS) domains outcome in time was investigated using a general linear model for repeated measurements. A result was considered significant at a P-value <0.05. The analysis was performed by using the GraphPad Prism version 5.0 software (GraphPad Software, San Diego, CA, USA).
Results
There was no statistical difference in serum levels of IL-9, IL-31 and IL-33 between ASDs and normotypic controls (results not shown).
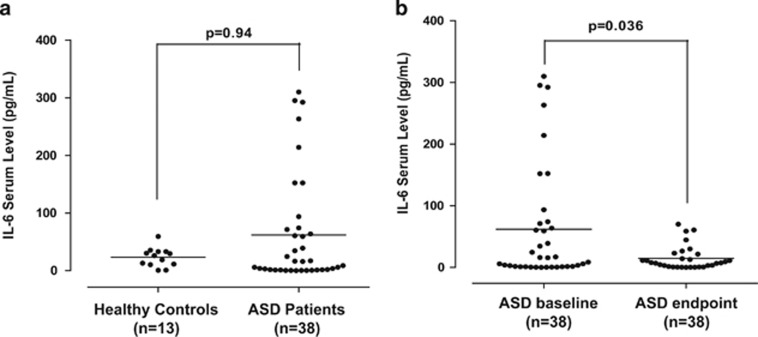
Serum IL-6 levels were elevated (61.95±94.76 pg ml−1) in children with ASDs as compared with normotypic controls (23.20±16.31 pg ml−1), but this increase did not reach statistical significance (Figure 1a).
Figure 1.
(a) Comparison of serum IL-6 levels in normal and ASDs children. (b) Serum IL-6 levels in children with ASDs before and after treatment with a luteolin-containing dietary formulation. Symbols represent individual data points, and the horizontal line represents the mean for each group. ASD, autism spectrum disorder; IL, interleukin.
Nevertheless, serum IL-6 levels were significantly (P=0.036) lower (14.68±19.22 pg ml−1) in children with ASDs after treatment with luteolin in comparison to their levels before the beginning of treatment (61.95±94.76 pg ml−1) (Figure 1b).
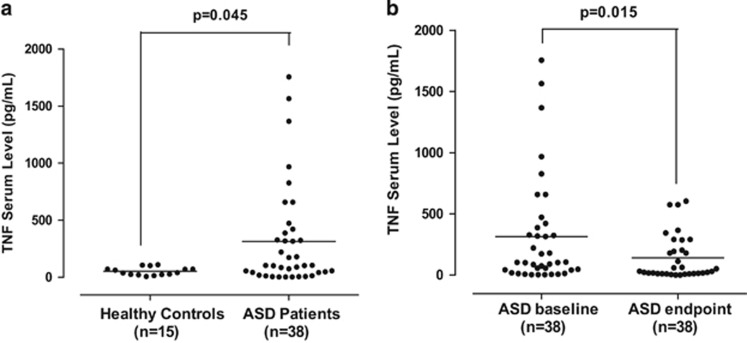
Serum TNF levels were significantly (P=0.045) elevated (313.8±444.3 pg ml−1) in children with ASDs as compared with normotypic controls (52.78±34.62 pg ml−1) (Figure 2a).
Figure 2.
(a) Comparison of serum TNF levels in normal and ASDs children. (b) Serum TNF levels in children with ASDs before and after treatment with a luteolin-containing dietary formulation. Symbols represent individual data points, and the horizontal line represents the mean for each group. ASD, autism spectrum disorder; TNF, tumor necrosis factor.
These elevated serum TNF levels were significantly (P=0.015) lower (139.6±181.5 pg ml−1) in children with ASDs after treatment with luteolin in comparison to their levels before the beginning of treatment (313.8±444.3 pg ml−1) (Figure 2b).
There were two clusters of ASD children with low and high serum IL-6 and TNF levels indicating two subgroups. Low IL-6 and TNF levels are those below the mean, while high IL-6 and TNF levels are those above the mean. The ASDs children who had both high serum IL-6 and TNF levels were the same (n=10).
The improvement in the VABS age-equivalent scores for these 10 ASDs children was significant (P<0.05) for all domains (Table 1). The VABS composite score was also significantly higher at the end of the study. These data indicate a positive effect of the luteolin dietary supplement on the adaptive functioning of this subgroup of ASDs children. More specifically these children gained 9.73 months in the communication domain, 6.64 months in daily living skills and 8.09 months in the social domain.
Table 1. Effectiveness of the study luteolin formulation in ASDs children with high serum IL-6 and TNF levels (n=10).
| Vineland adaptive behavior scales (VABS) | 0 week | 26 week | Change | Effect sizea | P-value |
|---|---|---|---|---|---|
| Communication AE | 38.91 (25.47)b | 48.64 (31.60) | 9.73 | 0.38 | 0.008 |
| Daily living skills AE | 38.45 (19.11) | 45.09 (21.00) | 6.64 | 0.35 | 0.0003 |
| Social AE | 36.55 (18.04) | 44.64 (20.93) | 8.09 | 0.45 | 0.001 |
| Composite score | 37.97 (19.68) | 46.12 (23.19) | 8.15 | 0.42 | 0.001 |
Abbreviations: AE, age equivalent; ASD, autism spectrum disorder; IL, interleukin; TNF, tumor necrosis factor; VABS, Vineland Adaptive Behavior Scale.
(26 week–0 week)/0 week s.d.
VABS scores (s.d.).
Discussion
Our study shows two clusters of ASD children with low and high serum IL-6 and TNF levels, indicating two subgroups. Moreover, the ASD children who had both high serum IL-6 and TNF levels were the same (n=10). We further show that the children with ASDs in which the elevated serum IL-6 and TNF levels decreased at the end of the treatment period with a luteolin formulation, were the ones whose behavior improved the most.
There is evidence indicating that certain cytokines can impair neurodevelopment and behavior13, 51 and that microglia activation and inflammation is involved in the pathogenesis of neuropsychiatric diseases13, 51 including ASDs.52, 53 IL-6 can directly alter neuronal activity, proliferation and survival that may impact behavior.54 IL-6 has also physiological and pathological effects on learning and memory.55 Increased gene expression of IL-6 was noted in postmortem specimens of the temporal cortex of the brain of individuals with ASDs56 and increased protein level of IL-6 was found in the brain and cerebrospinal fluid of individuals with ASDs.57 In agreement with these findings, IL-6 was significantly increased in the frontal cortex and cerebellum of ASD patients as compared with the age-matched controls.58 IL-6 may derive from microglia cells, which are activated in ASDs.20 Consistent with our results is a previously reported meta-analysis of increased IL-6 concentrations in peripheral blood in ASD participants compared with healthy controls.59 It is of interest that acute restraint stress of mice led to increased serum IL-6, which was entirely MC dependent.60 IL-1β can stimulate selective release of IL-6 from MCs.61
In the present study, we also found significantly increased serum TNF levels in children with ASDs in comparison to healthy controls. Another study showed increased TNF production in peripheral blood mononuclear cells of autistic subjects after stimulation with polyhydroxyalkanoates and tetanus.62 TNF was increased almost 50 times in the cerebrospinal fluid of ASD children.63 Brain MCs can secrete TNF.64 Both TNF and IL-6 expression has been documented in brains of children with ASDs65 and IL-6 has been implicated in an animal ‘model’ of autism.7 In this context, it is particularly important that MCs are the only cells that store preformed TNF, which they can secrete rapidly.66 MCs are the only cells that release IL-6 in response to stress.60 Preformed TNF is secreted from MCs67 and stimulates T-cell activation.42, 68 TNF has been linked with neurite growth and the regulation of homeostatic synaptic plasticity in the hippocampus.69
One study also showed increased serum levels of IL-17 in children with ASDs.70 Another study showed increased plasma IL-1β and IL-17, but only in children with ASD and regression; children with ASD and GI issues had higher plasma IL-1β and IL-6, but not TNF. TNF and IL-17 seem to act together in perpetuating the inflammatory process.71, 72 MC-derived IL-6 and transforming growth factor beta (TGFβ) induce the development of Th-17 cells through dendritic cell maturation;73 Moreover, MCs secrete IL-17, themselves.74
Here we report that treatment with a luteolin-containing dietary formulation normalized serum IL-6 and TNF in those children that showed the most benefit from the use of luteolin.
We recently reported that the structural analog of luteolin 3′,4′,5,7-tetramethoxy flavone was more potent than luteolin in its ability to inhibit mediator release from human MCs.35
Luteolin is structurally closely related to 7,8-dihydroflavone, which was shown to have brain-derived neurotrophic factor (BDNF)-like activity.75 In fact, absence of BDNF was associated with autistic-like-behavior in mice, while 7,8-dihydroflavone administration reduced symptoms in a mouse model of Rett syndrome,76 most patients with which have symptoms of ASDs.77
We believe this is the first time that objective biomarkers can (a) distinguish a subgroup of children with ASDs and (b) their reduced level correlate with a favorable clinical outcome, following administration of a natural anti-inflammatory compound. Flavonoids are considered generally safe78, 79, 80 and are being discussed as possible treatment of central nervous system disorders81 that may involve brain inflammation in response to environmental triggers. One obvious question is how much of the luteolin may reach the brain because flavonoids purely absorb orally and are extensively metabolized.82, 83, 84 Unfortunately, children with ASDs are prescribed many other supplements and psychotropic drugs that may have unwanted drug interactions.85 One way to deliver luteolin directly to the brain would be through intranasal administration through the cribriform plexus as shown before for another compound.86
Acknowledgments
This study was supported in part by the Autism Research Institute, the Jane Botsford Johnson Foundation and the BHARE Foundation. We thank Dr AK Theoharides for collecting the human normotypic serum samples and Mrs Smaro Panagiotidou for her word processing skills.
Disclaimer
TCT is the inventor of US patent No. 8,268,365 and US patent No. 20110027397 A1 on methods of treating autism spectrum disorders and compositions for same.
Footnotes
The authors declare no conflict of interest.
References
- Fombonne E. Epidemiology of pervasive developmental disorders. Pediatr Res 2009; 65: 591–598. [DOI] [PubMed] [Google Scholar]
- Johnson CP, Myers SM. Identification and evaluation of children with autism spectrum disorders. Pediatrics 2007; 120: 1183–1215. [DOI] [PubMed] [Google Scholar]
- McPartland J, Volkmar FR. Autism and related disorders. Handb Clin Neurol 2012; 106: 407–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahams BS, Geschwind DH. Advances in autism genetics: on the threshold of a new neurobiology. Nat Rev Genet 2008; 9: 341–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. CDC estimates 1 in 68 children has been identified with autism spectrum disorder, 2014. http://wwwcdcgov/media/releases/2014/p0327-autism-spectrum-disorder html.
- Hornig M, Weissenbock H, Horscroft N, Lipkin WI. An infection-based model of neurodevelopmental damage. Proc Natl Acad Sci USA 1999; 96: 12102–12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao EY, McBride SW, Chow J, Mazmanian SK, Patterson PH. Modeling an autism risk factor in mice leads to permanent immune dysregulation. Proc Natl Acad Sci USA 2012; 109: 12776–12781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blenner S, Reddy A, Augustyn M. Diagnosis and management of autism in childhood. BMJ 2011; 343: d6238. [DOI] [PubMed] [Google Scholar]
- Deth R, Muratore C, Benzecry J, Power-Charnitsky VA, Waly M. How environmental and genetic factors combine to cause autism: a redox/methylation hypothesis. NeuroToxicol 2008; 29: 190–201. [DOI] [PubMed] [Google Scholar]
- Herbert MR. Contributions of the environment and environmentally vulnerable physiology to autism spectrum disorders. Curr Opin Neurol 2010; 23: 103–110. [DOI] [PubMed] [Google Scholar]
- Rossignol DA, Genuis SJ, Frye RE. Environmental toxicants and autism spectrum disorders: a systematic review. Transl Psychiatry 2014; 4: e360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanni KE, Schupp CW, Simon D, Corbett BA. Verbal ability, social stress, and anxiety in children with autistic disorder. Autism 2012; 16: 123–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theoharides TC, Weinkauf C, Conti P. Brain cytokines and neuropsychiatric disorders. J Clin Psychopharmacol 2004; 24: 577–581. [DOI] [PubMed] [Google Scholar]
- Buie T, Campbell DB, Fuchs GJ III, Furuta GT, Levy J, Vandewater J et al. Evaluation, diagnosis, and treatment of gastrointestinal disorders in individuals with ASDs: a consensus report. Pediatrics 2010; 125: S1–18. [DOI] [PubMed] [Google Scholar]
- Rossignol DA, Frye RE. Mitochondrial dysfunction in autism spectrum disorders: a systematic review and meta-analysis. Mol Psychiatry 2012; 17: 290–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelidou A, Alysandratos KD, Asadi S, Zhang B, Francis K, Vasiadi M et al. Brief report: ‘allergic symptoms’ in children with autism spectrum disorders. More than meets the eye? J Autism Dev Disord 2011; 41: 1579–1585. [DOI] [PubMed] [Google Scholar]
- Gurney JG, McPheeters ML, Davis MM. Parental report of health conditions and health care use among children with and without autism: National Survey of Children's Health. Arch Pediatr Adolesc Med 2006; 160: 825–830. [DOI] [PubMed] [Google Scholar]
- Theoharides TC, Zhang B. Neuro-Inflammation, blood-brain barrier, seizures and autism. J Neuroinflamm 2011; 8: 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg H, Gressens P, Mallard C. Inflammation during fetal and neonatal life: implications for neurologic and neuropsychiatric disease in children and adults. Ann Neurol 2012; 71: 444–457. [DOI] [PubMed] [Google Scholar]
- Gupta S, Ellis SE, Ashar FN, Moes A, Bader JS, Zhan J et al. Transcriptome analysis reveals dysregulation of innate immune response genes and neuronal activity-dependent genes in autism. Nat Commun 2014; 5: 5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaper SD, Facci L, Giusti P. Mast cells, glia and neuroinflammation: partners in crime? Immunology 2014; 141: 314–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theoharides TC, Singh LK, Boucher W, Pang X, Letourneau R, Webster E et al. Corticotropin-releasing hormone induces skin mast cell degranulation and increased vascular permeability, a possible explanation for its pro-inflammatory effects. Endocrinology 1998; 139: 403–413. [DOI] [PubMed] [Google Scholar]
- Theoharides TC. Autism spectrum disorders and mastocytosis. Int J Immunopathol Pharmacol 2009; 22: 859–865. [DOI] [PubMed] [Google Scholar]
- Theoharides TC, Valent P, Akin C. Mast cells, mastocytosis and related diseases. N Engl J Med 2015; 373: 163–172. [DOI] [PubMed] [Google Scholar]
- Angelidou A, Francis K, Vasiadi M, Alysandratos K-D, Zhang B, Theoharides A et al. Neurotensin is increased in serum of young children with autistic disorder. J Neuroinflamm 2010; 7: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carraway R, Leeman SE. The isolation of a new hypotensive peptide, neurotensin, from bovine hypothalami. J Biol Chem 1973; 248: 6854–6861. [PubMed] [Google Scholar]
- Mustain WC, Rychahou PG, Evers BM. The role of neurotensin in physiologic and pathologic processes. Curr Opin Endocrinol Diabetes Obes 2011; 18: 75–82. [DOI] [PubMed] [Google Scholar]
- Tsilioni I, Dodman N, Petra AI, Taliou A, Francis K, Moon-Fanelli AA et al. Elevated serum neurotensin and CRH levels in children with autistic spectrum disorders and tail-chasing bull terriers with a phenotype similar to autism. Transl Psychiatry 2014; 4: e466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donelan J, Boucher W, Papadopoulou N, Lytinas M, Papaliodis D, Theoharides TC. Corticotropin-releasing hormone induces skin vascular permeability through a neurotensin-dependent process. Proc Natl Acad Sci USA 2006; 103: 7759–7764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theoharides TC, Stewart JM, Panagiotidou S, Melamed I. Mast cells, brain inflammation and autism. Eur J Pharmacol 2015. pii: S0014-2999(15)00398-2. [DOI] [PubMed]
- Kritas SK, Saggini A, Cerulli G, Caraffa A, Antinolfi P, Pantalone A et al. Corticotropin-releasing hormone, microglia and mental disorders. Int J Immunopathol Pharmacol 2014; 27: 163–167. [DOI] [PubMed] [Google Scholar]
- Theoharides TC. Is a subtype of autism an ‘allergy of the brain’? Clin Ther 2013; 35: 584–591. [DOI] [PubMed] [Google Scholar]
- Theoharides TC, Angelidou A, Alysandratos KD, Zhang B, Asadi S, Francis K et al. Mast cell activation and autism. Biochim Biophys Acta 2012; 1822: 34–41. [DOI] [PubMed] [Google Scholar]
- Middleton EJ, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease and cancer. Pharmacol Rev 2000; 52: 673–751. [PubMed] [Google Scholar]
- Weng Z, Patel A, Panagiotidou S, Theoharidess TC. The novel flavone tetramethoxyluteolin is a potent inhibitor of human mast cells. J Allergy Clin Immunol 2014; 14: 1044–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempuraj D, Madhappan B, Christodoulou S, Boucher W, Cao J, Papadopoulou N et al. Flavonols inhibit proinflammatory mediator release, intracellular calcium ion levels and protein kinase C theta phosphorylation in human mast cells. Br J Pharmacol 2005; 145: 934–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HH, Lee S, Son HY, Park SB, Kim MS, Choi EJ et al. Flavonoids inhibit histamine release and expression of proinflammatory cytokines in mast cells. Arch Pharm Res 2008; 31: 1303–1311. [DOI] [PubMed] [Google Scholar]
- Kimata M, Shichijo M, Miura T, Serizawa I, Inagaki N, Nagai H. Effects of luteolin, quercetin and baicalein on immunoglobulin E-mediated mediator release from human cultured mast cells. Clin Exp Allergy 2000; 30: 501–508. [DOI] [PubMed] [Google Scholar]
- Kritas SK, Saggini A, Varvara G, Murmura G, Caraffa A, Antinolfi P et al. Luteolin inhibits mast cell-mediated allergic inflammation. J Biol Regul Homeost Agents 2013; 27: 955–959. [PubMed] [Google Scholar]
- Jang S, Kelley KW, Johnson RW. Luteolin reduces IL-6 production in microglia by inhibiting JNK phosphorylation and activation of AP-1. Proc Natl Acad Sci USA 2008; 105: 7534–7539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker-Athill E, Luo D, Bailey A, Giunta B, Tian J, Shytle RD et al. Flavonoids, a prenatal prophylaxis via targeting JAK2/STAT3 signaling to oppose IL-6/MIA associated autism. J Neuroimmunol 2009; 217: 20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempuraj D, Tagen M, Iliopoulou BP, Clemons A, Vasiadi M, Boucher W et al. Luteolin inhibits myelin basic protein-induced human mast cell activation and mast cell dependent stimulation of Jurkat T cells. Br J Pharmacol 2008; 155: 1076–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HQ, Jin ZY, Wang XJ, Xu XM, Deng L, Zhao JW. Luteolin protects dopaminergic neurons from inflammation-induced injury through inhibition of microglial activation. Neurosci Lett 2008; 448: 175–179. [DOI] [PubMed] [Google Scholar]
- Sharma V, Mishra M, Ghosh S, Tewari R, Basu A, Seth P et al. Modulation of interleukin-1beta mediated inflammatory response in human astrocytes by flavonoids: implications in neuroprotection. Brain Res Bull 2007; 73: 55–63. [DOI] [PubMed] [Google Scholar]
- Dirscherl K, Karlstetter M, Ebert S, Kraus D, Hlawatsch J, Walczak Y et al. Luteolin triggers global changes in the microglial transcriptome leading to a unique anti-inflammatory and neuroprotective phenotype. J Neuroinflamm 2010; 7: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S, Dilger RN, Johnson RW. Luteolin inhibits microglia and alters hippocampal-dependent spatial working memory in aged mice. J Nutr 2010; 140: 1892–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao TK, Ou YC, Lin SY, Pan HC, Song PJ, Raung SL et al. Luteolin inhibits cytokine expression in endotoxin/cytokine-stimulated microglia. J Nutr Biochem 2011; 22: 612–624. [DOI] [PubMed] [Google Scholar]
- Asadi S, Zhang B, Weng Z, Angelidou A, Kempuraj D, Alysandratos KD et al. Luteolin and thiosalicylate inhibit HgCl(2) and thimerosal-induced VEGF release from human mast cells. Int J Immunopathol Pharmacol 2010; 23: 1015–1020. [DOI] [PubMed] [Google Scholar]
- Franco JL, Posser T, Missau F, Pizzolatti MG, Dos Santos AR, Souza DO et al. Structure-activity relationship of flavonoids derived from medicinal plants in preventing methylmercury-induced mitochondrial dysfunction. Environ Toxicol Pharmacol 2010; 30: 272–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taliou A, Zintzaras E, Lykouras L, Francis K. An open-label pilot study of a formulation containing the anti-inflammatory flavonoid luteolin and its effects on behavior in children with autism spectrum disorders. Clin Ther 2013; 35: 592–602. [DOI] [PubMed] [Google Scholar]
- Kempuraj D, Konstantinidou A, Boscolo P, Ferro F, Di Giannantonio M, Conti CM et al. Cytokines and the brain. Int J Immunopathol Pharmacol 2004; 17: 229–232. [DOI] [PubMed] [Google Scholar]
- Xu N, Li X, Zhong Y. Inflammatory cytokines: potential biomarkers of immunologic dysfunction in autism spectrum disorders. Mediators Inflamm 2015; 2015: 531518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonhajzerova I, Ondrejka I, Mestanik M, Mikolka P, Hrtanek I, Mestanikova A et al. Inflammatory activity in autism spectrum disorder. Adv Exp Med Biol 2015; 861: 93–98. [DOI] [PubMed] [Google Scholar]
- Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah I, Van de WJ. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav Immun 2011; 25: 40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun 2011; 25: 181–213. [DOI] [PubMed] [Google Scholar]
- Garbett K, Ebert PJ, Mitchell A, Lintas C, Manzi B, Mirnics K et al. Immune transcriptome alterations in the temporal cortex of subjects with autism. Neurobiol Dis 2008; 30: 303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol 2005; 57: 67–81. [DOI] [PubMed] [Google Scholar]
- Li X, Chauhan A, Sheikh AM, Patil S, Chauhan V, Li XM et al. Elevated immune response in the brain of autistic patients. J Neuroimmunol 2009; 207: 111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masi A, Quintana DS, Glozier N, Lloyd AR, Hickie IB, Guastella AJ. Cytokine aberrations in autism spectrum disorder: a systematic review and meta-analysis. Mol Psychiatry 2015; 20: 440–446. [DOI] [PubMed] [Google Scholar]
- Huang M, Pang X, Karalis K, Theoharides TC. Stress-induced interleukin-6 release in mice is mast cell-dependent and more pronounced in Apolipoprotein E knockout mice. Cardiovasc Res 2003; 59: 241–249. [DOI] [PubMed] [Google Scholar]
- Kandere-Grzybowska K, Kempuraj D, Letourneau L, Asare A, Athanasiou A, Theoharides TC. IL-1 induces differential release of IL-6 form human mast cells. FASEB J 2002; 16: A332. [Google Scholar]
- Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah IN, Van de WJ. Altered T cell responses in children with autism. Brain Behav Immun 2011; 25: 840–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chez MG, Dowling T, Patel PB, Khanna P, Kominsky M. Elevation of tumor necrosis factor-alpha in cerebrospinal fluid of autistic children. Pediatr Neurol 2007; 36: 361–365. [DOI] [PubMed] [Google Scholar]
- Cocchiara R, Bongiovanni A, Albeggiani G, Azzolina A, Geraci D. Evidence that brain mast cells can modulate neuroinflammatory responses by tumor necrosis factor-α production. Neuroreport 1998; 9: 95–98. [DOI] [PubMed] [Google Scholar]
- Zimmerman AW, Jyonouchi H, Comi AM, Connors SL, Milstien S, Varsou A et al. Cerebrospinal fluid and serum markers of inflammation in autism. Pediatr Neurol 2005; 33: 195–201. [DOI] [PubMed] [Google Scholar]
- Zhang B, Alysandratos KD, Angelidou A, Asadi S, Sismanopoulos N, Delivanis DA et al. Human mast cell degranulation and preformed TNF secretion require mitochondrial translocation to exocytosis sites: Relevance to atopic dermatitis. J Allergy Clin Immunol 2011; 127: 1522–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski MB, Groot AJ, Dastych J, Knol EF. TNF trafficking to human mast cell granules: mature chain-dependent endocytosis. J Immunol 2007; 178: 5701–5709. [DOI] [PubMed] [Google Scholar]
- Nakae S, Suto H, Kakurai M, Sedgwick JD, Tsai M, Galli SJ. Mast cells enhance T cell activation: Importance of mast cell-derived TNF. Proc Natl Acad Sci USA 2005; 102: 6467–6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore JH, Jarskog LF, Vadlamudi S. Maternal poly I:C exposure during pregnancy regulates TNF alpha, BDNF, and NGF expression in neonatal brain and the maternal-fetal unit of the rat. J Neuroimmunol 2005; 159: 106–112. [DOI] [PubMed] [Google Scholar]
- Al-Ayadhi LY, Mostafa GA. Elevated serum levels of interleukin-17 A in children with autism. J Neuroinflamm 2012; 9: 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napolioni V, Ober-Reynolds B, Szelinger S, Corneveaux JJ, Pawlowski T, Ober-Reynolds S et al. Plasma cytokine profiling in sibling pairs discordant for autism spectrum disorder. J Neuroinflamm 2013; 10: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin GK, Newton G, Tarrio ML, Bu DX, Maganto-Garcia E, Azcutia V et al. IL-17 and TNF-alpha sustain neutrophil recruitment during inflammation through synergistic effects on endothelial activation. J Immunol 2012; 188: 6287–6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudeck A, Suender CA, Kostka SL, von SE, Maurer M. Mast cells promote Th1 and Th17 responses by modulating dendritic cell maturation and function. Eur J Immunol 2011; 41: 1883–1893. [DOI] [PubMed] [Google Scholar]
- Kenna TJ, Brown MA. The role of IL-17-secreting mast cells in inflammatory joint disease. Nat Rev Rheumatol 2013; 9: 375–379. [DOI] [PubMed] [Google Scholar]
- Jang SW, Liu X, Yepes M, Shepherd KR, Miller GW, Liu Y et al. A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proc Natl Acad Sci USA 2010; 107: 2687–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RA, Lam M, Punzo AM, Li H, Lin BR, Ye K et al. 7,8-dihydroxyflavone exhibits therapeutic efficacy in a mouse model of Rett syndrome. J Appl Physiol 2012; 112: 704–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theoharides T, Athanassiou M, Panagiotidou S, Doyle R. Dysregulated brain immunity and neurotrophin signaling in Rett syndrome and autism spectrum disorders. J Neuroimmunol 2015; 279: 33–38. [DOI] [PubMed] [Google Scholar]
- Harwood M, nielewska-Nikiel B, Borzelleca JF, Flamm GW, Williams GM, Lines TC. A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem Toxicol 2007; 45: 2179–2205. [DOI] [PubMed] [Google Scholar]
- Kawanishi S, Oikawa S, Murata M. Evaluation for safety of antioxidant chemopreventive agents. Antioxid Redox Signal 2005; 7: 1728–1739. [DOI] [PubMed] [Google Scholar]
- Li L, Gu L, Chen Z, Wang R, Ye J, Jiang H. Toxicity study of ethanolic extract of Chrysanthemum morifolium in rats. J Food Sci 2010; 75: T105–T109. [DOI] [PubMed] [Google Scholar]
- Jager AK, Saaby L. Flavonoids and the CNS. Molecules 2011; 16: 1471–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Zheng S, Li L, Jiang H. Metabolism of flavonoids in human: a comprehensive review. Curr Drug Metab 2014; 15: 48–61. [DOI] [PubMed] [Google Scholar]
- Hollman PC, Katan MB. Absorption, metabolism and health effects of dietary flavonoids in man. Biomed Pharmacother 1997; 51: 305–310. [DOI] [PubMed] [Google Scholar]
- Hollman PC, de Vries JH, van Leeuwen SD, Mengelers MJ, Katan MB. Absorption of dietary quercetin glycosides and quercetin in healthy ileostomy volunteers. Am J Clin Nutr 1995; 62: 1276–1282. [DOI] [PubMed] [Google Scholar]
- Theoharides TC, Asadi S. Unwanted interactions among psychotropic drugs and other treatments for autism spectrum disorders. J Clin Psychopharmacol 2012; 32: 437–440. [DOI] [PubMed] [Google Scholar]
- Zhuang X, Xiang X, Grizzle W, Sun D, Zhang S, Axtell RC et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther 2011; 19: 1769–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]