Abstract
Two 4-week repeated-dose toxicity studies were conducted to evaluate the potential toxicity of l-cysteine and d-cysteine. In one study, three groups of 6 male rats were each administered l-cysteine once daily by gavage at doses of 500, 1,000, or 2,000 mg/kg/day for 28 consecutive days. The control group was administered a 0.5% methylcellulose vehicle solution. The other study followed a similar protocol except that the experimental groups received d-cysteine. Toxicological observations showed that the l-cysteine-treated groups exhibited renal injuries such as basophilic tubules with eosinophilic material in the lumen, and there were increased numbers of basophilic tubules in all treated groups. In 1,000 or 2,000 mg/kg/day-treated groups, salivation and necropsy findings indicative of focal erosion in the stomach mucosa were found. Increases in reticulocyte counts were observed in the 2,000 mg/kg/day-treated group. Toxicological findings obtained for the d-cysteine-treated groups included anemia and renal injuries such as basophilic tubules with eosinophilic material in the lumen, increased numbers of basophilic tubules, and crystal deposition in the medulla in the 2,000 mg/kg/day-treated group. Additional findings included sperm granuloma in the epididymis, necropsy findings suggestive of focal erosion in the stomach mucosa, and salivation in the 1,000 or 2,000 mg/kg/day-treated groups. One rat in the 2,000 mg/kg/day-treated group died due to renal failure. In conclusion, the no-observed-adverse-effect levels (NOAELs) were estimated to be less than 500 mg/kg/day for l-cysteine and 500 mg/kg/day for d-cysteine under our study conditions. The toxicological profiles were similar for l-cysteine and d-cysteine; however, there were slight differences in the dose responses. The mechanisms underlying these differences remain to be determined.
Keywords: l-cysteine, d-cysteine, amino acid, toxicity, rat
Introduction
l-Cysteine, one of the amino acids that have sulfur in their chemical structures, is synthesized in the human body from l-methionine and l-serine. l-Cysteine is a nonessential amino acid in adults. However, it is considered a semi-essential amino acid in infants because they synthesize insufficient amounts; thus, l-cysteine can be lacking in this age group. The Joint FAO/WHO/UNU Expert Consultation recommends 4.1 mg/kg/day as the daily requirement of l-cysteine for healthy adults1.
l-Cysteine is a macronutrient, and it is normally found in proteins. According to a safety assessment of its use in food, there is no concern regarding the use of l-cysteine as a flavoring agent because exposure to l-cysteine through food intake is expected to be much greater than that through its use as a flavoring agent. Therefore, l-cysteine has been approved as a flavoring agent by the Joint FAO/WHO Expert Committee on Food Additives (JECFA)2. l-Cysteine is also considered “generally recognized as safe” (GRAS) by the Flavor and Extract Manufacturers Association (FEMA) as a flavor ingredient (FEMA No. 3263)3, and it is approved by the U.S. Food and Drug Administration (FDA) as a nutrient and dough strengthener, with usage limitations4, 5. l-Cysteine monohydrochloride is included in the list of designated additives in Japan by the Ministry of Health, Labour and Welfare, and it is used as an antioxidant in natural juice and as a food manufacturing agent in bread6.
d-Cysteine, the other optical isomer of cysteine, is currently not used as a food additive or flavoring agent. However, because l-cysteine is susceptible to racemization when treated with heat and under alkaline conditions, it can be assumed that substantial amounts of d-cysteine exist in processed foods7, 8.
A previous 4-week intravenous repeated-dose toxicity study of l-cysteine performed with male rats reported suppressed body weight gain, slight anemia, and histopathological findings in the cerebellum, kidney, and epididymis following l-cysteine adminstration9. Only one study regarding l-cysteine oral toxicity has been conducted. The study was a 108-week drinking-water carcinogenicity study of l-cysteine hydrochloride in rats10. It reported that l-cysteine monohydrochloride did not induce any neoplasms when administered orally to rats, but it did induce nonneoplastic lesions indicative of renal papillary necrosis. Thus, the toxicity of l-cysteine after oral administration has not been fully elucidated. By contrast, there is no information about the toxicity of d-cysteine. It has been reported that l-cysteine cannot be replaced by d-cysteine nutritionally and that replacement of l-cysteine with d-cysteine in food causes growth depression in mice under certain test conditions11.
Recently, the International Council of Amino Acids Science (ICAAS) stated that insufficient safety information was available for some amino acids and that further toxicity studies are needed. Consequently, novel toxicity studies of glycine, l-phenylalanine, l-tyrosine, l-alanine, l-threonine, and l-methionine were published12,13,14,15,16,17. As part of the safety evaluation of cysteine, we report the results of two 4-week repeated-dose oral toxicity studies in male rats administered l-cysteine or d-cysteine.
Materials and Methods
Study designs
Two 4-week repeated-dose oral toxicity studies in rats were conducted: one on l-cysteine and one on d-cysteine. We consulted the Guidelines for Toxicity Studies of Drugs18 for both study designs, although we did not completely follow the guidelines with respect to sex ratio, animal numbers, and organs tested in histopathology.
Animals and animal husbandry
Male Crl:CD(SD)IGS rats were obtained from Charles River Laboratories Japan (Yokohama, Japan) and allowed free access to tap water and a certified rodent diet sterilized by gamma irradiation (CRF-1, Oriental Yeast Co., Ltd., Tokyo, Japan). The contents of l-cysteine and d-cysteine derived from feed protein were not determinable because cysteine in the feed was converted to cystine in the analysis process. The cystine content of the feed protein was 0.34 g per 100 g of rodent diet, as reported by the manufacturer. The animals were housed individually in suspended, stainless steel cages in an animal room under the following conditions: temperature of 20 to 26°C, relative humidity of 30 to 70%, air ventilation 10 to 15 times/hour (all-fresh ventilation), and illumination for 12 hours per day (from 7 a.m. to 7 p.m.). The rats were acclimatized for 6 days prior to random allocation. At the initiation of treatment, the animals were 6 weeks old and weighed 207 to 227 g. All animals were treated humanely according to institutional guidelines, and the experimental procedure was approved by the institutional ethics committee.
Test articles
l-Cysteine (CAS 52-90-4, lots 105XW24 and 105XW29) supplied by Ajinomoto Co., Inc. (Kawasaki, Japan) and d-cysteine (lot 13033/1) supplied by BIOSYNTH AG (Switzerland) were stored at room temperature under light-shielded conditions. Water for injection (lots 2I99, IG94, and 2F74, Otsuka Pharmaceutical Factory Inc., Tokushima, Japan) and methylcellulose (lot M8G3001, Nacalai Tesque, Inc., Tokyo, Japan) were used to prepare the vehicles for l-cysteine and d-cysteine.
Dosage preparation
For the vehicle control, 0.5% methylcellulose solution was prepared with water for injection. l-cysteine and d-cysteine at 50, 100, or 200 mg/mL were formulated using the vehicle. l-cysteine and d-cysteine are soluble at 50 and 100 mg/mL but not at 200 mg/mL; therefore, we prepared l-cysteine and d-cysteine in solutions at 50 and 100 mg/mL and in suspensions at 200 mg/mL.
Group designation and treatment
Animals were assigned to treatment groups according to a randomized complete block design with body weight stratification performed using a toxicological data processing system (MiTOX, Mitsui Zosen Systems Research Inc., Chiba, Japan). Each animal in the three treatment groups (6 males per group) was administered l-cysteine once daily by gavage at doses of 500, 1,000, or 2,000 mg/kg/day in a dose volume of 10 mL/kg for 28 consecutive days. Another group of 6 rats was administered the vehicle alone; this group served as the negative control group for l-cysteine treatment. For the study of d-cysteine, the same dosage groups were used as for the l-cysteine study.
Clinical observations
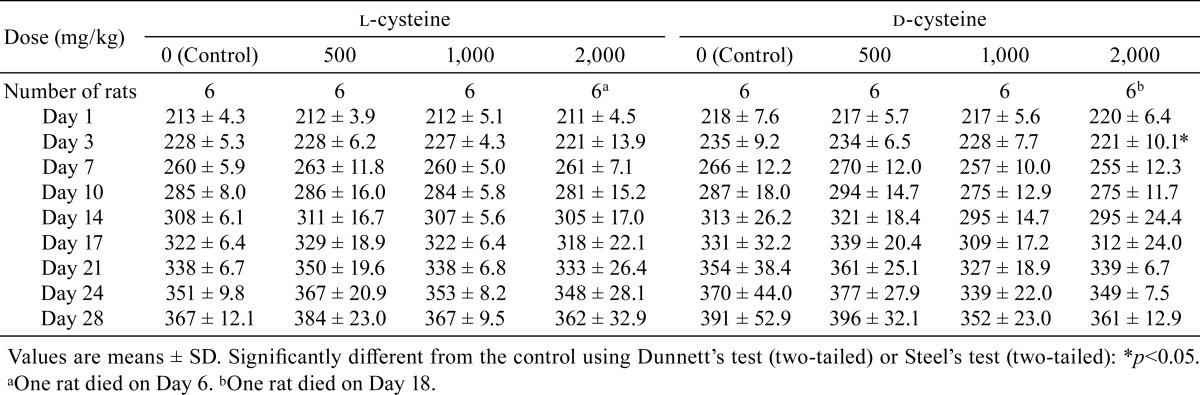
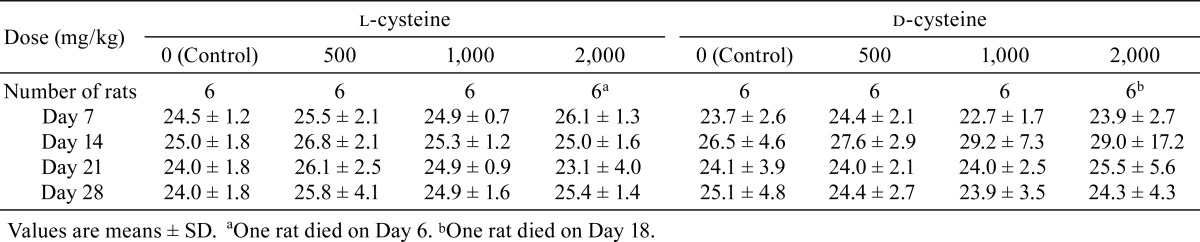
The animals were observed twice daily (before and after dosing) for clinical signs, including appearance/posture, behavior, feces/urine, body surface, fluid secretion/excretion, and body temperature. Individual body weight data were recorded on Days 1, 3, 7, 10, 14, 17, 21, 24, and 28, with the first day of administration defined as Day 1. Individual food and water consumption were measured on Days 7, 14, 21, and 28.
Clinical pathology
Urine samples were collected from each animal on Day 28 for approximately 18 hours and examined for pH, glucose, protein, occult blood, ketones, bilirubin, and urobilinogen using Multistix urinalysis (Siemens Healthcare Diagnostics K.K., Tokyo, Japan). Urinary volume (U. Vol) was measured, and specific gravity was determined by employing a Digital Urine Specific Gravity Refractometer (UG-D, Atago Co., Ltd., Tokyo, Japan). The urine samples were also analyzed via an electrolyte analyzer (EA06T, A&T Corporation, Tokyo, Japan) to determine the sodium ion (Na), potassium ion (K), and chloride ion (CI) contents. Hematology and clinical chemistry parameters were evaluated on the day of the scheduled necropsy. The animals were fasted overnight and then anesthetized with ether before blood was collected from the caudal vena cava. Blood samples containing potassium EDTA as the anticoagulant were analyzed with a total hematology system (THMS H1E, Bayer Sankyo Co. Ltd., Tokyo, Japan) to determine the red blood cell count, hemoglobin, hematocrit, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), platelet count, and white blood cell count. The white blood cell differential count included neutrophils, lymphocytes, monocytes, eosinophils, and basophils. Reticulocyte counts (per 1,000 red cells) were measured using an automated blood cell differential counter (Model 8200, Hitachi Ltd., Tokyo, Japan) in the study of l-cysteine and an automated reticulocyte counter (Sysmex CA-5000, Sysmex Corporation, Kobe, Japan) in the study of d-cysteine. The blood samples that had sodium citrate as the anticoagulant were processed with a fully automated blood coagulation analyzer (Sysmex CA-5000, Sysmex Corporation, Kobe, Japan) to determine prothrombin time (PT), activated partial thromboplastin time (APTT), and fibrinogen. Heparin-anticoagulated plasma samples were evaluated with an automated clinical chemistry analyzer (TBA-120FR, Toshiba Medical Systems, Inc., Tokyo, Japan) to determine lactate dehydrogenase (LDH), creatine phosphokinase (CPK), aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), total bilirubin, creatinine, blood urea nitrogen (BUN), triglycerides, total cholesterol, phospholipids, glucose, Na, K, Cl, Ca, inorganic phosphorus, and total protein. Protein fractionation, including the albumin/globulin ratio, albumin, α1 globulin, α2 globulin, β globulin, and γ globulin, was measured using an automated electrophoresis system (Rapid ElectroPhoresis, Helena Laboratories, Beaumont, TX, USA).
Postmortem evaluation
On Day 29, all surviving animals were euthanized after an overnight fast by exsanguination from the caudal vena cava under ether anesthesia. They were then subjected to complete gross pathological examination, including the external appearance of the carcass in addition to organs and tissues in the abdominal, thoracic, and cranial cavities. Although the use of ether as an anesthetic in research animals is now strongly discouraged, the experiment in this report was performed in 2002–2003, when ether was a more widely used general anesthetic than it is today. After necropsy, the following organs were weighed: the brain, thymus, heart, lungs (only in the l-cysteine-treated groups and corresponding control group), liver, spleen, kidneys (right and left [R/L]), adrenal glands (R/L), testes (R/L), and epididymides (R/L; only in the d-cysteine-treated groups and corresponding control group). Then, relative organ weights (weight per 100 g body weight) were calculated. The following tissues or representative samples were collected and preserved in phosphate buffered 10 vol% formalin: the brain, spinal cord, cervical lymph node, lungs (R/L), thymus, heart, liver, spleen, kidneys (R/L), adrenal glands (R/L), stomach, duodenum, jejunum, ileum, cecum, colon, pancreas, mesenteric lymph nodes, testes (R/L), epididymides (R/L; epididymides were collected and examined only in the d-cysteine-treated groups and corresponding control group), and gross lesions. However, the testes were fixed initially with formalin acetic acid solution and then preserved in phosphate buffered 10 vol% formalin. Organs and tissues from each animal were embedded in paraffin, sectioned, and stained with hematoxylin and eosin. Specimens from all animals were examined microscopically. Animals that were found dead were also examined macroscopically and histopathologically.
Data analysis
Body weight, food consumption, water consumption, urinalysis, hematology, clinical chemistry, and organ weight data were recorded using a MiTOX RDT system. Numerical data obtained during the study were used to calculate group mean values and standard deviations. Urinalysis, hematology, clinical chemistry, and organ weight data from the animals that died were not obtained. Group variances for the appropriate parameters were compared using Bartlett’s method (significant at p<0.01 in two-tailed tests)19. Where group variances did not significantly differ, Dunnett’s multiple comparison method was applied to determine the significance of differences between the control group and each l-cysteine- or d-cysteine-treated group (significant at p<0.05 in two-tailed tests)20. If the Bartlett’s test indicated significant differences among group variances for a given parameter, that parameter was compared among groups using the Steel’s multiple comparison method for mean ranking (significant at p<0.05 in two-tailed tests)21.
Results
Clinical observations
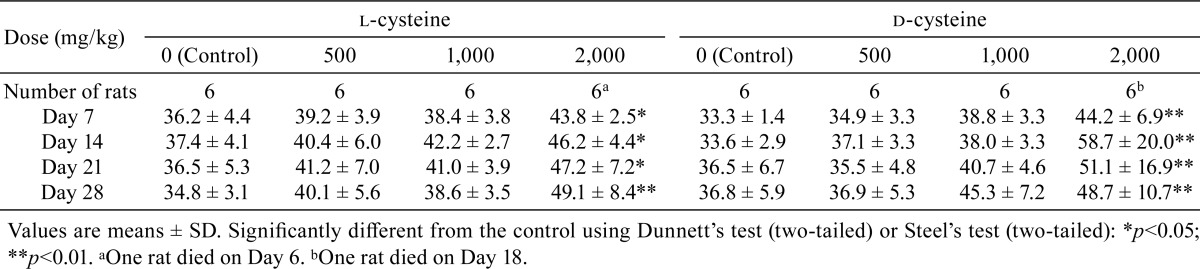
Transient salivation was sporadically observed post administration in rats that received 1,000 or 2,000 mg/kg/day in the l-cysteine-treated groups and in all d-cysteine-treated groups. No significant differences in body weight were recorded between the l-cysteine-treated rats and the controls, whereas body weight was significantly lower in the 2,000 mg/kg/day d-cysteine-treated group on Day 3 than in the control group. In addition, a tendency toward suppression of body weight increase was noted in the 1,000 or 2,000 mg/kg/day d-cysteine-treated group after Day 3 (Table 1). No significant difference in food consumption was observed between the l-cysteine or d-cysteine groups and their corresponding control groups (Table 2). Water intake was significantly greater in both the l-cysteine- and d-cysteine-treated groups administered doses of 2,000 mg/kg/day than in the respective control groups. In addition, a tendency toward increased water intake was noted in the l-cysteine-treated groups receiving 500 or 1,000 mg/kg/day and in the d-cysteine group receiving 1,000 mg/kg/day (Table 3).
Table 1. Summary of Body Weights (g).
Table 2. Summary of Food Consumption (g/day).
Table 3. Summary of Water Intake (g/day).
One rat in the l-cysteine group receiving 2,000 mg/kg/day died on Day 6. Based on the results of necropsy and histopathological examination, this death was considered to be caused by accidental swallowing aspiration due to a technical error. Furthermore, a rat in the d-cysteine group receiving 2,000 mg/kg/day exhibited decreased movement and soiled fur in the perioral region on Day 18, and it died on Day 19. As described later, the results of histopathological examination suggested that this death was due to renal failure.
Clinical pathology
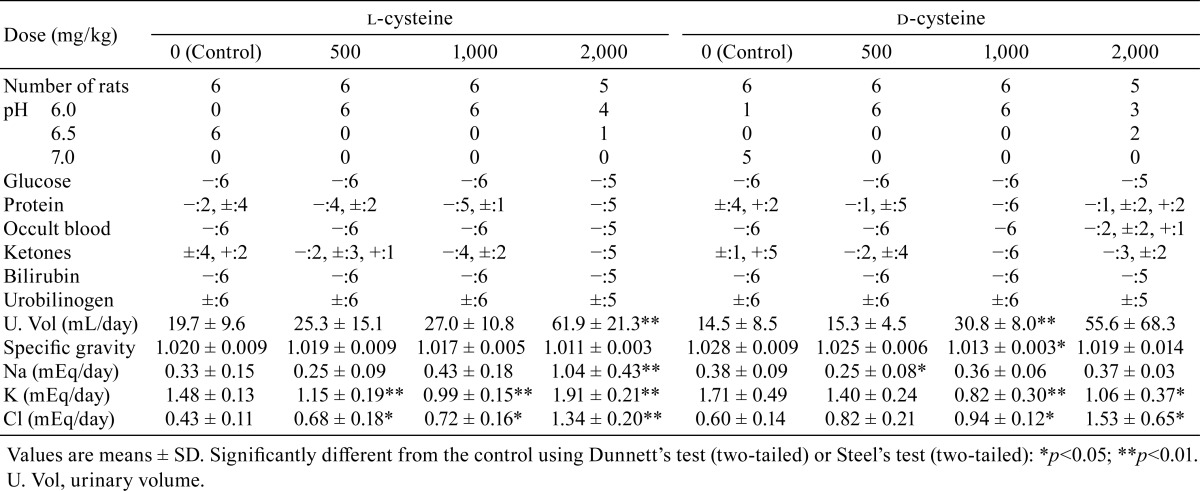
In the urinalysis, a decreasing trend was recorded for urine pH in all l-cysteine- and d-cysteine-treated groups (Table 4). Significant increases or increasing trends were noted for urinary volume in all l-cysteine treated groups and in the d-cysteine-treated groups receiving 1,000 or 2,000 mg/kg/day. Significant decreases or decreasing trends were noted for specific gravity in rats receiving 2,000 mg/kg/day l-cysteine and 1,000 or 2,000 mg/kg/day d-cysteine. Occult blood was observed in the urine in the d-cysteine group administered 2,000 mg/kg/day. Regarding electrolyte excretion, significant increases in Cl excretion were noted in all l-cysteine-treated groups and in rats receiving 1,000 or 2,000 mg/kg/day in the d-cysteine-treated groups. Na and K excretion increased significantly in the rats receiving 2,000 mg/kg/day in the l-cysteine-treated group, whereas decreased K was noted in the rats receiving 1,000 or 2,000 mg/kg/day in the d-cysteine-treated groups. The decreased K excretion in the l-cysteine-treated groups administered 500 or 1,000 mg/kg/day and decreased Na excretion in the d-cysteine-treated group administered 500 mg/kg/day were considered incidental owing to the lack of a dose response.
Table 4. Summary of Urinalysis.
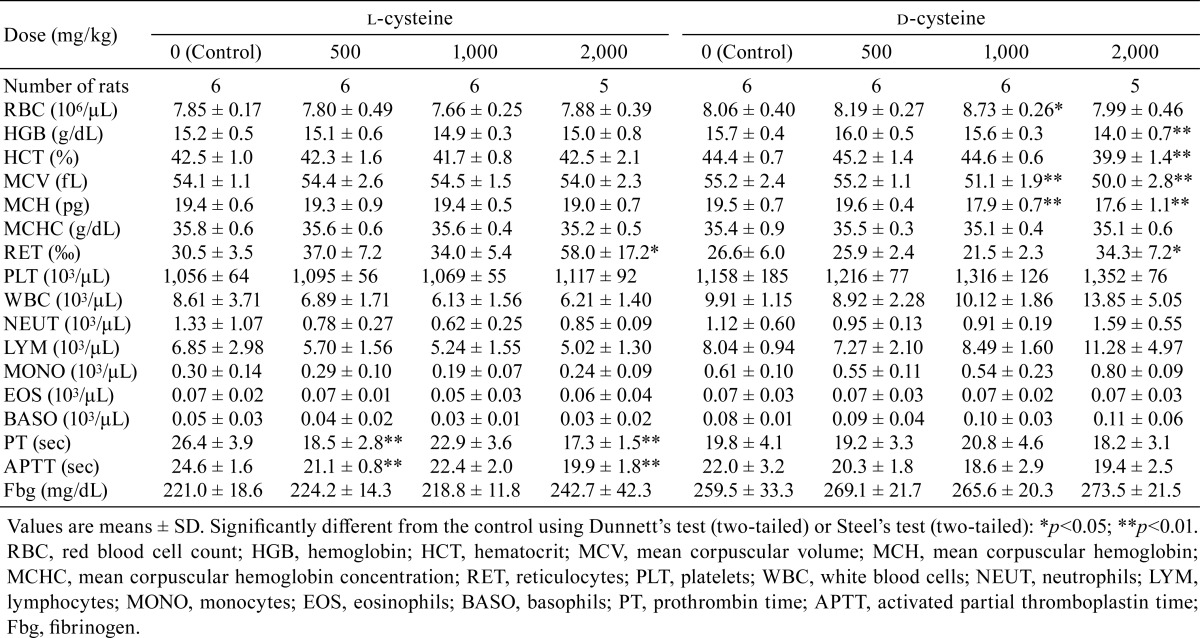
In the hematology analyses, significant decreases in MCV and MCH were observed in the rats receiving 1,000 or 2,000 mg/kg/day in the d-cysteine-treated groups (Table 5). Significant decreases in hemoglobin and hematocrit and an increase in reticulocyte counts were observed in the d-cysteine-treated group receiving 2,000 mg/kg/day. Significant increases in reticulocyte count were observed in the l-cysteine-treated group receiving 2,000 mg/kg/day. Decreases or decreasing trends in PT and APTT were observed in all l-cysteine-treated groups. The increased red blood cell count observed in rats receiving 1,000 mg/kg/day in the d-cysteine-treated group was considered incidental owing to the lack of a dose response.
Table 5. Summary of Hematology.
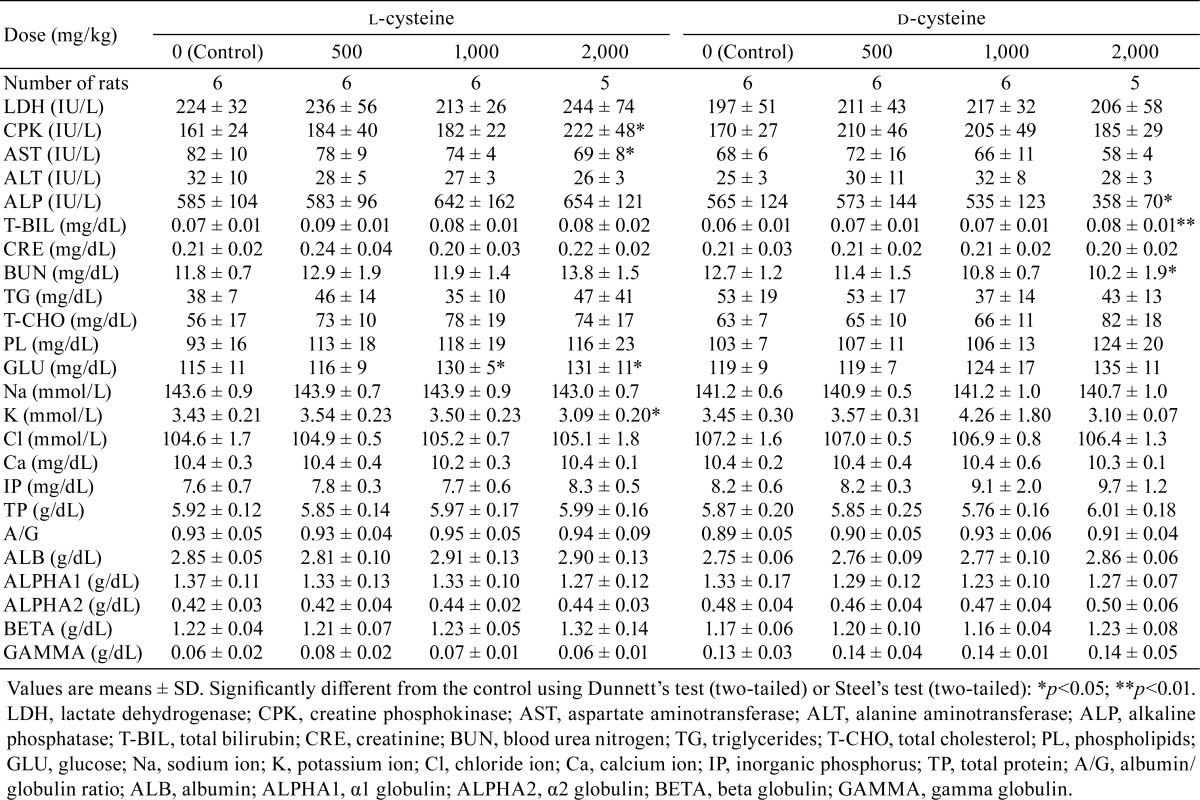
Regarding blood chemistry, increases in glucose were significant in the l-cysteine-treated groups receiving 1,000 or 2,000 mg/kg/day (Table 6). Increases in CPK and decreases in AST and K were significant in the l-cysteine-treated group administered 2,000 mg/kg/day. Increases in total bilirubin and decreases in ALP and BUN were significant in the d-cysteine-treated group receiving 2,000 mg/kg/day.
Table 6. Summary of Blood Chemistry and Plasma Protein Fractions.
Pathology
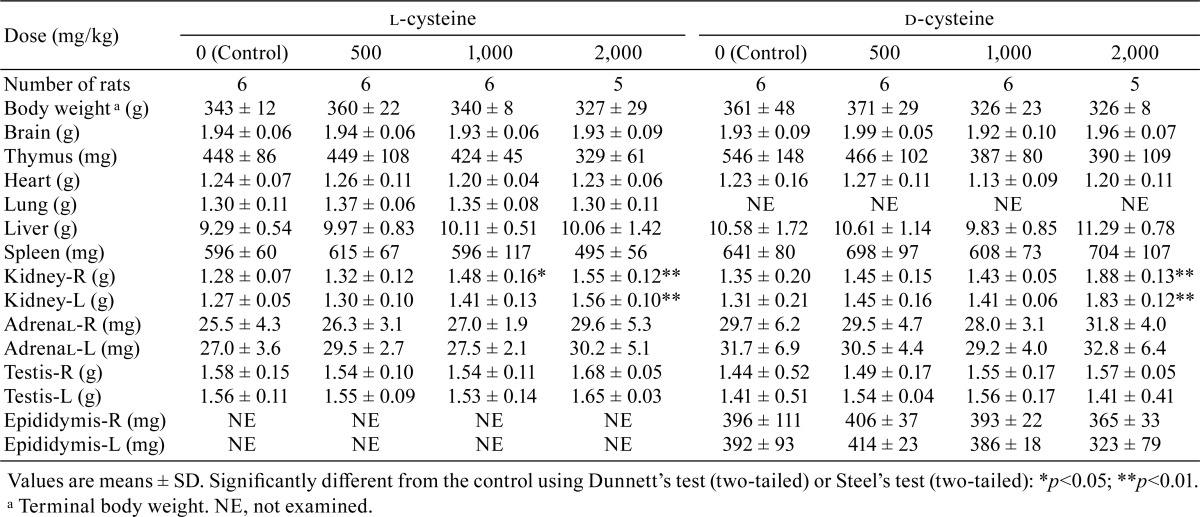
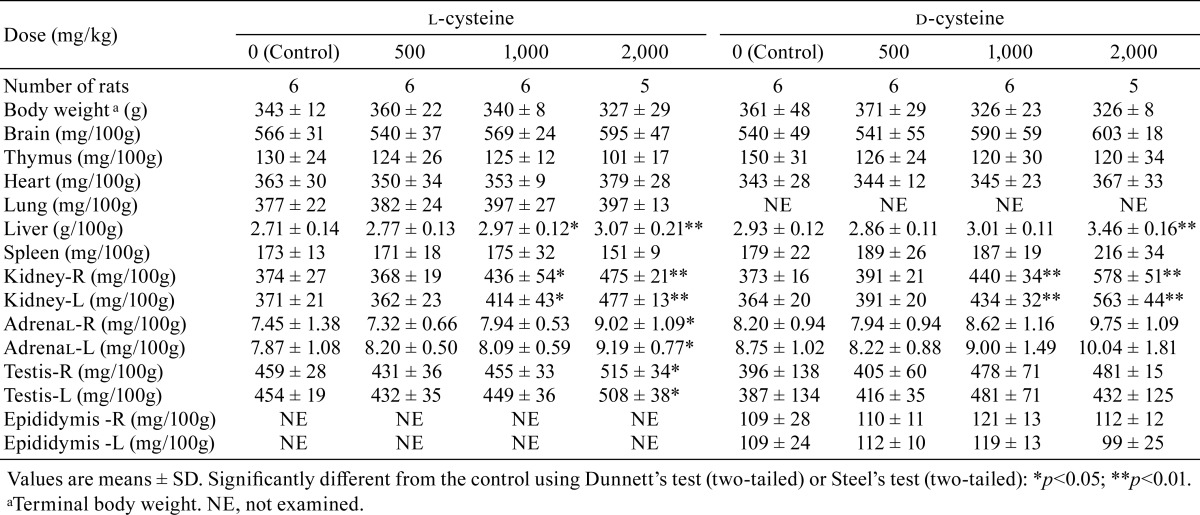
Increases in kidney weights (absolute weights or relative weights) were recorded in the rats receiving 1,000 or 2,000 mg/kg/day of l-cysteine and in the rats receiving 1,000 or 2,000 mg/kg/day of d-cysteine (Table 7 and 8). Increases in relative liver weight were observed in rats receiving 1,000 or 2,000 mg/kg/day in the l-cysteine study and in rats receiving 2,000 mg/kg/day in the d-cysteine study, and increases in the relative weights of both the adrenals and testes were noted in the rats receiving 2,000 mg/kg/day l-cysteine. Decreasing trends were observed for thymus weights in the rats receiving 2,000 mg/kg/day of l-cysteine and in all d-cysteine-treated groups. Decreasing tendencies were recorded for spleen weights in rats receiving 2,000 mg/kg/day of l-cysteine.
Table 7. Summary of Absolute Organ Weights.
Table 8. Summary of Relative Organ Weights.
Treatment-related gross findings were observed in the stomach in the l-cysteine- and d-cysteine-treated groups. Dark red areas of the mucosa in the glandular stomach or around the limiting ridge of the stomach were observed in 1/6 and 5/5 rats receiving 1,000 or 2,000 mg/kg/day of l-cysteine, respectively. A brown focus and brown lines were observed in the glandular stomach mucosa in 1/6 and 1/5 rats receiving 1,000 or 2,000 mg/kg/day of d-cysteine, respectively. Treatment-related gross findings were also observed in the kidney, urinary bladder, and epididymis in the d-cysteine-treated groups. Enlargement of the kidneys was observed in 3/6 and 5/5 rats receiving 1,000 or 2,000 mg/kg/day of d-cysteine, respectively. White lines in the renal papilla and scattered cysts were also observed in the kidneys in 1/5 rats receiving 2,000 mg/kg/day of d-cysteine. A distended urinary bladder with urine retention was observed in 1/5 rats receiving 2,000 mg/kg/day of d-cysteine. White nodules and enlargement of the epididymides were observed in 2/6 and 1/5 rats receiving 1,000 or 2,000 mg/kg/day of d-cysteine, respectively. Other gross findings observed in the treated groups were considered spontaneous or incidental based on the incidence or properties.
The following test article-related histopathological findings were observed in the l-cysteine-treated groups and d-cysteine treated groups (Table 9).
Table 9. Summary of Histopathology.
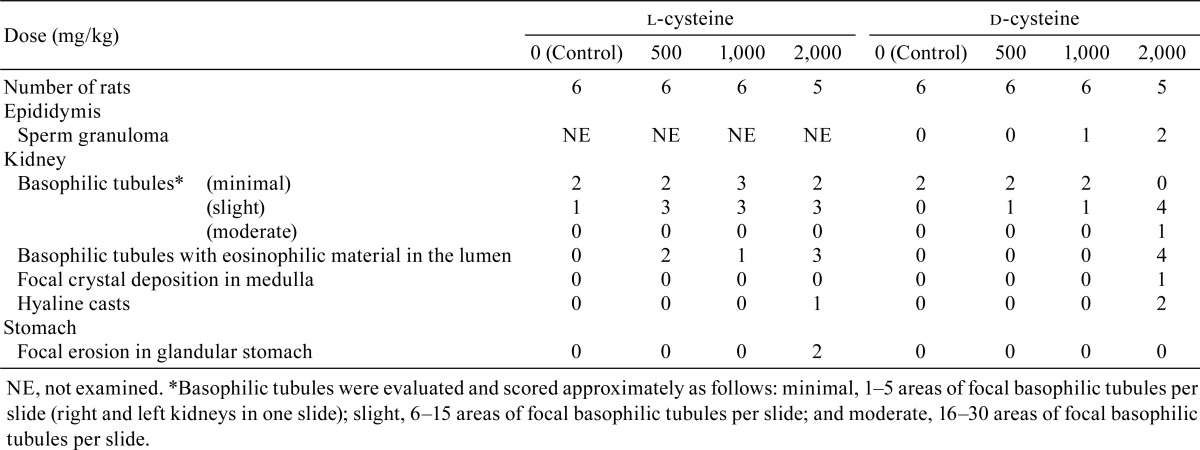
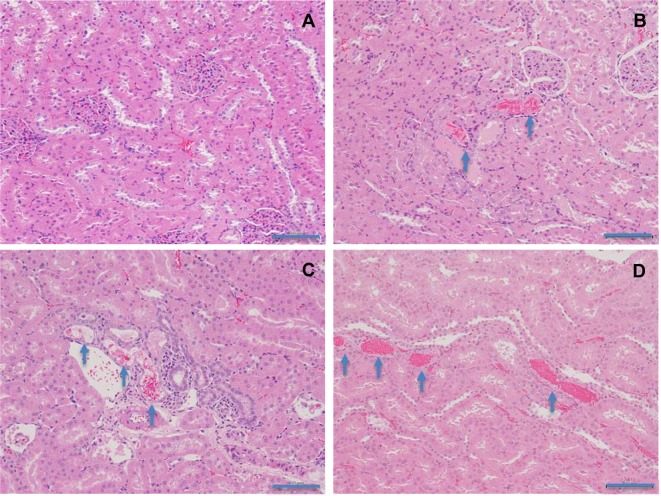
Kidney: Basophilic tubules with eosinophilic material in the lumen (Fig. 1) were observed in all l-cysteine-treated groups and in the d-cysteine-treated group receiving 2,000 mg/kg/day. Crystal deposition was observed in the medulla (Fig. 2) in one d-cysteine-treated animal receiving 2,000 mg/kg/day. Increased numbers of animals exhibited basophilic tubules in all l-cysteine-treated groups and in the d-cysteine-treated group receiving 2,000 mg/kg/day compared with the numbers in the corresponding control groups. In addition, hyaline casts were observed in rats receiving 2,000 mg/kg/day in both the l-cysteine-treated and d-cysteine-treated groups.
Fig. 1.
Histopathological appearances of basophilic tubules with eosinophilic material in the lumen in the kidney in rats treated with l-cysteine or d-cysteine for 4 weeks. A, Control; B, 1,000 mg/kg/day l-cysteine; C, 2,000 mg/kg/day l-cysteine; D, 2,000 mg/kg/day d-cysteine. Basophilic tubules with eosinophilic material in the lumen are shown (arrows) (×200). HE staining. Scale bars in 100 μm.
Fig. 2.
Histopathological appearances of crystal deposition in the renal medulla in rats treated with d-cysteine for 4 weeks. A, Control; B, 2,000 mg/kg/day. Crystal deposition is shown in the renal medulla (asterisk) (×100). HE staining. Scale bars in 200 μm.
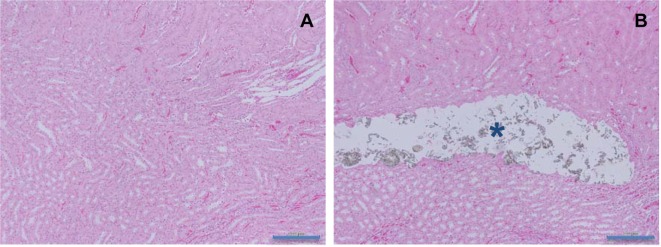
Stomach: Focal erosion in the glandular stomach (Fig. 3) was observed in the l-cysteine group receiving 2,000 mg/kg/day. Although gross findings were observed in the glandular stomach mucosa in the 1,000 mg/kg/day l-cysteine-treated group and 1,000 or 2,000 mg/kg/day d-cysteine-treated groups, abnormalities were not confirmed in the histopathological examination due to the narrow area of gross lesions.
Fig. 3.
Histopathological appearances of focal erosion in the glandular stomach in rats treated with l-cysteine for 4 weeks. A, Control; B, 2,000 mg/kg/day. Focal erosion is shown in the glandular stomach (arrow) (×100). HE staining. Scale bars in 200 μm.
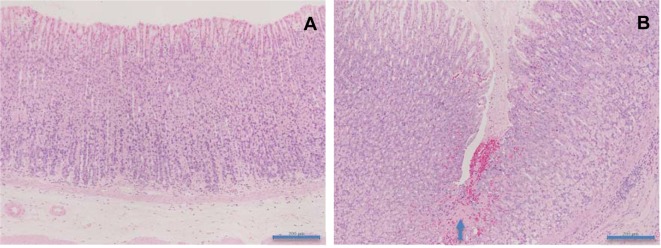
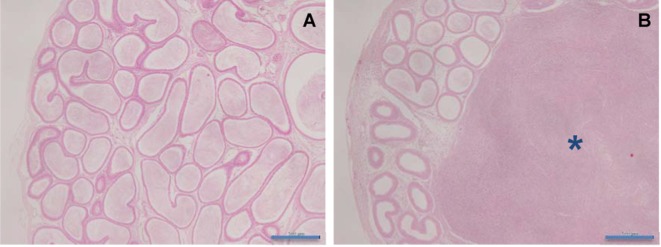
Epididymis: Sperm granuloma (Fig. 4) was observed in the d-cysteine-treated groups receiving 1,000 or 2,000 mg/kg/day.
Fig. 4.
Histopathological appearances of sperm granuloma in the epididymis in rats treated with d-cysteine for 4 weeks. A, Control; B, 2,000 mg/kg/day. Sperm granuloma is shown in the epididymis (asterisk) (×40). HE staining. Scale bars in 500 μm.
In addition, the findings for the dead animal in the d-cysteine group administered 2,000 mg/kg/day included diffuse tubular dilatation and pelvis dilatation in the kidneys.
Other histopathological findings observed in the treated groups were considered spontaneous or incidental based on the incidence or properties.
Discussion
As a part of the safety evaluation of cysteine, two separate 4-week repeated-dose oral toxicity rat studies were conducted with l-cysteine and d-cysteine. Three groups of 6 Crl:CD(SD)IGS male rats were each administered l-cysteine once daily by gavage at doses of 500, 1,000, or 2,000 mg/kg/day for 28 consecutive days. The control group was similarly administered 0.5% methylcellulose vehicle solution. Similar dosage and control groups were used for l-cysteine as for d-cysteine.
Treatment-related histopathological findings were observed in the kidneys in all l-cysteine-treated groups and in the d-cysteine group receiving 2,000 mg/kg/day. The eosinophilic material in the lumen of basophilic tubules was considered to be of erythrocyte origin (red blood cell [RBC] casts) based on the reddish rods or orbicular shapes of the eosinophilic material and the occult blood in urine in the d-cysteine group administered 2,000 mg/kg/day. These findings might indicate either damage to the interstitial blood vessels and epithelial basal lamina of proximal tubules or damage to the glomeruli; however, the precise mechanism is not clear22. Crystal deposition in the medulla was observed in one rat in the d-cysteine group receiving 2,000 mg/kg/day. The crystal component was inferred to be cystine based on the following considerations: cysteine is oxidized to cystine in the blood and under other normoxic conditions23, a previous report has shown that urinary excretion of inorganic sulfate was increased after oral administration of d-cysteine24, and high concentrations of cystine in urine cause cystine calculi. Diffuse tubular dilatation and pelvis dilatation were observed in the kidneys of the dead animal in the d-cysteine group receiving 2,000 mg/kg/day. Although no crystals were observed in this animal, these features might be the results of obstruction of the nephrons or lower urinary tract by crystalluria. In conjunction with renal lesions in histopathology, changes in kidney weight and urinalysis parameters, decreased K in blood chemistry, and increased water intake were observed. These changes are also considered toxicologically significant. Increased urinary volume and changes in excretion of electrolytes were considered to be due to impaired tubular reabsorption. Increases in water intake were considered to be related to increased urinary volume. Decreased K in blood chemistry for the rats in the 2,000 mg/kg/day l-cysteine-treated group was considered a result of increased K excretion. The decrease in urinary pH in all l-cysteine- and d-cysteine-treated groups was considered to be induced by the excretion of sulfate and was not considered toxicologically significant25. Basophilic tubules with eosinophilic materials in the lumen were also reported in a previous intravenous study of l-cysteine9. However, the necrosis and calcification of the renal papilla and the hyperplasia of the transitional epithelium in the renal pelvis that were reported in a previous carcinogenicity study in rats administered a treatment via drinking water10 were not observed in the present study, which was likely due to the shorter length of administration in the present study.
Sperm granuloma was observed in the epididymis in rats administered 1,000 or 2,000 mg/kg/day in the d-cysteine treated animals. No histopathological examination of the epididymis was performed in the l-cysteine-treated groups because there were no gross findings in the epididymis at necropsy. However, sperm granuloma caused by l-cysteine was reported in a 28-day intravenous study and intraperitoneal study with l-cysteine in rats5, 26. Additional histopathological examination of the epididymis of the l-cysteine-treated groups would be necessary to confirm this finding. The mechanism of development of sperm granuloma in the epididymis is thought to involve an increase in vascular permeability and exaggerated edema in the interstitium of the epididymis during exposure to high doses of l-cysteine, with increased intraluminal pressure causing rupture of the epididymal ducts27.
Hemorrhagic findings in the glandular stomach or around the limiting ridge of the stomach (focal erosions in histopathology and a dark red area or brown focus at necropsy) were observed in rats administered 1,000 or 2,000 mg/kg/day in both the l-cysteine- and d-cysteine-treated groups. These lesions were considered a result of direct damage to the mucous membrane by the test articles because no abnormalities were detected in a stomach in the 28-day intravenous study with l-cysteine in rats5. It has been reported that the thiol group decreases the viscosity of mucoproteins by cleaving the disulfide bond between the macromolecules28, 29. The decreased viscosity of gastric mucus after l-cysteine and d-cysteine treatment might affect gastric mucosal protection and might have caused the stomach injuries described above. In addition, there is also a possibility of direct cytotoxicity of l-cysteine in the mucous membrane according to a recent study reporting that l-cysteine at concentrations of 5–10 mmol/L reduced cell viability in intestinal epithelial cells in vitro30.
The decreases in hemoglobin, hematocrit, MCV, and MCH, increased reticulocyte counts, and increased total bilirubin in rats receiving 2,000 mg/kg/day of d-cysteine all suggest mild anemia. This anemic change was accompanied by the decreases in MCV and MCH, which indicate microcytic anemia. The common cause for microcytic anemia is iron deficiency. In this study, there were hemorrhagic changes at necropsy in stomachs and RBC casts in histopathology of kidneys, which might have induced prolonged hemorrhage and iron deficiency. In rats receiving 1,000 mg/kg/day of d-cysteine, although decreases in MCV and MCH were observed, there were no changes in hemoglobin, hematocrit, and red blood cell count, and thus these changes were considered not toxicologically significant. Increased reticulocyte counts were observed in rats receiving 2,000 mg/kg/day of l-cysteine. Given that there were hemorrhagic changes in the stomachs and RBC casts in the kidneys in the rats receiving 2,000 mg/kg/day of l-cysteine, the increased reticulocyte counts probably indicate a compensatory change caused by potential anemia, and thus were considered toxicologically significant. In addition, anemic findings were observed in an intravenous study of l-cysteine in rats receiving 1,000 mg/kg/day5, which indicates that higher exposure to l-cysteine induces anemia.
Slight suppression of body weight increase was recorded from Day 3 until the end of the dosing period in the d-cysteine groups receiving 1,000 or 2,000 mg/kg/day; however, no significant suppression of body weight increase was subsequently observed after Day 3, and the body weights of the animals receiving 1,000 or 2,000 mg/kg of d-cysteine were mostly within the range observed in the control group on Day 28 (control group, 328–474 g; 1,000 mg/kg/day d-cysteine-treated group, 324–385 g; and 2,000 mg/kg/day d-cysteine-treated group, 346–378 g). Thus, we considered this change to not be toxicologically significant. Previous reports have shown that oral administration of l-cysteine at a higher dose range induced reductions of body weight gain and food intake in rats31,32,33; however, in our study, such changes were not observed at doses up to 2,000 mg/kg/day.
Transient salivation post administration was observed in the l-cysteine groups receiving 1,000 and 2,000 mg/kg/day and in all d-cysteine-treated groups. Salivation was considered to be due to oral irritation or a CNS effect of l- and d-cysteine because l- and d-cysteine have a sulfurous taste34 and salivation was observed in most of the rats receiving 1,000 mg/kg/day in a 28-day intravenous study with l-cysteine in rats5. There were no histopathological changes in the brain in the present study and no histopathological changes in the salivary glands in a previous intravenous study5. In the rats receiving 500 mg/kg/day of d-cysteine, salivation was only observed in one rat on 2 days during the administration period. We conclude that there is no toxicological significance of salivation after 500 mg/kg/day d-cysteine administration and that there is toxicological significance of salivation after 1,000 or 2,000 mg/kg/day l-cysteine or d-cysteine administration.
Other changes in clinical pathology parameters or organ weights were not considered toxicologically significant because these changes were minimal and because no related changes were observed in the necropsy and histopathology findings or in other related parameters.
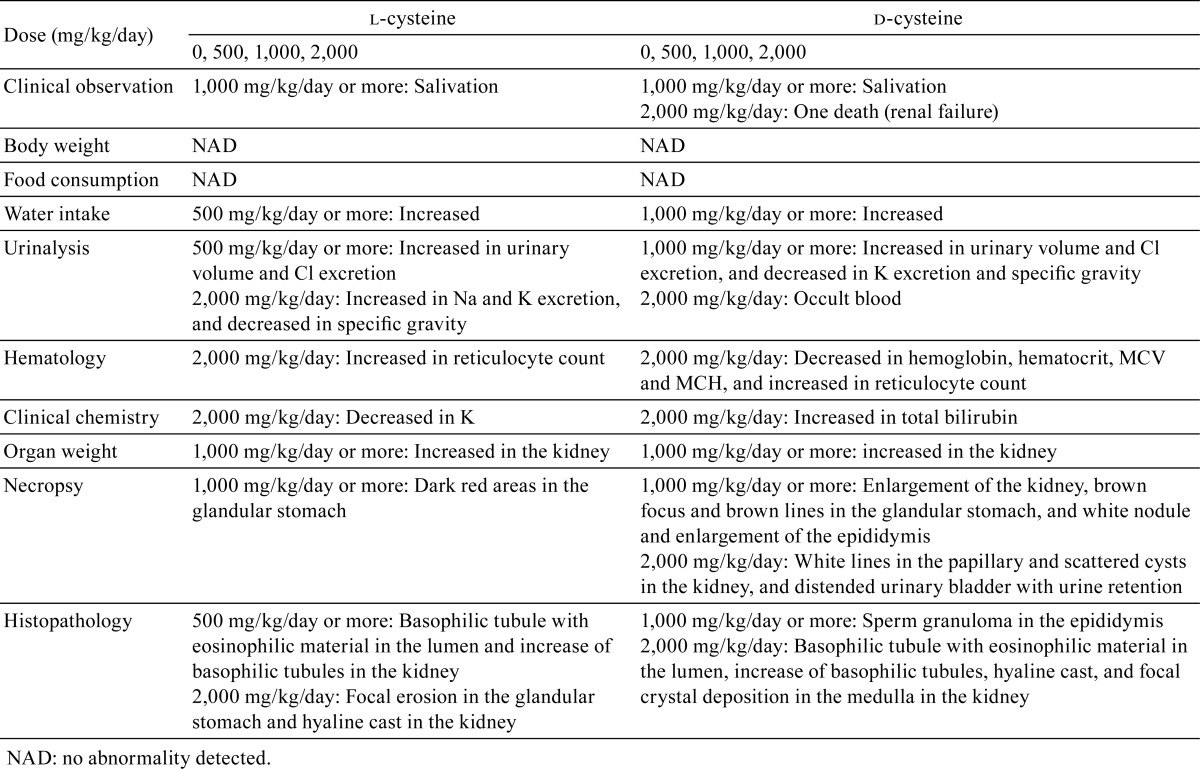
The findings that were considered toxicologically significant are summarized and compared in Table 10.
Table 10. Summary of Results of 4-week Oral Toxicity Studies with l-cysteine and d-cysteine in Male Rats.
The toxicological findings were similar between l-cysteine and d-cysteine; however, there were slight differences between them in terms of the dose responses. Anemia was observed with d-cysteine treatment but not with l-cysteine treatment. However, increased reticulocyte counts, which might indicate potential anemia, were also observed in rats receiving 2,000 mg/kg/day of l-cysteine. In addition, anemia has been observed following intravenous administration of l-cysteine5. By contrast, histopathological findings in the kidney were observed at the lower doses of l-cysteine but not at the lower doses of d-cysteine. It has been reported that the plasma cystine concentration is higher post d-cysteine administration than post l-cysteine administration24. In addition, the metabolic pathways differ between l-cysteine and d-cysteine, and fewer enzymes are associated with d-cysteine metabolism than with l-cysteine metabolism7, 23, 35, 36. The differences in the dose responses in our study potentially reflect differences in the metabolism of l-cysteine and d-cysteine. However, additional toxicokinetic studies of l-cysteine and d-cysteine are needed to clarify the mechanism.
We note that although we consulted the Guidelines for Toxicity Studies of Drugs18, we did not completely follow the guidelines with respect to sex ratio, the number of animals, and the organs examined in histopathology.
In conclusion, l-cysteine-related toxicological effects were observed in the kidney and stomach, and d-cysteine-related toxicological effects were observed in the kidney, epididymis, and stomach. In addition, salivation and mild anemia were observed in the d-cysteine-treated groups. Increased reticulocyte counts and salivation were observed in the l-cysteine-treated groups. Under our study conditions, the no-adverse-effect levels (NOAELs) were determined to be less than 500 mg/kg/day for l-cysteine and 500 mg/kg/day for d-cysteine. Additional toxicokinetic studies of l-cysteine and d-cysteine are needed to clarify the mechanisms underlying the differences in toxicological dose responses between l- and d-cysteine.
Footnotes
Disclosure of Potential Conflict of Interest: The authors declare that there is no conflict of interest in this study.
References
- 1.Joint FAO/WHO/UNU Expert Consultation. Protein and amino acid requirements in human nutrition: report of a joint FAO/WHO/UNU expert consultation. 2007, from website: http://www.who.int/nutrition/publications/nutrientrequirements/WHO_TRS_935/en/
- 2.JECFA. L-cysteine. 2004, from website: http://apps.who.int/food-additives-contaminants-jecfa-database/chemical.aspx?chemID=4381.
- 3.Oser BL, and Hall RL. Recent progress in the consideration of flavoring ingredients under the food additives amendment. 5. GRAS substances. Food Technol. 26: 35–42. 1972. [Google Scholar]
- 4.FDA. Code of Federal Regulations Title 21. 1. B. Food for Human Consumption (Continued). 172. Food Additives Permitted for Direct Addition to Food for Human Consumption, from website: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=172.
- 5.FDA. Code of Federal Regulations Title 21. 1. B. Food for Human Consumption (Continued). 184. Direct Food Substances Affirmed as Generally Recognized as Safe, from website: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=184.
- 6.The Japan Food Chemical Research Foundation. List of Designated Additives. 2016, from website: http://www.ffcr.or.jp/zaidan/FFCRHOME.nsf/pages/list-desin.add-x.
- 7.Man EH, and Bada JL. Dietary D-amino acids. Annu Rev Nutr. 7: 209–225. 1987. [DOI] [PubMed] [Google Scholar]
- 8.Csapo J, Albert C, and Csapo-Kiss Z. The D-amino acid content of foodstuffs. Acta Univ Sapientiae Aliment. 2: 5–30. 2009. [Google Scholar]
- 9.Sawamoto O, Kyo S, Kaneda S, Harada M, Kishimoto S, Koshitani O, Kurisu K, and Nakashima Y. Four-week intravenous repeated dose toxicity study of L-cysteine in male rats. J Toxicol Sci. 28: 95–107. 2003. [DOI] [PubMed] [Google Scholar]
- 10.Kitahori Y, Konishi N, Nakagawa Y, Cho M, Naitoh H, Yamamoto K, Matsui E, and Hiasa Y. Lack of carcinogenicity of L-cysteine monohydrochloride in Fischer 344 rats. J Toxicol Pathol. 10: 83–89. 1997. [Google Scholar]
- 11.Friedman M, and Levin CE. Nutritional and medicinal aspects of D-amino acids. Amino Acids. 42: 1553–1582. 2012. [DOI] [PubMed] [Google Scholar]
- 12.Shibui Y, Miwa T, Yamashita M, Chin K, and Kodama T. A 4-week repeated dose toxicity study of glycine in rats by gavage administration. J Toxicol Pathol. 26: 405–412. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shibui Y, Miwa T, Kodama T, and Gonsho A. 28-day dietary toxicity study of L-phenylalanine in rats. Fund Toxicol Sci. 1: 29–38. 2014. [Google Scholar]
- 14.Shibui Y, Manabe Y, Kodama T, and Gonsho A. 13-week repeated dose toxicity study of l-tyrosine in rats by daily oral administration. Food Chem Toxicol. 87: 55–64. 2016. [DOI] [PubMed] [Google Scholar]
- 15.Aoki M, Mochizuki M, Okamura T, Hatayama K, Nakamura A, and Morishita K. A 4-week oral toxicity study of L-alanine in rats with a recovery period of 2 weeks. Fund Toxicol Sci. 1: 63–72. 2014. [Google Scholar]
- 16.Aoki M, Ishida S, Fukuzumi H, and Morishita K. A 13-week feeding toxicity study of L-threonine in rats with a recovery period of 5 weeks. Fund Toxicol Sci. 1: 49–62. 2014. [Google Scholar]
- 17.Chin K, Toue S, Kawamata Y, Watanabe A, Miwa T, Smriga M, and Sakai R. A 4-week toxicity study of methionine in male rats. Int J Toxicol. 34: 233–241. 2015. [DOI] [PubMed] [Google Scholar]
- 18.MHLW. Guidelines for Toxicity Studies of Drugs. Pharmaceutical and Food Safety Bureau (PFSB) Notification No. 655. 1999.
- 19.Snedecor GW, and Cochran WG. Statistical Methods, eighth ed. Iowa State University Press, Ames. 1989. [Google Scholar]
- 20.Dunnett CW. A multiple comparison procedure for comparing several treatments with a control. J Am Stat Assoc. 50: 1096–1121. 1955. [Google Scholar]
- 21.Steel RGD. A multiple comparison rank sum test: Treatments versus control. Biometrics. 15: 560–572. 1959. [Google Scholar]
- 22.Frazier KS, Seely JC, Hard GC, Betton G, Burnett R, Nakatsuji S, Nishikawa A, Durchfeld-Meyer B, and Bube A. Proliferative and nonproliferative lesions of the rat and mouse urinary system. Toxicol Pathol. 40(Suppl): 14S–86S. 2012. [DOI] [PubMed] [Google Scholar]
- 23.Yin J, Ren W, Yang G, Duan J, Huang X, Fang R, Li C, Li T, Yin Y, Hou Y, Kim SWL, and Wu G. L-Cysteine metabolism and its nutritional implications. Mol Nutr Food Res. 60: 134–146. 2016. [DOI] [PubMed] [Google Scholar]
- 24.Krijgsheld KR, Glazenburg EJ, Scholtens E, and Mulder GJ. The oxidation of L- and D-cysteine to inorganic sulfate and taurine in the rat. Biochim Biophys Acta. 677: 7–12. 1981. [DOI] [PubMed] [Google Scholar]
- 25.Stipanuk MH. Metabolism of sulfur-containing amino acids. Annu Rev Nutr. 6: 179–209. 1986. [DOI] [PubMed] [Google Scholar]
- 26.Sawamoto O, Yamate J, Kuwamura M, Kotani T, and Kurisu K. Development of sperm granulomas in the epididymides of L-cysteine-treated rats. Toxicol Pathol. 31: 281–289. 2003. [DOI] [PubMed] [Google Scholar]
- 27.Sawamoto O, Kurisu K, Kuwamura M, Kotani T, and Yamate J. Relationship of interstitial edema with L-cysteine-induced sperm granulomas in the pubertal rat epididymis. Exp Toxicol Pathol. 55: 121–127. 2003. [DOI] [PubMed] [Google Scholar]
- 28.Sheffner AL. The reduction in vitro in viscosity of mucoprotein solutions by a new mucolytic agent, N-acetyl-L-cysteine. Ann N Y Acad Sci. 106: 298–310. 1963. [DOI] [PubMed] [Google Scholar]
- 29.Snary D, Allen A, and Pain RH. Structural studies on gastric mucoproteins: lowering of molecular weight after reduction with 2-mercaptoethanol. Biochem Biophys Res Commun. 40: 844–851. 1970. [DOI] [PubMed] [Google Scholar]
- 30.Ji Y, Wu Z, Dai Z, Sun K, Zhang Q, and Wu G. Excessive L-cysteine induces vacuole-like cell death by activating endoplasmic reticulum stress and mitogen-activated protein kinase signaling in intestinal porcine epithelial cells. Amino Acids. 48: 149–156. 2016. [DOI] [PubMed] [Google Scholar]
- 31.Lee S, Han KH, Nakamura Y, Kawakami S, Shimada K, Hayakawa T, Onoue H, and Fukushima M. Dietary L-cysteine improves the antioxidative potential and lipid metabolism in rats fed a normal diet. Biosci Biotechnol Biochem. 77: 1430–1434. 2013. [DOI] [PubMed] [Google Scholar]
- 32.McGavigan AK, O’Hara HC, Amin A, Kinsey-Jones J, Spreckley E, Alamshah A, Agahi A, Banks K, France R, Hyberg G, Wong C, Bewick GA, Gardiner JV, Lehmann A, Martin NM, Ghatei MA, Bloom SR, and Murphy KGL. L-cysteine suppresses ghrelin and reduces appetite in rodents and humans. Int J Obes. 39: 447–455. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muramatsu K, Odagiri H, Morishita S, and Takeuchi H. Effect of excess levels of individual amino acids on growth of rats fed casein diets. J Nutr. 101: 1117–1125. 1971. [DOI] [PubMed] [Google Scholar]
- 34.Solms J. [The taste of amino acids, peptides and proteins]. Int Z Vitaminforsch. 39: 320–322. 1969. [in German] [PubMed] [Google Scholar]
- 35.Huang J, Khan S, and O’Brien PJ. The glutathione dependence of inorganic sulfate formation from L- or D-cysteine in isolated rat hepatocytes. Chem Biol Interact. 110: 189–202. 1998. [DOI] [PubMed] [Google Scholar]
- 36.Shibuya N, Koike S, Tanaka M, Ishigami-Yuasa M, Kimura Y, Ogasawara Y, Fukui K, Nagahara N, and Kimura H. A novel pathway for the production of hydrogen sulfide from D-cysteine in mammalian cells. Nat Commun. 4: 1366 2013. [DOI] [PubMed] [Google Scholar]