Abstract
Objectives
Malignant external otitis (MEO) is a potentially fatal infection of the external auditory canal, temporal bone, and skull base. Despite treatment with modern antibiotics, MEO can lead to skull base osteomyelitis. Until now, there have been few studies on the prognostic factors of MEO.
Methods
We performed a retrospective study to identify prognostic factors of MEO, and a meta-analysis of other articles investigating MEO. On the basis of disease progression the 28 patients in our study were divided into ‘controlled’ and ‘uncontrolled’ groups, consisting of 12 and 16 patients, respectively. We identified three categories of prognostic factors: those related to patient, disease, and treatment. We compared these prognostic factors between the controlled and uncontrolled groups.
Results
In our study, the duration of diabetes mellitus (DM), presence of inflammatory markers (C-reactive protein and erythrocyte sedimentation rate), and computed tomography or magnetic resonance imaging findings influenced the prognosis of MEO. In contrast, prognosis was unrelated to age, gender, mean glucose level, hemoglobin A1c level, pathogen, comorbidity, or cranial nerve involvement. No factor related to treatment modality was correlated with prognosis, such as surgery, steroid therapy, or interval to the first appropriate treatment. Cranial nerve involvement has been proven to be associated with disease progression, but the relationship between cranial nerve involvement and the prognosis of MEO remains controversial. As a part of this study, we conducted a meta-analysis of cranial nerve involvement as a prognostic factor of MEO. We found that cranial nerve involvement has a statistically significant influence on the prognosis of MEO.
Conclusion
We found that glycemic control in diabetes mellitus, cranial nerve involvement, and the extent of disease determined from various imaging modalities influence the prognosis of MEO. We suggest that significant prognostic factors should be monitored to determine the prognosis of patients with MEO.
Keywords: Otitis Externa, Malignant, Prognosis, Meta-Analysis
INTRODUCTION
Malignant external otitis (MEO) is a potentially fatal infection of the external auditory canal (EAC), temporal bone, and skull base. MEO tends to affect the elderly as well as patients with diabetes mellitus (DM) or other conditions resulting in immunodeficiency, such as human immunodeficiency virus (HIV) infection and chemotherapy. The most common causative organism is Pseudomonas aeruginosa (>90%) [1]. Clinical manifestations include deep otalgia persisting for longer than 1 month, chronic otorrhea, headache, and cranial nerve involvement. Before the discovery of effective antibiotics, MEO had a mortality rate of up to 50%. Since the introduction of ciprofloxacin and other antipseudomonal agents in the 1990’s, the survival rate has improved [2]. Nevertheless, MEO can be fatal if the disease continues to progress despite modern antibiotic treatment.
MEO begins in the EAC, and then spreads to the skull base and the jugular bulb via the fissures of Santorini and the stylomastoid foramen. Venous channels and fascial planes facilitate the spread of infection along the dural sinuses, eventually resulting in extension to the petrous apex [3]. Multiple studies have shown that cranial nerve involvement, primarily the facial nerve, is associated with advanced infection and progression of the disease. As the infection advances to the medial skull base, it reaches the jugular foramen, leading to involvement of the glossopharyngeal, vagus, and spinal accessory nerves [4]. The hypoglossal nerve may be involved at its location within the hypoglossal canal, and with the progression of the infection, the nerves in the cavernous sinus could be affected as well. Severe complications, such as cranial nerve involvement and skull base osteomyelitis, are associated with increased risk of mortality.
Many factors were believed to affect the prognosis of MEO, such as a medical history of DM, glucose level, cranial nerve involvement, and the extent of disease determined from various imaging modalities. However, further studies showed that these factors were not actually related to the outcome of MEO. For example, Mani et al. [5] reported that the presence of cranial nerve involvement did not affect patient survival rate under an optimized treatment plan. As reported by Soudry et al. [6], facial nerve involvement indicated progression of MEO, but did not, by itself, worsen prognosis. There is an ongoing debate regarding the various factors predicting the outcome for MEO. It is unclear which factor(s) lead to a poorer prognosis. Therefore, we analyzed prognostic factors in the hope of optimizing treatment strategy for this disease. We investigated the controversial prognostic factors and performed a meta-analysis of other articles investigating MEO retrospectively.
MATERIALS AND METHODS
All cases of MEO diagnosed and treated by Department of Otolaryngology, Severance Hospital, Yonsei University Health System between January 2000 and March 2014 were identified. The diagnosis of MEO was based on the criteria set forth by Cohen and Friedman [7]. We reviewed the records of 28 patients who met these criteria (Table 1). We examined the patients’ basic data, including interval to first intravenous antibiotic administration, presence of underlying disease, results of ear-nosethroat and neurologic examinations, results of imaging studies including computed tomography (CT) and magnetic resonance imaging (MRI), blood glucose measurements and level of glycemic control, treatment regimen, any surgeries performed, and the erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels. We divided the patients into two groups based on the outcome of treatment: controlled and uncontrolled groups. The controlled group included patients who recovered fully from MEO; the uncontrolled group included patients who did not recover or who died. All patients in the uncontrolled group were deceased at the time of the study. The clinical investigations were all conducted according to the principles expressed in the Declaration of Helsinki. This study was approved by the Institutional Review Board of Severance Hospital, Yonsei University Health System (4-2016-0268).
Table 1.
The diagnostic criteria of malignant external otitis [7]
| Major (obligatory) signs |
| 1) Pain |
| 2) Exudate |
| 3) Edema |
| 4) Granulations |
| 5) Microabscesses |
| 6) Positive Technetium-99 (99Tc) scan of failure of local treatment after more than 1 week |
| Minor (occasional) signs |
| 7) Pseudomonas |
| 8) Positive radiograph |
| 9) Diabetes mellitus |
| 10) Cranial nerve involvement |
| 11) Debilitating conditions |
| 12) Old age |
The diagnostic criteria of malignant external otitis (MEO) was divided into two categories: obligatory and occasional. All of the obligatory criteria must be present in order to establish the diagnosis. The presence of occasional criteria alone does not establish it.
The two groups were compared using Student t-test, Fisher test, and the chi-squared test, as indicated; SAS ver. 9.2 (SAS Institute Inc., Cary, NC, USA) and Microsoft Office Excel 2003.
A meta-analysis of other articles related to MEO was also performed. Several accepted sources were searched in order to identify primary studies published between 1974 and 2013. We primarily searched the Medline database, using the terms “malignant external otitis,” “malignant otitis externa,” “skull base osteomyelitis,” “necrotizing otitis externa,” and “infective external otitis.” Alternate spellings and an explicit search strategy were used for each source. All terms were searched in English.
The literature search was extensive and was designed to obtain a large number of hits for MEO. In total, 368 articles were identified. After eliminating duplicates and articles on subjects obviously different from MEO, 342 publications remained for the period from January 1974 to April 2014 (Fig. 1). Most studies evaluating the prognosis for MEO were retrospective, so the meta-analysis was conducted on retrospective studies only. After eliminating articles with methodological restrictions and data limitations, six articles met our criteria for inclusion (retrospective study, complete reporting, and complete dichotomous outcome data). We used only the data on cranial or facial nerve involvement as a prognostic factor. We compared the outcomes for MEO using dichotomous data (controlled vs. uncontrolled group) from the retrospective studies.
Fig. 1.
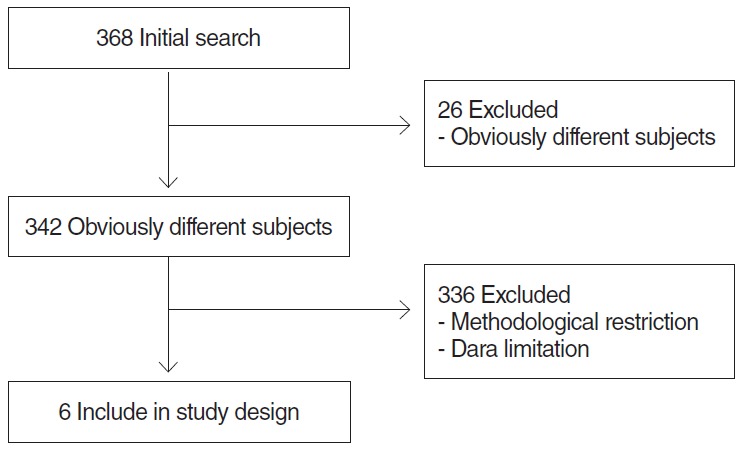
Flow chart of search procedure.
The data were analyzed using Stata ver. 8.2 (Stata Co., College Station, TX, USA). Our analysis was based on a random-effects model, which generates a wider 95% confidence interval (CI) for the pooled data. The relationship between cranial nerve involvement and the prognosis for MEO effect sizes were calculated as the natural log of the odds ratio (OR). Effect sizes are depicted with their respective 95% CI.
RESULTS
We identified 28 patients with a diagnosis of MEO. There were 22 males (78.6%) and 6 females (21.4%), with a mean age of 65±13 years (range, 33 to 81 year). Twelve patients (42.9%) experienced complete remission from MEO and were included in the controlled group. Sixteen patients (57.1%) survived with the disease (12 patients, 42.9%) or died from the disease (4 patients, 14.3%) and were included in the uncontrolled group. Basic data at the time of presentation and results of statistical analyses are summarized in Table 2.
Table 2.
Characteristics of the malignant external otitis patients
| Characteristic | Controlled group (n=12) | Uncontrolled group (n=16) | P-value |
|---|---|---|---|
| Gender | 0.690 | ||
| Male | 9 | 13 | |
| Female | 3 | 3 | |
| Age (yr), mean±standard deviation | 62±16 | 68±9 | 0.207 |
| Skull vase osteomyelitis | 3 | 16 | <0.001* |
| Diabetes mellitus | 7 | 16 | 0.004* |
| Comorbidity | 4 | 12 | 0.027* |
| Cranial involvement | 6 | 7 | 0.743 |
| First intravenous antibiotic administration (day) | 29.0 | 32.6 | 0.615 |
| Steroid therapy | 8 | 12 | 0.629 |
| Surgery | 3 | 6 | 0.483 |
Significant differences were found in skull base osteomyelitis, hospitalization, comorbidity, and diabetes mellitus. No significant differences were found for any other comparison.
Skull base osteomyelitis was found in 19 patients (67.9%); 9 out of 19 patients underwent surgery. Surgery was performed on patients whose condition failed to respond to medical treatment, or was performed for local debridement, and to collect biopsy specimen for histological confirmation of the disease.
Prognostic factors related to the condition of the patient
Patients in the controlled group (12 patients) with a mean age of 62±16 years recovered fully from MEO. Meanwhile patients in the uncontrolled group (16 patients) with a mean age of 68±9 years either remained alive with the disease or died from progression of the disease (P=0.207). Three patients died in hospital as a result of aspiration pneumonia related to MEO, and another patient died from sepsis. In both groups, the majority of the patients were male (9 of 12 patients in the controlled group, and 13 of 16 patients in the uncontrolled group); however, this did not significantly affect disease outcome.
Twenty-three patients (82.1%) had DM. DM is a common disease associated with MEO, and it was significantly related to disease progression in 7 patients (25.0%) in the controlled group and 16 patients (57.1%) in the uncontrolled group (P=0.008) (Table 3). Duration of disease also differed significantly between the two groups; patients in the controlled group suffered from DM for an average of 7.1 years prior to the diagnosis of MEO and patients in the uncontrolled group for an average of 21.8 years (P=0.001). Hemoglobin A1c (HbA1c; glycated hemoglobin) reflects the 3-month average of plasma glucose concentration. This was checked in all patients but was not a prognostic indicator for MEO (P=0.054).
Table 3.
Underlying disease of the MEO patients
| Patient’s underlying disease | Controlled group (n=12) | Uncontrolled group (n=16) | P-value |
|---|---|---|---|
| DM | 7 | 16 | 0.008* |
| History (yr) | 7.1 | 21.8 | 0.001* |
| HbA1c (%) | 6.49±0.97 | 7.46±1.44 | 0.054 |
| Glucose level | 121.3±39.6 | 150.8±38.7 | 0.053 |
| Comorbidity | 4 | 12 | 0.053 |
| Hypertension | 2 | 6 | NS |
| Ischemic heart disease | 0 | 2 | NS |
| Chronic renal failure | 0 | 2 | NS |
| DM retinopathy | 1 | 1 | NS |
| Cerebrovascular accident | 1 | 1 | NS |
MEO, malignant external otitis; DM, diabetes mellitus; HbA1c, hemoglobin A1c; NS, not significant.
Statistically significant.
Other underlying diseases identified in this study included hypertension (8 patients), chronic renal failure (3 patients), ischemic heart disease (2 patients), DM retinopathy (2 patients), and cerebrovascular accident (2 patients). There was no significant difference between the controlled and uncontrolled groups with respect to the presence of the above comorbidities (P=0.053).
The treatment outcomes for patients who were responsive (fasted morning blood glucose <126 mg/dL) and unresponsive (≥126 mg/dL) to treatment with antidiabetic medication were compared (Fig. 2). In the MEO controlled group, 10 patients were responsive to antidiabetic medication and 2 patients were unresponsive. By contrast, 4 patients in the MEO uncontrolled group were responsive to treatment with antidiabetic medication while 12 patients were unresponsive (P<0.05).
Fig. 2.
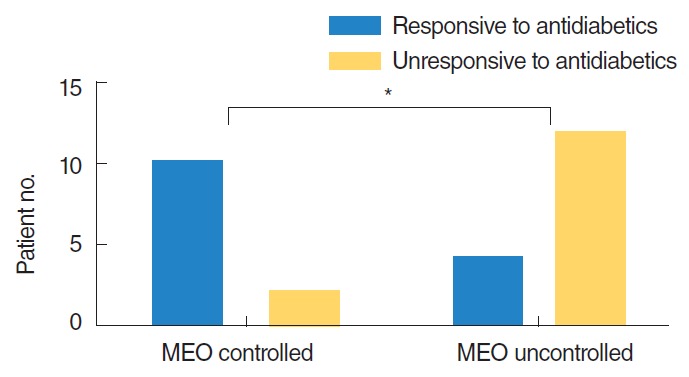
Comparison of glycemic control between MEO controlled group and uncontrolled group. *Statistically significant (P<0.05). MEO, malignant external otitis.
ESR and CRP levels increased with disease progression and were higher in the uncontrolled group. The ESR and CRP levels used for comparison between the two groups were the highest levels measured during the course of disease for each individual. A mean value of 34.8±30.5 mm/hr was obtained for the ESR level in the controlled group, and a mean value of 96.1±30.8 mm/hr was obtained for the uncontrolled group (P<0.001). A mean value of 5.33±3.21 mg/L was measured for the CRP level in the controlled group and a mean value of 15.46±5.70 mg/L was measured for the uncontrolled group (P<0.001). Both inflammatory markers were closely related to outcome for the MEO patients (Fig. 3). Additionally, they changed with the disease status in the individual patients.
Fig. 3.
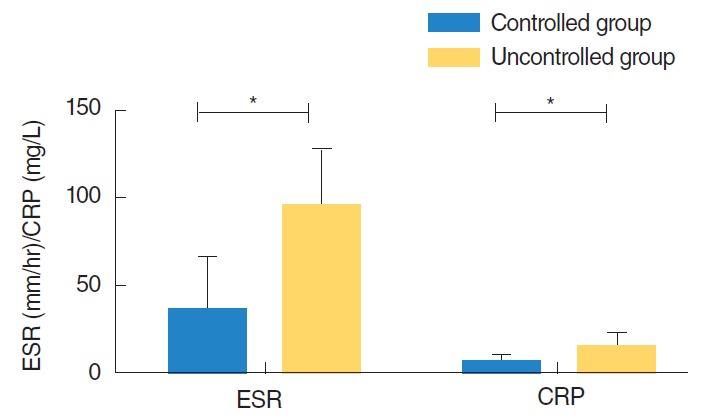
Comparison of inflammatory marker (ESR, CRP) between two groups. ESR, erythrocyte sedimentation rate; CRP, C-reactive protein. *Statistically significant (P<0.001, P<0.001; Student t-test).
P. aeruginosa was the most commonly isolated bacterial pathogen (13 patients), followed by methicillin resistant Staphylococcus aureus (MRSA, 10 patients). Table 4 provides a detailed list of isolated microorganisms. There was no significant difference between the controlled and uncontrolled groups with respect to the infecting microorganism.
Table 4.
Microorganisms isolated
| Microorganism | Patient no. |
|---|---|
| Bacteria | |
| Pseudomonas aeruginosa | 13 |
| MRSA | 10 |
| Enterobacter | 3 |
| Klebsiella pneumoniae | 2 |
| Proteus | 1 |
| Fungi | |
| Aspergillus fumigatus | 2 |
| Polymicrobial infection | 4 |
MRSA, methicillin resistant staphylococcus aureus.
Prognostic factors related to disease extent
We classified the extent of disease present according to the expected course of the disease. The infection typically starts in the EAC and spreads to the stylomastoid foramen and then to the mastoid tip and the jugular foramen. Finally, the septic process extends to the petrous apex and the middle cranial fossa (6). CT or MRI imaging was performed in all patients. All 28 patients had EAC involvement, 13 patients had stylomastoid foramen involvement (6 controlled, 7 uncontrolled), and 5 patients had jugular foramen and lateral venous sinus involvement (0 controlled, 5 uncontrolled). Seven patients had petrous apex involvement (1 controlled, 6 uncontrolled); one of these had cavernous sinus involvement, presenting as abducens nerve palsy. In 5 of the patients with petrous apex or cavernous sinus involvement, the infection arose from the jugular foramen. In the remaining 2 patients, the jugular foramen was not involved and the disease did not follow its typical course.
The CT or MRI findings were categorized according to the extent of the disease. Patients who had jugular foramen and petrous apex involvement had a tendency for higher morbidity and mortality than patients without involvement of these structures (P=0.045, P=0.005). There was no association between disease extent and glucose level, suggesting that involvement of the jugular foramen and petrous apex independently affected the outcome of MEO (Fig. 4).
Fig. 4.
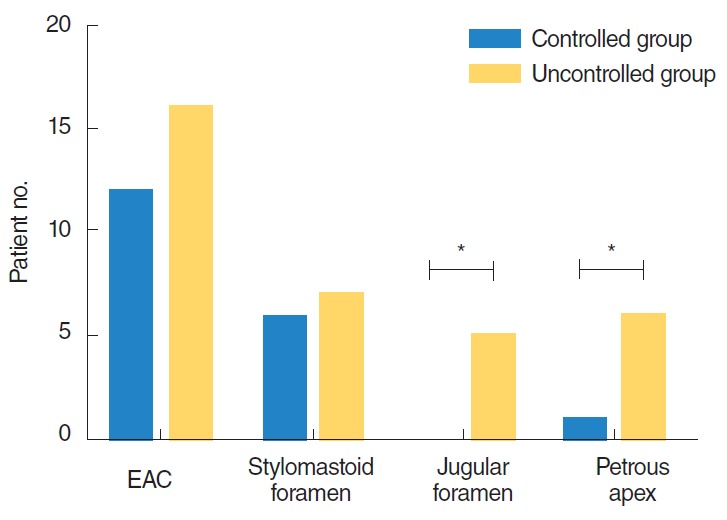
Comparison of disease extent in imaging modalities between controlled group and uncontrolled group. *Statistically significant (P<0.05; chi-squared test).
Cranial nerve involvement was observed in 13 patients (46.4%). All 13 patients had facial nerve (VII) involvement; 4 patients also had involvement of the lower cranial nerves (IX, X, and XI) and 2 patients had involvement of the hypoglossal nerve (XII). The abducens nerve (VI) was affected in only 1 patient (Table 5).
Table 5.
Cranial nerve involvement in the MEO patients
| Cranial nerve | Controlled group (n=12) | Uncontrolled group (n=16) | P-value |
|---|---|---|---|
| CN involvement | 6 | 7 | 0.742 |
| Facial nerve (VII) | 6 | 7 | 0.743 |
| Lower cranial nerve (IX, X, XI) | 0 | 4 | 0.113 |
| IX | 0 | 3 | NS |
| X | 0 | 3 | NS |
| XI | 0 | 2 | NS |
| Hypoglossal nerve (XII) | 0 | 2 | NS |
| Abducens nerve (VI) | 0 | 1 | NS |
| Multiple CN involvement | 0 | 4 | 0.113 |
MEO, malignant external otitis; CN, cranial nerve; NS, not significant.
The facial nerve was the cranial nerve most commonly affected due to its proximity to the EAC, but there was no significant difference in occurrence between the controlled and uncontrolled groups (P=0.702). None of the patients showing involvement of the other cranial nerves belonged to the controlled group, but the difference was not significant (P=0.113). Single or multiple cranial nerve involvement was not related to prognosis for the patients with MEO in our study.
Prognostic factors related to the treatment
After diagnosing MEO, treatment with an antibiotic to which the microorganism is susceptible is important; these include fluoroquinolone and third-generation cephalosporins for P. aeruginosa and vancomycin or teicoplanin for MRSA. Intravenous or oral steroid therapy was also used for skull base osteomyelitis or intractable otorrhea for its anti-inflammatory properties. When cranial nerve involvement gave rise to facial or vocal cord palsy, high-dose steroid therapy was instituted for the recovery of cranial nerve function. Because most of the patients in this study also had DM, steroid therapy was used only with careful monitoring. The use of steroid therapy was unrelated to outcome for patients with MEO (P=0.630). Fig. 5 provides a comparison between treatment modalities for the two groups.
Fig. 5.
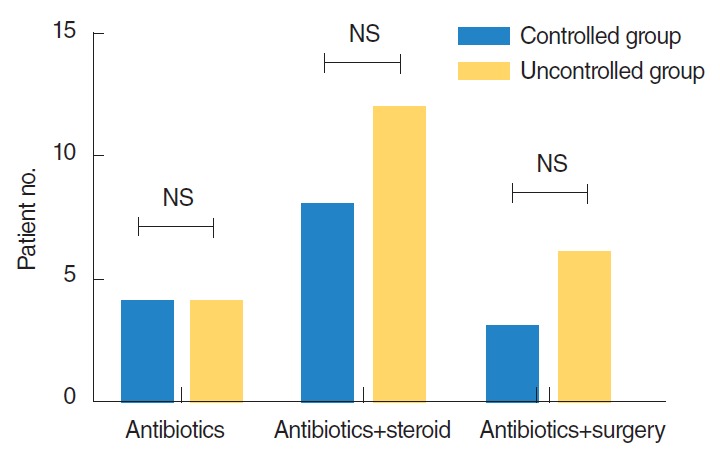
Comparison on treatment modality between controlled group and uncontrolled group. NS, not significant.
Nine patients underwent surgery: 3 in the controlled group and 6 in the uncontrolled group. These were all patients who did not respond to medical treatment alone. In the controlled group, 2 patients underwent a radical mastoidectomy, facial nerve decompression, and mastoid obliteration. In the other patient incision and drainage of a localized mastoid tip abscess was performed. All 3 patients showed resolution of the disease, although recovery from the facial palsy was not complete. In the uncontrolled group, 5 patients underwent radical mastoidectomy and facial nerve decompression and the remaining patient required skull base surgery via an infratemporal fossa approach, type A. Although surgery only seemed to benefit the 3 patients in the controlled group, there was no significant difference between the controlled and uncontrolled groups with respect to surgery (P=0.687).
Meta-analysis of prognostic factors
A meta-analysis was performed due to an ongoing debate with respect to cranial nerve involvement as a prognostic factor. The baseline characteristics of the included trials including study population, cranial nerve involvement, and statistical evaluation were comparable (Table 6) [5,6,8-12].
Table 6.
Summary of the studies describing cranial nerve involvement in patients with MEO
| Trial | CN involved | Patient no. (%) | P-value* | |
|---|---|---|---|---|
| Chandler [12] | Controlled (n=26) | Uncontrolled (n=12) | ||
| FN | 8 (50) | 8 (50) | ||
| Multiple CN | 1 (20) | 4 (80) | ||
| Chen et al. [11] | Survival (n=21) | Mortality (n=5) | ||
| Single CN | 7 | 4 | 0.058 | |
| Multiple CN | 2 | 3 | 0.034 | |
| Franco-Vidal et al. [10] | FN | 22.2% | 77.8% | 0.023 |
| Lee et al. [9] | CN | HR, 0.28; 95% CI, 0.03−0.93 | P <0.05 | |
| Mani et al. [5] | Controlled (n=21) | Uncontrolled (n=2) | NS† | |
| CN | 8 | 2 | ||
| Soudry et al. [6] | Controlled (n=29) | Uncontrolled (n=19) | NS† | |
| FN | 7 | 1 | ||
| Ali et al. [8] | n=37 | |||
| FN | 15 (40) | |||
| Multiple CN | 9 | |||
| IX | 4 | |||
| X | 5 | |||
| XI | 3 | |||
| XII | 3 | |||
MEO, malignant external otitis; CN, cranial nerve; FN, facial nerve; HR, hazard ratio; CI, confidence interval.
Statistical evaluation by the author of the trial.
No significant difference found between groups.
All of the included trials reported the number of patients in each group, with the exception of Ali et al. [8]. Of the six remaining articles, four reported significantly different prognoses depending on presence of cranial nerve involvement. Three articles described only facial nerve involvement while the others described the involvement of multiple cranial nerves (especially the facial nerve and lower cranial nerves).
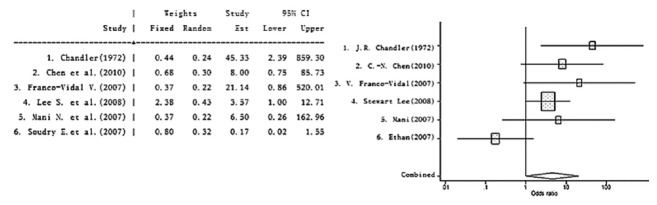
Outcomes for MEO were analyzed in terms of cranial nerve involvement regardless of the specific cranial nerve affected; these outcomes are shown in Fig. 6. The evaluated studies did not specifically define controlled and uncontrolled groups, but did compare mortality. We deduced which patients belonged in either the complete remission or the uncontrolled groups from review of the papers. A statistical evaluation was not included for all trials; therefore, we based our calculations on the original data reported.
Fig. 6.
Meta-analysis for the outcome cranial nerve involvement vs. noncranial nerve involvement in malignant external otitis.
Using the provided dichotomous data (controlled vs. uncontrolled groups), the combined OR (random effect model) was 4.537 (95% CI, 1.015 to 20.277). This suggests that cranial nerve involvement has a statistically significant effect on the prognosis for MEO. However, this result must be viewed with caution. First, the I2 analysis showed a variability of 0.581 and the combined OR (random effect model) showed a trend towards cranial nerve involvement being associated with the prognosis for MEO. Second, there is no consensus regarding measurement of outcome in MEO. As mentioned above, the “outcome” was frequently not clarified, so we made deductions from the original data in the studies we reviewed. Third, we calculated the P-value if possible. For the study performed by Lee et al. [9], statistical significance was deduced from the hazard ratio and the P-value was presumed to be 0.05.
DISCUSSION
MEO has become a treatable disease with the advent of new antibiotics. However, despite the treatment, the prognosis worsens once skull base osteomyelitis or other complications develop.
The duration of DM and the patient’s serum glucose levels were believed to be related to the prognosis of MEO. As reported by Joshua et al. [13], MEO diagnosed on the basis of all obligatory clinical and radiological criteria was associated with a higher rate of current oral anti-diabetic treatment and history of diabetic complications, all of which led to significantly longer treatment and shorter survival times. In contrast, Franco-Vidal et al. [10] reported that the presence of diabetes was not a significant prognostic factor in and of itself. Chen et al. [11] also suggested that the duration of DM and the degree of glycemic control did not affect survival. However, the results of this study demonstrated that the existence of DM, both currently and in the medical history, were important prognostic factors for MEO. Although mean serum glucose and HbA1c levels were unrelated to survival, responsiveness to antidiabetic therapy was closely correlated with the course of disease. Thus, strict glycemic control is necessary. Our data did not show a significant difference in prognosis between patients treated with oral antidiabetic medications and those treated with insulin.
It has not been well established why MEO has a tendency to affect diabetic patients; however, microangiopathy is presumed to be the predisposing factor. DM leads to microangiopathy which results in poor microcirculation and impaired polymorphonuclear cell function [14,15]. Furthermore, chemotaxis of leukocytes and the mechanisms of phagocytosis and intracellular digestion are impaired, and the effectiveness of antibiotic therapy is also reduced [16,17]. This occurs because the transport of antibiotics to the infected region is compromised by small vessel obliteration and the resultant hypoperfusion. We suggest that rigorous glycemic control and the administration of appropriate antibiotics are key factors in the treatment of MEO.
Skull base osteomyelitis is a life-threatening complication of MEO, which begins as a soft tissue infection in the EAC and spreads via the fissures of Santorini and the tympanomastoid suture to involve the cranial base [18]. The facial nerve is the most commonly affected cranial nerve due to its proximity to the EAC. As the disease advances to the medial skull base, it involves the jugular foramen and hypoglossal canal, so that the glossopharyngeal, vagus, spinal accessory, and hypoglossal nerves are also affected. As Nadol reported, the disease spreads to the central skull base via four checkpoints: EAC, stylomastoid foramen, jugular foramen, and petrous apex [3]. CT and MRI findings were helpful for diagnosing MEO at admission, but did not predict the prognosis for the disease, as reported by Sudhoff et al. [19]. In our series, there was a significant difference in outcome when the disease extended to the jugular foramen and petrous apex. The prognosis was poorer when greater disease extension was seen in imaging studies. We suggest that involvement of the jugular foramen and petrous apex are checkpoints for predicting the outcome and the course of disease for MEO from imaging studies. However, Kondziella and Skagervik [20] reported an atypical presentation of MEO showing extensive cranial neuropathy in the absence of facial paralysis. Therefore, the disease does not always spread via the pathway described above. In our series, while most of the cases followed the described course of disease, the jugular foramen was unaffected in 2 patients who had petrous apex involvement. We propose that CT and MRI are useful to help to predict the prognosis for MEO, and that the jugular foramen and petrous apex are critical points in the progression of MEO. There is a debate regarding the best imaging modality for the initial diagnosis of and monitoring of MEO. Some authors have recommended that a Ga-67 citrate scan is a good indicator of disease resolution; it has also been suggested that negative results can provide useful information regarding the appropriate time to stop treatment [21,22].
Cranial nerve involvement is believed to be related to the outcome of MEO, although it is a controversial prognostic factor [12]. We found no significant difference in the outcome of MEO with respect to any specific cranial nerve involvement or the involvement of multiple cranial nerves. Several studies have suggested that cranial nerve involvement is closely correlated with the outcome of the disease. Franco-Vidal et al. [10] and Lee et al. [9] reported that facial paralysis significantly influenced survival, while Mani et al. [5] reported that cranial nerve involvement did not affect patient survival rate with optimized medical treatment. Chen et al. [11] reported that in patients with MEO, mortality was not related to involvement of a single cranial nerve, but was related to the involvement of multiple cranial nerves. Soudry reported that although facial nerve involvement was a sign of MEO progression, it did not, by itself, worsen the prognosis in their case series [6]. Because of these differences in opinion, we performed a meta-analysis of cranial nerve involvement and prognosis. The results showed that cranial nerve involvement tended to affect the prognosis for MEO. However, our meta-analysis was limited by the fact that it analyzed the data dichotomously, and only a few of the articles evaluated described cranial nerve involvement and the number of survivors with disease progression. The small number of patients in the present study could have led to the discrepancies between our results and those obtained from the meta-analysis.
In our series, disease outcome was closely correlated with both ESR and CRP levels. Changes in ESR and CRP levels can help to monitor the response to antibiotic therapy. Once MEO is suspected, laboratory data including ESR and CRP levels should be checked. The levels should then be followed regularly until complete remission is achieved.
We performed surgery in 9 of our 28 patients. This involved either a radical mastoidectomy with facial nerve decompression, or infratemporal fossa or skull base debridement of soft tissue or bone. In the controlled group, 1 of the 3 patients requiring surgery underwent incision and drainage of a mastoid tip abscess; in the other 2 patients, a radical mastoidectomy with facial nerve decompression was performed. In the uncontrolled group, 1 patient had skull base surgery via a type-A intratemporal fossa approach. Although necrotic bone and soft tissue were debrided, the infection could not be halted in the uncontrolled group. In contrast, patients in the controlled group recovered fully after surgery; therefore, surgery may be an alternative to unsuccessful medical treatment. Regardless, surgery was not directly related to prognosis in the two groups. No factor relating to treatment affected prognosis; this included surgery, steroid therapy, and interval to the first appropriate treatment.
Various surgical treatments have been proposed for patients with MEO who are refractory to medical treatment. Radical mastoidectomies have been recommended for patients with inflammation that cannot be completely excised (e.g., cochlear fistula, disease tracking into the petrous apex) [23]. Facial nerve decompression has been recommended for patients with disease involving the facial nerve in the stylomastoid foramen region [24]. The infratemporal fossa approach is recommended for patients with disease involving the petrous apex and intralabyrinthine areas [25].
In conclusion, all the major factors (responsiveness to antidiabetic medication and disease extent determined from CT or MRI imaging) were related to the prognosis of MEO. Furthermore, meta-analysis of cranial nerve involvement showed that exerted a statistically significant influence on MEO prognosis. We propose that responsiveness to antidiabetic medications in the treatment of DM, cranial nerve involvement, and disease extent determined from imaging could be useful in predicting the outcome of MEO.
HIGHLIGHTS
▪ The duration of diabetes mellitus, presence of inflammatory markers, and imaging findings influenced the prognosis of external otitis (MEO).
▪ None of the treatment-related factors, including surgery, steroid therapy, and the interval to the first appropriate treatment, were correlated with the prognosis.
▪ The meta-analysis of cranial nerve involvement as a prognostic factor showed that cranial nerve involvement had a statistically significant influence on the prognosis of MEO.
Acknowledgments
This work was supported by Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT & Future Planning (2013R1A1A 1061080) and Soonchunhyang University Research Fund.
Footnotes
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Rubin J, Yu VL. Malignant external otitis: insights into pathogenesis, clinical manifestations, diagnosis, and therapy. Am J Med. 1988 Sep;85(3):391–8. doi: 10.1016/0002-9343(88)90592-x. [DOI] [PubMed] [Google Scholar]
- 2.Chandler JR. Malignant external otitis and facial paralysis. Otolaryngol Clin North Am. 1974 Jun;7(2):375–83. [PubMed] [Google Scholar]
- 3.Nadol JB., Jr Histopathology of Pseudomonas osteomyelitis of the temporal bone starting as malignant external otitis. Am J Otolaryngol. 1980 Nov;1(5):359–71. doi: 10.1016/s0196-0709(80)80016-0. [DOI] [PubMed] [Google Scholar]
- 4.Rowlands RG, Lekakis GK, Hinton AE. Masked pseudomonal skull base osteomyelitis presenting with a bilateral Xth cranial nerve palsy. J Laryngol Otol. 2002 Jul;116(7):556–8. doi: 10.1258/002221502760132700. [DOI] [PubMed] [Google Scholar]
- 5.Mani N, Sudhoff H, Rajagopal S, Moffat D, Axon PR. Cranial nerve involvement in malignant external otitis: implications for clinical outcome. Laryngoscope. 2007 May;117(5):907–10. doi: 10.1097/MLG.0b013e318039b30f. [DOI] [PubMed] [Google Scholar]
- 6.Soudry E, Joshua BZ, Sulkes J, Nageris BI. Characteristics and prognosis of malignant external otitis with facial paralysis. Arch Otolaryngol Head Neck Surg. 2007 Oct;133(10):1002–4. doi: 10.1001/archotol.133.10.1002. [DOI] [PubMed] [Google Scholar]
- 7.Cohen D, Friedman P. The diagnostic criteria of malignant external otitis. J Laryngol Otol. 1987 Mar;101(3):216–21. doi: 10.1017/s0022215100101562. [DOI] [PubMed] [Google Scholar]
- 8.Ali T, Meade K, Anari S, ElBadawey MR, Zammit-Maempel I. Malignant otitis externa: case series. J Laryngol Otol. 2010 Aug;124(8):846–51. doi: 10.1017/S0022215110000691. [DOI] [PubMed] [Google Scholar]
- 9.Lee S, Hooper R, Fuller A, Turlakow A, Cousins V, Nouraei R. Otogenic cranial base osteomyelitis: a proposed prognosis-based system for disease classification. Otol Neurotol. 2008 Aug;29(5):666–72. doi: 10.1097/MAO.0b013e318179972f. [DOI] [PubMed] [Google Scholar]
- 10.Franco-Vidal V, Blanchet H, Bebear C, Dutronc H, Darrouzet V. Necrotizing external otitis: a report of 46 cases. Otol Neurotol. 2007 Sep;28(6):771–3. doi: 10.1097/MAO.0b013e31805153bd. [DOI] [PubMed] [Google Scholar]
- 11.Chen CN, Chen YS, Yeh TH, Hsu CJ, Tseng FY. Outcomes of malignant external otitis: survival vs mortality. Acta Otolaryngol. 2010;130(1):89–94. doi: 10.3109/00016480902971247. [DOI] [PubMed] [Google Scholar]
- 12.Chandler JR. Malignant external otitis. Laryngoscope. 1968 Aug;78(8):1257–94. doi: 10.1288/00005537-196808000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Joshua BZ, Sulkes J, Raveh E, Bishara J, Nageris BI. Predicting outcome of malignant external otitis. Otol Neurotol. 2008 Apr;29(3):339–43. doi: 10.1097/MAO.0b013e3181661879. [DOI] [PubMed] [Google Scholar]
- 14.Geerlings SE, Hoepelman AI. Immune dysfunction in patients with diabetes mellitus (DM) FEMS Immunol Med Microbiol. 1999 Dec;26(3-4):259–65. doi: 10.1111/j.1574-695X.1999.tb01397.x. [DOI] [PubMed] [Google Scholar]
- 15.O’Sullivan TJ, Dickson RI, Blokmanis A, Roberts FJ, Kaan K. The pathogenesis, differential diagnosis, and treatment of malignant otitis externa. J Otolaryngol. 1978 Aug;7(4):297–303. [PubMed] [Google Scholar]
- 16.Lucente FE, Parisier SC, Som PM, Arnold LM. Malignant external otitis: a dangerous misnomer? Otolaryngol Head Neck Surg. 1982 Mar-Apr;90(2):266–9. doi: 10.1177/019459988209000223. [DOI] [PubMed] [Google Scholar]
- 17.Lucente FE, Parisier SC, Som PM. Complications of the treatment of malignant external otitis. Laryngoscope. 1983 Mar;93(3):279–81. doi: 10.1288/00005537-198303000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Bernheim J, Sade J. Histopathology of the soft parts in 50 patients with malignant external otitis. J Laryngol Otol. 1989 Apr;103(4):366–8. doi: 10.1017/s0022215100108977. [DOI] [PubMed] [Google Scholar]
- 19.Sudhoff H, Rajagopal S, Mani N, Moumoulidis I, Axon PR, Moffat D. Usefulness of CT scans in malignant external otitis: effective tool for the diagnosis, but of limited value in predicting outcome. Eur Arch Otorhinolaryngol. 2008 Jan;265(1):53–6. doi: 10.1007/s00405-007-0416-8. [DOI] [PubMed] [Google Scholar]
- 20.Kondziella D, Skagervik I. Malignant external otitis with extensive cranial neuropathy but no facial paralysis. J Neurol. 2007 Sep;254(9):1298–9. doi: 10.1007/s00415-006-0516-1. [DOI] [PubMed] [Google Scholar]
- 21.Stokkel MP, Takes RP, van Eck-Smit BL, Baatenburg de Jong RJ. The value of quantitative gallium-67 single-photon emission tomography in the clinical management of malignant external otitis. Eur J Nucl Med. 1997 Nov;24(11):1429–32. doi: 10.1007/s002590050172. [DOI] [PubMed] [Google Scholar]
- 22.Okpala NC, Siraj QH, Nilssen E, Pringle M. Radiological and radionuclide investigation of malignant otitis externa. J Laryngol Otol. 2005 Jan;119(1):71–5. doi: 10.1258/0022215053222978. [DOI] [PubMed] [Google Scholar]
- 23.Stevens SM, Lambert PR. Mastoidectomy: surgical techniques. In: Flint PW, Haughey BH, Lund VJ, Niparko JK, Robbins KT, Thomas JR, et al., editors. Cummings otolaryngology: head and neck surgery. 6th ed. Philadelphia (PA): Elsevier Saunders; 2015. pp. 2188–99. [Google Scholar]
- 24.Brant JA, Ruckenstein MJ. Infections of the external ear. In: Flint PW, Haughey BH, Lund VJ, Niparko JK, Robbins KT, Thomas JR, et al., editors. Cummings otolaryngology: head and neck surgery. 6th ed. Philadelphia (PA): Elsevier Saunders; 2015. pp. 2115–22. [Google Scholar]
- 25.Walvekar RR, Culicchia F, Nuss DW. Surgery of the anterior and middle cranial base. In: Flint PW, Haughey BH, Lund VJ, Niparko JK, Robbins KT, Thomas JR, et al., editors. Cummings otolaryngology: head and neck surgery. 6th ed. Philadelphia (PA): Elsevier Saunders; 2015. pp. 2671–700. [Google Scholar]