Abstract
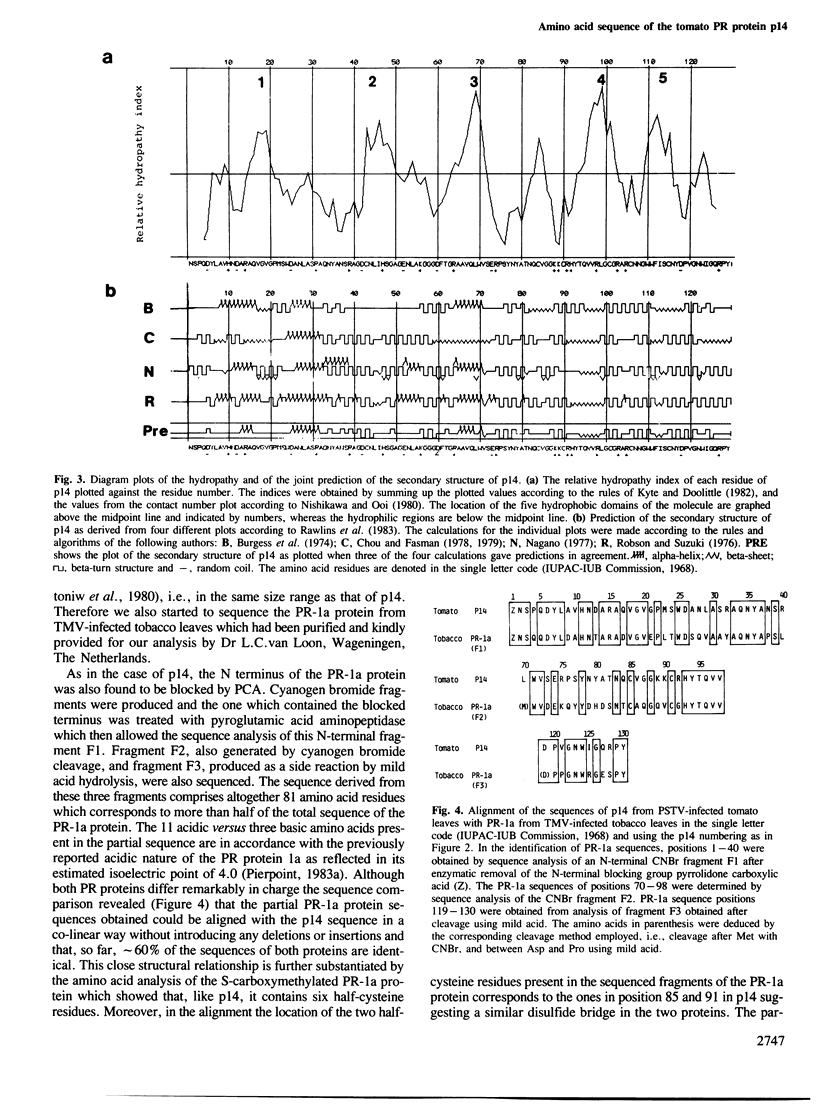
We have established the complete sequence of the 130 amino acid residues of the pathogenesis-related (PR) protein p14 accumulating in tomato leaves infected with the viroid of the spindle tuber disease of potato (PSTV) and partial sequences of the PR protein 1a which accumulates in tobacco mosaic virus-infected tobacco leaves. Both PR proteins are closely related to each other. However, no homology could be found between the sequence of p14 and any of the 3061 published protein sequences compiled in the protein sequence database at present. Thus, p14 represents not only the first completely sequenced PR plant protein but also a new type of structurally unfamiliar proteins whose biological function in the diseased plant remains to be elucidated.
Keywords: disease-stimulated protein, pathogenesis-related protein, plant protein, tomato leaf protein
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham G. N., Podell D. N. Pyroglutamic acid. Non-metabolic formation, function in proteins and peptides, and characteristics of the enzymes effecting its removal. Mol Cell Biochem. 1981 Aug 11;38(Spec No)(Pt 1):181–190. doi: 10.1007/BF00235695. [DOI] [PubMed] [Google Scholar]
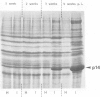
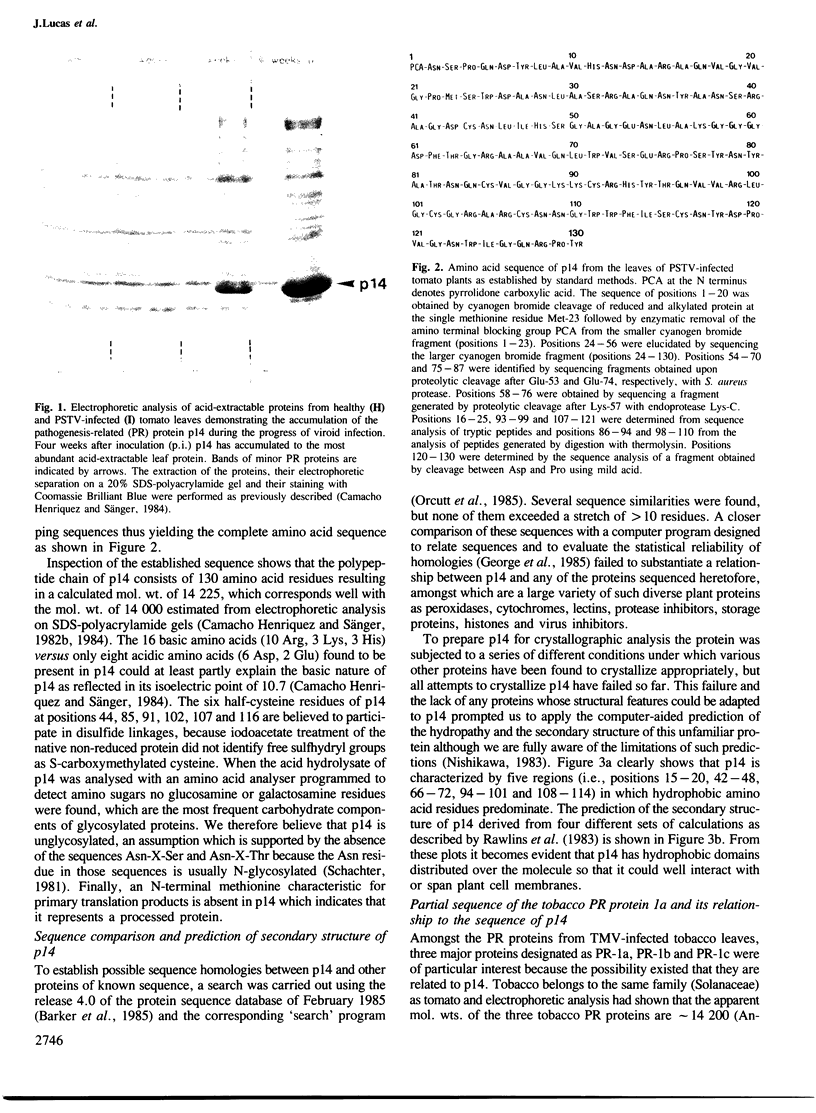
- Camacho Henriquez A., Sänger H. L. Analysis of acid-extractable tomato leaf proteins after infection with a viroid, two viruses and a fungus and partial purification of the "pathogenesis-related" protein p 14. Arch Virol. 1982;74(2-3):181–196. doi: 10.1007/BF01314711. [DOI] [PubMed] [Google Scholar]
- Camacho Henriquez A., Sänger H. L. Purification and partial characterization of the major "pathogenesis-related" tomato leaf protein P14 from potato spindle tuber viroid (PSTV)-infected tomato leaves. Arch Virol. 1984;81(3-4):263–284. doi: 10.1007/BF01309998. [DOI] [PubMed] [Google Scholar]
- Chang J. Y. N-terminal sequence analysis of polypeptide at the picomole level. Biochem J. 1981 Dec 1;199(3):557–564. doi: 10.1042/bj1990557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of beta-turns. Biophys J. 1979 Jun;26(3):367–383. doi: 10.1016/S0006-3495(79)85259-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Edman P., Begg G. A protein sequenator. Eur J Biochem. 1967 Mar;1(1):80–91. doi: 10.1007/978-3-662-25813-2_14. [DOI] [PubMed] [Google Scholar]
- Gianinazzi S., Martin C., Vallée J. C. Hypersensibilité aux virus, température et protéines soubles chez le Nicotiana Xanthi n.c. Apparition de nouvelles macromolécules lors de la répression de la synthèse virale. C R Acad Sci Hebd Seances Acad Sci D. 1970 May 11;270(19):2383–2386. [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Nagano K. Triplet information in helix prediction applied to the analysis of super-secondary structures. J Mol Biol. 1977 Jan 15;109(2):251–274. doi: 10.1016/s0022-2836(77)80033-8. [DOI] [PubMed] [Google Scholar]
- Nishikawa K. Assessment of secondary-structure prediction of proteins. Comparison of computerized Chou-Fasman method with others. Biochim Biophys Acta. 1983 Oct 28;748(2):285–299. doi: 10.1016/0167-4838(83)90306-0. [DOI] [PubMed] [Google Scholar]
- Nishikawa K., Ooi T. Prediction of the surface-interior diagram of globular proteins by an empirical method. Int J Pept Protein Res. 1980 Jul;16(1):19–32. doi: 10.1111/j.1399-3011.1980.tb02931.x. [DOI] [PubMed] [Google Scholar]
- Nover L., Scharf K. D. Synthesis, modification and structural binding of heat-shock proteins in tomato cell cultures. Eur J Biochem. 1984 Mar 1;139(2):303–313. doi: 10.1111/j.1432-1033.1984.tb08008.x. [DOI] [PubMed] [Google Scholar]
- Podell D. N., Abraham G. N. A technique for the removal of pyroglutamic acid from the amino terminus of proteins using calf liver pyroglutamate amino peptidase. Biochem Biophys Res Commun. 1978 Mar 15;81(1):176–185. doi: 10.1016/0006-291x(78)91646-7. [DOI] [PubMed] [Google Scholar]
- Rawlings N., Ashman K., Wittmann-Liebold B. Computerised version of the Chou and Fasman protein secondary structure predictive method. Int J Pept Protein Res. 1983 Nov;22(5):515–524. doi: 10.1111/j.1399-3011.1983.tb02124.x. [DOI] [PubMed] [Google Scholar]
- Robson B., Suzuki E. Conformational properties of amino acid residues in globular proteins. J Mol Biol. 1976 Nov 5;107(3):327–356. doi: 10.1016/s0022-2836(76)80008-3. [DOI] [PubMed] [Google Scholar]
- Scharf K. D., Nover L. Heat-shock-induced alterations of ribosomal protein phosphorylation in plant cell cultures. Cell. 1982 Sep;30(2):427–437. doi: 10.1016/0092-8674(82)90240-9. [DOI] [PubMed] [Google Scholar]
- van Loon L. C. Induction by 2-chloroethylophosphonic acid of viral-like lesions, associated proteins, and systemic resistance in tobacco. Virology. 1977 Jul 15;80(2):417–420. doi: 10.1016/s0042-6822(77)80016-0. [DOI] [PubMed] [Google Scholar]
- van Loon L. C., van Kammen A. Polyacrylamide disc electrophoresis of the soluble leaf proteins from Nicotiana tabacum var. "Samsun" and "Samsun NN". II. Changes in protein constitution after infection with tobacco mosaic virus. Virology. 1970 Feb;40(2):190–211. doi: 10.1016/0042-6822(70)90395-8. [DOI] [PubMed] [Google Scholar]