Abstract
In today’s society, every individual is subjected to stressful stimuli with different intensities and duration. This exposure can be a key trigger in several mental illnesses greatly affecting one’s quality of life. Yet not all subjects respond equally to the same stimulus and some are able to better adapt to them delaying the onset of its negative consequences. The neural specificities of this adaptation can be essential to understand the true dynamics of stress as well as to design new approaches to reduce its consequences. In the current work, we employed ex vivo high field diffusion magnetic resonance imaging (MRI) to uncover the differences in white matter properties in the entire brain between Fisher 344 (F344) and Sprague–Dawley (SD) rats, known to present different responses to stress, and to examine the effects of a 2-week repeated inescapable stress paradigm. We applied a tract-based spatial statistics (TBSS) analysis approach to a total of 25 animals. After exposure to stress, SD rats were found to have lower values of corticosterone when compared with F344 rats. Overall, stress was found to lead to an overall increase in fractional anisotropy (FA), on top of a reduction in mean and radial diffusivity (MD and RD) in several white matter bundles of the brain. No effect of strain on the white matter diffusion properties was observed. The strain-by-stress interaction revealed an effect on SD rats in MD, RD and axial diffusivity (AD), with lower diffusion metric levels on stressed animals. These effects were localized on the left side of the brain on the external capsule, corpus callosum, deep cerebral white matter, anterior commissure, endopiriform nucleus, dorsal hippocampus and amygdala fibers. The results possibly reveal an adaptation of the SD strain to the stressful stimuli through synaptic and structural plasticity processes, possibly reflecting learning processes.
Introduction
Stress is a major risk factor to the development of severe mental illnesses, including major depression, anxiety,1, 2 bipolar disorders and schizophrenia (for review, see Walker et al.3) and overall one of the more common factors in eliciting dynamic changes in brain states.4 Stress is known to trigger the activation of the hypothalamus–pituitary–adrenal axis, culminating in the production of glucocorticoids by the adrenals5, 6 that will in turn generate, depending on the individual and the stress stimulus characteristics, adaptive or maladaptive psychoneuroendocrine responses to the stressful stimulus.7 In patients with major depressive disorder, dysregulation of the hypothalamus–pituitary–adrenal axis elicits specific and long-lasting functional and structural changes on a network of regions encompassing the hippocampus,8, 9, 10 the medial prefrontal cortex11, 12 and amygdala.13, 14 Subjects with ultrahigh risk for psychosis are particularly sensitive to social stress, life events and daily hassles, which have the potential to trigger psychiatric symptoms; they have an increased basal cortisol level15, 16 and a smaller hippocampal volume.17, 18 Moreover, stressful life events in non-psychiatric subjects are associated with a gray matter volume decrease in a network encompassing the anterior cingulate cortex, the hippocampus and the parahippocampal gyrus that was observed within a 3-month period.19
Animal models have confirmed the drastic effects that stress can have on the brain, including changes in dendritic trees, synaptic plasticity inhibition in the hippocampus and the hippocampal-to-prefrontal pathway,12, 20, 21 decreased neurogenesis in the hippocampus22 and apoptosis, involving corticosteroids and glutamate receptors.23 Taken together, these findings support the effect of stress on structural changes within networks of spatially distributed gray matter regions.
In addition to these regional changes, increasing evidence suggests that stress may also disrupt the structural and functional connectivity within neural networks.24, 25, 26, 27, 28 Diffusion magnetic resonance imaging (dMRI) is an advanced technique for examining white matter (WM) anatomy providing insights on the pathway microstructure within neural networks.29 A commonly used feature in dMRI studies is fractional anisotropy (FA), which estimates the degree to which tissue organization limits diffusion of water molecules in brain WM.30 In animals, different recent dMRI studies investigated changes in diffusion signal associated to chronic stress exposure. Delgado y Palacios et al.31 were the first to report the effects of stress using in vivo dMRI in rats: using diffusion kurtosis imaging (DKI), hippocampus microstructure was revealed to be altered in chronically stressed rats, independently of the hedonic state. More recently, the same team evaluated the mean kurtosis in the PFC, caudate–putamen (CPu) and amygdala in anhedonic-like and resilient rats and found a decrease in the CPu in the anhedonic-like.32 In addition, using a similar chronic mild stress (CMS) model, Kumar et al.33 showed increases in axial diffusion (AD) and radial diffusion (RD) specifically in the CPu and the amygdala of stressed rats. Another study using in vivo dMRI showed an increase in the mean diffusion (MD) in the lateral ventricles of chronically stressed rats, although no other changes were found.27 Finally, using a mice and a social defeat stress paradigm, Anacker et al.28 have shown correlations between diffusion metrics and social avoidance correlating positively with FA in the hypothalamus and hippocampus.
Here, we used dMRI and the tract-based spatial statistics (TBSS) approach34 adapted to brain rat to investigate the WM microstructure on the entire brain. We selected two strains of rats, Fischer 344 (F344) and Sprague–Dawley (SD), known to have differential response to stress,35, 36 and compared their WM microstructure, assessed by four complementary dMRI measures (FA, MD, AD and RD), after exposure to repeated inescapable stress. Repeated exposure to the same stressor very often results in habituation, which leads to a decrease in the hypothalamus–pituitary–adrenal axis response.37 In contrast to SD rats, F344 rats show virtually no habituation or adaptation of the corticosterone stress response during repeated stress but an exaggerated acute stress-induced corticosterone secretion35, 36 and increased anxiety-related behaviors38 with increased amygdala volume.39 Such a design allowed studying the effect, but also the responsivity, to stress.
Materials and methods
Animals
Experiments were performed with male adult SD (n=14) and Fisher 344 (F344; n=14) rats (Charles River, Saint-Germain-sur-l'Arbresle, France) at 8 weeks’ age (average of 200 g for SD and 180 g for F344). Rats were housed in groups of two animals with ad libitum access to food and water and maintained in a temperature-controlled room, with a light/dark cycle of 12/12 h (lights on at 0600 hours). For each strain, rats were randomly assigned to stressed (N=14) and non-stressed (N=14) groups. Two animals of the SD strain of the control group were killed before the end of the 2 weeks. The protocols have been approved by the Comité d' Éthique en Expérimentation Animale du Commissariat à l' Énergie Atomique et aux Energies Alternatives—Direction des Sciences du Vivant Ile de France (CETEA/CEA/DSV IdF) under protocol ID 12-058. All procedures were conducted in conformity with National (JO 887–848) and European (86/609/EEC) rules for animal experimentation.
Stress protocol
The behavioral stress protocol has been previously described elsewhere.20 Briefly, rats were placed on an elevated and unsteady platform for 30 min. The platform was positioned 1 m above the ground and illuminated with a high-intensity light source (1500 Lux). While on the platform, animals showed urination, defecation, grooming and freezing. This inescapable stress exposure (called a session) was repeated daily during 15 days between 0900 and 1200 hours.40 We measured corticosterone levels for all animals in control condition and after the end of the stress session. Animals were randomly chosen to be stressed or not. This protocol was chosen as we previously demonstrated that it causes with a similar sample size a disruption of synaptic plasticity in the hippocampal-to-prefrontal cortex pathway20 and changes in regional brain volumes that are associated with an increase in plasma corticosterone levels.39
Corticosterone immunoassay
The plasma level of corticosterone was assessed as a biomarker of stress in all experiments. Blood samples were collected from the tail under quick anesthesia in basal conditions on day 0 (D0) and 10 min after the end of the stress session at different times (acute stress: D1; repeated stress: D15) for the group exposed to stress. Blood samples for the control group were taken at D15. Anesthesia was induced with 5% isoflurane mixed with oxygen, using a calibrated vaporizer maintained at 2% during the sampling. Samples were centrifuged at 1000 g for 15 min, and serum stored at −20 °C. Plasma corticosterone was assessed by immunoassay (Corticosterone Immunoassay, Enzo Life Sciences, Villeurbane, France).
Tissue preparation
Twenty-four hours after the last day of repeated inescapable stress or after daily handling in control animals, rats were anesthetized with sodium pentobarbital (100–150 mg kg−1, intraperitoneally (i.p.)), followed by intracardiac perfusion with physiological NaCl solution and 4% cold paraformaldehyde in 0.01 M phosphate-buffered saline (pH=7.4). After perfusion, the brain was harvested maintaining integrity and stored in 4% PFA in phosphate-buffered saline at 4 °C. Before MRI, the brains were washed into phosphate-buffered saline for 24 h to remove the fixation solution and then placed into a custom-built MRI-compatible tube. The tube was filled with Fluorinert, an MRI susceptibility-matching fluid (Sigma-Aldrich, St Louis, MO, USA).
Acquisition of diffusion MRI data
Diffusion ex vivo data with high spatial and angular resolution were acquired to quantify the subtle changes in the WM microstructure within the entire rat brain. The ex vivo MRI acquisitions were performed on a 7 T preclinical scanner (PharmaScan, Bruker, Ettlingen, Germany) using a home‐made quadrature birdcage coil (inside diameter=28 mM). Diffusion images were acquired using a Spin-Echo Multi Shot Echo Planar Imaging (repetition time (TR)=26 s, echo time (TE)=29 ms, 90° excitation pulse followed by a 180° refocusing pulse, 4 segments, 4 averages, total time=24 h16 m 03 s). One hundred four interleaved slices with 0.25 mM thickness were acquired, with a matrix size of 106 × 106, a field-of-view of 25.44 × 25.44 mM and an in-plane resolution of 0.24 × 0.24 mM. Following 10 acquisitions with no diffusion sensitization (b=0 s mm−2), diffusion-weighted images were acquired along 200 noncollinear directions (b=4000 s mm−2). The physicist performing the MRI acquisition was blind to the group allocation (stress versus no stress).
Preprocessing of diffusion MRI
The dMRI images were reconstructed using an in-house script and visually inspected for brain lesions and artefacts, after which two subjects (one F344 and one SD from the control group) were excluded because of the presence of artefacts. All the data were pre-processed using the FMRIB Software Library41 (FSL, http://fsl.fmrib.ox.ac.uk/fsl/) v5.0.6 using the following steps: bias field correction using FAST,42 correction of the field inhomogeneity, estimated from b0 images, on all volumes; eddy current distortions and movement correction with fsl ‘eddy_correct’ command-line tool (the first volume without diffusion sensitization was chosen as the reference volume for the affine registration); segmentation of the brain signal using BET:43 BET was applied to the mean of the images without diffusion sensitization, with the resulting mask being applied to all volumes. The gradient vector directions were rotated for each subject according to the eddy correct output.44
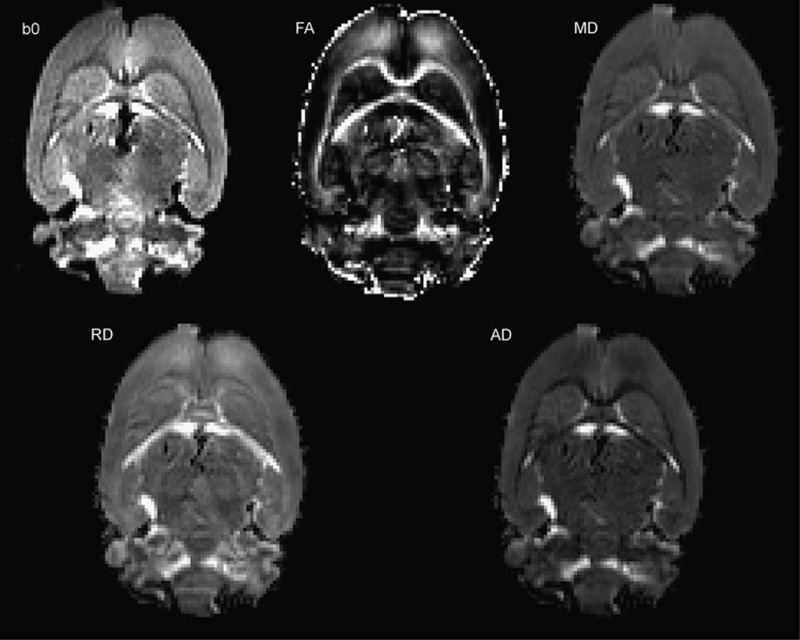
Tensor fitting and scalar maps were calculated using FSL FDT ‘dtifit’ command line45, 46 using the corrected vector directions. These maps were used to obtain the FA, AD, MD and RD maps (see Figure 1). These indexes derived from dMRI provide complementary information of WM microstructure. Although discussed, FA is classically considered to reflect the degree of myelination and axonal density.47, 48, 49, 50 AD measures diffusivity parallel to axonal fibers and AD decreases are thought to reflect pathology of the axon itself, such as from trauma or ischemic changes.47 RD measures diffusivity perpendicular to axonal fibers and appears to be more strongly correlated with myelin abnormalities, like demyelination, as observed in multiple sclerosis.51
Figure 1.
Representative image of the diffusion MRI data (b0 map) and diffusion metrics (FA, MD, RD, AD) in a rat brain. AD, axial diffusivity; FA, fractional anisotropy; MD, mean diffusivity; MRI, magnetic resonance imaging; RD, radial diffusivity.
TBSS
Whole-brain voxel-based statistical analysis was performed using the TBSS approach34 distributed as part of FSL adapted to the rat brain. The FA maps of all subjects obtained in the tensor-fitting step were aligned into a common space using a study-dedicated template and the nonlinear registration tool FNIRT.52 The template was defined as the most representative animal, calculated during the TBSS pipeline as the one that minimizes transformations. Next, all the FA images were averaged and thinned in order to create the mean FA skeleton. A threshold of 0.3 was applied to this skeleton in order to restrict the analysis to the WM tracts, and thus defining the final voxels for analysis. The AD, MD and RD maps of all animals were then warped into this skeleton map using the nonlinear transformations previously calculated for the FA maps.
Statistical analysis for the skeletonized maps of FA, AD, MD and RD was performed using the ‘randomise’ fsl command-line tool, yielding a non-parametric test based on randomization methods. A total of 10 000 random permutations were used with threshold-free cluster enhancement,53 and multiple comparison corrections for family-wise error results were considered significant at P<0.05. Six different contrasts were calculated, testing for the effect of stress (‘Stress>No stress’ ‘Stress<No stress’), strain (‘SD>F344’ ‘SD<F344’) and stress-by-strain interaction.
Labeling of significant clusters in the FA skeleton was based on the standard Paxinos and Watson atlas54 and cross-validated by visual inspection (Figure 2). Descriptive statistics were then calculated separately for each WM bundles.
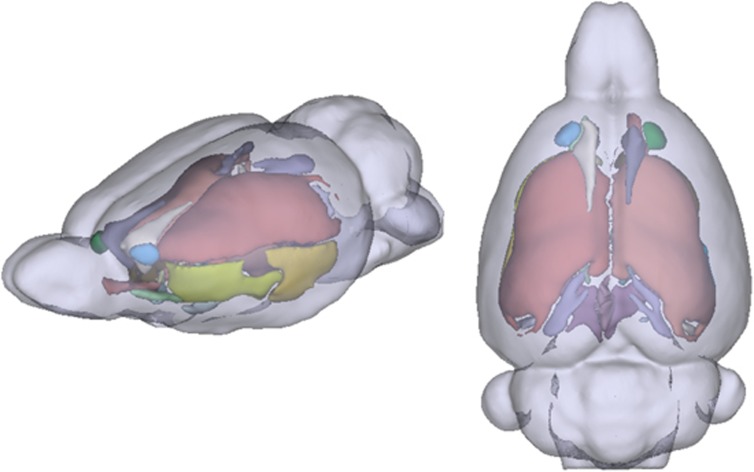
Figure 2.
Three-dimensional reconstructions of the white matter skeleton used for TBSS analyses (lateral and top views). White matter tracts were color-coded based on the Paxinos and Watson atlas.54 TBSS, tract-based spatial statistics.
Results
Corticosterone plasma level
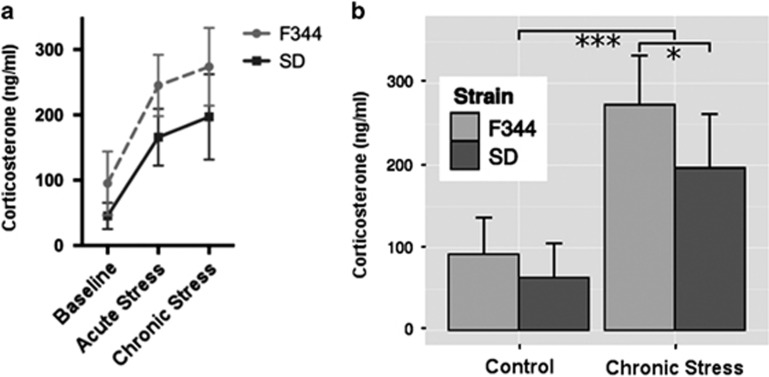
As expected, we found a significant main effect of stress on corticosterone plasma levels (F(25, 1)=54.87, P=2.7 × 10−7) after chronic stress exposure (increase) in both strains ((SD rats: n=6, 197±65.34 ng ml−1 and F344 rats: n=8, 273.75±59.50 ng ml−1) when compared with non-stressed rats (SD rats: n=6, 63.66±41.86 ng ml−1 and F344 rats: n=6, 92.50±44.01 ng ml−1). There was also a significant difference between the two strains after 15 days of stress exposure with a higher plasma corticosterone level in F344 rats compared with SD rats (T(11)=2.18, P=0.02; Figure 3). There was no variance difference in corticosterone plasma levels between strains (SD versus F344) in control and stressed animals nor difference between conditions (stress versus no stress) in SD and F344 (Fligner–Killeen non-parametric test of homogeneity of variances, all P-values>0.2).
Figure 3.
Plasma levels of corticosterone (mean±s.d.) obtained in control and after stress in F344 and SD rats. (a) Longitudinal data at baseline (D0), after acute stress (D1) and after chronic stress (D15). (b) Comparison between strains before stress (D0) and after chronic stress (D15). Significance levels: *P<0.05, ***P<0.001. F344, Fischer 344; SD, Sprague–Dawley.
White matter microstructure
The final number of animals involved in the analysis was as follows: 11 SD rats (four control and seven stressed) and 13 F344 (six control and seven stressed).
The WM skeleton in which statistical tests were conducted was constituted by a total of 6254 voxels. All the statistical tests were done with 23 degrees of freedom.
TBSS analyses revealed no significant main effect of strain (‘F344’ versus ‘SD’) in FA, AD, MD or RD maps (Pcorrected>0.05).
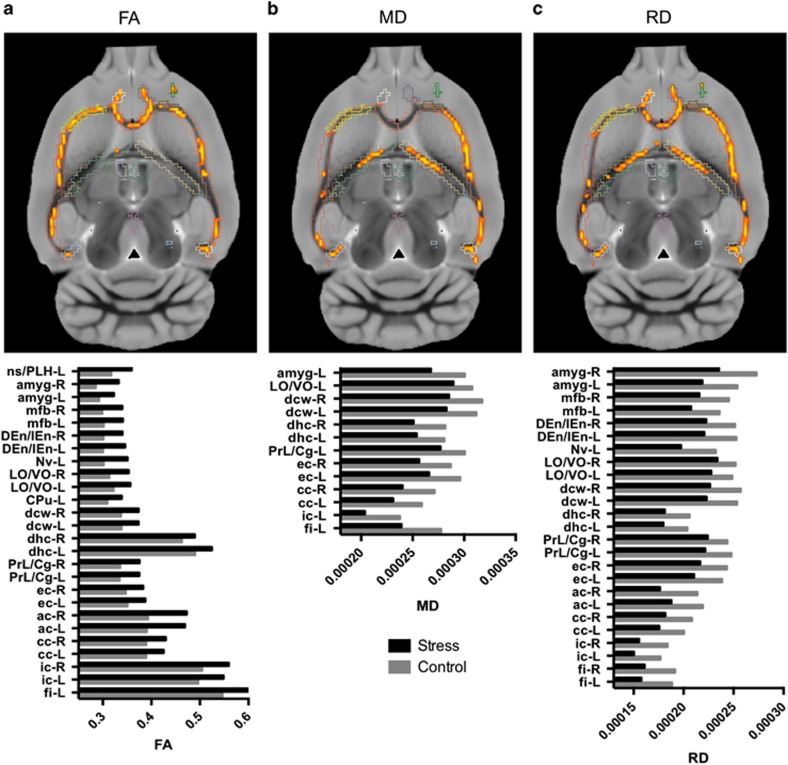
In contrast, we found a main effect of stress (‘control’ versus ‘stress’) in several WM bundles, with increased FA (peak T-value=5.720, peak-corrected P-value=0.012, cluster size of 3126 voxels) and decreased RD (peak T-value=4.621, peak-corrected P-value=0.011, cluster size of 3480 voxels) and MD (peak T-value=4.598, peak-corrected P-value=0.037, cluster size of 1515 voxels) in stressed animals compared with controls (Figure 4). These stress-related differences were distributed over the entire brain and involved WM bundles in posterior and anterior areas, on both hemispheres (Table 1).
Figure 4.
White matter tracts with microstructural differences between control rats and stressed rats. Top panel represents an axial brain slice with voxels with significant main effect of stress (red–yellow scale) superimposed on the white matter skeleton used for TBSS analyses. Bottom panel provides histogram of white matter tracts microstructure (FA, MD or RD) in control (black) and stressed (light gray) rats. Only tracts with significant main effect of stress on FA (a), MD (b) or RD (c) are represented. For illustration purpose, the tracts were slightly dilated. FA, fractional anisotropy; MD, mean diffusivity; RD, radial diffusivity; TBSS, tract-based spatial statistics.
Table 1. Abbreviations of the white matter tracts investigated in the study.
| Abbreviation | White matter tract |
|---|---|
| ac | Anterior commissure |
| amygfib | Amygdala fibers |
| cc | Corpus callosum |
| dcw | Deep cerebral white matter |
| denien | Dorsal and intermediate endopiriform nucleus fibers |
| dhc | Dorsal hippocampus commissure |
| ec | External capsule |
| fi | Fimbria of the hippocampus |
| ic | Internal capsule |
| inwh | Intermediate white layer |
| lovo | Lateral orbital cortex/ventral orbital cortex |
| mfb | Medial forebrain bundle |
| nsplh | Nigrostriatal bundle/peduncular part of the lateral hypothalamus |
| nv | Navicular nu basal forebrain |
| opt | Optic tract |
| optot | Olivary pretectal nu/nu of the optic tract |
| prlcg | Prelimbic cortex/cingulate cortex |
| strfibers | Striatum fibers |
| strmlfr | Superior thalamic radiation/medial lemniscus/fasciculus retroflexus |
White matter tracts were labeled based on the Paxinos and Watson atlas.54
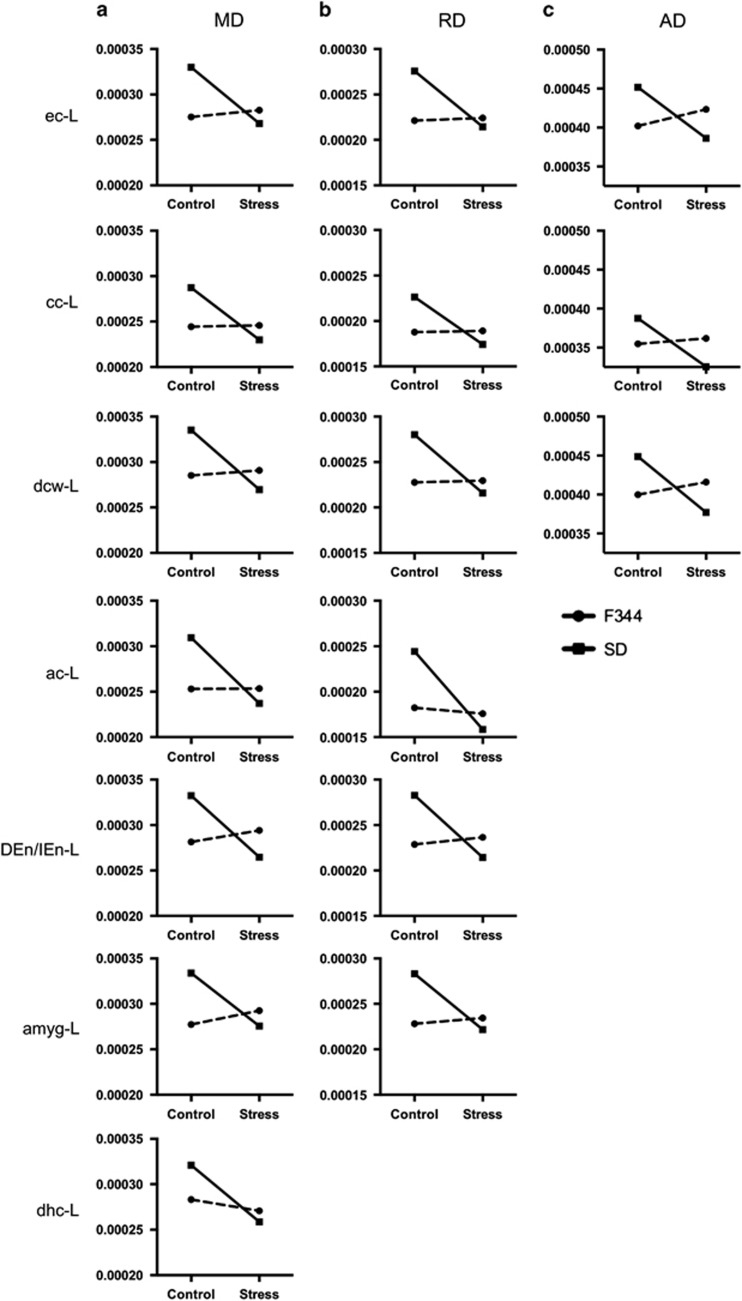
Finally, significant strain-by-stress interactions were found in MD, RD and AD maps (Figure 5), with a stress-related decrease in SD rats and an absence of change in F344 rats. Significant interactions involved WM bundles in the left hemisphere and included the following: the corpus callosum (cc), external capsule (ec) and deep cerebral WM (dcw) for MD, RD and AD measures; the anterior commissure (ac), dorsal and intermediate endopiriform nucleus (DEn/IEn) and amygdala for MD and RD measures; and dorsal hippocampus commissure (dhc) on MD. All statistics related to these results can be found on Table 2.
Figure 5.
White matter tract changes in microstructure associated with maladaptative response to stress. Interaction graphs provide the mean values of white matter tract microstructure - FA (a), MD (b) or RD (c) - in control (black) and stressed (light gray) animals, in F344 (circles with solid lines) and SD (squares with dotted lines) rats. ac, anterior commissure; amygFib, amygdala fiber; cc, corpus callosum; dcw, deep cerebral white matter; denien, dorsal and intermediate endopiriform nucleus fiber; dhc, dorsal hippocampus commisure; ec, external capsule; FA, fractional anisotropy; F344, Fischer 344; MD, mean diffusivity; RD, radial diffusivity; SD, Sprague–Dawley.
Table 2. Statistics of the white matter tracts associated with maladaptive response to stress.
| White matter tract | MD | RD | AD |
|---|---|---|---|
| External capsule (ec) | |||
| Peak T-value | 3.62 | 3.55 | 3.35 |
| Peak P-value | 0.041 | 0.048 | 0.043 |
| # Voxels | 219 | 26 | 205 |
| Corpus callosum (cc) | |||
| Peak T-value | 4.17 | 3.66 | 3.65 |
| Peak P-value | 0.041 | 0.035 | 0.049 |
| # Voxels | 104 | 114 | 15 |
| Deep cerebral white matter (dcw) | |||
| Peak T-value | 3.89 | 3.264 | 4.39 |
| Peak P-value | 0.041 | 0.043 | 0.048 |
| # Voxels | 199 | 154 | 117 |
| Anterior commissure (ac) | |||
| Peak T-value | 4.05 | 4.41 | — |
| Peak P-value | 0.043 | 0.044 | — |
| # Voxels | 23 | 23 | — |
| Dorsal and intermediate endopiriform nucleus fibers (denien) | |||
| Peak T-value | 2.97 | 2.69 | — |
| Peak P-value | 0.046 | 0.048 | — |
| # Voxels | 8 | 7 | — |
| Amygdala fibers (amyg) | |||
| Peak T-value | 2.56 | 2.50 | — |
| Peak P-value | 0.042 | 0.045 | — |
| # Voxels | 1 | 2 | — |
| Dorsal hippocampus commissure (dhc) | |||
| Peak T-value | 2.72 | — | — |
| Peak P-value | 0.049 | — | — |
| # Voxels | 2 | — | — |
Abbreviations: AD, axial diffusivity; MD, mean diffusivity; RD, radial diffusivity.
The peak T-value, the corresponding peak P-value and the number of voxels in the cluster are provided for white matter tracts with significant strain-by-stress interactions on MD, RD and AD.
Discussion
This diffusion MRI study reveals that 15 days of repeated exposure to the same inescapable stressor in rats leads to microstructural WM changes—increased FA and decreased MD and RD—of several WM bundles distributed in the entire brain. Furthermore, differential stress effects were observed in SD and F344 rat strains, which are known to have a different behavioral and physiological habituation to repeated stress.35, 36
Several WM bundles reported in this study (including amygdala fibers, dcw, DEn/IEn fibers, dorsal hippocampus, fimbria of the hippocampus, external capsule and corpus callosum) connect brain areas associated with emotion formation and processing, attention, and learning and memory.14, 55, 56, 57 Of note, some of these bundles interconnect the hippocampus (dhc and fi) or connect the hippocampus to the amygdala, prefrontal cortex and anterior thalamic nuclei (ec, cc, StrlCg and dcw), regions consistently reported to be affected by stress.7, 9, 10, 12, 14 Changes found in ac, proximal to the olfactory bulb and in Denien58 may indicate a stress-related alteration in sensory circuits, possibly because of a readjustment of the perception of their surroundings.
To our knowledge, this is the first study to show the effects of repeated acute stress exposure in two strains with different stress sensitivity and habituation. Indeed, we show decreased MD, RD and AD in several brain bundles in SD rats, whereas no such differences were observed in F344 rats. This is particularly relevant, as SD rats were able to adjust their stress response to the repeated exposure to acute stress (resilience), therefore, showing an adaptive response that may be triggered by the acquisition of coping mechanisms that are paralleled by the decreases in MD, RD and AD, despite the overall increase in FA. In contrast, F344 (nonresilient) rats, which display a maladaptive response, do not reveal significant changes in these parameters. These findings suggest that differential response to repeated acute stressors may be revealed by or are associated with the ability to trigger structural plastic events in WM.
A few preclinical dMRI studies previously reported measurable effects of stress on several brain regions, and in all cases addressing the impact of chronic stress. Indeed, a significant decrease in the mean and radial kurtosis in the hippocampus was detected following CMS in rats.31 More recently, the same team reported significant stress-related increases in AD and RD in the CPu and in the amygdala, respectively, along with a mean kurtosis decrease in the CPu in anhedonic-like animals compared with resilient animals.32 Such effects were interpreted as the result of axonal degeneration and demyelination within WM bundles with disrupted microstructural spatial coherence. A FA decrease interpreted as a potential loss of myelin sheath was also found in the corpus callosum, bilateral frontal cortex and bilateral hypothalamus in rats after a similar CMS protocol.33
Such contrasting results are likely to reflect the temporal dynamics of the stress response (and its successful, or not, adaptation). Yet, we cannot exclude that other methodological differences may also explain the difference in FA change direction, including the stress paradigm (repeated acute stress versus CMS), image acquisition (in vivo dMRI versus ex vivo data with higher spatial resolution and higher signal-to-noise ratio) or image analysis (measure in a priori preselected regions of interest, mostly within gray matter structures versus voxel-wise analysis on the whole WM tracts).
Increased WM FA has been repeatedly associated to learning59, 60, 61 via neuronal plasticity processes (for example, synaptogenesis and dendritic branching) and glial remodeling (for example, modification of astrocyte processes).62 An increased FA was found in the corpus callosum after a spatial learning task and such increase was supported by significant increases in immune reactivity for a myelin marker, suggesting an increase in the cellular organization and packing of axons or myelin.59, 63 More recently, TBSS analysis also revealed higher FA in skilled learning rats in comparison with control64 that could be explained by increases in myelination. On the other hand, a reduction in MD was found in both rat hippocampi before and after learning a hippocampal-dependent spatial navigation task.61 Data from both human and animal studies indicate the potential for rapid changes in dMRI indices,61, 65 suggesting changes in structural plasticity in specific brain regions. The patterns of FA increase/RD decrease are likely related to a tissue density increase due to reshaping of neuronal or glial processes, and/or enhancement of tissue organization, including strengthening of axonal or dendritic backbones and surrounding tissue.66 Myelination, known to be modified by experience and maturation,67, 68 may also partly explain the RD decrease observed in the stressed rats as RD increases have previously been associated with demyelination processes.69, 70 Of note, an activity-dependent myelination has been recently proposed in a human study of motor training, where the FA change in WM was accompanied by adjacent gray matter density alterations.71
This study presents some limitations that should be considered. Here the susceptible/resilient differences are achieved by using different strains. We cannot discard the possibility that the mechanisms that lead to different responses to stress within a single strain are different, or if the results found are specific to the SD strain, making its generalization harder. In addition, corticosterone was the only measure used to access the stress response, and although it is known to be one of the more representative markers of stress, the use of complementary behavioral assessment could be beneficial. Other limitations include the lack of direct histological correlations between DTI indices and morphological markers due to the exclusive ex vivo approach. In vivo longitudinal measurements would have allowed comparisons before and after stress, however, at the expense of signal-to-noise ratio and diffusion MRI spatial and angular resolution.
To conclude, we identified microstructural changes in the key WM tracts like the corpus callosum and the amygdala fibers linked to the frontolimbic circuitry with a functional relevance for cognitive performance and emotional response. Our data demonstrate that SD rats able to adjust to repeated exposure to an acute stress leads to significant changes in dMRI indices. These changes are not well understood, but we demonstrate that dMRI may offer a novel measure of microstructural remodeling occurring in response to stress to further explore the neural basis of adaptive and maladaptive response to stress in rodents and provide quantitative biomarkers to evaluate novel treatments to the protection of stress effects.
Acknowledgments
We thank Neurospin (high field MRI center CEA Saclay) for providing its support for MRI acquisition. JB was supported by grants from Fondation pour la Recherche Médicale (FRM) and Groupe Pasteur Mutualité (GPM). This work was supported by a grant from ANR (SIGMA). This work was performed on a platform of France Life Imaging (FLI) network partly funded by the grant ANR-11-INBS-0006. This work and RM were supported by a fellowship of the project FCT-ANR/NEU-OSD/0258/2012 founded by FCT/MEC (www.fct.pt) and by Fundo Europeu de Desenvolvimento Regional (FEDER). AC was supported by a grant from the Fondation NRJ.
Footnotes
The authors declare no conflict of interest.
References
- Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry 1999; 156: 837–841. [DOI] [PubMed] [Google Scholar]
- Grillon C, Lissek S, Rabin S, McDowell D, Dvir S, Pine DS. Increased anxiety during anticipation of unpredictable but not predictable aversive stimuli as a psychophysiologic marker of panic disorder. Am J Psychiatry 2008; 165: 898–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker E, Mittal V, Tessner K. Stress and the hypothalamic pituitary adrenal axis in the developmental course of schizophrenia. Annu Rev Clin Psychol 2008; 4: 189–216. [DOI] [PubMed] [Google Scholar]
- Sousa N. The dynamics of the stress neuromatrix. Mol Psychiatry 2016; 21: 302–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitnall MH. Regulation of the hypothalamic corticotropin-releasing hormone neurosecretory system. Prog Neurobiol 1993; 40: 573–629. [DOI] [PubMed] [Google Scholar]
- Jacobson L. Hypothalamic-pituitary-adrenocortical axis regulation. Endocrinol Metab Clin N Am 2005; 34: 271–292, vii. [DOI] [PubMed] [Google Scholar]
- McEwen BS. The neurobiology of stress: from serendipity to clinical relevance. Brain Res 2000; 886: 172–189. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Mittler BL, Mintun MA. The hippocampus and depression. Eur Psychiatry 2002; 17: 300–305. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Mood disorders and allostatic load. Biol Psychiatry 2003; 54: 200–207. [DOI] [PubMed] [Google Scholar]
- Sousa N, Lukoyanov NV, Madeira MD, Almeida OF, Paula-Barbosa MM. Erratum to ‘Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement’. Neuroscience 2000; 101: 483. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C et al. Deep brain stimulation for treatment-resistant depression. Neuron 2005; 45: 651–660. [DOI] [PubMed] [Google Scholar]
- Cerqueira JJ, Mailliet F, Almeida OFX, Jay TM, Sousa N. The prefrontal cortex as a key target of the maladaptive response to stress. J Neurosci 2007; 27: 2781–2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Dedovic K, Pruessner M, Lord C, Buss C, Collins L et al. Stress regulation in the central nervous system: evidence from structural and functional neuroimaging studies in human populations—2008 Curt Richter Award Winner. Psychoneuroendocrinology 2010; 35: 179–191. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci 2009; 10: 423–433. [DOI] [PubMed] [Google Scholar]
- Tessner KD, Mittal V, Walker EF. Longitudinal study of stressful life events and daily stressors among adolescents at high risk for psychotic disorders. Schizophr Bull 2011; 37: 432–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaumette B, Kebir O, Mam-Lam-Fook C, Morvan Y, Bourgin J, Godsil BP et al. Salivary cortisol in early psychosis: new findings and meta-analysis. Psychoneuroendocrinology 2016; 63: 262–270. [DOI] [PubMed] [Google Scholar]
- Aiello G, Horowitz M, Hepgul N, Pariante CM, Mondelli V. Stress abnormalities in individuals at risk for psychosis: a review of studies in subjects with familial risk or with ‘at risk’ mental state. Psychoneuroendocrinology 2012; 37: 1600–1613. [DOI] [PubMed] [Google Scholar]
- Collip D, Habets P, Marcelis M, Gronenschild E, Lataster T, Lardinois M et al. Hippocampal volume as marker of daily life stress sensitivity in psychosis. Psychol Med 2013; 43: 1377–1387. [DOI] [PubMed] [Google Scholar]
- Papagni SA, Benetti S, Arulanantham S, McCrory E, McGuire P, Mechelli A. Effects of stressful life events on human brain structure: a longitudinal voxel-based morphometry study. Stress 2010; 14: 227–232. [DOI] [PubMed] [Google Scholar]
- Rocher C, Spedding M, Munoz C, Jay TM. Acute stress-induced changes in hippocampal/prefrontal circuits in rats: effects of antidepressants. Cereb Cortex 2004; 14: 224–229. [DOI] [PubMed] [Google Scholar]
- Shakesby AC, Anwyl R, Rowan MJ. Overcoming the Effects of stress on synaptic plasticity in the intact hippocampus: rapid actions of serotonergic and antidepressant agents. J Neurosci 2002; 22: 3638–3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Tanapat P, McEwen BS, Flügge G, Fuchs E. Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proc Natl Acad Sci USA 1998; 95: 3168–3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci 1999; 22: 105–122. [DOI] [PubMed] [Google Scholar]
- Sousa N, Almeida OF. Disconnection and reconnection: the morphological basis of (mal)adaptation to stress. Trends Neurosci 2012; 35: 742–751. [DOI] [PubMed] [Google Scholar]
- Soares JM, Sampaio A, Ferreira LM, Santos NC, Marques F, Palha JA et al. Stress-induced changes in human decision-making are reversible. Transl Psychiatry 2012; 2: e131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares JM, Sampaio A, Ferreira LM, Santos NC, Marques P, Marques F et al. Stress impact on resting state brain networks. PLoS One 2013; 8: e66500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henckens MJ, van der Marel K, van der Toorn A, Pillai AG, Fernandez G, Dijkhuizen RM et al. Stress-induced alterations in large-scale functional networks of the rodent brain. NeuroImage 2015; 105: 312–322. [DOI] [PubMed] [Google Scholar]
- Anacker C, Scholz J, O’Donnell KJ, Allemang-Grand R, Diorio J, Bagot RC et al. Neuroanatomic differences associated with stress susceptibility and resilience. Biol Psychiatry 2015; 79: 840–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares JM, Marques P, Alves V, Sousa N. A hitchhiker’s guide to diffusion tensor imaging. Front Neurosci 2013; 7: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B 1996; 111: 209–219. [DOI] [PubMed] [Google Scholar]
- Delgado y Palacios R, Campo A, Henningsen K, Verhoye M, Poot D, Dijkstra J et al. Magnetic resonance imaging and spectroscopy reveal differential hippocampal changes in anhedonic and resilient subtypes of the chronic mild stress rat model. Biol Psychiatry 2011; 70: 449–457. [DOI] [PubMed] [Google Scholar]
- Delgado y Palacios R, Verhoye M, Henningsen K, Wiborg O, Van der Linden A. Diffusion kurtosis imaging and high-resolution MRI demonstrate structural aberrations of caudate putamen and amygdala after chronic mild stress. PLoS One 2014; 9: e95077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemanth Kumar BS, Mishra SK, Trivedi R, Singh S, Rana P, Khushu S. Demyelinating evidences in CMS rat model of depression: A DTI study at 7T. Neuroscience 2014; 275C: 12–21. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 2006; 31: 1487–1505. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, McEwen BS, Spencer RL. Adaptation to prolonged or repeated stress—comparison between rat strains showing intrinsic differences in reactivity to acute stress. Neuroendocrinology 1997; 65: 360–368. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, Miller AH, McEwen BS, Spencer RL. Differential activation of adrenal steroid receptors in neural and immune tissues of Sprague Dawley, Fischer 344, and Lewis rats. J Neuroimmunol 1995; 56: 77–90. [DOI] [PubMed] [Google Scholar]
- Natelson BH, Ottenweller JE, Cook JA, Pitman D, McCarty R, Tapp WN. Effect of stressor intensity on habituation of the adrenocortical stress response. Physiol Behav 1988; 43: 41–46. [DOI] [PubMed] [Google Scholar]
- Uchida S, Nishida A, Hara K, Kamemoto T, Suetsugi M, Fujimoto M et al. Characterization of the vulnerability to repeated stress in Fischer 344 rats: possible involvement of microRNA-mediated down-regulation of the glucocorticoid receptor. Eur J Neurosci 2008; 27: 2250–2261. [DOI] [PubMed] [Google Scholar]
- Bourgin J, Cachia A, Boumezbeur F, Djemaï B, Bottlaender M, Duchesnay E et al. Hyper-responsivity to stress in rats is associated with a large increase in amygdala volume. A 7T MRI study. Eur Neuropsychopharmacol 2015; 25: 828–835. [DOI] [PubMed] [Google Scholar]
- Storey JD, Robertson DAF, Beattie JE, Reid IC, Mitchell SN, Balfour DJK. Behavioural and neurochemical responses evoked by repeated exposure to an elevated open platform. Behav Brain Res 2006; 166: 220–229. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004; 23: S208–S219. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging 2001; 20: 45–57. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp 2002; 17: 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leemans A, Jones DK. The B‐matrix must be rotated when correcting for subject motion in DTI data. Magn Reson Med 2009; 61: 1336–1349. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? NeuroImage 2007; 34: 144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TE, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S et al. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med 2003; 50: 1077–1088. [DOI] [PubMed] [Google Scholar]
- Song S-K, Sun S-W, Ju W-K, Lin S-J, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. NeuroImage 2003; 20: 1714–1722. [DOI] [PubMed] [Google Scholar]
- Song S-K, Sun S-W, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. NeuroImage 2002; 17: 1429–1436. [DOI] [PubMed] [Google Scholar]
- Arfanakis K, Haughton VM, Carew JD, Rogers BP, Dempsey RJ, Meyerand ME. Diffusion tensor MR imaging in diffuse axonal injury. Am J Neuroradiol 2002; 23: 794–802. [PMC free article] [PubMed] [Google Scholar]
- Harsan LA, Poulet P, Guignard B, Steibel J, Parizel N, Loureiro de Sousa P et al. Brain dysmyelination and recovery assessment by noninvasive in vivo diffusion tensor magnetic resonance imaging. J Neurosci Res 2006; 83: 392–402. [DOI] [PubMed] [Google Scholar]
- Song S-K, Yoshino J, Le TQ, Lin S-J, Sun S-W, Cross AH et al. Demyelination increases radial diffusivity in corpus callosum of mouse brain. NeuroImage 2005; 26: 132–140. [DOI] [PubMed] [Google Scholar]
- Andersson JLR, Jenkinson M, Smith S Non-linear registration aka spatial normalisation. FMRIB Analysis Group of the University of Oxford: Oxford, UK, 2007. FMRIB technical report TR07JA2..
- Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 2009; 44: 83–98. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press: New York, NY, USA, 1998. [Google Scholar]
- Goldman-Rakic PS. Architecture of the prefrontal cortex and the central executive. Ann N Y Acad Sci 1995; 769: 71–83. [DOI] [PubMed] [Google Scholar]
- Cerqueira JJ, Pêgo J, Taipa R, Bessa JM, Almeida OFX, Sousa N. Morphological correlates of corticosteroid-induced changes in prefrontal cortex-dependent behaviors. J Neurosci 2005; 25: 7792–7800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay TM, Rocher C, Hotte M, Naudon L, Gurden H, Spedding M. Plasticity at hippocampal to prefrontal cortex synapses is impaired by loss of dopamine and stress: importance for psychiatric diseases. Neurotox Res 2004; 6: 233–244. [DOI] [PubMed] [Google Scholar]
- Brunjes PC, Illig KR, Meyer EA. A field guide to the anterior olfactory nucleus (cortex). Brain Res Brain Res Rev 2005; 50: 305–335. [DOI] [PubMed] [Google Scholar]
- Blumenfeld-Katzir T, Pasternak O, Dagan M, Assaf Y. Diffusion MRI of structural brain plasticity induced by a learning and memory task. PLoS One 2011; 6: e20678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding AY, Li Q, Zhou IY, Ma SJ, Tong G, McAlonan GM et al. MR diffusion tensor imaging detects rapid microstructural changes in amygdala and hippocampus following fear conditioning in mice. PLoS One 2013; 8: e51704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagi Y, Tavor I, Hofstetter S, Tzur-Moryosef S, Blumenfeld-Katzir T, Assaf Y. Learning in the fast lane: new insights into neuroplasticity. Neuron 2012; 73: 1195–1203. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Fields RD, Johansen-Berg H. Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nat Neurosci 2012; 15: 528–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerch JP, Yiu AP, Martinez-Canabal A, Pekar T, Bohbot VD, Frankland PW et al. Maze training in mice induces MRI-detectable brain shape changes specific to the type of learning. NeuroImage 2011; 54: 2086–2095. [DOI] [PubMed] [Google Scholar]
- Sampaio-Baptista C, Khrapitchev AA, Foxley S, Schlagheck T, Scholz J, Jbabdi S et al. Motor skill learning induces changes in white matter microstructure and myelination. J Neurosci 2013; 33: 19499–19503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofstetter S, Tavor I, Tzur Moryosef S, Assaf Y. Short-term learning induces white matter plasticity in the fornix. J Neurosci 2013; 33: 12844–12850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaf Y, Pasternak O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: a review. J Mol Neurosci 2008; 34: 51–61. [DOI] [PubMed] [Google Scholar]
- Markham JA, Greenough WT. Experience-driven brain plasticity: beyond the synapse. Neuron Glia Biol 2004; 1: 351–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez I, Hassinger L, Paskevich PA, Shine HD, Nixon RA. Oligodendroglia regulate the regional expansion of axon caliber and local accumulation of neurofilaments during development independently of myelin formation. J Neurosci 1996; 16: 5095–5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klawiter EC, Schmidt RE, Trinkaus K, Liang HF, Budde MD, Naismith RT et al. Radial diffusivity predicts demyelination in ex vivo multiple sclerosis spinal cords. NeuroImage 2011; 55: 1454–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun SW, Liang HF, Le TQ, Armstrong RC, Cross AH, Song SK. Differential sensitivity of in vivo and ex vivo diffusion tensor imaging to evolving optic nerve injury in mice with retinal ischemia. NeuroImage 2006; 32: 1195–1204. [DOI] [PubMed] [Google Scholar]
- Scholz J, Klein MC, Behrens TEJ, Johansen-Berg H. Training induces changes in white-matter architecture. Nat Neurosci 2009; 12: 1370–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]