Abstract
Multiple lines of evidence link the incidence of diabetes to the development of Alzheimer’s Disease (AD). Patients with diabetes have a 50 to 75% increased risk of developing AD. In parallel, AD patients have a higher than normal tendency to develop type 2 diabetes or impaired fasting glucose. Tau is the major component of neurofibrillary tangles (NFT), one of the hallmarks of AD pathology. The current study examined the effect of hyperglycemia on tau modification. Glucose treatment of rat embryonic cortical neurons results in concentration-dependent apoptosis and caspase-3 activation. These changes are well correlated with glucose time- and concentration-dependent tau cleavage. Aβ treatment induces tau cleavage and when added together with glucose there is an additive effect on caspase activation, apoptosis and tau cleavage. Tau cleavage is partially blocked by the caspase inhibitor, ZVAD. Cleaved tau displays a punctate staining along the neurites and colocalizes with cleaved caspase-3 in the cytoplasm. Both type 1 and type 2 diabetic mice display increased tau phosphorylation in the brain. In agreement with the effects of glucose on tau modifications in vitro, there is increased tau cleavage in the brains of ob/ob mice; however, tau cleavage is not observed in type 1 diabetic mouse brains. Our study demonstrates that hyperglycemia is one of major factors that induce tau modification in both in vitro and in vivo models of diabetes. We speculate that tau cleavage in diabetic conditions (especially in type 2 diabetes) may be a key link for the increased incidence of AD in diabetic patients.
Keywords: Alzheimer’s disease, diabetes, hyperglycemia, tau
INTRODUCTION
Alzheimer's disease (AD) affects 4.5 million Americans and the incidence is expected to reach over 13 million by 2050 [1]. At the same time, over 20 million Americans have diabetes and this incidence is increasing by 5% per year [2]. Multiple studies report that patients with diabetes have a 50 to 75% increased risk of developing AD compared to age- and gender-matched control groups [3–7]. In parallel, a recent study of the Mayo Clinic AD Patient Registry reveals that 80% of AD patients have either type 2 diabetes or impaired fasting glucose [8].
Two of the most prominent pathological characteristics of AD are the accumulation of β-amyloid (Aβ) in extracellular plaques and the appearance of intracellular neurofibrillary tangles (NFT) containing hyperphosphorylated tau [9, 10]. Tau undergoes various post-translational modifications, hyperphosphorylation being the most prominent and studied changes [10]. Mounting evidence suggests that not only the hyperphosphorylation, but also tau cleavage plays an important role in the progression of AD [11, 12]. N- and C-terminal fragmentation induces toxic tau aggregation in N2a cells [13]. When cleaved at Asp421 by caspase, tau assembles more rapidly and more extensively into tau filaments in vitro [14], suggesting that cleaved tau enhances polymerization kinetics and serves as a nucleation center, promoting the pathologic assembly of tau filaments [15, 16]. Caspases [11, 12, 17], the ubiquitin-proteasome system [18], and calpains [19, 20] are all implicated in tau cleavage.
One of the most prominent characteristics of diabetes is elevated blood glucose levels [21, 22]. Our laboratory has consistently demonstrated that hyperglycemia results in neuronal injuries, leading to eventual death of neurons and supporting Schwann cells and the development of diabetic neuropathy [reviewed in [21, 23]]. Hyperglycemia is also associated with impaired cognitive performance and an increased number of mental subtraction errors in individuals with diabetes [6].
Recently we reported age-dependent tau modification in the diabetic mouse brain [24]. This current study expands our previous study and examines the effect of hyperglycemia using embryonic cortical neurons as an in vitro model. We also confirm our findings using different mouse models of type 1 and type2 diabetes. We report here that glucose treatment of E15 rat embryonic cortical neuron cultures results in concentration- and time-dependent tau cleavage and neuronal apoptosis. When the cortical neurons are treated with both glucose and Aβ together there is an additive effect on apoptosis and tau cleavage. Inhibition of caspases, but not calpain or the proteasome, prevents apoptosis and tau cleavage. Tau cleavage was also observed in brains from type 2, but not type 1 diabetic mice. Our results demonstrating hyperglycemia-induced tau cleavage provide a novel mechanism for the increased incidence of AD in diabetic patients.
MATERIALS AND METHODS
Antibodies and chemicals
Polyclonal antibodies against phosphorylated tau (pTau, pS199/202, pS396, pT231) were purchased from Biosource International (Camillo, CA). Anti-amyloid β (Aβ1-42) was also from Biosource. AT8 monoclonal antibody detecting phosphorylated Ser202 was from Pierce (Rockford, IL) and anti-TauC3 (detecting cleaved tau) was from Millipore (Billerica, MA). Tau1 (recognizing dephosphorylated tau), Tau5 (for total tau) and anti-GAPDH antibodies were from Chemicon (Temecula, CA). Anti-tau46 (detecting total tau) was from Abcam (Cambridge, MA). The antibody against cleaved caspase-3 was purchased from Cell Signaling (Beverly, MA).
Inhibitors of caspases, calpain and the proteasome were purchased from Calbiochem (La Jolla, CA). D-(+)-glucose and 2-deoxy-D-glucose (a non-metabolizable glucose analog) were purchased from Sigma (St. Louis, MO). All other chemicals were purchased from either Sigma or Fisher Scientific (Fair Lawn, NJ).
Cortical neuron preparation
Cortical neurons were prepared from E15 embryos of Sprague Dawley rats. The cortex was dissected and dissociated using trypsin and plated in 12-well tissue culture plates coated with poly-L-lysine (PLL). For immunohistochemistry (IHC), cells were plated on glass cover slide coated with poly-L-lysine (PLL) in 24-well culture plates. Cells were maintained in feed media (Neurobasal media, Invitrogen, Grand Island, NY) supplemented with 1X B27 without antioxidant (Invitrogen), antibiotics (penicillin, streptomycin, and neomycin; Sigma), 2.5 µg/ml albumin, 10 µg/ml apo-transferin, 0.1 µg/ml biotin, 15 µg/ml D-galactose, 7 ng/ml progesterone, 16 µg/ml putrescine, 4 ng/ml selenium, 3 ng/ml β-estradiol, 4 ng/ml hydrocortisone, 3 µg/ml catalase and 2.5 µg/ml SOD. Cells were cultured for 6 days before being used in experiments. Culture media was changed to treatment media (feed media without B27 and antibiotics) for 3–4 h prior to glucose, Aβ, and/or inhibitor treatment.
Induction of diabetes and mouse brain preparation
Wild type C57Bl/6J and ob/ob mice (B6.V-Lepob/J, JAX Mice # 000632) were purchased from Jackson Laboratory (Bar Harbor, ME) and used as a model of type 2 diabetes. Mice were sacrificed at 12 wk of age (approximately 8 wk of diabetes). Type 1 diabetes was induced by streptozotocin (STZ) injection when mice (DBA/2J, JAX Mice #000671) reached a weight of 25 g (~13 wk old). STZ was injected at the concentration of 50 mg/kg for 5 consecutive days (http://www.amdcc.org/shared/Protocols.aspx) [25]. Mice were sacrificed at 38 wk of age (25 wk diabetes). At least 6 animals were used for each group. Fasting blood glucose levels were measured every 4 wk using a standard Glucometer (OneTouch; LifeScan Inc., Milpitas, CA). All mice were housed in a pathogen-free environment, and cared for following the University of Michigan Committee on the Care and Use of Animals guidelines.
The mice were euthanized per our published protocols with an overdose of sodium pentobarbital. For Western blotting analyses, brains were cut in half and the cortex and hippocampus separated and then snap frozen in liquid nitrogen and stored at −80°C until use. For immunohistochemistry (IHC), mice were perfused intracardially with 30 ml of 2% PLP (paraformaldehyde-lysine-periodate) following euthanasia. After perfusion, whole brains were removed and processed for the cryosecton as previously described [22, 26]. Brain sections were mounted on SuperFrost glass slides (Fisher Scientific, Pittsburgh, PA) and stored at −20°C until use.
Western immunoblotting
Western immunoblotting was performed as described previously [27]. Cortical neuron cultures were lysed in RIPA buffer (Pierce) containing 1 µg/ml of aprotinin and leupeptin and 100 µg/ml phenylmethylsulfonyl fluoride (PMSF). Lysates were collected, briefly sonicated and centrifuged at 13,000 rpm for 15 min at 4 °C. Mouse cortex and hippocampus were homogenized using a plastic pestle in a microcentrifuge tube in T-PER tissue protein extraction reagent (Pierce) containing aprotinin, leupeptin and PMSF. Samples were briefly sonicated and centrifuged at 13,000 rpm for 20 min at 4°C and supernatants were collected. Protein concentration of the cortical neuron or brain lysates was measured by DC protein assay reagent (Bio-Rad Laboratory, Hercules, CA). The lysates were separated by SDS-PAGE and transferred to nitrocellulose (NC) membranes. After blocking with Superblock solution (Pierce) with 0.1% Tween-20, NC membranes were incubated with the appropriate primary antibodies followed by secondary antibodies conjugated with horseradish peroxidase (Santa Cruz). The incubations with primary and secondary antibodies were carried out either at RT for 2 h or at 4°C overnight. The signal was visualized using enhanced chemiluminescence reagents (ECL, Amersham Bioscience, Piscataway, NJ) or SuperSignal West Femto Maximum Sensitivity Substrate (Pierce) depending on the signal strength. Images were captured using the Chemidoc XRS system and analyzed by Quantity One software (Bio-Rad Laboratory). In some experiments, the NC membranes were incubated at 60°C for 15 min in stripping solution (2% SDS, 100 mM dithiothreitol and 100 mM Tris, pH6.8) whereupon they were utilized for immunoblotting with another antibody. All experiments were repeated at least 3 times and representative results are presented in the figures.
Immunohistochemistry (IHC)
The slides with brain sections were heated on a slide warmer (55°C) for 10 min followed by hydration in PBS. Cortical neurons grown on cover slides were fixed with 2 % paraformaldehyde (PFA) in PBS for 10 min. The slides were permeabilized and incubated in primary antibody diluted in PBS with 1% BSA/1% normal goat serum/0.1 % Triton X-100 in a humidified chamber at RT overnight. After rinsing with PBS 3 times for 5 min each, the cells were incubated with the appropriate secondary antibody conjugated with AlexaFluor 594 or 488 (Molecular Probes, Eugene, OR) for 2 h at RT. After rinsing with PBS, the samples were mounted with ProLong Gold anti-fade mounting media containing DAPI (Molecular Probes). The digital images were captured using a Spot-RT camera (Diagnostic Instruments Inc., Sterling Heights, MI) attached to a Nikon Microphot-FXA microscope.
Measurement of apoptosis, TdT mediated dUTP-biotin nick end labeling (TUNEL)
TUNEL staining labels fragmented DNA, which is a characteristic of apoptotic cells. Cortical neurons grown on glass cover slides were fixed in 4% paraformaldehyde prior to staining. Samples were labeled with digoxygenin-dUTP and followed by horseradish peroxidase-conjugated anti-digoxygenin antibody using a kit according to the manufacturer’s instructions (Intergen, Gaithersburg, MD).
Measurement of apoptosis, Annexin V staining
During apoptosis, phosphatidylserine (PS), which is normally located on the cytoplasmic surface, translocates to the outer leaflet of the plasma membrane [28]. Annexin V binds to the exposed PS in a Ca++-dependent manner [29]. Annexin V conjugated with Alexa Fluor 488 was purchased from Molecular Probes. After treatment, cells were labeled with Annexin V (1:20 dilution) in binding buffer (10 mM HEPES, pH7.5, 150 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2) for 10 min at RT. The resulting fluorescence was measured using a Fluoroskan Ascent FL fluorimeter (Labsystems, Thermo Fisher Scientific Inc., Waltham, MA).
RESULTS
Tau cleavage by high glucose and Aβ in primary rat embryonic cortical neurons
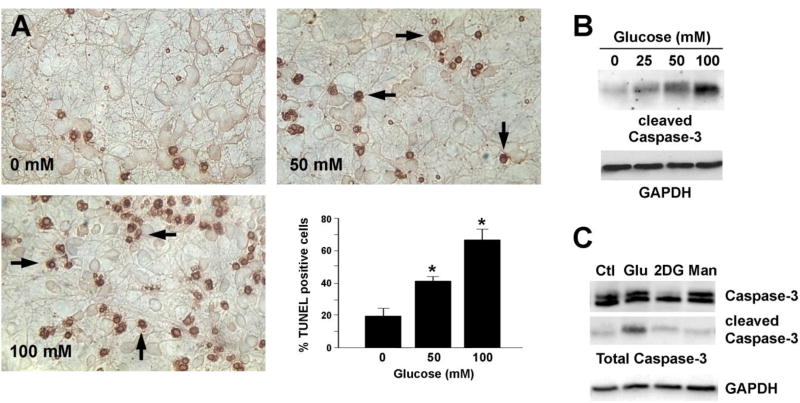
Our laboratory demonstrated that hyperglycemia mimicking diabetes induces diabetic neuropathy in vivo [21, 23] as well as neuronal apoptosis in vitro in both neuroblastoma cell lines and primary neuron cultures [26, 30, 31]. Recent reports demonstrate tau dysfunction in both type 1 and type 2 diabetic animal models [32–34], suggesting that tau modification is a link between increased AD in diabetic patients. Therefore, we examined whether high glucose treatment induces neuronal apoptosis and tau modification in vitro using primary rat embryonic cortical neurons as the model system. Consistent with our previous reports, glucose treatment resulted in a concentration-dependent increase in neuronal apoptosis (Fig 1A) detected by TUNEL staining. Activation of the caspase family of cell death proteases is essential for most types of apoptosis [35]. We and others have shown that activation of caspase-3 by a variety of neuropathological insults, including hyperglycemia, plays an important role in apoptosis in neuronal cells [30, 36]. In agreement with the apoptosis results, there was a glucose concentration-dependent increase in caspase-3 cleavage (i.e. activation) after 24 h of hyperglycemia (Fig 1B). Treatment with an equimolar concentration of mannitol, however, failed to induce caspase-3 activation (Fig 1C). Treatment of the cortical neurons with as much as 250 mM mannitol or 2DG resulted only minimal caspase-3 cleavage, suggesting that the effect of glucose was not the result of hyperosmotic stress.
Fig 1. Glucose-mediated apoptosis of cortical neurons.
(A) E15 rat embryonic cortical neurons cultured in vitro for 6 d were treated with 0, 50 or 100 mM glucose for 24 h and apoptosis was measured using TUNEL staining. Arrows show the apoptotic cells. Glucose treatment significantly increased the number of TUNEL positive cells. p<0.05 by t-test. (B, C) Cortical neurons were treated with the indicated concentrations of glucose (Glu), 2-deoxyglucose (2DG, 100 mM) or mannitol (Man, 100 mM) for 24 h. Cell lysates were prepared in RIPA buffer and immunoblotted with an antibody against cleaved caspase-3 (B) or total caspase-3 (C). The blots were stripped and reprobed with an anti-GAPDH antibody. These experiments were repeated at least 3 times and a representative result is shown.
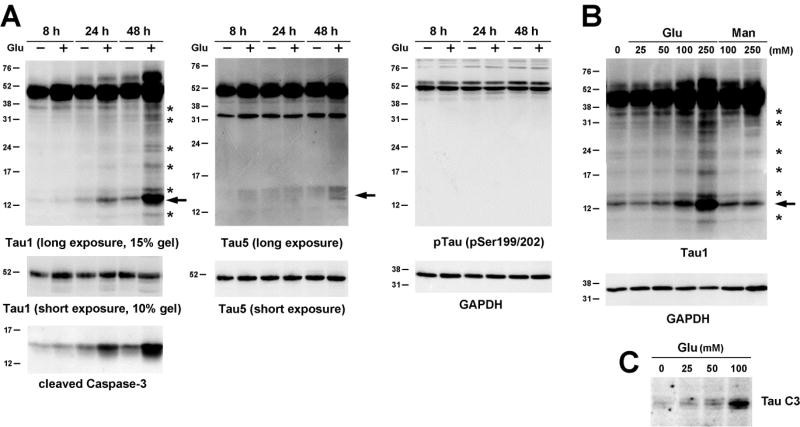
We next examined tau modification during glucose-mediated apoptosis. Tau cleavage was detected by the appearance of cleavage products recognized by Tau1 immunoblotting. Treatment of the embryonic cortical neurons with 100 mM glucose resulted in a time-dependent increase in tau cleavage (Fig 2A) with the appearance of the prominent ~14 kDa fragment starting at 24 h (arrow). Glucose treatment for 48 h resulted in the appearance of more cleavage fragments ranging from ~40 kDa to ~10 kDa (Fig 2A, asterisks). The time course of tau cleavage was very well correlated with caspase-3 cleavage (Fig 2A), with continued increase in both tau and caspase-3 cleavage at 48 h. However, the levels of tau and caspaspe-3 cleavage in cells not exposed to hyperglycemic conditions were also increased at 48 h. The cleaved tau at 14 kDa was also detected by the Tau5 antibody when the blot was overexposed (Fig 2A); however, the immunoreactivity was not as prominent when detected by the Tau1 antibody. Total tau levels, detected by short exposure with the Tau5 antibody, or tau phosphorylation, detected by the antibody against phosphorylation at the Ser199/202 residues, were not significantly changed at 48 h of glucose treatment. The effect of glucose on the appearance of cleaved tau fragments was also concentration-dependent up to 250 mM glucose treatment (Fig 2B, arrow) and was well-correlated with apoptosis and caspase-3 cleavage (Fig 1). In agreement with the effect on caspase-3 cleavage, mannitol treatment up to 250 mM had a minimal effect on the appearance of the 14 kDa tau fragment even though 40 kDa fragments were present (Fig 2B, asterisks). Tau cleavage was also confirmed by TauC3 immunoblotting (Fig 2C), which detects tau cleaved at Asp421.
Fig 2. Tau cleavage by glucose treatment.
(A) E15 rat embryonic cortical neurons were treated with 100 mM glucose for 8, 24 or 48 h. Cell lysates were immunoblotted with the indicated antibodies. For Tau1 and Tau5 antibodies the blots were exposed for a short time using ECL or a longer time using SuperSignal West Femto to examine various cleavage products (asterisks). Arrow indicates the major cleavage products of 14 kDa. (B) Cortical neurons were treated with increasing concentrations of glucose or mannitol for 24 h. Tau cleavage was examined as in (A). Tau cleavage also confirmed by TauC3 antibody immunoblotting (C). The Western blots were repeated at least 3 times and representative results are shown.
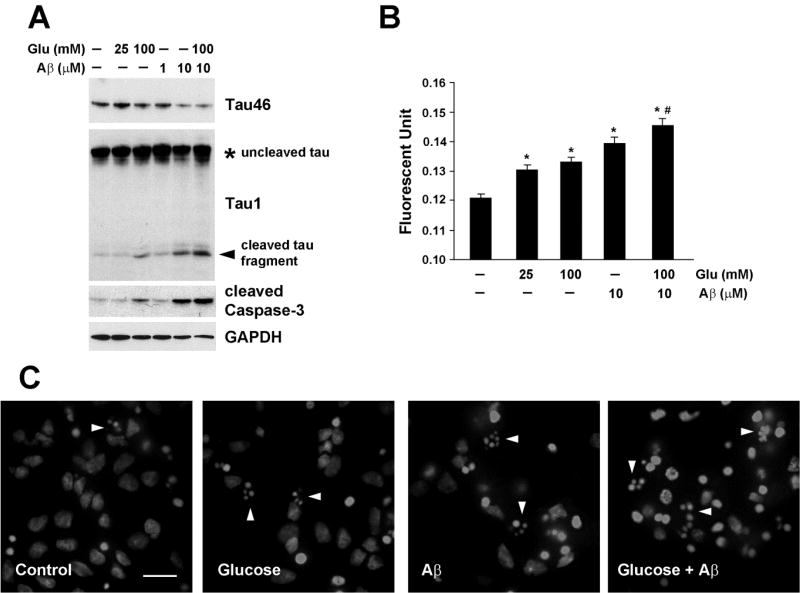
Studies demonstrate that Aβ treatment results in apoptosis of embryonic cortical or hippocampal neurons [17, 37]. We next tested the hypothesis that hyperglycemia accelerates neuronal injury induced by Aβ. Treatment of cortical neurons with glucose or Aβ for 24 h resulted in concentration-dependent tau cleavage as seen by the decrease in Tau46 immunoreactivity (Fig 3A upper panel) and the appearance of cleaved fragments (Fig. 3A, arrowhead), and an increase in cleaved caspase-3. When the cells were treated with both glucose and Aβ there was an additive effect on tau cleavage and caspase-3 activation (Fig 3A). Even though there is a tendency for increased total tau levels by glucose and/or Aβ, the changes were not statistically significant (data not shown). In agreement with caspase-3 activation, cell death was also increased when examined by Annexin V staining, with an additive effect when glucose and Aβ were added together (Fig 3B). Apoptosis was also measured by examining fragmented apoptotic nuclei after staining the cells with DAPI. The cells treated with glucose or Aβ display increased staining of fragmented nuclei (Fig 3C, arrowheads), which became more frequent when both treatments were added together.
Fig 3. Glucose and Aβ-mediated apoptosis and tau cleavage in primary cortical neurons.
E15 rat embryonic cortical neurons were cultured for 6 d prior to treatment with glucose, Aβ or both for 24 h. (A) The cell lysates were immunoblotted with the indicated antibodies. The arrowhead indicates the 14 kDa cleaved tau fragment. These experiments were repeated at least 3 times and representative results are shown. (B) Apoptosis was measured by Annexin V staining. *, p<0.05 compared to no treatment. #, p<0.05 compared to 100 mM glucose or 10 µM Aβ treatment. (C) The cortical neurons were grown on PLL-coated glass coverslips before treatment. The cells were fixed with 2% PFA and mounted with ProLong Gold anti-fade mounting media containing DAPI. The arrowheads indicate apoptotic nuclei. The bar represents 15 µm.
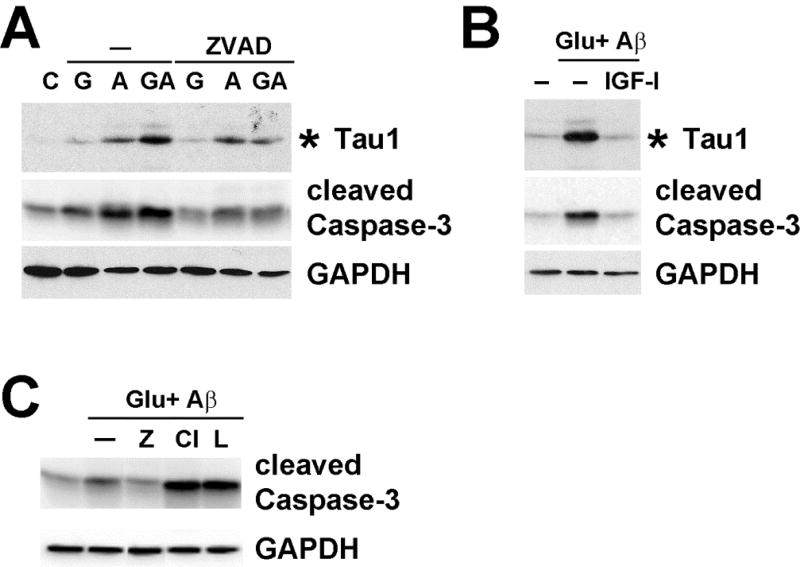
Caspases [11, 17], the proteasome [18] and calpain [19, 20] are all implicated in tau cleavage during AD progression. Therefore, we next examined the possible proteases responsible for tau cleavage during glucose/Aβ treatment using protease inhibitors. Glucose- and Aβ-mediated tau cleavage and caspase-3 activation were partially blocked by the pan-caspase inhibitor, ZVAD, and almost completely blocked by IGF-I (Fig 4A), which was used as a positive control. This result suggests the involvement of other proteases. Calpain inhibitor III and lactacystin (proteasome inhibitor), however, could not prevent tau cleavage since they augmented caspase activation and cell death (Fig 4B). A recent study also reported similar results with calpain inhibitor (MDL28170)-treated SH-SY5Y cells [38]. We also observed similar results using other calpain (ALLN) and proteasome (MG-132) inhibitors (data not shown).
Fig 4. Tau cleavage in cortical neurons is prevented by caspase inhibitors.
(A) E15 rat embryonic cortical neurons were treated without (C) or with 100 mM glucose (G), 10 µM Aβ (A) or both (GA) for 24 h in the presence or absence of 20 µM ZVAD. The cell lysates were immunoblotted for the indicated proteins. The asterisk indicates the 14 kDa fragment of tau recognized by the Tau1 antibody. (B) Cortical neurons were treated without or with 100 mM glucose + 10 µM Aβ for 24 h in the presence or absence of 10 nM IGF-I. The cell lysates were immunoblotted for the indicated proteins. The asterisk indicates the 14 kDa fragment of tau recognized by Tau1 antibody. (C) Cortical neuron lysates were treated with glucose and Aβ in the presence of 20 µM ZVAD (Z), calpain inhibitor III (Cl), or lactacystin (L) for 24 h and the cell lysates were immunoblotted with the indicated antibodies. These experiments were repeated at least 3 times and representative results are shown.
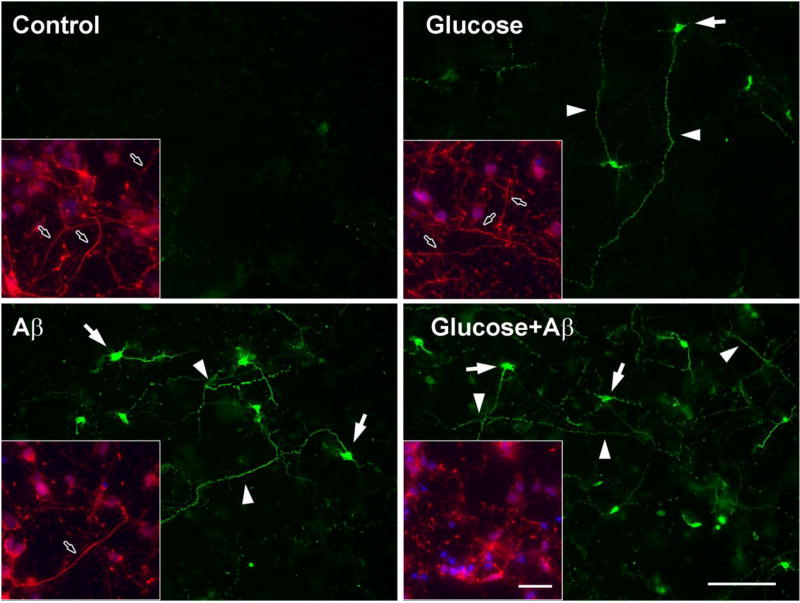
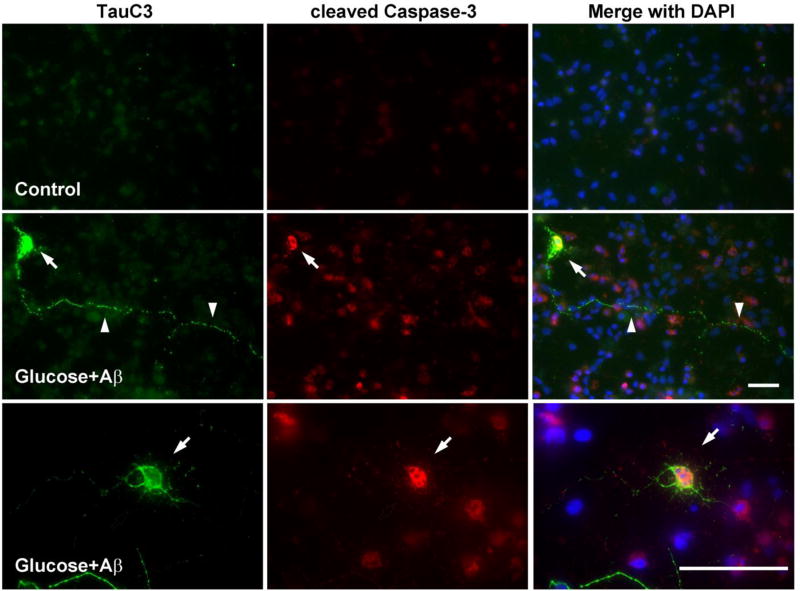
We conducted immunohistochemistry (IHC) to better understand the subcellular changes of tau modification during glucose-mediated apoptosis. Embryonic cortical neurons grown on cover slips were treated with glucose, Aβ, or both for 24 h. The cells were fixed with 2% PFA and then stained with the TauC3 antibody to examine the distribution of cleaved tau. In agreement with the increase in apoptosis and caspase expression (Fig 3 & 4) there was a decrease in the number of neurites in the cells treated with glucose or Aβ and it was more prominent when the cells were exposed to both glucose and Aβ (Fig 5, insets). In contrast there was a strong labeling of TauC3 in the cytoplasm of apoptotic neurons (Fig 5, arrows). TauC3 staining also displayed a punctate pattern of labeling along the neurites (Fig 5, arrowheads), which was more numerous when glucose and Aβ were treated together. We next examined the distribution of cleaved caspase-3 after glucose and Aβ treatment in the cortical neurons. There was increased immunostaining for cleaved caspase-3 in the cytoplasm when the cells were treated with glucose and Aβ. TauC3 IHC showed labeling in the cytoplasm (Fig 6, arrows) as well as a punctate pattern along the neurites (Fig 6, arrowheads), which were not detected in control cells. When the images were merged we could detect the colocalization of cleaved caspase-3 with cleaved tau in the cytoplasm (Fig 6, arrows). There was similar colocalization when the cells were treated with glucose or Aβ alone (data not shown). These results, along with the biochemical data, strongly suggest that caspase is the key protease involved in tau cleavage during glucose- and Aβ-mediated neuronal apoptosis.
Fig 5. TauC3 immunostaining of rat embryonic cortical neurons.
E15 rat embryonic cortical neurons were grown on PLL-coated glass coverslips for 6 days and then treated with 100 mM glucose, 10 µM Aβ or both for 24 h. The cells were fixed with 2% PFA and stained for TauC3. Arrowheads indicate TauC3 staining along the neurites and arrows indicate staining in the cytoplasm. Insets: cells were stained with anti-neurofilament antibody (red) and DAPI (blue). Arrows in insets indicate the neurites. The bar represents 100 µm.
Fig 6. TauC3 is colocalized with cleaved caspase-3.
E15 rat embryonic cortical neurons were treated without (top panel) or with 100 mM glucose + 10 µM Aβ (bottom 2 panels) for 24 h. The cells were simultaneously stained for TauC3 (green) and cleaved caspase-3 (red). Nuclei are stained with DAPI (blue). Arrowheads indicate the TauC3 staining along the neurites and arrows indicate the colocalization of TauC3 and cleaved caspase-3 in the cytoplasm. The bars represent 50 µm.
Tau hyperphosphorylation and cleavage in diabetic mice
Our results from the embryonic cortical neurons strongly suggest the direct role of hyperglycemia on tau modification. Recently we reported age-dependent tau phosphorylation and cleavage in type 1 (STZ-injected) and type 2 (db/db) diabetic mice [24]. Here we employ different models of diabetes to confirm our previous reports as well as the in vitro results.
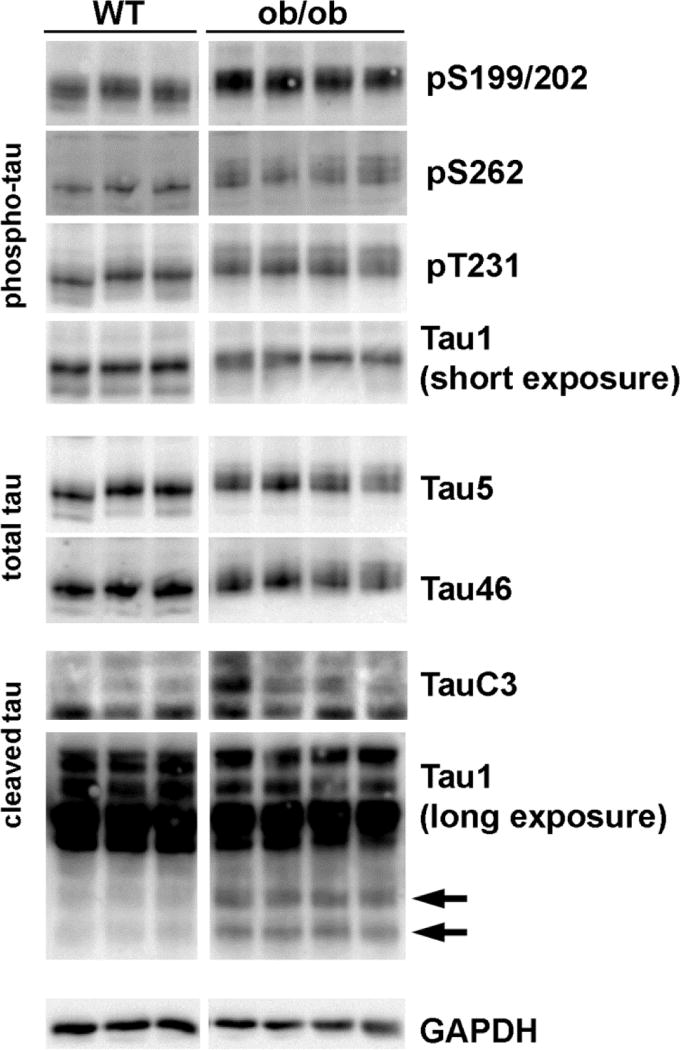
B6.V-Lepob/J mice, commonly known as ob/ob, are used as a type 2 diabetes model. ob/ob mice express a homozygous mutation of leptin and their characteristics are well described in the Jackson laboratory web site (http://jaxmice.jax.org/strain/000632.html). Wild type (WT) C57Bl/6J mice are used as controls. We examined tau phosphorylation in 12 wk WT and ob/ob brains. We observed increased tau phosphorylation in the cortex of ob/ob mice compared to WT at several residues including Ser199/202, Ser262 and Thr231 (Fig 7). We also detect a decreased immunoreactivity by Tau1 antibody, which detects dephosphorylated tau. Total tau levels, examined with Tau5 and Tau46 antibody, were not different between WT and ob/ob. We observed, however, a decrease in the mobility and increased diffusion of the tau band from ob/ob mouse brains in SDS-PAGE, which represent high Ser/Thr phosphorylation levels in these animals.
Fig 7. Increased Tau hyperphosphorylation and cleavage in 12 wk ob/ob mouse cortex.
Cortex lysates from 12 wk old wild type (WT) and ob/ob male mice were immunoblotted with the indicated antibodies for phosphorylated, total or cleaved tau. Tau1 immunoblot was exposed for a short time to detect tau (de)phosphorylation or overexposed to detect tau cleavage (arrows). The blots were stripped and reprobed with the anti-GAPDH antibody as a loading control. We observe similar results using hippocampus lysates (data not shown).
Mounting evidence suggests that not only the hyperphosphorylation, but also tau cleavage plays an important role in the progression of AD [11]. Therefore, we next examined whether tau cleavage is enhanced in ob/ob mice. The tauC3 antibody detects tau cleaved at Asp421 [14]. When the brain lysates were immunoblotted with the TauC3 antibody, we detected an increased expression of a band representing cleaved tau in the cortex ob/ob mice (Fig 7). In addition, we detected a smaller band by longer exposure of the Tau1 immunoblots, which may represent a cleaved fragment of tau. These results from TauC3 and Tau1 antibodies are in agreement with our previous report using db/db mice [24]. We observed similar changes in tau phosphorylation and cleavage in the hippocampus (data not shown).
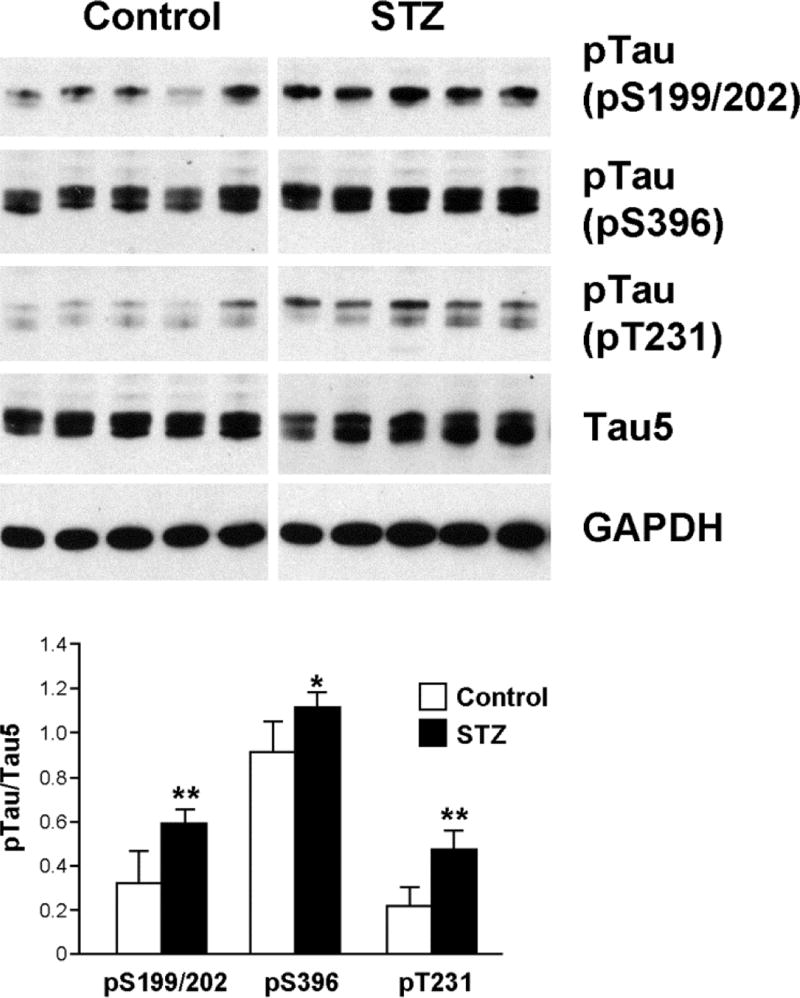
STZ-injected DBA/2J mice were used as a type 1 diabetes model. Fasting glucose levels were significantly higher at 38 wks of age (25 wks of diabetes) in STZ injected groups (501.5 ± 77.7 mg/dL) compared to control (112.6 ± 23.4 mg/dL). In agreement with the published reports [24, 33, 34], we detected increased tau phosphorylation in the cortices of STZ-injected mice at all residues examined (Fig. 8). In contrast to ob/ob mice, however, we did not detect tau cleavage in STZ-injected mouse brains (data not shown).
Fig 8. Tau phosphorylation is increased in the cortices of STZ-injected mice.
Brains were prepared from DBA/2J mice 25 wk after STZ injection (38 wk of age). The cortex was homogenized in T-PER buffer and the lysates were immunoblotted with the indicated antibodies. The relative density of phosphorylated tau over total tau (Tau5) was measured after immunoblotting. *, p<0.05 and **, p<0.01 by t-test
DISCUSSION
The abnormal aggregation of tau into NFT is one of the hallmarks of AD [9]. Both the cleavage and hyperphosphorylation of tau lead to aggregation and NFT formation [11, 13]. When cleaved by caspases, tau aggregates more easily and extensively in vitro [14]. In the current report, we present results strongly suggesting that caspase-mediated cleavage of tau due to hyperglycemic conditions is a potential mechanism for the increased risk of AD in diabetic patients [6, 7].
Our laboratory has reported that hyperglycemia resembling the high blood glucose levels in diabetes results in neuronal apoptosis in vivo and in vitro [21–23]. Consistent with our theory, glucose treatment induced concentration-dependent apoptosis in rat embryonic cortical neuron cultures. Apoptosis was well-correlated with the appearance of cleaved caspase-3 (i.e. activation), suggesting the involvement of caspases during apoptosis. Equimolar concentrations of mannitol or 2DG did not induce caspase-3 activation, demonstrating that the effect of glucose is not due to hyperosmorality.
Previous studies demonstrated modulation of tau phosphorylation and cleavage during apoptosis in cortical neurons [39, 40] and our current results clearly show that tau is altered by glucose-mediated apoptosis. Tau cleavage was glucose time- and concentration-dependent and well-correlated with the appearance of cleaved caspase-3 and apoptosis. Our IHC data of TauC3 staining further support glucose-mediated tau cleavage in cortical neurons. A similar pattern of the TauC3 immunostaining along neurites has been demonstrated in hippocampal/cortical neuron cultures during staurosporine-induced apoptosis [11]. Consistent with the apoptosis results, mannitol or 2DG did not induce tau cleavage, demonstrating a specific effect of glucose. To our knowledge, our data are the first to demonstrate glucose-mediated tau cleavage both in vitro and in vivo.
In our study, tau cleavage is demonstrated by the appearance of fragments using a Tau1 antibody both for in vitro (cortical neuron) and in vivo (ob/ob mice brain). Tau cleavage was also confirmed by the increase in TauC3 immunoreactivity. Despite the significant increase in Tau1 immunoreactivity, tau phosphorylation was not significantly changed, suggesting tau cleavage precedes changes in tau phosphorylation, at least in vitro. However, the sequence of tau cleavage and phosphorylation during neuronal apoptosis remains controversial and depends on the experimental model or apoptotic stimuli used [41–43].
Studies demonstrate that treatment of embryonic cortical or hippocampal neurons with Aβ results in apoptosis [17], tau cleavage [14, 41], and activation of proteases, including caspases [19, 44] and calpain [19, 20, 45], all findings present in AD [46, 47]. In transgenic animal models, tau pathology is exacerbated by coexpression of Aβ [48–50]. Asp421-cleaved tau expression in primary cortical neurons enhances mitochondrial dysfunction induced by Aβ treatment [51]. Our results also demonstrate Aβ-induced tau cleavage, caspase activation, and apoptosis by Western immunoblotting and IHC. In addition, we show the additive effects of glucose and Aβ on these changes, suggesting that glucose- and Aβ-induced neuronal injury are mediated by parallel pathways. Our results clearly demonstrate that glucose directly induces tau cleavage and supports our hypothesis that hyperglycemia, in combination with Aβ toxicity, augments AD pathology by accelerating tau modifications.
The current study does not elucidate the exact nature of the tau cleavage products; however, studies show the formation of various sizes of tau fragments during neuronal apoptosis [38, 52–54]. One study demonstrated the generation of a similar size tau fragment to our current study in hippocampal neurons during Aβ-mediated apoptosis using the same Tau1 antibody [55]. The cleavage fragments, when directly added to the culture, are toxic to the neurons and induce apoptosis [38, 55]. Truncated tau, without mutation or hyperphosphorylation, can facilitate NFT formation in vivo [16]. Therefore, it is possible that glucose and Aβ induce the apoptotic pathway, generating tau fragments that facilitate the process by a feed-forward mechanism.
Several proteases including caspases [11, 17], proteases associated with the proteasome [18], and calpains [19, 20] are implicated in tau cleavage. Tau cleaved at Asp421 by caspase-3 is detected in AD brains and neurons treated with Aβ [56, 57]. Calpain is abnormally activated in AD patients compared to age-matched controls [58] and colocalized with NFT in AD [59]. The role of the proteasome in tau degradation remains controversial [60, 61]. We demonstrate in this report the activation of caspase-3 during glucose and Aβ treatment, which show an additive effect when both are used together. Furthermore, our IHC data demonstrating the colocalization of cleaved tau with active capase-3 strongly support the important role of caspase-3 in tau cleavage. Similar colocalization of active caspase-3 and cleaved tau has been reported from post-mortem AD brains [41]. However, the pan-caspase inhibitor, ZVAD, did not completely inhibit caspase-3 activation or tau cleavage as much as IGF-I treatment did, suggesting the involvement of other proteases. Unfortunately, calpain or proteasome inhibitors augmented cell death and increased caspase-3 activation. A recent report by Caliaano and colleagues showed similar results using a different calpain inhibitor, MDL28170 [38]. Therefore, we could not exclude the possibility of the contribution of calpains or the proteasome. These results support the idea that different and/or additional proteases may be operational in vivo.
In agreement with in vitro results, tau cleavage was also observed in the brains of the ob/ob mouse model of type 2 model of diabetes, but not in WT mice, confirming our recently published report [24]. Unlike the effect of glucose on cortical neuron culture, tau cleavage in ob/ob mouse brains was accompanied by increased tau phosphorylation. Our results are in agreement with several recent reports of AD-like changes in type 1 and type 2 diabetic animal models [32, 33, 62]; however, all studies so far focus on tau hyperphosphorylation. Our current and previous [24] results show clear evidence of increased tau cleavage in type 2 diabetic animals. In spite of the increased glucose level and tau phosphorylation, however, tau cleavage was not observed in STZ-injected DBA/2J (current study) or C57Bl/6J mice [24]. These results suggest that type 1 and type 2 diabetes affect tau modifications differently regardless of genetic background. The difference of tau cleavage in type 1 and type2 diabetes may be due to the IR observed in type 2 diabetes. Insulin resistance is the major feature of the cluster of related physiologic dysfunctions known as the metabolic syndrome (glucose intolerance, obesity, dyslipidemia and hypertension) [63, 64]. IR and metabolic syndrome are major risk factors for the development of type 2 diabetes [65]. We recently published a series of manuscripts demonstrating the development of IR in neurons [66–68]. We proved that, like the peripheral tissues, neurons develop IR upon chronic hyperinsulinemia which is observed only in type 2 diabetes. We further demonstrated the neuronal IR disrupts Akt-mediated signaling, which is critical for cell survival and glucose transport [69, 70]. Therefore, even though our in vitro results clearly demonstrate that hyperglycemia is critical for tau cleavage with acute treatment, additional factors (such as IR) may be required for chronic long term effects in vivo for tau modification.
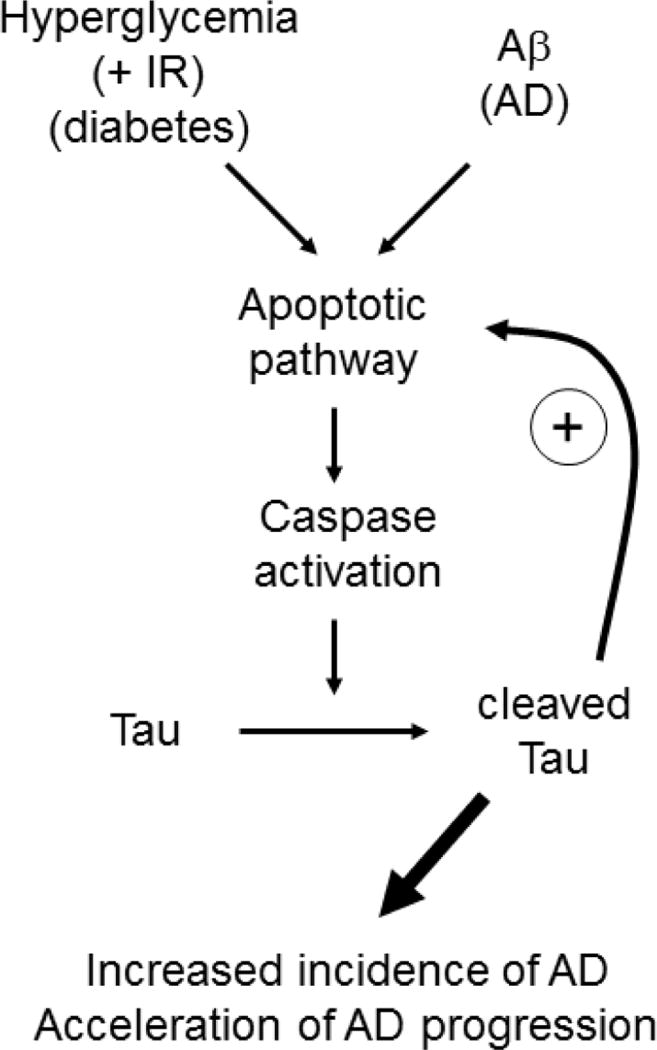
In summary, we demonstrate the possible contribution hyperglycemia on tau modification in vitro and in vivo. Our hypothesis is presented in Figure 9. Hyperglycemic conditions in diabetes (along with IR in type 2 diabetes) may initiate the apoptotic pathway, including caspase activation, which leads to tau cleavage. This process may prime the neurons to be more susceptible for later Aβ insults. The combined effects of glucose and Aβ further facilitate the apoptotic pathway, generating toxic tau cleavage fragments, which can augment the process by a positive feedback mechanism. Recent reports demonstrate a clear correlation between AD and diabetes. Considering the steady increase in the number of diabetic and AD patients, it is becoming more important to elucidate the mechanisms linking these two diseases. Our results present a new mechanism for increased AD incidence in diabetic patients: hyperglycemia-induced tau cleavage, in combination with Aβ pathology, facilitates AD pathogenesis.
Fig 9. Model.
Hyperglycemia, possibly along with IR (diabetes pathology) may initiate the apoptotic pathway, including caspase activation, and tau cleavage. This process may prime neurons to be more susceptible to Aβ insults (AD pathology). The combined effects of glucose and Aβ further facilitate the apoptotic pathway, generating toxic tau cleavage fragments which can augment the process by a positive feedback mechanism.
Acknowledgments
This work was supported by the grants from the Michigan Diabetes Research and Training Center (NIH 5P60-DK020572), Animal Models of Diabetic Complications Consortium (NIH U01-DK076160), the Taubman Institute, and the Program for Neurology Research and Discovery. The authors greatly appreciate the helpful discussion from Dr. K. A. Sullivan and technical support from Ms. Lisa McLean and Mr. John Hayes.
References
- 1.Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003;60:1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 2.Feldman EL. Oxidative stress and diabetic neuropathy: a new understanding of an old problem. J Clin Invest. 2003;111:431–433. doi: 10.1172/JCI17862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology. 1999;53:1937–1942. doi: 10.1212/wnl.53.9.1937. [DOI] [PubMed] [Google Scholar]
- 4.Peila R, Rodriguez BL, Launer LJ. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: The Honolulu-Asia Aging Study. Diabetes. 2002;51:1256–1262. doi: 10.2337/diabetes.51.4.1256. [DOI] [PubMed] [Google Scholar]
- 5.Biessels GJ, Kappelle LJ. Increased risk of Alzheimer's disease in Type II diabetes: insulin resistance of the brain or insulin-induced amyloid pathology? Biochem Soc Trans. 2005;33:1041–1044. doi: 10.1042/BST0331041. [DOI] [PubMed] [Google Scholar]
- 6.Cox DJ, Kovatchev BP, Gonder-Frederick LA, Summers KH, McCall A, Grimm KJ, Clarke WL. Relationships between hyperglycemia and cognitive performance among adults with type 1 and type 2 diabetes. Diabetes Care. 2005;28:71–77. doi: 10.2337/diacare.28.1.71. [DOI] [PubMed] [Google Scholar]
- 7.Brands AM, Biessels GJ, de Haan EH, Kappelle LJ, Kessels RP. The effects of type 1 diabetes on cognitive performance: a meta-analysis. Diabetes Care. 2005;28:726–735. doi: 10.2337/diacare.28.3.726. [DOI] [PubMed] [Google Scholar]
- 8.Janson J, Laedtke T, Parisi JE, O'Brien P, Petersen RC, Butler PC. Increased risk of type 2 diabetes in Alzheimer disease. Diabetes. 2004;53:474–481. doi: 10.2337/diabetes.53.2.474. [DOI] [PubMed] [Google Scholar]
- 9.Johnson GV, Stoothoff WH. Tau phosphorylation in neuronal cell function and dysfunction. J Cell Sci. 2004;117:5721–5729. doi: 10.1242/jcs.01558. [DOI] [PubMed] [Google Scholar]
- 10.Martin L, Latypova X, Terro F. Post-translational modifications of tau protein: implications for Alzheimer's disease. Neurochem Int. 2011;58:458–471. doi: 10.1016/j.neuint.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 11.Cotman CW, Poon WW, Rissman RA, Blurton-Jones M. The role of caspase cleavage of tau in Alzheimer disease neuropathology. J Neuropathol Exp Neurol. 2005;64:104–112. doi: 10.1093/jnen/64.2.104. [DOI] [PubMed] [Google Scholar]
- 12.Zilka N, Kovacech B, Barath P, Kontsekova E, Novak M. The self-perpetuating tau truncation circle. Biochem Soc Trans. 2012;40:681–686. doi: 10.1042/BST20120015. [DOI] [PubMed] [Google Scholar]
- 13.Wang YP, Biernat J, Pickhardt M, Mandelkow E, Mandelkow EM. Stepwise proteolysis liberates tau fragments that nucleate the Alzheimer-like aggregation of full-length tau in a neuronal cell model. Proc Natl Acad Sci U S A. 2007;104:10252–10257. doi: 10.1073/pnas.0703676104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gamblin TC, Chen F, Zambrano A, Abraha A, Lagalwar S, Guillozet AL, Lu M, Fu Y, Garcia-Sierra F, LaPointe N, Miller R, Berry RW, Binder LI, Cryns VL. Caspase cleavage of tau: linking amyloid and neurofibrillary tangles in Alzheimer's disease. Proc Natl Acad Sci U S A. 2003;100:10032–10037. doi: 10.1073/pnas.1630428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yin H, Kuret J. C-terminal truncation modulates both nucleation and extension phases of tau fibrillization. FEBS Lett. 2006;580:211–215. doi: 10.1016/j.febslet.2005.11.077. [DOI] [PubMed] [Google Scholar]
- 16.Zilka N, Filipcik P, Koson P, Fialova L, Skrabana R, Zilkova M, Rolkova G, Kontsekova E, Novak M. Truncated tau from sporadic Alzheimer's disease suffices to drive neurofibrillary degeneration in vivo. FEBS Lett. 2006;580:3582–3588. doi: 10.1016/j.febslet.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 17.Chong YH, Shin YJ, Lee EO, Kayed R, Glabe CG, Tenner AJ. ERK1/2 activation mediates Abeta oligomer-induced neurotoxicity via caspase-3 activation and tau cleavage in rat organotypic hippocampal slice cultures. J Biol Chem. 2006;281:20315–20325. doi: 10.1074/jbc.M601016200. [DOI] [PubMed] [Google Scholar]
- 18.Oddo S. The ubiquitin-proteasome system in Alzheimer's disease. J Cell Mol Med. 2008;12:363–373. doi: 10.1111/j.1582-4934.2008.00276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park SY, Tournell C, Sinjoanu RC, Ferreira A. Caspase-3- and calpain-mediated tau cleavage are differentially prevented by estrogen and testosterone in beta-amyloid-treated hippocampal neurons. Neuroscience. 2007;144:119–127. doi: 10.1016/j.neuroscience.2006.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reinecke JB, DeVos SL, McGrath JP, Shepard AM, Goncharoff DK, Tait DN, Fleming SR, Vincent MP, Steinhilb ML. Implicating calpain in tau-mediated toxicity in vivo. PLoS ONE. 2011;6:e23865. doi: 10.1371/journal.pone.0023865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Figueroa-Romero C, Sadidi M, Feldman EL. Mechanisms of disease: the oxidative stress theory of diabetic neuropathy. Rev Endocr Metab Disord. 2008;9:301–314. doi: 10.1007/s11154-008-9104-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sullivan KA, Hayes JM, Wiggin TD, Backus C, Su Oh S, Lentz SI, Brosius F, 3rd, Feldman EL. Mouse models of diabetic neuropathy. Neurobiol Dis. 2007;28:276–285. doi: 10.1016/j.nbd.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vincent AM, Edwards JL, Sadidi M, Feldman EL. The antioxidant response as a drug target in diabetic neuropathy. Curr Drug Targets. 2008;9:94–100. doi: 10.2174/138945008783431754. [DOI] [PubMed] [Google Scholar]
- 24.Kim B, Backus C, Oh S, Hayes JM, Feldman EL. Increased tau phosphorylation and cleavage in mouse models of type 1 and type 2 diabetes. Endocrinology. 2009;150:5294–5301. doi: 10.1210/en.2009-0695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wiggin TD, Kretzler M, Pennathur S, Sullivan KA, Brosius FC, Feldman EL. Rosiglitazone treatment reduces diabetic neuropathy in streptozotocin-treated DBA/2J mice. Endocrinology. 2008;149:4928–4937. doi: 10.1210/en.2008-0869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vincent AM, Russell JW, Sullivan KA, Backus C, Hayes JM, McLean LL, Feldman EL. SOD2 protects neurons from injury in cell culture and animal models of diabetic neuropathy. Exp Neurol. 2007;208:216–227. doi: 10.1016/j.expneurol.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leventhal PS, Shelden EA, Kim B, Feldman EL. Tyrosine phosphorylation of paxillin and focal adhesion kinase during insulin-like growth factor-I-stimulated lamellipodial advance. J Biol Chem. 1997;272:5214–5218. doi: 10.1074/jbc.272.8.5214. [DOI] [PubMed] [Google Scholar]
- 28.van Engeland M, Nieland LJ, Ramaekers FC, Schutte B, Reutelingsperger CP. Annexin V-affinity assay: a review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry. 1998;31:1–9. doi: 10.1002/(sici)1097-0320(19980101)31:1<1::aid-cyto1>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 29.Andree HA, Reutelingsperger CP, Hauptmann R, Hemker HC, Hermens WT, Willems GM. Binding of vascular anticoagulant alpha (VAC alpha) to planar phospholipid bilayers. J Biol Chem. 1990;265:4923–4928. [PubMed] [Google Scholar]
- 30.Leinninger GM, Backus C, Sastry AM, Yi YB, Wang CW, Feldman EL. Mitochondria in DRG neurons undergo hyperglycemic mediated injury through Bim, Bax and the fission protein Drp1. Neurobiol Dis. 2006;23:11–22. doi: 10.1016/j.nbd.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 31.Russell JW, Berent-Spillson A, Vincent AM, Freimann CL, Sullivan KA, Feldman EL. Oxidative injury and neuropathy in diabetes and impaired glucose tolerance. Neurobiol Dis. 2008;30:420–429. doi: 10.1016/j.nbd.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li ZG, Zhang W, Sima AA. Alzheimer-like changes in rat models of spontaneous diabetes. Diabetes. 2007;56:1817–1824. doi: 10.2337/db07-0171. [DOI] [PubMed] [Google Scholar]
- 33.Clodfelder-Miller BJ, Zmijewska AA, Johnson GV, Jope RS. Tau is hyperphosphorylated at multiple sites in mouse brain in vivo after streptozotocin-induced insulin deficiency. Diabetes. 2006;55:3320–3325. doi: 10.2337/db06-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Planel E, Tatebayashi Y, Miyasaka T, Liu L, Wang L, Herman M, Yu WH, Luchsinger JA, Wadzinski B, Duff KE, Takashima A. Insulin dysfunction induces in vivo tau hyperphosphorylation through distinct mechanisms. J Neurosci. 2007;27:13635–13648. doi: 10.1523/JNEUROSCI.3949-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krieglstein K. Cell death in the nervous system. Adv Exp Med Biol. 2006;557:1–10. doi: 10.1007/0-387-30128-3_1. [DOI] [PubMed] [Google Scholar]
- 36.Vincent AM, McLean LL, Backus C, Feldman EL. Short-term hyperglycemia produces oxidative damage and apoptosis in neurons. FASEB J. 2005;19:638–640. doi: 10.1096/fj.04-2513fje. [DOI] [PubMed] [Google Scholar]
- 37.Nelson TJ, Alkon DL. Protection against beta-amyloid-induced apoptosis by peptides interacting with beta-amyloid. J Biol Chem. 2007;282:31238–31249. doi: 10.1074/jbc.M705558200. [DOI] [PubMed] [Google Scholar]
- 38.Corsetti V, Amadoro G, Gentile A, Capsoni S, Ciotti MT, Cencioni MT, Atlante A, Canu N, Rohn TT, Cattaneo A, Calissano P. Identification of a caspase-derived N-terminal tau fragment in cellular and animal Alzheimer's disease models. Mol Cell Neurosci. 2008;38:381–392. doi: 10.1016/j.mcn.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 39.Yoon S, Choi J, Huh JW, Hwang O, Kim D. Calpain activation in okadaic-acid-induced neurodegeneration. Neuroreport. 2006;17:689–692. doi: 10.1097/01.wnr.0000214398.04093.7f. [DOI] [PubMed] [Google Scholar]
- 40.Wen Y, Yang S, Liu R, Simpkins JW. Transient cerebral ischemia induces site-specific hyperphosphorylation of tau protein. Brain Res. 2004;1022:30–38. doi: 10.1016/j.brainres.2004.05.106. [DOI] [PubMed] [Google Scholar]
- 41.Rissman RA, Poon WW, Blurton-Jones M, Oddo S, Torp R, Vitek MP, LaFerla FM, Rohn TT, Cotman CW. Caspase-cleavage of tau is an early event in Alzheimer disease tangle pathology. J Clin Invest. 2004;114:121–130. doi: 10.1172/JCI20640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mondragon-Rodriguez S, Basurto-Islas G, Santa-Maria I, Mena R, Binder LI, Avila J, Smith MA, Perry G, Garcia-Sierra F. Cleavage and conformational changes of tau protein follow phosphorylation during Alzheimer's disease. Int J Exp Pathol. 2008;89:81–90. doi: 10.1111/j.1365-2613.2007.00568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rametti A, Esclaire F, Yardin C, Terro F. Linking alterations in tau phosphorylation and cleavage during neuronal apoptosis. J Biol Chem. 2004;279:54518–54528. doi: 10.1074/jbc.M408186200. [DOI] [PubMed] [Google Scholar]
- 44.Lee KY, Koh SH, Noh MY, Kim SH, Lee YJ. Phosphatidylinositol-3-kinase activation blocks amyloid beta-induced neurotoxicity. Toxicology. 2008;243:43–50. doi: 10.1016/j.tox.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 45.Chen X, Huang T, Zhang J, Song J, Chen L, Zhu Y. Involvement of calpain and p25 of CDK5 pathway in ginsenoside Rb1's attenuation of beta-amyloid peptide(25-35)-induced tau hyperphosphorylation in cortical neurons. Brain Res. 2008;1200C:99–106. doi: 10.1016/j.brainres.2007.12.029. [DOI] [PubMed] [Google Scholar]
- 46.Sorrentino G, Bonavita V. Neurodegeneration and Alzheimer's disease: the lesson from tauopathies. Neurol Sci. 2007;28:63–71. doi: 10.1007/s10072-007-0789-x. [DOI] [PubMed] [Google Scholar]
- 47.Chun W, Johnson GV. The role of tau phosphorylation and cleavage in neuronal cell death. Front Biosci. 2007;12:733–756. doi: 10.2741/2097. [DOI] [PubMed] [Google Scholar]
- 48.Lewis J, Dickson DW, Lin WL, Chisholm L, Corral A, Jones G, Yen SH, Sahara N, Skipper L, Yager D, Eckman C, Hardy J, Hutton M, McGowan E. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science. 2001;293:1487–1491. doi: 10.1126/science.1058189. [DOI] [PubMed] [Google Scholar]
- 49.Gotz J, Chen F, van Dorpe J, Nitsch RM. Formation of neurofibrillary tangles in P301l tau transgenic mice induced by Abeta 42 fibrils. Science. 2001;293:1491–1495. doi: 10.1126/science.1062097. [DOI] [PubMed] [Google Scholar]
- 50.Oddo S, Caccamo A, Kitazawa M, Tseng BP, LaFerla FM. Amyloid deposition precedes tangle formation in a triple transgenic model of Alzheimer's disease. Neurobiol Aging. 2003;24:1063–1070. doi: 10.1016/j.neurobiolaging.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 51.Quintanilla RA, Dolan PJ, Jin YN, Johnson GV. Truncated tau and Abeta cooperatively impair mitochondria in primary neurons. Neurobiol Aging. 2012;33:619 e625–635. doi: 10.1016/j.neurobiolaging.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Canu N, Dus L, Barbato C, Ciotti MT, Brancolini C, Rinaldi AM, Novak M, Cattaneo A, Bradbury A, Calissano P. Tau cleavage and dephosphorylation in cerebellar granule neurons undergoing apoptosis. J Neurosci. 1998;18:7061–7074. doi: 10.1523/JNEUROSCI.18-18-07061.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garcia-Sierra F, Mondragon-Rodriguez S, Basurto-Islas G. Truncation of tau protein and its pathological significance in Alzheimer0027s disease. J Alzheimers Dis. 2008;14:401–409. doi: 10.3233/jad-2008-14407. [DOI] [PubMed] [Google Scholar]
- 54.McMillan PJ, Kraemer BC, Robinson L, Leverenz JB, Raskind M, Schellenberg G. Truncation of tau at E391 promotes early pathologic changes in transgenic mice. J Neuropathol Exp Neurol. 2011;70:1006–1019. doi: 10.1097/NEN.0b013e31823557fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Park SY, Ferreira A. The generation of a 17 kDa neurotoxic fragment: an alternative mechanism by which tau mediates beta-amyloid-induced neurodegeneration. J Neurosci. 2005;25:5365–5375. doi: 10.1523/JNEUROSCI.1125-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chung CW, Song YH, Kim IK, Yoon WJ, Ryu BR, Jo DG, Woo HN, Kwon YK, Kim HH, Gwag BJ, Mook-Jung IH, Jung YK. Proapoptotic effects of tau cleavage product generated by caspase-3. Neurobiol Dis. 2001;8:162–172. doi: 10.1006/nbdi.2000.0335. [DOI] [PubMed] [Google Scholar]
- 57.Gamblin TC, Berry RW, Binder LI. Tau polymerization: role of the amino terminus. Biochemistry. 2003;42:2252–2257. doi: 10.1021/bi0272510. [DOI] [PubMed] [Google Scholar]
- 58.Saito K, Elce JS, Hamos JE, Nixon RA. Widespread activation of calcium-activated neutral proteinase (calpain) in the brain in Alzheimer disease: a potential molecular basis for neuronal degeneration. Proc Natl Acad Sci U S A. 1993;90:2628–2632. doi: 10.1073/pnas.90.7.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Adamec E, Mohan P, Vonsattel JP, Nixon RA. Calpain activation in neurodegenerative diseases: confocal immunofluorescence study with antibodies specifically recognizing the active form of calpain 2. Acta Neuropathol. 2002;104:92–104. doi: 10.1007/s00401-002-0528-6. [DOI] [PubMed] [Google Scholar]
- 60.David DC, Layfield R, Serpell L, Narain Y, Goedert M, Spillantini MG. Proteasomal degradation of tau protein. J Neurochem. 2002;83:176–185. doi: 10.1046/j.1471-4159.2002.01137.x. [DOI] [PubMed] [Google Scholar]
- 61.Feuillette S, Blard O, Lecourtois M, Frebourg T, Campion D, Dumanchin C. Tau is not normally degraded by the proteasome. J Neurosci Res. 2005;80:400–405. doi: 10.1002/jnr.20414. [DOI] [PubMed] [Google Scholar]
- 62.Freude S, Plum L, Schnitker J, Leeser U, Udelhoven M, Krone W, Bruning JC, Schubert M. Peripheral hyperinsulinemia promotes tau phosphorylation in vivo. Diabetes. 2005;54:3343–3348. doi: 10.2337/diabetes.54.12.3343. [DOI] [PubMed] [Google Scholar]
- 63.Bruce KD, Hanson MA. The developmental origins, mechanisms, and implications of metabolic syndrome. J Nutr. 2010;140:648–652. doi: 10.3945/jn.109.111179. [DOI] [PubMed] [Google Scholar]
- 64.Petersen KF, Shulman GI. Etiology of insulin resistance. Am J Med. 2006;119:S10–16. doi: 10.1016/j.amjmed.2006.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Taubes G. Insulin resistance. Prosperity's plague. Science. 2009;325:256–260. doi: 10.1126/science.325_256. [DOI] [PubMed] [Google Scholar]
- 66.Kim B, Feldman EL. Insulin resistance in the nervous system. Trends Endocrinol Metab. 2012;23:133–141. doi: 10.1016/j.tem.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim B, McLean LL, Philip SS, Feldman EL. Hyperinsulinemia induces insulin resistance in dorsal root ganglion neurons. Endocrinology. 2011;152:3638–3647. doi: 10.1210/en.2011-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim B, Sullivan KA, Backus C, Feldman EL. Cortical neurons develop insulin resistance and blunted Akt signaling: a potential mechanism contributing to enhanced ischemic injury in diabetes. Antioxid Redox Signal. 2011;14:1829–1839. doi: 10.1089/ars.2010.3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hay N. Akt isoforms and glucose homeostasis - the leptin connection. Trends Endocrinol Metab. 2011;22:66–73. doi: 10.1016/j.tem.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zdychova J, Komers R. Emerging role of Akt kinase/protein kinase B signaling in pathophysiology of diabetes and its complications. Physiol Res. 2005;54:1–16. doi: 10.33549/physiolres.930582. [DOI] [PubMed] [Google Scholar]