Abstract
Allergic conjunctivitis (AC), which is characterized by ocular itching, hyperemia, and edema, deteriorates quality of life. In this study, effects of anti-allergic drugs were evaluated by assessing eye-scratching behavior, the number of eosinophils in conjunctiva epithelial tissues, and concentrations of chemical mediators in the tears of the guinea pig model of ovalbumin (OA)-induced AC.
Methodology
On day 0, 3-week-old guinea pigs were sensitized by OA subconjunctival injections. On days 15, 17, and 19, OA solution was administered. Anti-allergic eye drops were administered 5 and 15 min before the final OA challenge on day 19. Scratching behavior within 1 h after OA exposure was studied. Eosinophils in the conjunctiva were stained with Giemsa reagent. Histamine and substance P (SP) concentrations in tears were measured using ELISA.
Results
Subconjunctivally injected guinea pigs were observed for clinical symptoms. Scratching responses significantly reduced with ketotifen or olopatadine treatment. Eosinophil numbers reduced in animals treated with ketotifen, levocabastine, or tranilast. Histamine and/or SP concentrations in tears were inhibited by some of these anti-allergic drugs.
Conclusions
It is important to assess the anti-allergic AC drugs objectively because there are several of these drugs currently available. This model allows for an objective evaluation of anti-allergic drugs for AC.
Keywords: Allergic conjunctivitis, Anti-allergic drugs, Histamine, Substance P
1. Introduction
Allergic conjunctivitis (AC) is one of the most common immune-mediated diseases of the eye. Ocular itching and nasal symptoms can adversely affect the quality of life of patients. Additionally, the incidence of AC has been rapidly increasing during the past 30 years [1].
AC can be categorized into acute and late phase disease. The acute phase is clinically characterized by ocular itching, hyperemia, and edema, which is followed by cellular infiltration into the conjunctiva. The late phase reaction is activated by immunoglobulins and characterized by the presence of inflammatory sequelae and clinical symptoms that manifest several hours after the acute phase. The acute phase can be induced by allergen interactions with mast cell-bound antigen- specific immunoglobulin E (IgE), which triggers mast cell degranulation in the conjunctiva and the release of inflammatory mediators. Histamine is the dominant mediator of allergic reactions [2, 3]. Histamine eye drops had a significant effect on eye scratching behavior and allergy-like symptoms in mice [4, 5]. Histamine can be recognized by four different histamine receptors: H1, H2, H3, and H4 receptors. The acute phase of AC can induce binding of histamine to the H1 receptor. Accordingly, histamine H1 receptor antagonists are being developed for the treatment of various allergic diseases, including AC.
Substance P (SP), a member of the tachykinin peptide family, has also been reported to be a key factor in the development of allergic disorders [6]. It is a sensory neuropeptide involved in the pathogenesis of an allergic response, contributing to tissue damage and/or chronicity [7, 8]. SP enhances lymphocyte proliferation and cytokine secretion from lymphocytes, monocytes, macrophages, and mast cells, and induces the release of inflammatory mediators such as cytokines, oxygen radicals, and arachidonic acid derivatives.
Eosinophils, which are generally absent in the non-allergic normal conjunctiva, intensively infiltrate tissues during the inflammatory process and are thought to be key cells involved in the pathophysiology of AC [9, 10]. The functions of these compounds are well known in the late phase of AC. In the late phase, eosinophils produce toxic cationic proteins (i.e., eosinophilic cationic protein; ECP), oxygen metabolites, and histamine, and may be responsible for chronic symptoms and damage to the conjunctival epithelium [11, 12]. Eosinophils are activated 6 h after allergen exposure and may induce allergy [13]. However, eosinophils appear not only in the late phase, but also in the acute phase of an allergen-induced reaction [13]. We hypothesized that the number of eosinophils could be used as a clinical diagnostic marker to monitor the severity of allergic diseases. Here, we report that the number of these cells can act as a marker of the effects of an anti-allergic drug.
Several anti-allergic drugs are being developed that have H1 receptor antagonist and/or suppressive activities. However, it has been difficult to assess anti-allergic drugs either objectively or relatively, because ocular itching and chronic pain are based on individual patient assessments. This present study suggests that some anti-allergic drugs can be evaluated based on counting eosinophils in the conjunctiva of allergic animals, quantifying instances of scratching behavior, and measuring concentrations of histamine and SP in the tears of animals with AC.
2. Materials and methods
2.1. Reagents
The following reagents were purchased: phosphate buffered saline (PBS) solution (Nissui Pharmaceutical Co., Tokyo, Japan); ovalbumin (OA), aluminum hydroxide gel, isoflurane inhalation, May-Grunwald staining solution, and Giemsa’s staining solution (Wako Pure Chemical Industries, Osaka, Japan).
2.2. Drugs
Ketotifen fumarate 0.05% (Novartis pharmaceuticals, Basel, Switzerland), olopatadine hydrochloride 0.1% (Alcon Laboratories, Fort Worth, TX, USA), levocabastine hydrochloride 0.025% (Santen Pharmaceutical Co., Osaka, Japan), and tranilast 0.5% (Kissei Pharmaceutical Co., Nagano, Japan) were used in these experiments.
2.3. Animals
Male 3-week-old Hartley guinea pigs were purchased from Sankyo Labo Service Corporation (Tokyo, Japan). The Keio University Animal Research Committee approved all animal procedures used in this study. Guinea pigs were euthanized by an overdose of isoflurane inhalation. All animals in this study were treated according to the Association for Research in Vision and Ophthalmology (ARVO) statement for the Use of Animals in Ophthalmic and Vision Research.
2.4. Sensitization and antigen challenge
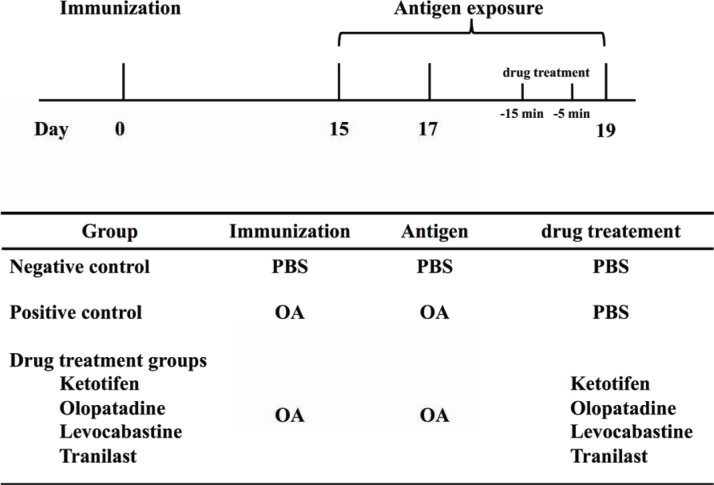
To actively sensitize 3-week-old guinea pigs, subconjunctival injections of 2µg of OA with 2 mg of aluminum hydroxide gel were administered on day 0. On days 15, 17, and 19, 20 µL of 2.5% OA solution was instilled into the conjunctival sac as a challenge for the positive control and drug treatment groups, or PBS was instilled into the conjunctival sac for the control group. On day 19, 20 µL of each drug was administered to conjunctival sac of the animals 5 and 15 min before the final challenge with OA (Figure 1).
Figure 1.
Experimental protocol.
On day 0, three-week-old guinea pigs were injected subconjunctivally with 2µgof OA and 2 mg of aluminum hydroxide gel. On days 15, 17, and 19, AC animals were challenged with OA. On day 19, anti-allergic drug was administered in the drug-treatment groups, and PBS was used in the AC positive control group at 5 and 15 min before OA challenge.
2.5. Counting the instances of scratching behavior and calculating the scratching response
Scratching behavior was evaluated according to the method described by Kuraishi et al, with minor modifications [14]. Animals were housed in a cage in the observation room to acclimatize for 2 h before the challenge with 2.5% OA, after which their behavior was recorded.
Animals were recorded by video and monitored for scratching reactions. Scratching behavior was defined as an interrupted cluster of rapid hind limb movements that were precisely directed to the eye. Scratching behavior was counted within 1 h from OA exposure on days 15 and 19. Scratching responses were calculated based on the number of such behaviors on day 19 divided by the number of these behaviors recorded on day 15 for the same individual. This experiment was independently performed six times.
2.6. Eosinophil staining
Eosinophils were stained and measured according to the method described by Kari and Saari with minor modifications [15]. Briefly, conjunctival epithelial cells were collected from the upper palpebral conjunctiva using a cotton-wool swab 4 h after the final OA challenge on day 19. Swab samples were spread on glass slides and fixed, and then Giemsa’s staining was carried out using May-Grunwald and Giemsa’s staining solutions. This experiment was independently performed six times.
2.7. Measurements of histamine and SP concentrations in tears
On day 19, 5 µL of tears was sampled from the eyes of animals 15 min after OA challenge, and were rapidly analyzed for histamine or SP concentrations.
Histamine concentrations were determined using a commercial histamine measurement kit (Kikkoman Co., Chiba) according to the manufacturer’s instructions. Sampled cells were quantified based on absorbance at 470 nm by using a UV-1280 spectrophotometer (Shimadzu Co., Kyoto, Japan). SP concentrations in tears were measured using a commercial Substance P ELISA (Caymen Chemical Co., Ann Arbor, MI) based on the competitive binding technique and according to the manufacturer’s instructions. ELISA plates were washed using PBS, and absorbance at 412 nm for each well was measured using an infinite M1000 microplate reader (TECAN, Månnedorf, Switzerland).
2.8. Statistical analysis
Experiments were repeated at least three times. Data were expressed as mean ± S.E.M. values. For comparisons of two means, an unpaired t-test was used. Means of groups of four samples were compared using a one-way analysis of variance (ANOVA) test, which was performed to identify any significant differences between each paired combination of sample means. To analyze the clinical symptoms, a non-parametric multiple comparison test was used. A value of P < 0.05 was accepted to indicate a statistically significant difference.
3. Results
3.1. OA-induced experimental AC
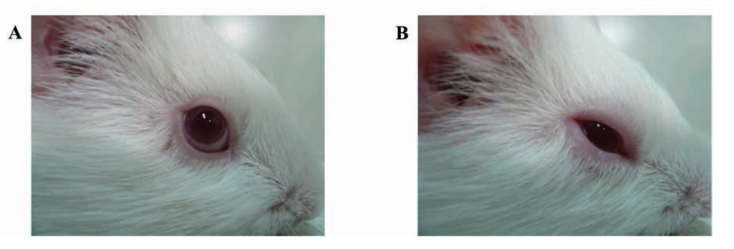
To investigate the effects of topical anti-allergic drugs on AC, guinea pigs were sensitized by subconjunctival injection of OA and aluminum hydroxide gel on day 0. On days 15, 17, and 19, 2.5% OA solution was administered. At 5 and 15 min before the final OA challenge on day 19, an anti-allergic drug or PBS was administrated to animals (Figure 1). OA-sensitized guinea pigs treated with PBS (positive control animals) showed significant clinical symptoms, such as eye redness, edema, and sensory eye activation, which resulted in more itching compared with that observed in the negative controls (Figures 2A and 2B).
Figure 2.
Clinical symptoms in OA-induced AC animals.
On day 19, 30 min after OA challenge, the eye appearance was noted in the (A) negative and (B) positive control groups. The positive control animals clearly displayed symptoms of AC.
3.2. Scratching responses
Scratching reactions were counted for 1 h after exposure to OA on days 15 and 19. They were calculated as the number of reactions on day 19 divided by the number of reactions on day 15 in the same animal. Scratching responses in the positive control group were significantly higher than those in the negative control group. However, after treatment with ketotifen or olopatadine eye drop treatment, scratching responses significantly reduced in the AC model guinea pigs compared with those in the non-treatment group (i.e., the positive control group; Figure 3).
Figure 3.
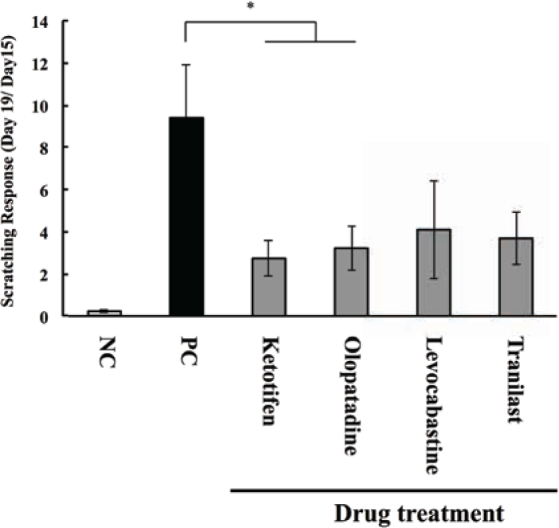
Effects of anti-allergic drugs on the scratching response.
Guinea pigs were sensitized by injecting OA and aluminum hydroxide gel into the conjunctival sac. AC was elicited by the instillation of OA solution on days 15, 17, and 19, and scratching behavior was counted for 1 h on days 15 and 19. Scratching responses were calculated as follows: (the number of scratching behaviors on day 19) / (the number of scratching behaviors on day 15); * P < 0.05 vs. positive control group. This experiment was independently performed six times (n = 12 - 14).
3.3. Quantification of eosinophils in conjunctival epithelial tissues
On day 19, 4 h after the final OA exposure, eosinophils were collected from the upper palpebral conjunctival epithelial tissues. After harvest, samples were fixed in 95% methanol and Giemsa staining was performed. The number of eosinophils was 5.25 ± 4.67 in the conjunctiva of the negative control group compared with 180.5 ± 40.2 in the OA-induced AC model animals (i.e., the positive control group). The number of eosinophils among conjunctival epithelial cells reduced after drug treatment: there were 66.3 ± 13.5, 110.3 ± 25.1, 48.3 ± 12.0, and 31.11 ± 10.1 eosinophils in AC guinea pigs treated with ketotifen, olopatadine, levocabastine, and tranilast, respectively (Figure 4).
Figure 4.
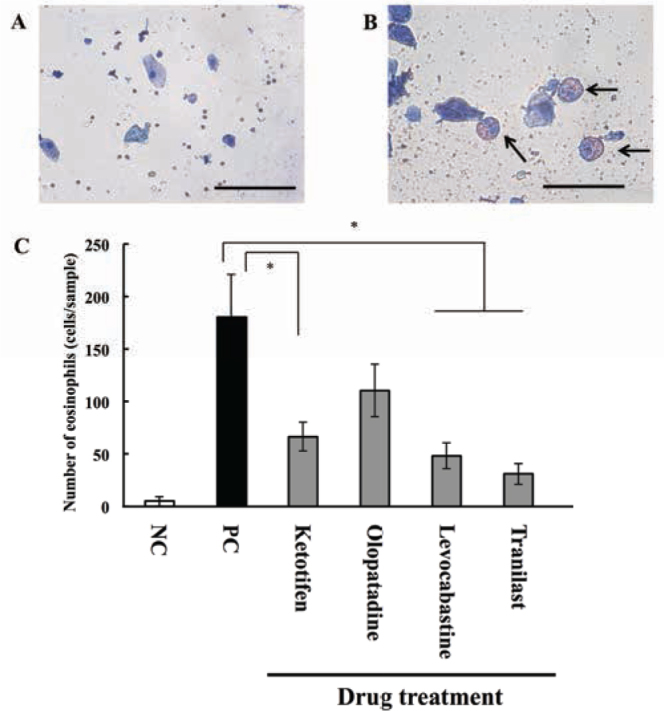
Effects of anti-allergic drugs on eosinophils in conjunctiva.
Guinea pigs were sensitized by injecting OA and aluminum hydroxide gel into the conjunctival sac. AC was elicited by the instillation of OA solution on days 15, 17, and 19. At 4 h after the final challenge (i.e., day 19), eosinophils were collected from the palpebral conjunctival epithelial tissue. Samples from the (A) negative and (B) positive control groups were stained using Giemsa’s solution (scale bars, 50 µm). (C) The number of eosinophils in conjunctival epithelial tissues from AC animals with or without eye drop drug treatment. * P < 0.05 vs. positive control group. Data represent means ± S.E.M from three independent experiments (n = 9 in the negative control group; and n = 12 in the positive control and drug treatment groups).
3.4. Histamine concentrations in tears
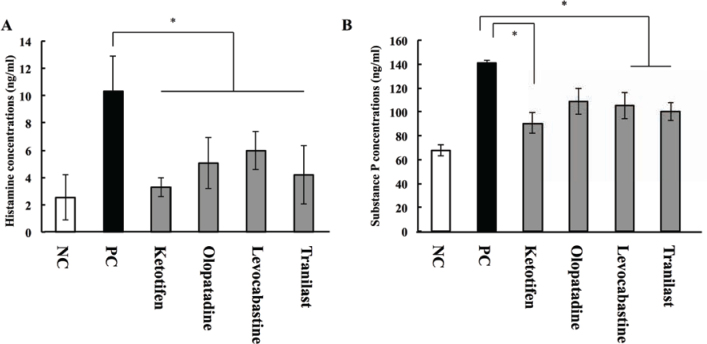
The concentration of histamine in tears was measured 15 min after the final OA challenge on day 19 using a histamine measurement kit. Concentrations in the negative and positive control animals were 2.52 ± 1.65 and 10.33 ± 2.56 ng/mL, respectively. After treatment with anti-allergic drugs, histamine concentrations were significantly lower. In AC model animals treated with ketotifen, olopatadine, levocabastine, and tranilast, concentrations of histamine were 3.28 ± 0.68, 5.04 ± 1.85, 5.96 ± 1.38, and 4.19 ± 2.13 ng/mL, respectively (Figure 5A). No significant differences were detected between the different drug treatment groups.
Figure 5.
Effects of anti-allergic drugs on histamine and substance P concentrations in tears.
On day 19, 15 min after the final challenge, concentrations of (A) histamine and (B) substance P were measured by ELISA. * P < 0.05 vs. positive control group. Data represent means ± S.E.M from three independent experiments (n = 12 per group).
3.5. SP concentrations in tears
Concentrations of SP in tears on day 19 were measured by ELISA. The concentration of SP was 67.7 ± 4.76 ng/ mL in the negative control group, and 140.9 ± 2.13 ng/ mL in OA-induced allergic animals that were not treated with drugs (positive control group). SP concentrations decreased after anti-allergic treatment: they were 90.5 ± 8.44, 109.0 ± 10.9, 104.8 ± 11.0, and 100.0 ± 7.36 ng/mL after treatment of AC animals with ketotifen, olopatadine, levocabastine, and tranilast, respectively (Figure 5B). No significant difference in SP concentrations was detected between the groups.
4. Discussion
AC affects 15–20% of the population in developed countries. In humans, AC is characterized by intense ocular itching, hyperemia, and edema that impair the quality of life of patients. Ocular itching and/or pain are subjectively estimated by individuals, so it is important to identify objective measurements to evaluate the effects of anti-allergic eye drops for AC.
Guinea pigs in the OA-induced AC model were observed for clinical symptoms that resembled human AC and eosinophils in conjunctival epithelial tissues were quantified (Figure 2). We used this animal model to objectively evaluate the effects of anti-allergic drugs on the scratching response, number of eosinophils in the conjunctiva, and concentrations of histamine and SP in tears.
Histamine and SP are well established to be associated with allergic reactions that involve the nasal mucosa and conjunctiva. Recently, novel allergen mice were reported in a study on the interaction between nasal and conjunctiva inflammation in allergic rhinoconjunctivitis [16]. They reported that chemical mediators were increased in both conjunctiva and trigeminal ganglion following nasal allergen challenge, and these concentrations were reduced after ketotifen treatment. In our present study, concentrations of chemical mediators, such as histamine and SP, were decreased in tears of AC animals treated with anti-allergic eye drops, and this reduction was correlated with changes in scratching behavior.
Histamine is significantly elevated in the acute phase after allergen exposure. The concentration of histamine first peaks at 10 min and then peaks again at 4 h, after which it returns to baseline at 8 h [17]. Histamine depolarizes the sensory nerves that contain histamine H1 receptors, and the resulting neural wave of depolarization is centrally converted to a secretovasometer. Itch centers in the brain and nerve impulses are transmitted to autonomic nerves that innervate the walls of vessels and submucosal glands, which can induce the secretion of various neurotransmitters from nerve endings. These neurotransmitters can trigger vasodilation, increased vascular permeability, and hypersecretion by submucosal glands, producing symptoms of an allergic reaction [18]. Intravenous histamine administration can induce allergic reactions in guinea pigs [19]. SP is also elevated during the acute phase and is widely distributed in the immune [20] and hematopoietic [21, 22] systems. Moreover, SP has been reported to be a chemical mediator involved in allergic inflammation that contributes to the severity of ocular allergy symptoms [6, 23]. Thus, blockade of the H1 receptor and/or release of histamine and SP could potentially inhibit downstream reactions from centers of the brain.
According to our data, scratching behavior was significantly inhibited by ketotifen and olopatadine treatment (Figure 3). The concentration of histamine in tears was significantly reduced by treatment with each of the anti-allergic drugs used in this study, while the concentration of SP was reduced after ketotifen and tranilast treatments (Figure 5). Ketotifen and olopatadine utilize independent pharmacological mechanisms to antagonize the histamine H1 receptor and act as a suppressor of chemical mediators releasing from mast cells [24, 25]. By contrast, levocabastine acts as a selective H1 receptor antagonist [26] and tranilast can inhibit the degranulation and release of histamine from mast cells [27]. Thus, we found that anti-allergic drugs with H1 receptor antagonist and chemical mediator suppressive activities efficiently inhibited scratching behavior and reduced concentrations of histamine and SP in tears.
IgE is known to play a major role in allergic disease, and exerts activities such as eosinophil recruitment and activation [28]. We attempted to alter the levels of OA-specific IgE antibody in the serum; however, levels of OA-specific IgE antibody were not significantly different between the negative control, positive control, and drug treatment groups (data not shown). Moreover, eye drop administration of anti-allergic drug could significantly prevent the appearance of eosinophils in the conjunctiva (Figure 4). Our findings suggested that eye drop drugs could specifically affect the conjunctiva, but not the whole body.
AC reduces the quality of vision in patients and considerably increases economic expenses, even though it is considered the most benign condition among all ocular allergic diseases. There are several anti-allergic AC drugs currently available, and it is important to be able to objectively assess these drugs. Here, we could clearly evaluate the objective effect of anti-allergic drugs on AC. This model will be useful for optimizing and expediting drug development for AC.
Abbreviation list
- AC
allergic conjunctivitis
- OA
ovalbumin
- SP
substance P
Footnotes
Conflict of interest: Authors state no conflict of interest.
References
- [1].Sears M.R.. Epidemiology of childhood asthma. Lancet. 1997;350:1015–1020. doi: 10.1016/S0140-6736(97)01468-2. [DOI] [PubMed] [Google Scholar]
- [2].Proud D., Sweet J., Stein P., Settipane R.A., Kagey-Sobotka A., Friedlaender M.H.. et al. Inflammatory mediator release on conjunctival provocation of allergic subjects with allergen. J. Allergy Clin. Immunol. 1990;85:896–905. doi: 10.1016/0091-6749(90)90075-F. [DOI] [PubMed] [Google Scholar]
- [3].Woodward D.F., Nieves A.L., Spada C.S., Williams L.S., Tuckett R.P.. Characterization of a behavioral model for peripherally evoked itch suggests platelet-activating factor as a potent pruritogen. J. Pharmacol. Exp. Ther. 1995;272:758–765. [PubMed] [Google Scholar]
- [4].Nakano Y., Takahashi Y., Ono R., Kurata Y., Kagawa Y., Kamei C.. Role of histamine H(4) receptor in allergic conjunctivitis in mice. Eur. J. Pharmacol. 2009;608:71–75. doi: 10.1016/j.ejphar.2009.02.035. [DOI] [PubMed] [Google Scholar]
- [5].Tomljenovic D., Baudoin T., Megla Z.B., Vagic D., Hellings P., Kalogjera L.. Nasal and ocular responses after specific and nonspecific nasal challenges in seasonal allergic rhinitis. Ann. Allergy Asthma. Immunol. 2016;116:199–205. doi: 10.1016/j.anai.2015.12.022. [DOI] [PubMed] [Google Scholar]
- [6].Fujishima H., Takeyama M., Takeuchi T., Saito I., Tsubota K.. Elevated levels of substance P in tears of patients with allergic conjunctivitis and vernal keratoconjunctivitis. Clin. Exp. Allergy. 1997;27:372–378. doi: 10.1111/j.1365-2222.1997.tb00721.x. [DOI] [PubMed] [Google Scholar]
- [7].Taylor A.W.. Ocular immunosuppressive microenvironment. Chem. Immunol. Allergy. 2007;92:71–85. doi: 10.1159/000099255. [DOI] [PubMed] [Google Scholar]
- [8].Scott J.R., Muangman P., Gibran N.S.. Making sense of hypertrophic scar: a role for nerves. Wound Repair Regen. 2007;15:S27–S31. doi: 10.1111/j.1524-475X.2007.00222.x. [DOI] [PubMed] [Google Scholar]
- [9].Ono S.J., Abelson M.B.. Allergic conjunctivitis: update on pathophysiology and prospects for future treatment. J. Allergy Clin. Immunol. 2005;115:118–122. doi: 10.1016/j.jaci.2004.10.042. [DOI] [PubMed] [Google Scholar]
- [10].Choi S.H., Bielory L.. Late-phase reaction in ocular allergy. Curr. Opin. Allergy Clin. Immunol. 2008;8:438–444. doi: 10.1097/ACI.0b013e32830e6b3a. [DOI] [PubMed] [Google Scholar]
- [11].Tomassini M., Magrini L., De Petrillo G., Adriani E., Bonini S., Balsano F.. et al. Serum levels of eosinophil cationic protein in allergic diseases and natural allergen exposure. J. Allergy Clin. Immunol. 1996;97:1350–1355. doi: 10.1016/S0091-6749(96)70204-X. [DOI] [PubMed] [Google Scholar]
- [12].Cook E.B.. Tear cytokines in acute and chronic ocular allergic inflammation. Curr. Opin. Allergy Clin. Immunol. 2004;4:441–445. doi: 10.1097/00130832-200410000-00018. [DOI] [PubMed] [Google Scholar]
- [13].Malm-Erjefält M., Greiff L., Ankerst J., Andersson M., Wallengren J., Cardell L.O.. et al. Circulating eosinophils in asthma, allergic rhinitis, and atopic dermatitis lack morphological signs of degranulation. Clin. Exp. Allergy. 2005;35:1334–1340. doi: 10.1111/j.1365-2222.2005.02335.x. [DOI] [PubMed] [Google Scholar]
- [14].Kuraishi Y., Nagasawa T., Hayashi K., Satoh M.. Scratching behavior induced by pruritogenic but not algesiogenic agents in mice. Eur. J. Pharmacol. 1995;275:229–233. doi: 10.1016/0014-2999(94)00780-B. [DOI] [PubMed] [Google Scholar]
- [15].Kari O., Saari K.M.. Clinical measurement of eosinophil numbers in eosinophilic conjunctivitis. Methods Mol, Biol. 2014;1178:197–202. doi: 10.1007/978-1-4939-1016-8_17. [DOI] [PubMed] [Google Scholar]
- [16].Callebaut I., De Vries A., Steelant B., Hox V., Bobic S., Van Gerven L.. et al. Nasal allergen deposition leads to conjunctival mast cell degranulation in allergic rhinoconjunctivitis. Am. J. Rhinol. Allergy. 2014;28:290–296. doi: 10.2500/ajra.2014.28.4052. [DOI] [PubMed] [Google Scholar]
- [17].Helleboid L., Khatami M., Wei Z.G., Rockey J.H.. Histamine and prostacyclin. Primary and secondary release in allergic conjunctivitis. Invest. Ophthalmol. Vis. Sci. 1991;32:2281–2289. [PubMed] [Google Scholar]
- [18].Chaen T., Watanabe N., Mogi G., Mori K., Takeyama M.. Substance P and vasoactive intestinal peptide in nasal secretions and plasma from patients with nasal allergy. Ann. Otol. Rhinol. Laryngol. 1993;102:16–21. doi: 10.1177/000348949310200104. [DOI] [PubMed] [Google Scholar]
- [19].Van Oosterhout A.J., van Ark I., Hofman G., Van Der Linde H.J., Fattah D., Nijkamp F.P.. Role of interleukin-5 and substance P in development of airway hyperreactivity to histamine in guinea-pigs. Eur. Respir. J. 1996;9:493–499. doi: 10.1183/09031936.96.09030493. [DOI] [PubMed] [Google Scholar]
- [20].McGillis J.P., Mitsuhashi M., Payan D.G.. Immunomodulation by tachykinin neuropeptides. Ann. NY. Acad. Sci. 1990;594:85–94. doi: 10.1111/j.1749-6632.1990.tb40470.x. [DOI] [PubMed] [Google Scholar]
- [21].Rameshwar P., Ganea D., Gascón P.. In vitro stimulatory effect of substance P on hematopoiesis. Blood. 1993;81:391–398. [PubMed] [Google Scholar]
- [22].Rameshwar P., Gascón P.. Substance P (SP) mediates production of stem cell factor and interleukin-1 in bone marrow stroma: potential autoregulatory role for these cytokines in SP receptor expression and induction. Blood. 1995;86:482–490. [PubMed] [Google Scholar]
- [23].Foreman J.C.. Substance P and calcitonin gene-related peptide: effects on mast cells and in human skin. Int. Arch. Allergy Appl. Immunol. 1987;82:366–371. doi: 10.1159/000234229. [DOI] [PubMed] [Google Scholar]
- [24].Grant S.M., Goa K.L., Fitton A., Sorkin E.M.. Ketotifen. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in asthma and allergic disorders. Drugs. 1990;40:412–448. doi: 10.2165/00003495-199040030-00006. [DOI] [PubMed] [Google Scholar]
- [25].Brockman H., Graff G., Spellman J., Yanni J.. A comparison of the effects of olopatadine and ketotifen on model membranes. Acta Ophthalmol. Scand. Suppl. 2000;230:10–15. doi: 10.1034/j.1600-0420.2000.078s230010.x. [DOI] [PubMed] [Google Scholar]
- [26].Dechant K.L., Goa K.L.. Levocabastine. A review of its pharmacological properties and therapeutic potential as a topical antihistamine in allergic rhinitis and conjunctivitis. Drugs. 1991;41:202–224. doi: 10.2165/00003495-199141020-00006. [DOI] [PubMed] [Google Scholar]
- [27].Zampini P., Riviera A.P., Tridente G.. In vitro inhibition of histamine release from mouse mast cells and human basophils by an anthranilic acid derivative. Int. J. Immunopharmacol. 1983;5:431–435. doi: 10.1016/0192-0561(83)90019-x. [DOI] [PubMed] [Google Scholar]
- [28].Chen J., Zhang J., Zhao R., Jin J., Yu Y., Li W.. et al. Topical Application of Interleukin-28A Attenuates Allergic Conjunctivitis in an Ovalbumin-Induced Mouse Model. Invest. Ophthalmol. Vis. Sci. 2016;57:604–610. doi: 10.1167/iovs.15-18457. [DOI] [PubMed] [Google Scholar]