Abstract
Synthetically modified mRNA is a unique bioactive agent, ideal for use in therapeutic applications, such as cancer vaccination or treatment of single-gene disorders. In order to facilitate mRNA transfections for future therapeutic applications, there is a need for the delivery system to achieve optimal transfection efficacy, perform with durable stability, and provide drug safety. The objective of our study was to comprehensively analyze the use of 3β-[N-(N',N'-dimethylaminoethane) carbamoyl](DC-Cholesterol)/dioleoylphosphatidylethanolamine (DOPE) liposomes as a potential transfection agent for modified mRNAs. Our cationic liposomes facilitated a high degree of mRNA encapsulation and successful cell transfection efficiencies. More importantly, no negative effects on cell viability or immune reactions were detected posttransfection. Notably, the liposomes had a long-acting transfection effect on cells, resulting in a prolonged protein production of alpha-1-antitrypsin (AAT). In addition, the stability of these mRNA-loaded liposomes allowed storage for 80 days, without the loss of transfection efficacy. Finally, comprehensive analysis showed that these liposomes are fully hemocompatible with fresh human whole blood. In summary, we present an extensive analysis on the use of DC-cholesterol/DOPE liposomes as mRNA delivery vehicles. This approach provides the basis of a safe and efficient therapeutic strategy in the development of successful mRNA-based drugs.
Introduction
Modified mRNAs have recently gained enormous potential for therapeutic applications in the fields of vaccination1, 2 and disease treatments.3, 4, 5, 6 The use of synthetic mRNAs in therapy, aiming to induce production of a specific protein of interest, instead of gene therapy, was described more than two decades ago by Wolff et al.7 Under normal physiological conditions, this novel therapeutic strategy enables the cellular machinery to produce a specific protein, after the delivery of mRNA to the cytosol. Hence, the genome is not altered, which is a decisive advantage as compared to gene therapy.8 More recently, specific chemically modified mRNAs that encode for the specific proteins can be generated in large yields, and modifications can be performed to reduce immunogenicity, as well as to increase stability.9, 10
The use of therapeutic mRNA allows the treatment of diseases associated with missing or defective membrane or cytoplasmic proteins,11 which is not covered by current substitution therapies. Therefore, mRNA therapy will provide a new and highly promising therapeutic approach in the treatment of monogenetic diseases, such as familial hypercholesterolemia (FH) or alpha-1-antitrypsin deficiency (AATD). Many single-gene disorders are associated with early-onset but chronic diseases, which may lead to severe conditions and therefore necessitate expensive lifelong care. Until now, therapeutic treatment options have been limited for such single-gene disorders.12 Hence, mRNA therapy could be a highly beneficial alternative. It results in functional protein production by hepatocytes after intravenous (i.v.) injection of the complexed protein-encoding mRNA.13 In order to be effective, the requirement of such an mRNA-based drug should comprise higher safety, therapeutic efficiency, long availability, improved complexity of drug administration, and lower costs.
In order for the clinical translation of mRNA applications to occur, the engineering of a safe and effective delivery vehicle is required to guarantee protection against nucleases, cellular uptake, and prolonged availability of the mRNA for efficient translation, as well as compatibility with human blood. Another elegant approach would be a vehicle that is able to provide long-lasting and efficient translation of mRNA, as well as being fully compatible with human blood. In current DNA or small interfering RNA (siRNA) applications, cellular transfection has been achieved with both viral and non-viral vectors. However, viral vectors have limitations, including carcinogenic and immunogenic properties,14, 15 difficult production,16 and limited packaging capacity.17, 18 For non-viral approaches, DNA or RNA is frequently encapsulated with positively charged liposomes and polysomes to form lipoplexes or polyplexes, respectively.19 The cellular uptake is mainly mediated by either lipoplex endocytosis or endocytosis-like mechanisms.20, 21 Furthermore, liposome delivery systems have substantial advantages like low batch variability, easy synthesis, scalability, and biocompatibility.22 It has also been noted that drug delivery systems in the nano-range enhance the pharmacokinetic availability of the encapsulated drugs.22 Additionally, different drugs encapsulated within liposomes, like siRNA for liver cancer (Alnylam) or amphotericin B for fungal infection (Gilead), were tested by the Food and Drug Administration (FDA) in clinical trials.23
Many groups, including ours, understand that the translation of mRNA-based therapeutic agents is also dependent on an efficient delivery system, as well as a safe cellular uptake.17 Therefore, we developed a delivery system for the transfection of therapeutic mRNA using 3β-[N-(N',N'-dimethylaminoethane) carbamoyl](DC-Cholesterol)/dioleoylphosphatidylethanolamine (DOPE) liposomes. We also give a comprehensive analysis of the advantages of our mRNA-lipoplexes, looking at their transfection efficiency, immunogenicity, duration of protein expression, hemocompatibility, and storage stability. Our data show that the lipoplexes exhibit high transfection efficiencies and no adverse effects in vitro, demonstrating their safety for further in vivo investigations.
Results
Characterization and Transfection Evaluation of Liposomes
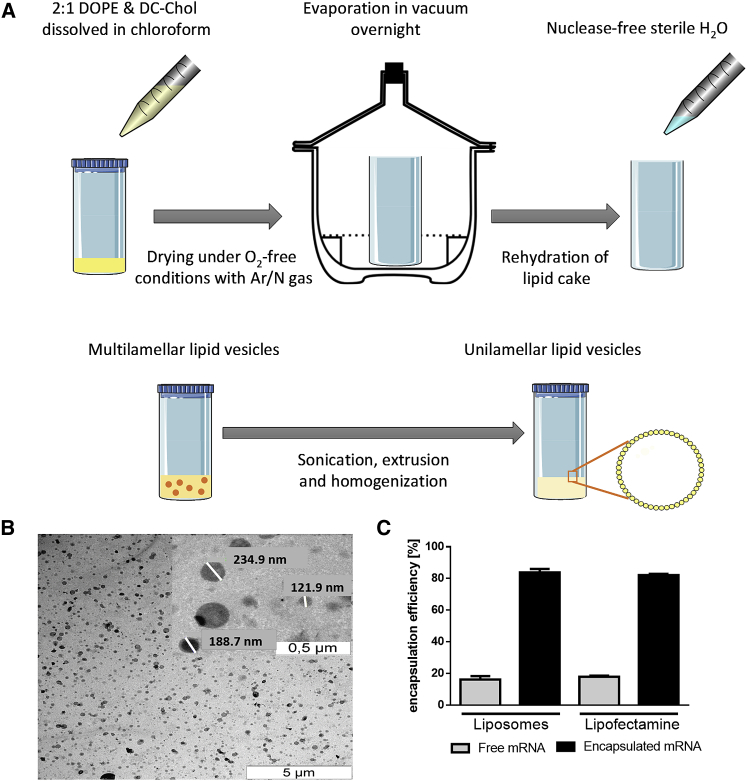
The liposomes were generated with the dry-film method and were extruded through a membrane with a pore size of 200 nm. This procedure creates a homogeneous unilamellar liposome solution (Figure 1A). The size and morphology of the liposomes were analyzed with transmission electron microscopy (TEM). The liposomes are round-shaped and are 181.8 ± 39.95 nm in size (Figure 1B). Additionally, the encapsulation efficacy of the liposomes was measured and compared with Lipofectamine 2000. Therefore, the samples were incubated with RNA-binding fluorescence dye, and the amount of free mRNA was calculated using a standard curve. It was shown that the liposomes have an equivalent capability to encapsulate mRNA like commercially available Lipofectamine 2000. After complexation of mRNA with liposomes, only 16.19% ± 3.58% of free mRNA was detected. This is comparable to the complexation of mRNA with Lipofectamine 2000, where 17.88% ± 1.07% of free mRNA was detected (Figure 1C).
Figure 1.
Preparation and Characterization of Liposomes
(A) Schematic overview of the liposome production process including lipid preparation and mixing, drying under O2-free condition, and evaporation overnight in vacuum. The developed lipid cake was rehydrated with water followed by sonification, extrusion, and homogenization to get unilamellar lipid vesicles. (B) Visualization of the liposomes using TEM after negative staining. (C) Analysis of the mRNA encapsulation efficacy of liposomes compared to Lipofectamine 2000. Data are shown as mean ± SEM (n = 5).
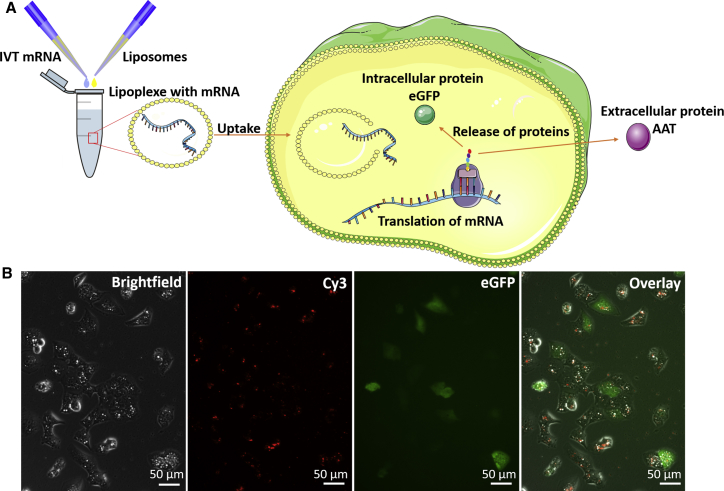
For successful cell transfection, the uptake of liposomes loaded with mRNA via endocytosis is a key factor and was analyzed after the generation of lipoplexes composed of liposomes and EGFP- or AAT-encoding mRNA. After the lipoplexes are incorporated into the cytosol and the lipid layer of the lipoplexes is degraded, the mRNA is released into the cytosol. Then, the translation of mRNA into protein by ribosomes begins. Afterward, the EGFP protein stays inside the cell, and the AAT protein is secreted into the extracellular space (Figure 2A). To prove the ability of liposomes to transfect cells with mRNA, cyanine 3 (Cy3)-labeled EGFP-encoding mRNA was used. Therefore, the labeled mRNA was encapsulated in liposomes, and the cells were transfected with lipoplexes for 24 hr. After that, fluorescence microscopy was performed to detect the Cy3-labeled mRNA and the translated EGFP protein inside the cells. Both Cy3-labeled mRNA and the EGFP protein were detected inside the cells 24 hr after transfection with lipoplexes (Figure 2B).
Figure 2.
Transfection of Cells with Liposomes for Induced Expression of Different Proteins
(A) The schematic overview shows the process of mRNA incorporation in liposomes and subsequent transfection and mRNA translation. During transfection, the lipoplexes are endocytosed by the cells of interest. Inside the cell, the lipid layer is degraded and mRNA is released. In the cytosol, the mRNA is translated by ribosomes and the protein is subsequently released intra- or extracellularly. (B) The presence of Cy3-labeled EGFP-encoding mRNA after liposome uptake and expression of EGFP protein in cells 24 hr posttransfection.
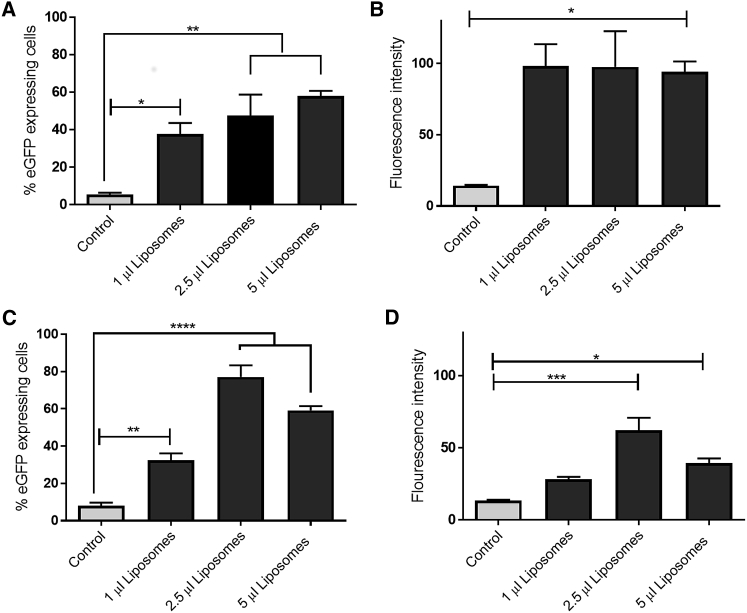
To determine the optimal ratio of liposomes to mRNA for best cellular transfection, we complexed different amounts of liposomes (1–5 μL) with 1 μg of EGFP mRNA and added them to the cells in full medium for 48 or 120 hr. The percentage of EGFP-expressing cells was significantly increased to 37.8% ± 7.2% at 48 hr after EGFP mRNA transfection with 1 μL of liposomes. However, the use of 2.5 or 5 μL of liposomes resulted in an even higher number of transfected cells, i.e., 47.7% ± 14.8% and 57.9% ± 3.7%, respectively (Figure 3A). The fluorescence intensity was similar in all tested liposome concentrations (Figure 3B). After 120 hr of transfection, the number of EGFP-expressing cells increased significantly to 32.6% ± 4.7% with 1 μL of liposomes to 77.1% ± 8.26% with 2.5 μL of liposomes and to 59.21% ± 2.98% using 5 μL of liposomes (Figure 3C). The highest fluorescence intensity was detected in cells transfected with 2.5 μL of liposomes (Figure 3D). Because the best transfection efficiencies were achieved when 2.5 μL of liposomes was used for the encapsulation of 1 μg of mRNA, this liposome volume was used for all further transfection experiments.
Figure 3.
Transfection Efficacy of Liposomes Containing EGFP mRNA at Different Time Points
(A–D) Flow cytometric analysis showing % eGFP-expressing cells (A) and fluorescence intensity (B) of A549 cells transfected with different amount of liposomes containing 1 μg eGFP-encoding mRNA 48 h posttransfection. In addition, % eGFP-expressing cells (C) and fluorescence intensity (D) were measured 120 h after transfection.
Data are shown as mean ± SEM (n = 5). *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
Effect of Liposomes on Immunogenic Reactions and Cell Viability after Long-Time Transfection
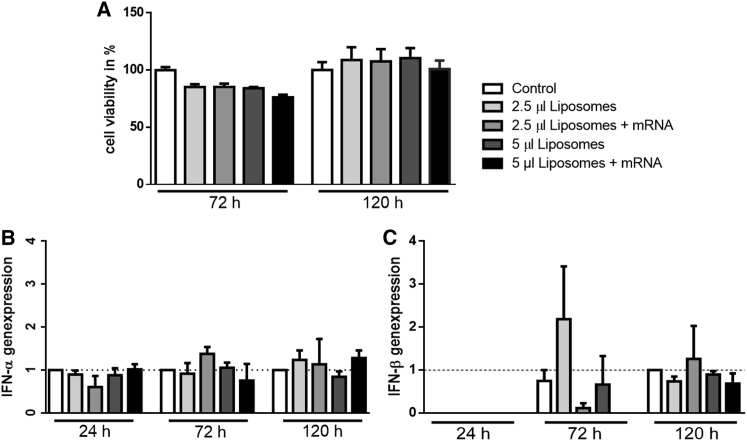
The viability and immunogenic reactions of cells were analyzed with the help of the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay and real-time qPCR, respectively. To detect effects of liposomes on cell viability, the cells were transfected with 2.5 and 5 μL of empty liposomes, as well as with 2.5 and 5 μL of liposomes containing 1 μg of EGFP mRNA in full media for 72 and 120 hr. Our data show that neither liposomes nor liposomes containing mRNA significantly influence cell viability (Figure 4A).
Figure 4.
Investigations of Immunogenic and Toxic Effects of Liposomes on Cells after Different Time Points
(A) Viability measurement of cells was performed after transfection for 72 or 120 hr with liposomes and lipoplexes. (B and C) Relative normalized gene expressions of IFN-α (B) or IFN-β (C) in cells after long-time transfection from 24 to 120 hr with liposomes were achieved using real-time qPCR. Data are shown as mean ± SEM (n = 5).
For the detection of possible immune reactions, cells were treated with empty liposomes or liposomes containing 1 μg of EGFP mRNA. After 24, 72, and 120 hr, the RNA of the cells was isolated and reverse transcribed into copy DNA. After that, the expression of early inflammatory markers, i.e., interferon-α (IFN-α), interferon-β (IFN-β), tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as a housekeeping gene, was analyzed using real-time qPCR. Only a very weak, insignificant increase in IFN-α and IFN-β was detected 72 hr after transfection of cells with 2.5 μL of empty as well as mRNA-loaded liposomes (Figures 4B and 4C). No increase in gene expression of TNF-α or IL-6 was observed at any time point (data not shown).
Usability of Liposomes for Therapeutic mRNA Applications
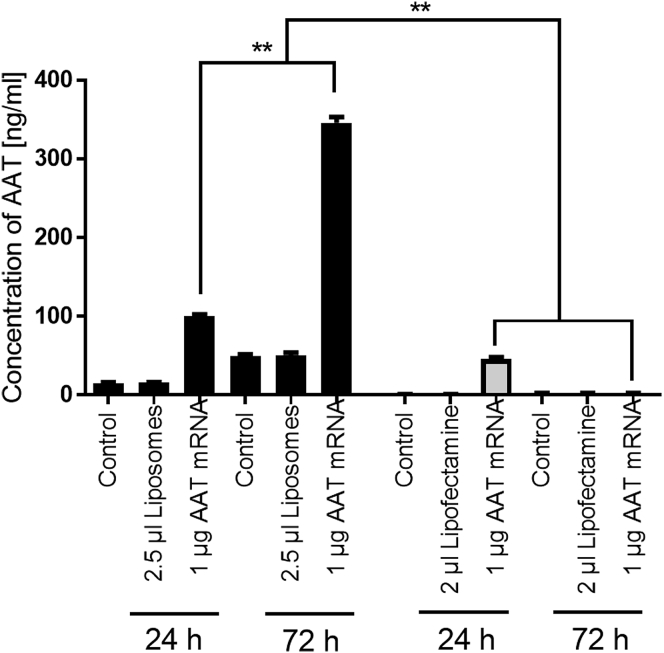
In order to analyze the ability of the liposomes for therapeutic applications, we used an AAT-encoding mRNA, which could also be employed for the effective treatment of AATD.3 In order to analyze the time of AAT production, which is a prerequisite for subsequent drug development, we transfected cells with either 2.5 μL of empty liposomes or 2.5 μL of liposomes loaded with 1 μg of AAT-encoding mRNA. Alongside liposome testing, cells were further treated with pure Lipofectamine 2000 or Lipofectamine 2000 containing AAT-encoding mRNA. The concentration of AAT protein in the cell supernatant of all samples was analyzed 24 and 72 hr posttransfection (Figure 5). Our data show that the cells treated with AAT-encoding mRNA encapsulated in liposomes as well as Lipofectamine 2000 produce a similar amount of AAT protein, which was significantly higher when compared to the corresponding controls 24 hr after transfection. Interestingly, an increase in AAT protein concentration of even more than 3.5 times (from 100.75 ± 2.37 ng/mL to 345.96 ± 8.52 ng/mL) was detected 72 hr after transfection. However, in the supernatants of cells transfected with AAT-encoding mRNA encapsulated with Lipofectamine 2000, no AAT protein was measured 72 hr posttransfection.
Figure 5.
Analysis of AAT Expression after Transfection with Liposomes or Lipofectamine 2000 Containing AAT-Encoding mRNA
Concentration of AAT protein in cell supernatants after transfection with AAT mRNA encapsulated in liposomes or Lipofectamine 2000 for 24 or 72 hr. Data are shown as mean ± SEM (n = 5). **p < 0.01.
Optimal Storage Conditions for mRNA Encapsulated in Liposomes
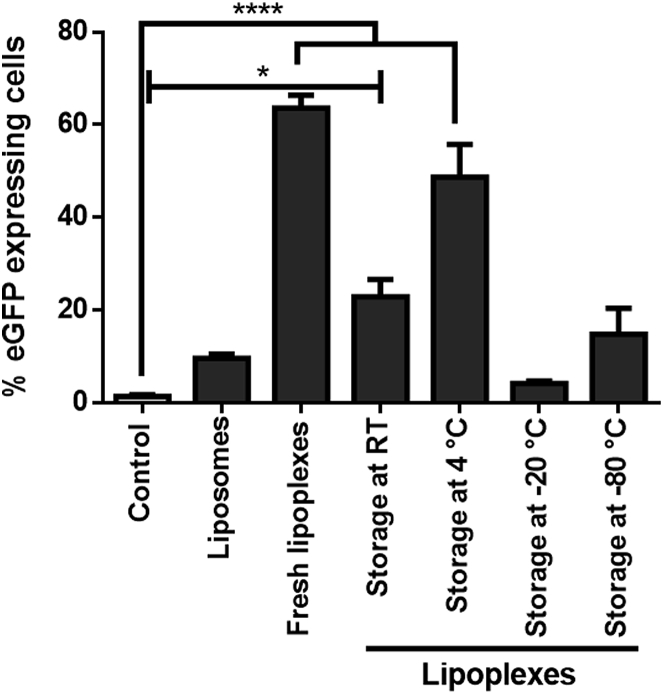
With regard to the storage requirements for a ready-to-use mRNA-based therapeutic agent, different storage conditions were tested. Therefore, EGFP mRNA was encapsulated into liposomes and stored at different temperature conditions (room temperature [RT], 4°C, −20°C, and −80°C) for 80 days. After storage, fresh lipoplexes as well as stored lipoplexes were added to the cells and incubated for 48 hr. Next, the amount of EGFP-expressing cells was measured. Our data show that the optimal storage temperature for lipoplexes is 4°C. The expression of EGFP is significantly higher (****p < 0.0001) compared to the control group, and the amount of EGFP-positive cells was not significantly different compared to cells transfected with freshly generated lipoplexes (Figure 6).
Figure 6.
Optimal Storage Conditions for Liposomes Containing mRNA
EGFP expression was investigated in cells after transfection with fresh and stored lipoplexes. Fresh lipoplexes as well as lipoplexes, which were stored at RT for 80 days, have a significantly higher EGFP expression than the control samples. Lipoplexes, which were stored at 4°C, induce nearly the same level of EGFP expression as fresh-generated lipoplexes. Data are shown as mean ± SEM (n = 3). *p < 0.05; ****p < 0.001.
Hemocompatibility of the Liposomes and Liposomes Containing mRNA with Human Whole Blood
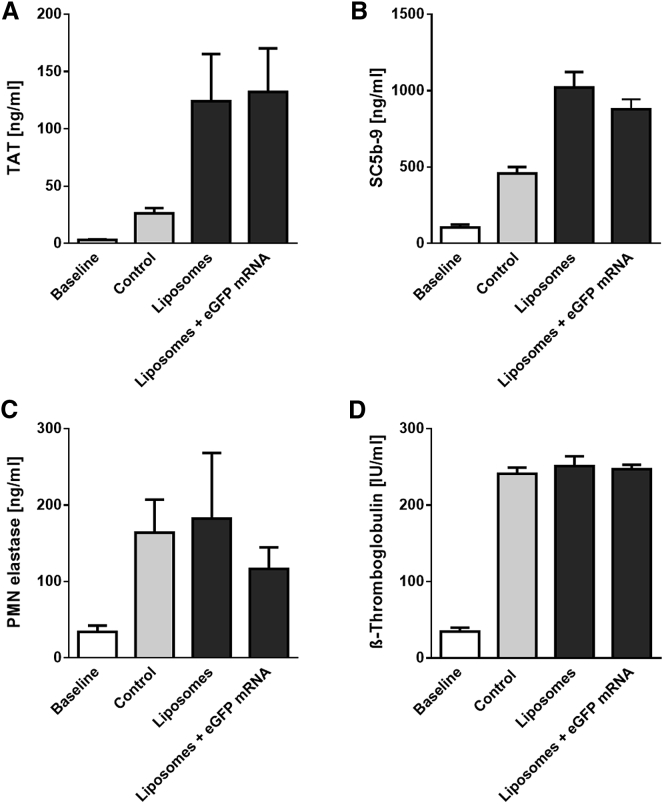
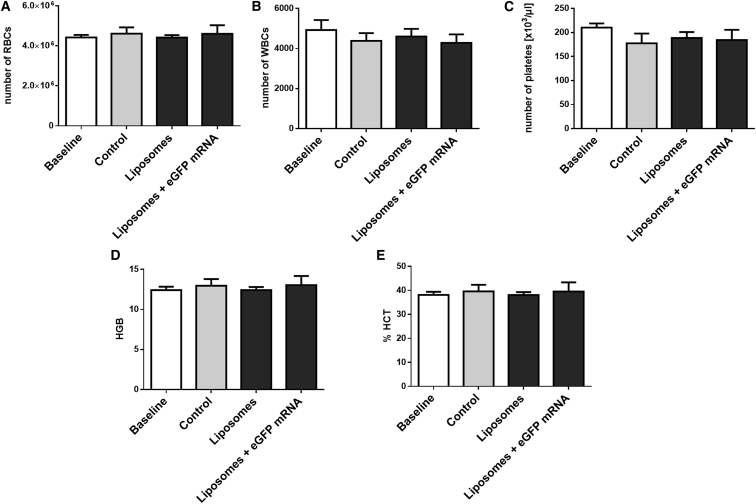
The hemocompatibility of the lipoplexes plays a pivotal role for potential clinical translation and its systemic i.v. application in patients; therefore, we analyzed various hematological markers in our study.24 After incubation of liposomes or lipoplexes with fresh human whole blood, various hematological markers indicating the activation of the coagulation and complement cascades, as well as activation of platelets and granulocytes, were measured in blood plasma samples using specific ELISAs. It was shown that neither the liposomes nor the lipoplexes have a negative impact on blood. The levels of thrombin-antithrombin (TAT) III formation (Figure 7A), as well as of the terminal complement complex SC5b-9 (Figure 7B), were not significantly different compared to the control and the liposome groups. Moreover, no difference in polymorphonuclear (PMN)-elastase (Figure 7C) and the platelet activation marker β-thromboglobulin (Figure 7D) was detectable between the groups after incubation. Additionally, blood cell counts, i.e., red blood cells (RBC), white blood cells (WBC), and platelets, were measured before and after incubation in all samples (Figures 8A–8C). Again, no differences were found. Likewise, no changes in hemoglobin (HGB) und hematocrit (HCT) values were measured (Figures 8D and 8E).
Figure 7.
Hemocompatibility of Liposomes and Lipoplexes Incubated in Human Whole Blood
Markers for the activation of blood coagulation (A; TAT) and the complement system (B; SC5b-9), as well as for neutrophils (C; PMN elastase) and platelets (D; β-thromboglobulin), were quantified in untreated human whole blood or blood treated with liposomes and lipoplexes after incubation in a dynamic flow model using ELISA. Data are shown as mean ± SEM (n = 5).
Figure 8.
Blood Cell Counts in Human Whole Blood before and after Incubation with Liposomes
Numbers of red blood cells (A), white blood cells (B), and platelets (C) per microliter of blood were measured. Additionally, hemoglobin (D) and hematocrit (E) values were quantified. Data are shown as mean ± SEM (n = 5).
Discussion
Our data illustrate the potential of DC-cholesterol/DOPE liposomes for mRNA transfection, showcasing their high encapsulation efficacy, complete hemocompatibility, as well as the absence of toxic and immunogenic effects. The DC-cholesterol/DOPE liposomes ensure the availability of mRNA inside the cells and keep the expression of the investigated therapeutic protein high over time. In addition, mRNA encapsulated into liposomes can be stored at 4°C for at least 80 days, without loss of function and transfection efficacy.
In line with previous studies, DC-cholesterol exhibits excellent biocompatibility and stability,25 whereas the neutral lipid DOPE was reported to increase transfection efficiencies.26 Farhood et al.26 and Ciani et al.27 reported that DC-cholesterol and DOPE are the most potent lipids for the formulation of liposomes. In addition, Zhang et al.28 showed that a 1:2 ratio of DC-cholesterol/DOPE in the formulation results in optimal cellular transfection efficiencies using other nucleic acids, like plasmid DNA and siRNA. Our TEM analysis indicates that the liposomes that have been generated are round and have a diameter of around 200 nm. In agreement with the study by Yang et al.,29 these liposomes were generated by the dry-film method; therefore, we also observed stable, homogeneous distribution in solution, and there was no formation of aggregates.
One of the most important properties for the therapeutic mRNA approach is the encapsulation efficacy of its vehicle system. In the case of DC-cholesterol/DOPE liposomes, which are positively charged, the formation of lipoplexes with the negatively charged mRNA occurs spontaneously.29 Our results demonstrated similar mRNA encapsulation efficacy of the generated DC-cholesterol/DOPE as compared to the commercially available transfection agent, Lipofectamine 2000.
A number of parameters, including liposomes/mRNA lipoplexes, cell surface fusion, internalization, exposition of mRNA from endosomes into the cytosol, and translation efficiency, are critical for successful protein expression.30 High positively charged amounts of liposomes are recognized by the cells and promote apoptosis and inflammation in cells through the activation of different pathways, like Toll-like receptors (TLRs) or caspase activation. This in turn can lead to the reduced translation of transfected mRNA inside the cells as observed in our study.
We recognized the ratio of liposomes/mRNA plays an important role for efficient transfection; therefore, to address the optimal liposomes/mRNA ratio for cellular transfection, we systematically tested different amounts and optimized our lipoplexes for maximum transfection efficiency.
The positive charge of liposomes also results in electrostatic interactions with the cell membrane leading to fusion or uptake by endocytosis.31, 32, 33 However, upon the recognition of a high density of positive charges, these interactions may lead to membrane destabilization, pro-apoptotic reactions, and pro-inflammatory reactions, which in turn lead to the activation of nuclear factor κB (NF-κB)-dependent and -independent pathways.21, 34 In both pathways, the expression of cytokines, such as TNF-α, IL-6, and IFN-β, are triggered.21, 35, 36 Hence, we analyzed the expression of these cytokines in the cells that were transfected with lipoplexes for 24, 72, or 120 hr. Our results show no expression in TNF-α and IL-6 genes and only weak, not significant, expression of IFN-α and IFN-β. Although some studies have noted that cationic lipids trigger apoptosis in cells via caspase-9 and caspase-8,37, 38 others have shown that this pro-apoptotic effect can be minimized with the addition of DOPE.39 There were also indications that the apoptotic effect depends on the ratio of neutral lipids in the liposome formulation, as well as on the size of the created liposomes.40 We did not observe any viability issues in the cells with our liposomes. This could be because of the low amount of liposomes needed for successful transfection and protein expression in our study.
A safe approach that can provide a long-lasting effect is necessary for the treatment of genetic diseases, such as AATD, because it will minimize the stress and burden of patients. As a proof of concept, we used an AAT-encoding mRNA to demonstrate the possible liposomal therapeutic potential of sustained AAT protein production and measured an increase in AAT supernatant concentration over 3 days. In the human body, the physiological level of AAT is 20–53 μmol/L.41 For effective lung protection of patients suffering with AATD, 60 mg/kg AAT protein should be supplemented on a weekly basis.42, 43 Previous studies showed that effective therapeutic levels of translated protein might be achieved using a low dosage of mRNA in different models. Pardi et al.’s group44 showed that i.v. injection of 1.4 mg/kg modified mRNA coding for HIV antibody into HIV-infected mice protected the mice against HIV challenge. It has also been shown that a single intratracheal application of 20 μg of a TLR coding mRNA could lead to better lung functions in asthmatic mice.45 Moreover, DeRosa and colleagues11 described a depot-creating effect of cationic lipids containing erythropoietin or factor-IX-encoding mRNA in cells resulting in high protein expression levels of human erythropoietin or factor IX for up to 7 days in vitro and in vivo. As an innovative mRNA-based therapy for AATD treatment, this encapsulated AAT-encoding mRNA could possibly be administered either by systemic i.v. injection, which would result in liver transfection,13 or through local aerosol application for lung targeting.46, 47, 48 The optimal dose of AAT lipoplexes as well as the frequency of treatment for therapeutic applications need to be further investigated in appropriate animal models. Furthermore, for efficient transfection of the liver, the size of the liposomes is critical, because the size of endothelial fenestrae in human liver is around 107 nm and hence extravasation can be reduced because of the use of larger liposomes.49 Therefore, in preparation for future experiments employing AAT mRNA, liposomes with different sizes should be analyzed to ensure efficient transfection of hepatocytes in vivo.
During blood circulation, the lipoplexes encounter major challenges, like macrophage-mediated clearance50 or aggregation of blood plasma proteins.51 Studies have shown that liposomes can be easily modified in order to achieve specific organ targeting22 or extend blood circulation time.50 Seeing that the hemocompatibility of the liposomes is an important attribute for a therapeutic application, we comprehensively investigated the hemocompatible properties of these mRNA-loaded liposomes using fresh human whole blood according to International Organization for Standardization (ISO) 10993-4. We demonstrated that neither the liposomes nor the lipoplexes altered any of the investigated blood parameters; hence, this mRNA delivery system fulfills the requirements of ISO 10993-4.
In order to advance this approach to future clinical use, factors such as drug stability, shelf life, and storage conditions are important and must be investigated. These results are in accordance with a study by Yang et al.,29 which demonstrated that their liposomes show no aggregation after 2 months. Maitani et al.52 also reported that their liposomes are stable and retain the ability to transfect cells for at least 6 months. Our data indicate that our lipoplexes can be safely stored at 4°C for at least 80 days and do not undergo a change in transfection efficiency.
In conclusion, the present study shows that DC-cholesterol/DOPE liposomes can be used as highly functional delivery vehicles for mRNA with high encapsulation efficiency. These lipoplexes result in high transfection efficiencies and sustained protein production, creating a depository effect inside the cells. The lipoplexes enable long-term storage without affecting their efficiency. More importantly, they were highly hemocompatible, and no adverse effects on cell viability or cellular immune response were observed. The generated DC-cholesterol/DOPE liposomes fulfill the necessary safety aspects facilitating the therapeutic delivery of mRNA in various therapeutic settings and representing a substantial contribution to the future development of novel mRNA-based therapeutics.
Materials and Methods
In Vitro Transcription of Nucleotide-Modified mRNA
The synthesis of modified mRNA via in vitro transcription (IVT) was described earlier.53, 54 Briefly, the plasmid DNA sequences of EGFP and AAT (Eurofins Medigenomix) were multiplied by using HotStar HiFidelity Polymerase Kit (QIAGEN), as well as a forward primer (5′-TTG GAC CCT CGT ACA GAA GCTA ATA CG-3′; Ella Biotech) and a reverse primer (poly T-tail of 120 thymidines [T120] 5′-CTT CCT ACT CAG GCT TTA TTC AAA GAC CA-3′; Ella Biotech). After subsequent purification (QIAquick PCR purification kit; QIAGEN) and gel electrophoresis, the DNA was used as a template for IVT using the MEGAscript T7 kit (Ambion). The following mRNA modifications were implemented: 3′-0-Me-m7G(5′)ppp(5′)G RNA cap structure analog (ARCA; New England Biolabs), pseudouridine-5′-triphosphate (TriLink Biotech), and 5-methylcytidine-5′-triphosphate (TriLink Biotech). For the fluorescent labeling of the mRNA, cy3-cytidine-triphosphate (PerkinElmer) was used. For RNase inhibition, an RNase inhibitor (Thermo Scientific) was added. The reaction mix was incubated for 4 hr at 37°C. Afterward, the mRNA was purified with RNeasy kit (QIAGEN), dephosphorylated using the Antarctic Phosphatase kit (New England Biolabs), purified again, and controlled with 1% agarose gel.
Generation of Liposomes
The liposomes were generated with the dry-film method.55, 56 Therefore, the cationic lipid DC-cholesterol (3β-[N-(N’,N’-dimethylaminoethane)-carbamoyl]cholesterol hydrochloride; Avanti Polar Lipids) and the neutral lipid DOPE (1,2-dioleoyl-sn-glycero-3-phosphoethanolamine; Avanti Lipids), both dissolved in chloroform, were mixed together in the ratio of 1:2 in a glass flask. Drying of the lipids took place under argon gas flow excluding O2. To remove chloroform completely, we put the glass flask with the formed lipid cake into an evaporator overnight. After the evaporation, the lipid cake was rehydrated with nuclease-free H2O (QIAGEN). After several minutes of vortexing, the glass flask with the formed multilamellar liposomes was placed in a sonication bath for 30 min to form unilamellar liposomes. Finally, the lipids were extruded with the mini extruder using a membrane with 200 nm pores (Avanti Polar Lipids).
Electron Microscopy
Liposomes from suspension are adsorbed onto a carbon-coated grid, washed, incubated with uranyl acetate, and visualized by negative staining using a Zeiss LIBRA 120 transmission electron microscope (Carl Zeiss) operating at 120 kV.
Encapsulation Efficiency of Liposomes
To test the capsulation efficiency of the liposomes, we used the Quant-iT RiboGreen assay (Invitrogen). Therefore, the standard curve and the samples were prepared. For the standard curve, EGFP mRNA was diluted in different concentrations in water ranging from 0 to 1,000 ng. Additionally, lipoplexes were formed by mixing 1 μg of EGFP mRNA and liposomes or Lipofectamine 2000 (Invitrogen) and incubated for 20 min at RT. Afterward, the RiboGreen fluorescent dye was added to the samples and incubated for 5 min. Fluorescence of samples was measured at 530 nm using a Mithras microplate reader (Berthold Technologies), and the concentration of each sample was calculated using a standard curve.
Transfection of A549 Cells
The cells were cultivated in full medium (DMEM with 10% fetal calf serum [FCS], 1% l-glutamine, 1% penicillin/streptomycin [P/S]). For transfection, A549 cells were seeded with a density of 150,000 cells/well into a 12-well plate. For transfections with a duration of more than 3 days, six-well plates were used. For the Lipofectamine 2000 transfection, 1,000 μL of Opti-MEM I (Invitrogen), 2 μL of Lipofectamine 2000 (Invitrogen), and 1 μg of AAT mRNA were mixed. The transfection complexes were formed during 20 min incubation at RT. For transfection, the cells were washed with PBS (without [w/o] Ca2+/Mg2+; GIBCO), and the complexes were added. After incubation of cells with the transfection complexes for 4 hr at 37°C and 5% CO2, the transfection medium was replaced by cell full medium. The analysis of the cells was performed 24 or 72 hr later. With regard to the transfection with liposomes, different amounts of liposomes (1–5 μL) were mixed with 1 μg of EGFP or AAT-encoding mRNA and incubated for 20 min at RT. After this incubation, the liposome complexes were added to the full cell culture medium. After different time points (24, 48, 72, or 120 hr) the cells were analyzed by flow cytometry (FACScan; Becton Dickinson) or the supernatants were collected and centrifuged at 3,000 × g and 4°C for 10 min.
AAT ELISA
To determine the concentration of human AAT in the supernatants of cells transfected with AAT-encoding mRNA encapsulated in liposomes or Lipofectamine 2000, we implemental a specific ELISA (Abcam) according to the manufacturer’s instructions.
Viability Assay
The MTT (AppliChem) assay was used to verify the viability of transfected A549 cells. Therefore, 150,000 cells/well were seeded in a six-well plate and transfected with different concentrations of mRNA/liposome complexes. After 72 and 120 hr, the cells were washed with PBS (without Ca2+/Mg2+; GIBCO) and incubated with 250 μg of MTT dissolved in RPMI 1640 (without phenol red; GIBCO) for 4 hr at 37°C. Subsequently, the MTT solution was removed and the cells were incubated for 10 min at 37°C with 500 μL of DMSO (dimethyl sulfoxide; Serva). The absorbance was measured at 540 nm using the Mithras microplate reader (Berthold).
Real-Time qPCR
The immune reaction of the cells that might be potentially triggered after liposomal transfection was investigated using real-time qPCR. Therefore, A549 cells were transfected with liposomes and lipoplexes containing 1 μg of EGFP mRNA as described above. After an incubation time of 24, 72, and 120 hr, total RNA was isolated from the cells with Aurum total RNA isolation kit (Bio-Rad), and the isolated RNA was converted to copy DNA using the iScript kit (Bio-Rad). Expression of IFN-α, IFN-β, TNF-α, and IL-6 was analyzed. GAPDH expression was used as reference. The real-time qPCR was performed using the CFX Connect Real-Time PCR detection system (Bio-Rad).
Storage
The lipoplexes were formed by mixing 1 μg of EGFP mRNA and 5 μL of liposomes and stored for 80 days at RT, −4°C, −20°C, or −80°C. After storage, A549 cells seeded 1 day before in 12-well plates were transfected with the respective samples. Fresh lipoplexes were generated and served as controls. After 48 hr of incubation, flow cytometric analysis was performed.
Hemocompatibility Assay
Blood samples were collected by venipuncture of five healthy volunteers. The procedures were approved by the Ethics Committee of the University Hospital of Tübingen. Written informed consent was obtained from all subjects before blood sampling, and the collected blood was anticoagulated with 1.5 IU/mL heparin (Rathiopharm). The following exclusion criteria to guarantee good blood quality have been strictly fulfilled: drug intake in the last 14 days before blood sampling, especially hemostasis-affecting agents like acetylsalicylic acid, oral contraceptives, and cigarettes.
To prove the compatibility of human blood with the generated liposomes and lipoplexes, we directly transferred the heparinized blood after blood sampling into EDTA, a mixture of citrate, theophylline, adenosine, and dipyridamole (CTAD), and citrate monovettes (Sarstedt AG) in order to get baseline values of various hemocompatibility markers. Liposomes (fc: 5 μL/mL) and EGFP mRNA encapsulated in liposomes (fc: 5 μL/mL lipoplexes and 1 μg/mL EGFP mRNA) were incubated in 12 mL of fresh human blood in round polypropylene bottom tubes (BD Falcon) at 37°C for 90 min under dynamic conditions. After incubation, blood was collected and transferred into the respective terminating media including EDTA, CTAD, and citrate. The EDTA-anticoagulated blood was used to analyze whole blood counts. Afterward, all blood samples were centrifugated followed by blood plasma collection and storage at −20°C or −70°C until the analysis of various hemocompatibility markers was performed as previously described.24
Statistics
Data are presented as means with error of the mean (SEM). The Kolmogorov-Smirnov test was used to test for normal distribution. To analyze differences between the groups, we executed different analysis tests. Data with normal distribution were analyzed using one-way ANOVA with Bonferroni’s multiple comparison test, and data with non-normal distribution were analyzed using the Kruskal-Wallis test. All analyses were performed using the statistical software package GraphPad Prism (version 6; GraphPad Software). Statistical significance was defined as p < 0.05.
Author Contributions
Conception and Design, T.M., M.-K.A., M.A.-A., C.S., K.P., H.P.W., X.W., S.K.; Collection, Analysis, and Interpretation of Data, T.M., D.L., S.R., M.L.S.M., J.K., M.S., X.W., S.K.; Manuscript Drafting, T.M., H.P.W., X.W., S.K.; Figure Preparation, T.M., S.K. The manuscript was finally approved by all authors.
Conflicts of Interest
The authors report no conflicts of interests.
Acknowledgments
The staining and imaging of liposomes with TEM were kindly provided by the work group of Prof. Martin Schaller. M.-K.A. is supported by the German research association (Deutsche Forschungsgemeinschaft). K.P. was supported by a National Health and Medical Research Council Principal Research Fellowship. X.W. is supported by a National Heart Foundation Postdoctoral Fellowship with the Paul Korner Innovation Award. S.K. was supported by the Margarete von Wrangell Habilitation Programme for Women of the Ministry of Science and Arts Baden-Wuerttemberg.
References
- 1.Diken M., Kranz L.M., Kreiter S., Sahin U. mRNA: a versatile molecule for cancer vaccines. Curr. Issues Mol. Biol. 2017;22:113–128. doi: 10.21775/cimb.022.113. [DOI] [PubMed] [Google Scholar]
- 2.Magini D., Giovani C., Mangiavacchi S., Maccari S., Cecchi R., Ulmer J.B., De Gregorio E., Geall A.J., Brazzoli M., Bertholet S. Self-amplifying mRNA vaccines expressing multiple conserved influenza antigens confer protection against homologous and heterosubtypic viral challenge. PLoS ONE. 2016;11:e0161193. doi: 10.1371/journal.pone.0161193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Michel T., Kankura A., Salinas Medina M.L., Kurz J., Behring A., Avci-Adali M., Nolte A., Schlensak C., Wendel H.P., Krajewski S. In vitro evaluation of a novel mRNA-based therapeutic strategy for the treatment of patients suffering from alpha-1-antitrypsin deficiency. Nucleic Acid Ther. 2015;25:235–244. doi: 10.1089/nat.2015.0537. [DOI] [PubMed] [Google Scholar]
- 4.Abraham M.K., Nolte A., Reus R., Behring A., Zengerle D., Avci-Adali M., Hohmann J.D., Peter K., Schlensak C., Wendel H.P., Krajewski S. In vitro study of a novel stent coating using modified CD39 messenger RNA to potentially reduce stent angioplasty-associated complications. PLoS ONE. 2015;10:e0138375. doi: 10.1371/journal.pone.0138375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahiny A.J., Karikó K. Measuring hematocrit in mice injected with in vitro-transcribed erythropoietin mRNA. Methods Mol. Biol. 2016;1428:297–306. doi: 10.1007/978-1-4939-3625-0_20. [DOI] [PubMed] [Google Scholar]
- 6.Hadas Y., Katz M.G., Bridges C.R., Zangi L. Modified mRNA as a therapeutic tool to induce cardiac regeneration in ischemic heart disease. Wiley Interdiscip. Rev. Syst. Biol. Med. 2017;9:e1367. doi: 10.1002/wsbm.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolff J.A., Malone R.W., Williams P., Chong W., Acsadi G., Jani A., Felgner P.L. Direct gene transfer into mouse muscle in vivo. Science. 1990;247:1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- 8.Sahin U., Karikó K., Türeci Ö. mRNA-based therapeutics—developing a new class of drugs. Nat. Rev. Drug Discov. 2014;13:759–780. doi: 10.1038/nrd4278. [DOI] [PubMed] [Google Scholar]
- 9.Karikó K., Muramatsu H., Ludwig J., Weissman D. Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA. Nucleic Acids Res. 2011;39:e142. doi: 10.1093/nar/gkr695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stepinski J., Waddell C., Stolarski R., Darzynkiewicz E., Rhoads R.E. Synthesis and properties of mRNAs containing the novel “anti-reverse” cap analogs 7-methyl(3′-O-methyl)GpppG and 7-methyl (3′-deoxy)GpppG. RNA. 2001;7:1486–1495. [PMC free article] [PubMed] [Google Scholar]
- 11.DeRosa F., Guild B., Karve S., Smith L., Love K., Dorkin J.R., Kauffman K.J., Zhang J., Yahalom B., Anderson D.G., Heartlein M.W. Therapeutic efficacy in a hemophilia B model using a biosynthetic mRNA liver depot system. Gene Ther. 2016;23:699–707. doi: 10.1038/gt.2016.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petrache I., Hajjar J., Campos M. Safety and efficacy of alpha-1-antitrypsin augmentation therapy in the treatment of patients with alpha-1-antitrypsin deficiency. Biologics. 2009;3:193–204. doi: 10.2147/btt.2009.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pardi N., Tuyishime S., Muramatsu H., Kariko K., Mui B.L., Tam Y.K., Madden T.D., Hope M.J., Weissman D. Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes. J. Control. Release. 2015;217:345–351. doi: 10.1016/j.jconrel.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baum C., Kustikova O., Modlich U., Li Z., Fehse B. Mutagenesis and oncogenesis by chromosomal insertion of gene transfer vectors. Hum. Gene Ther. 2006;17:253–263. doi: 10.1089/hum.2006.17.253. [DOI] [PubMed] [Google Scholar]
- 15.Bessis N., GarciaCozar F.J., Boissier M.C. Immune responses to gene therapy vectors: influence on vector function and effector mechanisms. Gene Ther. 2004;11(Suppl 1):S10–S17. doi: 10.1038/sj.gt.3302364. [DOI] [PubMed] [Google Scholar]
- 16.Bouard D., Alazard-Dany D., Cosset F.L. Viral vectors: from virology to transgene expression. Br. J. Pharmacol. 2009;157:153–165. doi: 10.1038/bjp.2008.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yin H., Kanasty R.L., Eltoukhy A.A., Vegas A.J., Dorkin J.R., Anderson D.G. Non-viral vectors for gene-based therapy. Nat. Rev. Genet. 2014;15:541–555. doi: 10.1038/nrg3763. [DOI] [PubMed] [Google Scholar]
- 18.Thomas C.E., Ehrhardt A., Kay M.A. Progress and problems with the use of viral vectors for gene therapy. Nat. Rev. Genet. 2003;4:346–358. doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
- 19.Düzgüneş N., De Ilarduya C.T., Simões S., Zhdanov R.I., Konopka K., Pedroso de Lima M.C. Cationic liposomes for gene delivery: novel cationic lipids and enhancement by proteins and peptides. Curr. Med. Chem. 2003;10:1213–1220. doi: 10.2174/0929867033457403. [DOI] [PubMed] [Google Scholar]
- 20.Khalil I.A., Kogure K., Akita H., Harashima H. Uptake pathways and subsequent intracellular trafficking in nonviral gene delivery. Pharmacol. Rev. 2006;58:32–45. doi: 10.1124/pr.58.1.8. [DOI] [PubMed] [Google Scholar]
- 21.Lonez C., Vandenbranden M., Ruysschaert J.M. Cationic lipids activate intracellular signaling pathways. Adv. Drug Deliv. Rev. 2012;64:1749–1758. doi: 10.1016/j.addr.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 22.Noble G.T., Stefanick J.F., Ashley J.D., Kiziltepe T., Bilgicer B. Ligand-targeted liposome design: challenges and fundamental considerations. Trends Biotechnol. 2014;32:32–45. doi: 10.1016/j.tibtech.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 23.Allen T.M., Cullis P.R. Liposomal drug delivery systems: from concept to clinical applications. Adv. Drug Deliv. Rev. 2013;65:36–48. doi: 10.1016/j.addr.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 24.Krajewski S., Prucek R., Panacek A., Avci-Adali M., Nolte A., Straub A., Zboril R., Wendel H.P., Kvitek L. Hemocompatibility evaluation of different silver nanoparticle concentrations employing a modified Chandler-loop in vitro assay on human blood. Acta Biomater. 2013;9:7460–7468. doi: 10.1016/j.actbio.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 25.Caracciolo G., Amenitsch H. Cationic liposome/DNA complexes: from structure to interactions with cellular membranes. Eur. Biophys. J. 2012;41:815–829. doi: 10.1007/s00249-012-0830-8. [DOI] [PubMed] [Google Scholar]
- 26.Farhood H., Serbina N., Huang L. The role of dioleoyl phosphatidylethanolamine in cationic liposome mediated gene transfer. Biochim. Biophys. Acta. 1995;1235:289–295. doi: 10.1016/0005-2736(95)80016-9. [DOI] [PubMed] [Google Scholar]
- 27.Ciani L., Casini A., Gabbiani C., Ristori S., Messori L., Martini G. DOTAP/DOPE and DC-Chol/DOPE lipoplexes for gene delivery studied by circular dichroism and other biophysical techniques. Biophys. Chem. 2007;127:213–220. doi: 10.1016/j.bpc.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y., Li H., Sun J., Gao J., Liu W., Li B., Guo Y., Chen J. DC-Chol/DOPE cationic liposomes: a comparative study of the influence factors on plasmid pDNA and siRNA gene delivery. Int. J. Pharm. 2010;390:198–207. doi: 10.1016/j.ijpharm.2010.01.035. [DOI] [PubMed] [Google Scholar]
- 29.Yang S., Chen J., Zhao D., Han D., Chen X. Comparative study on preparative methods of DC-Chol/DOPE liposomes and formulation optimization by determining encapsulation efficiency. Int. J. Pharm. 2012;434:155–160. doi: 10.1016/j.ijpharm.2012.05.041. [DOI] [PubMed] [Google Scholar]
- 30.Wasungu L., Hoekstra D. Cationic lipids, lipoplexes and intracellular delivery of genes. J. Control. Release. 2006;116:255–264. doi: 10.1016/j.jconrel.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 31.Ewert K.K., Evans H.M., Bouxsein N.F., Safinya C.R. Dendritic cationic lipids with highly charged headgroups for efficient gene delivery. Bioconjug. Chem. 2006;17:877–888. doi: 10.1021/bc050310c. [DOI] [PubMed] [Google Scholar]
- 32.Elouahabi A., Ruysschaert J.M. Formation and intracellular trafficking of lipoplexes and polyplexes. Mol. Ther. 2005;11:336–347. doi: 10.1016/j.ymthe.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 33.Hoekstra D., Rejman J., Wasungu L., Shi F., Zuhorn I. Gene delivery by cationic lipids: in and out of an endosome. Biochem. Soc. Trans. 2007;35:68–71. doi: 10.1042/BST0350068. [DOI] [PubMed] [Google Scholar]
- 34.Lonez C., Vandenbranden M., Ruysschaert J.M. Cationic liposomal lipids: from gene carriers to cell signaling. Prog. Lipid Res. 2008;47:340–347. doi: 10.1016/j.plipres.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 35.Kedmi R., Ben-Arie N., Peer D. The systemic toxicity of positively charged lipid nanoparticles and the role of Toll-like receptor 4 in immune activation. Biomaterials. 2010;31:6867–6875. doi: 10.1016/j.biomaterials.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 36.Vangasseri D.P., Cui Z., Chen W., Hokey D.A., Falo L.D., Jr., Huang L. Immunostimulation of dendritic cells by cationic liposomes. Mol. Membr. Biol. 2006;23:385–395. doi: 10.1080/09687860600790537. [DOI] [PubMed] [Google Scholar]
- 37.Iwaoka S., Nakamura T., Takano S., Tsuchiya S., Aramaki Y. Cationic liposomes induce apoptosis through p38 MAP kinase-caspase-8-Bid pathway in macrophage-like RAW264.7 cells. J. Leukoc. Biol. 2006;79:184–191. doi: 10.1189/jlb.0405181. [DOI] [PubMed] [Google Scholar]
- 38.Kusumoto K., Ishikawa T. Didodecyldimethylammonium bromide (DDAB) induces caspase-mediated apoptosis in human leukemia HL-60 cells. J. Control. Release. 2010;147:246–252. doi: 10.1016/j.jconrel.2010.07.114. [DOI] [PubMed] [Google Scholar]
- 39.Filion M.C., Phillips N.C. Toxicity and immunomodulatory activity of liposomal vectors formulated with cationic lipids toward immune effector cells. Biochim. Biophys. Acta. 1997;1329:345–356. doi: 10.1016/s0005-2736(97)00126-0. [DOI] [PubMed] [Google Scholar]
- 40.Takano S., Aramaki Y., Tsuchiya S. Physicochemical properties of liposomes affecting apoptosis induced by cationic liposomes in macrophages. Pharm. Res. 2003;20:962–968. doi: 10.1023/a:1024441702398. [DOI] [PubMed] [Google Scholar]
- 41.Hurley K., Lacey N., O’Dwyer C.A., Bergin D.A., McElvaney O.J., O’Brien M.E., McElvaney O.F., Reeves E.P., McElvaney N.G. Alpha-1 antitrypsin augmentation therapy corrects accelerated neutrophil apoptosis in deficient individuals. J. Immunol. 2014;193:3978–3991. doi: 10.4049/jimmunol.1400132. [DOI] [PubMed] [Google Scholar]
- 42.Campos M.A., Lascano J. α1 Antitrypsin deficiency: current best practice in testing and augmentation therapy. Ther. Adv. Respir. Dis. 2014;8:150–161. doi: 10.1177/1753465814542243. [DOI] [PubMed] [Google Scholar]
- 43.Wewers M.D., Casolaro M.A., Sellers S.E., Swayze S.C., McPhaul K.M., Wittes J.T., Crystal R.G. Replacement therapy for alpha 1-antitrypsin deficiency associated with emphysema. N. Engl. J. Med. 1987;316:1055–1062. doi: 10.1056/NEJM198704233161704. [DOI] [PubMed] [Google Scholar]
- 44.Pardi N., Secreto A.J., Shan X., Debonera F., Glover J., Yi Y., Muramatsu H., Ni H., Mui B.L., Tam Y.K. Administration of nucleoside-modified mRNA encoding broadly neutralizing antibody protects humanized mice from HIV-1 challenge. Nat. Commun. 2017;8:14630. doi: 10.1038/ncomms14630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zeyer F., Mothes B., Will C., Carevic M., Rottenberger J., Nürnberg B., Hartl D., Handgretinger R., Beer-Hammer S., Kormann M.S. mRNA-mediated gene supplementation of Toll-like receptors as treatment strategy for asthma in vivo. PLoS ONE. 2016;11:e0154001. doi: 10.1371/journal.pone.0154001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mays L.E., Ammon-Treiber S., Mothes B., Alkhaled M., Rottenberger J., Müller-Hermelink E.S., Grimm M., Mezger M., Beer-Hammer S., von Stebut E. Modified Foxp3 mRNA protects against asthma through an IL-10-dependent mechanism. J. Clin. Invest. 2013;123:1216–1228. doi: 10.1172/JCI65351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kormann M.S., Hasenpusch G., Aneja M.K., Nica G., Flemmer A.W., Herber-Jonat S., Huppmann M., Mays L.E., Illenyi M., Schams A. Expression of therapeutic proteins after delivery of chemically modified mRNA in mice. Nat. Biotechnol. 2011;29:154–157. doi: 10.1038/nbt.1733. [DOI] [PubMed] [Google Scholar]
- 48.Middleton P.G., Caplen N.J., Gao X., Huang L., Gaya H., Geddes D.M., Alton E.W. Nasal application of the cationic liposome DC-Chol:DOPE does not alter ion transport, lung function or bacterial growth. Eur. Respir. J. 1994;7:442–445. doi: 10.1183/09031936.94.07030442. [DOI] [PubMed] [Google Scholar]
- 49.Wisse E., Jacobs F., Topal B., Frederik P., De Geest B. The size of endothelial fenestrae in human liver sinusoids: implications for hepatocyte-directed gene transfer. Gene Ther. 2008;15:1193–1199. doi: 10.1038/gt.2008.60. [DOI] [PubMed] [Google Scholar]
- 50.Woodle M.C. Controlling liposome blood clearance by surface-grafted polymers. Adv. Drug Deliv. Rev. 1998;32:139–152. doi: 10.1016/s0169-409x(97)00136-1. [DOI] [PubMed] [Google Scholar]
- 51.Capriotti A.L., Caracciolo G., Caruso G., Cavaliere C., Pozzi D., Samperi R., Laganà A. Analysis of plasma protein adsorption onto DC-Chol-DOPE cationic liposomes by HPLC-CHIP coupled to a Q-TOF mass spectrometer. Anal. Bioanal. Chem. 2010;398:2895–2903. doi: 10.1007/s00216-010-4104-y. [DOI] [PubMed] [Google Scholar]
- 52.Maitani Y., Igarashi S., Sato M., Hattori Y. Cationic liposome (DC-Chol/DOPE=1:2) and a modified ethanol injection method to prepare liposomes, increased gene expression. Int. J. Pharm. 2007;342:33–39. doi: 10.1016/j.ijpharm.2007.04.035. [DOI] [PubMed] [Google Scholar]
- 53.Avci-Adali M., Behring A., Steinle H., Keller T., Krajeweski S., Schlensak C., Wendel H.P. In vitro synthesis of modified mRNA for induction of protein expression in human cells. J. Vis. Exp. 2014;(93):e51943. doi: 10.3791/51943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Avci-Adali M., Behring A., Keller T., Krajewski S., Schlensak C., Wendel H.P. Optimized conditions for successful transfection of human endothelial cells with in vitro synthesized and modified mRNA for induction of protein expression. J. Biol. Eng. 2014;8:8. doi: 10.1186/1754-1611-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Son K.K., Patel D.H., Tkach D., Park A. Cationic liposome and plasmid DNA complexes formed in serum-free medium under optimum transfection condition are negatively charged. Biochim. Biophys. Acta. 2000;1466:11–15. doi: 10.1016/s0005-2736(00)00176-0. [DOI] [PubMed] [Google Scholar]
- 56.Pozzi D., Marchini C., Cardarelli F., Amenitsch H., Garulli C., Bifone A., Caracciolo G. Transfection efficiency boost of cholesterol-containing lipoplexes. Biochim. Biophys. Acta. 2012;1818:2335–2343. doi: 10.1016/j.bbamem.2012.05.017. [DOI] [PubMed] [Google Scholar]