Abstract
The factors involved in maintaining a localized inflammatory state in psoriatic skin remain poorly understood. Here, we demonstrate through metabolomic and transcriptomic profiling marked suppression of glucocorticoid biosynthesis in the epidermis of psoriatic skin leading to localized deficiency of cortisol. Utilizing a 3D human epidermis model, we demonstrate that glucocorticoid biosynthesis is suppressed by pro-inflammatory cytokines and that glucocorticoid deficiency promotes inflammatory responses in keratinocytes. Finally, we show in vitro and in vivo that treatment with topical glucocorticoids leads to rapid restoration of glucocorticoid biosynthesis gene expression coincident with normalization of epidermal differentiation and suppression of inflammatory responses. Taken together, our data suggest that localized glucocorticoid deficiency in psoriatic skin interferes with epidermal differentiation and promotes a sustained and localized inflammatory response. This may shed new light on the mechanism of action of topical steroids, and demonstrates the critical role of endogenous steroid in maintaining both inflammatory and differentiation homeostasis in the epidermis.
INTRODUCTION
Psoriasis is a chronic inflammatory skin disorder accompanied by increased proliferation and altered differentiation of epidermal keratinocytes. Topical glucocorticoids are a mainstay for mild-to-moderate psoriasis (Schon and Boehncke, 2005). It has been assumed that the effect of topical glucocorticoids is through anti-inflammatory action largely on infiltrating immune cells, but the precise cellular targets and mechanism of action of glucocorticoids in psoriasis remain unclear. The skin has been known to express the genes for enzymes involved in glucocorticoid biosynthesis (Dumont et al., 1992; Rogoff et al., 2001; Slominski et al., 1996; Slominski et al., 2004), and elements of the hypothalamo-pituitary-adrenal axis (HPA) have been implicated as major regulators of glucocorticoid synthesis in skin (Slominski et al., 2007). Loss of glucocorticoid receptor activity results in impaired epidermal differentiation, decreased expression of late differentiation markers including filaggrin, and abnormal hair growth (Bayo et al., 2008), features that are commonly seen in cutaneous psoriasis (Rittie et al., 2016). Furthermore, glucocorticoid receptor inactivation in mouse skin results in increased expression of various inflammatory markers (Sevilla et al., 2013), many of which are shared with psoriasis. Interestingly, reduced nuclear translocation of glucocorticoid receptors is a feature of psoriatic skin and keratinocytes (Man et al., 2013; Pang et al., 2015) but the reason for this has been unclear. Here, we show that endogenous biosynthesis of glucocorticoids is suppressed in psoriatic skin resulting in localized steroid deficiency. We further demonstrate that this steroid depleted microenvironment impacts epidermal differentiation and leads to heightened epidermal inflammatory responses, which can be normalized by exogenous glucocorticoids leading to restoration of epidermal differentiation, steroid biosynthesis, and inflammatory responses.
RESULTS
Profiling reveals marked metabolomic shifts with localized glucocorticoid deficiency in psoriatic skin
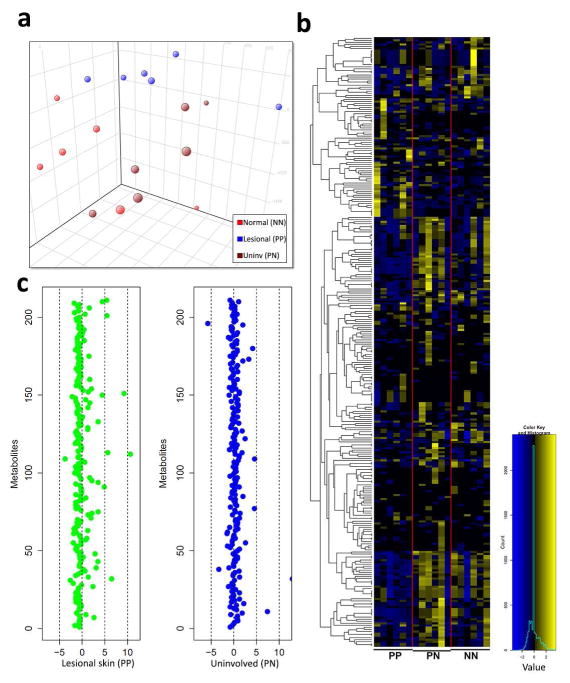
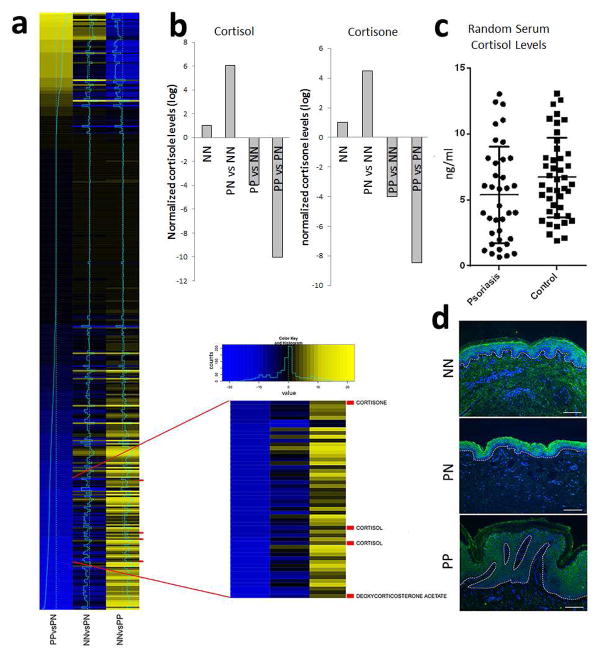
We performed untargeted metabolomic profiling using liquid chromatography followed by mass-spectrometry (LC-MS) on biopsies of lesional (PP) and uninvolved (PN) skin obtained from 6 psoriatic patients as well as normal (NN) skin from six healthy controls. Principal component analyses showed separation between lesional versus uninvolved and healthy control skin (Figure 1a). Among the 2724 metabolites examined, 377 were detected in at least two samples from each of the psoriatic and normal patients (Figure 1b). Amongst the most decreased compounds in PP skin were the glucocorticoids; cortisone and cortisol (357 and 1057-fold decreased, respectively, p<1×10−4) (Figure 2) (supplementary files 1 and 2). These data were validated for cortisol using a targeted steroid panel (p<1×10−3). Serum cortisol levels were slightly lower in psoriatic (n=37) compared to healthy controls (n=43), but this did not reach significance (p=0.08) (Figure 2c). Immunofluoresence (IF) showed decreased cortisol throughout the psoriatic epidermis compared to PN and NN skin (Figure 2d). These data demonstrate that psoriatic skin is in a locally confined state of chronic glucocorticoid deficiency. Other compounds dysregulated in psoriasis include taurine and homoserine (7,000- and 250- fold higher in psoriatic skin, respectively compared to uninvolved, p<1×10−4), and the B-vitamins riboflavin and pantothenate, which were 370- and 1600- fold lower, respectively, in psoriatic compared to uninvolved skin (p<1×10−4) (supplementary files 1 and 2).
Figure 1. Metabolomic profiling reveals differences between psoriatic, uninvolved and normal skin.
(a) Principal component analyses showed separation between lesional, uninvolved, and healthy control skin (n=6 for each). (b) Heatmap of named compounds identified in PP, PN, and NN skin. (c) Z-scores were calculated for both PP and PN skin for positively charged compounds.
Figure 2. Glucocorticoid levels are decreased in psoriatic skin.
(a) 377 metabolites were detected in at least two samples from each of the psoriatic and normal patients and significantly altered (p<0.05), and of the most decreased metabolites in psoriatic skin (both PP vs PN and PP vs NN) several were glucocorticoids including cortisone, cortisol and deoxycorticosterone acetate. (b) In comparison to normal healthy skin, glucocorticoid levels (both cortisol and cortisone) were markedly lower in psoriatic plaques compared to both NN and PN skin (log10 fold changes shown). (c) In contrast, random serum cortisol levels between psoriasis (n=37) and healthy controls (n=43) were slightly, but not significantly, lower in patients with psoriasis (p=0.08). (d) IF for cortisol showed decreased cortisol levels in psoriatic skin throughout the epidermis (n=3).
Suppression of glucocorticoid biosynthesis in psoriasis and other skin disorders
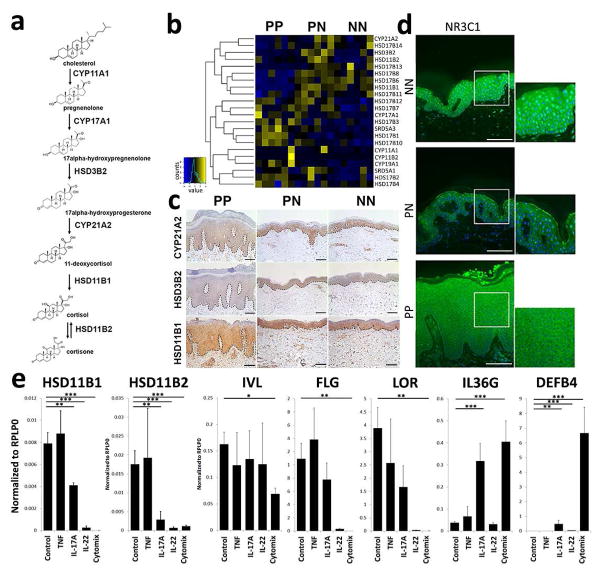
Using RNA-seq, we characterized the transcriptomes of biopsies from the same plaques submitted for metabolomic profiling (n=6 in each group) (Supplementary Figure 1). Five out of six critical enzymes involved in steroid biosynthesis (Figure 3a, b) had decreased mRNA expression in psoriatic skin compared to both PN and NN skin. These included HSD11B1, which was reduced by 12-fold in PP compared to PN skin (p=3.7×10−5), and HSD3B2, which was reduced by 9-fold (p=4×10−3) (Supplementary Figures 2 and 3). We confirmed these findings at the protein level by immunohistochemistry (Figure 3c). Consistent with a glucocorticoid depleted microenvironment, we also observed decreased mRNA expression (1.4-fold, p<0.05) and decreased nuclear localization of the glucocorticoid receptor in PP skin (Figure 3d). (Figure 3d). Of note, as mineralcorticoid receptor has been implicated in glucocorticoid responses in skin (Boix et al., 2016), mRNA expression of the mineralcorticoid receptor was also decreased in PP compared to PN and NN skin (2.2-fold decreased, p<0.01).
Figure 3. Glucocorticoid biosynthesis and steroid responses are suppressed in psoriatic skin.
RNA-sequencing was performed on psoriatic (PP), uninvolved (PN) and normal (NN) skin (n=6) matching patients analyzed by metabolic profiling. (a) Glucocorticoid biosynthetic pathway. (b–c) Suppression of gene and protein expression involving key enzymes in the glucocorticoid biosynthetic pathway. (d) Reduced nuclear localization of the glucocorticoid receptor in psoriatic skin (scale bar = 100μm). (e) IL-17A and IL-22 at 10ng/ml suppressed HSD11B1 and HSD11B2 expression, whereas TNF-α (10ng/ml) had no effect in 3D epidermal raft cultures. Combination of IL-17A/IL-22 and TNF-α (cytomix) suppressed HSD11B1 and HSD11B2 expression, accompanied by decreased expression of desmoglein 1 (DSG1), filaggrin (FLG) and loricrin (LOR), but increased expression of IL36G and DEFB4. (Data shown with SEM, n=2–10, **p<0.01, ***p<0.001, for IHC and IF representative figures are shown (n=3)).
To assess for involvement of the HPA axis, we looked at the mRNA and protein expression of several genes involved in this pathway (Supplementary Figure 4). mRNA expression of cortitropin releasing hormone (CRH) and CRH-receptor-1 (CRHR1) was unchanged in both PP, PN and NN skin. Expression of the pro-opiomelancortin (POMC) was similarly unchanged between PP, PN and NN skin. These findings argue against the HPA-axis being responsible for the cortisol deficiency observed in psoriatic skin.
To address the character of genes regulated by glucocorticoid receptor in psoriatic skin, we retrieved the genomic locations of the binding sites for the glucocorticoid receptor gene (NR3C1) and focused on genes within 10 kilobases of at least one NR3C1 binding site. Restricting the analysis to genes that are expressed in NN or PP skin, this generated a list of 3,453 genes (Heinz et al., 2010). Given the ubiquitous binding of the glucocorticoid receptor, we limited our functional enrichment analysis to genes with at least 3 NR3C1 binding sites. Highly enriched categories included cytokine signaling in immune system (p<1×10−4), response to type I interferons (p=3×10−4), response to IFN-γ (p=1.8×10−3), and differentiation (1.9×10−2). This is consistent with the well-known anti-inflammatory effect of glucocorticoids in skin and further suggests a role for this steroid in epidermal differentiation (Supplementary file 3) (Stojadinovic et al., 2007).
To address if suppression of glucocorticoid biosynthesis is unique to psoriasis we analyzed HSD11B1 expression, as previously described by our group (Swindell et al., 2016), across 41 datasets (17 psoriasis, 10 atopic dermatitis, 6 non-melanoma skin cancer, 6 wound healing, 2 acne, and discoid lupus). Decreased expression of HSD11B1 was most profoundly suppressed in psoriasis (p=5.4×10−32), but closely followed by atopic dermatitis (p=1.3×10−8), wound healing (p=1.1×10−6) and squamous cell carcinoma (p=2.26×10−9) (Supplementary Figure 5 and file 4).
Epidermal glucocorticoid biosynthesis and glucocorticoid consumption is dependent on differentiation stage
To gain a better understanding of the relative importance of local cortisol signaling axis in the epidermis, we utilized a 3D human skin tissue culture model comprised purely of normal human keratinocytes that differentiate with time into all of the stratified epidermal cell layers evident in vivo (Supplementary Figure 6). Importantly, this 3D tissue culture system is removed from any systemic hormone influence and is maintained in a growth medium that typically is supplemented with exogenous hydrocortisone (1000nM) (Arnette et al., 2016). Using this model, we found an increase in HSD11B1 and HSD11B2 mRNA expression with progression of epidermal differentiation (p<0.05) (Supplementary Figure 6) that strongly correlated with markers of differentiation (loricrin, r=0.55, p<0.003). Stimulation of the 3D rafts with pro-inflammatory cytokines, using TNF-α, IL-17A, and IL-22 individually, or in a combination for 72 hours led to decreased expression of both HSD11B1 and HSD11B2 mRNA with maximal suppression of HSD11B1 mRNA expression from baseline observed with combination of TNF-α/IL-17A, with HSD11B1 being suppressed 1000-fold and HSD11B2 about 15-fold (p<0.0001 and p<0.01 respectively) (Figure 3e). In contrast, no changes were seen with the same cytokines in monolayer keratinocyte cultures (data not shown), further supporting the role of epidermal differentiation in modulating inflammatory responses in context of glucocorticoid biosynthesis. Concomitant with the suppression of HSD11B1 and HSD11B2 expression, we observed decreased expression of epidermal differentiation markers, including involucrin (p<0.05), filaggrin (p<0.01) and loricrin (p<0.01), whereas inflammatory markers such as IL36G (p<0.001) and DEFB4 (p<0.001) were increased (Figure 3e). Coincident with these changes, we found a differentiation-dependent decrease in total cortisol levels (2.9-fold decrease compared to baseline, n=3, p<0.01; Supplementary Figure 7). As HSD11B1 regulates synthesis of cortisol and HSD11B2 facilitates conversion of biologically active cortisol to inactive cortisone, we determined the ratio between HSD11B1 and HSD11B2 in the 3D epidermal culture models as compared to patient skin. Consistent with the idea that cortisol synthesis is favored under steady-state conditions, HSD11B1:HSD11B2 ratio in normal skin was 4:1 while this ratio was markedly lower in psoriatic skin (0.4:1). In 3D epidermis, this ratio was lowest in undifferentiated tissue equivalents (0.1:1) and increased with progressive differentiation (0.5:1) albeit never to the levels of intact skin; this likely reflecting the actively regenerating state of these ex vivo culture models (Supplementary Figure 7). Collectively, these data indicate that cortisol consumption and expression of critical enzymes in glucocorticoid biosynthesis are tightly linked to the differentiation state of human epidermis.
Glucocorticoid deficiency alters epidermal differentiation and elicits heightened inflammatory responses
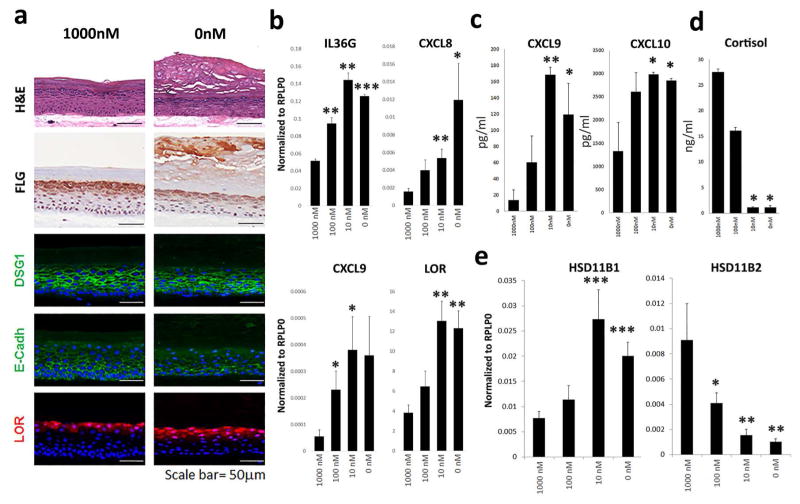
To address the impact of decreased glucocorticoids on epidermal differentiation, we grew 3D human epidermal cultures in the presence of progressively lower concentrations of hydrocortisone from the initial stages of their development at an air-liquid interface (1000nM, 100nM, 10nM and 0nM). A stratified epithelial tissue equivalent formed under all these conditions, but hydrocortisone depletion was associated with impaired epidermal morphogenesis. In particular, there was a marked disorganization of the epidermal cell layers as reflected by disrupted localization of junctional proteins involved in maintaining epidermal tissue integrity, including E-cadherin and desmoglein 1 (Dsg1), and late stage differentiation markers (filaggrin, loricrin). In contrast, glucocorticoid depletion was associated with aberrant development of the granular layer and abnormal stratum corneum (Figure 4a). Accordingly, there was a marked increase in filaggrin and loricrin mRNA transcript levels in 3D epidermal tissues grown in reduced hydrocortisone conditions. Glucocorticoid deficiency also led to increased mRNA expression and secretion of pro-inflammatory cytokines including IL36G, CXCL8/IL-8, CXCL9 and CXCL10 (p<0.05 for all) (Figure 4b–c), in addition to decreased cortisol levels (Figure 4d). Interestingly, HSD11B1 expression was increased (p<0.001) while HSD11B2 was significantly decreased (p<0.01), indicating that the 3-D epidermal cultures depleted of exogenous glucocorticoids respond by ramping up endogenous cortisol production machinery.
Figure 4. Glucocorticoid deficiency leads to abnormal epidermal differentiation and heightened pro-inflammatory responses.
(a) 3D-human epidermal tissue cultures were grown in the presence of normal and low concentrations of hydrocortisone (1000nM, 100nM, 10nM and 0 nM). Histologically, this was associated with thickening of the epidermis with increased prominence of the granular layer and thickening of the stratum corneum, and expression of loricrin (LOR), and abnormal intercellular distribution of desmoglein-1 (DSG1) and E-cadherin (E-cad). (b–c) mRNA expression and protein secretion of pro-inflammatory and differentiation markers was increased with progressively lower hydrocortisone supplementation. (d) There was progressive decrease in cortisol levels, and decreased expression of HSD11B2 (p<0.01), while (e) HSD11B1 expression was increased (p<0.001). (Data shown with SEM, n=4, *p<0.05, **p<0.01, ***p<0.001, for IHC and IF representative figures are shown (n=3)).
To confirm the importance of keratinocyte-derived cortisol biosynthesis for epidermal differentiation, we used a pharmacological inhibitor of HSD11B1 (PF915275) to block endogenous cortisol synthesis (Bhat et al., 2008). For this purpose, 3D epidermal cultures were differentiated in the presence of hydrocortisone (1000 nM) for 6 or 9 days and then switched to medium lacking hydrocortisone to mimic a steroid-depleted tissue microenvironment; the cultures were treated with PF915275 at this time point. Epidermal morphology and differentiation was largely unaffected by this more acute depletion of exogenous hydrocortisone alone whereas combination of glucocorticoid depletion with pharmacological blockade of HSD11B1 markedly impaired epidermal architecture and the distribution of structural and differentiation proteins, including E-cadherin, Dsg1, loricrin, and filaggrin (Figure 5a). Importantly, these changes were normalized by re-addition of exogenous cortisol (100nM). QRT-PCR demonstrated decreased expression of involucrin (IVL) and filaggrin (FLG) (p<0.05), and increased HSD11B1 but decreased HSD11B2 mRNA expression (p<0.05) (Figure 5b). These data highlight the importance of the endogenous cortisol metabolism machinery for maintaining epidermal differentiation.
Figure 5. Exogenous glucocorticoids suppress inflammatory response and restore epidermal differentiation.
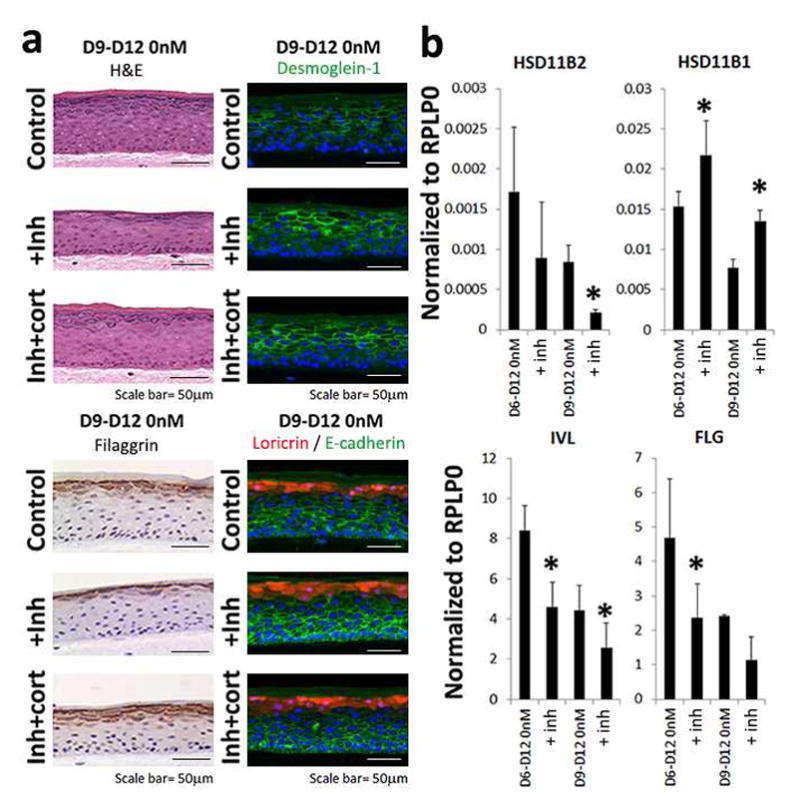
(a) We grew 3D-human epidermis in the presence of hydrocortisone (1000nM) for 6 (data not shown) to 9 days and then switched the cultures to steroid deficient medium (0nM hydrocortisone) +/− the HSD11B1 inhibitor PF915275 (inh). Whereas control cultures showed near normal epidermal differentiation, cultures treated with the HSD11B1 inhibitor showed marked epidermal changes including filaggrin, desmoglein-1 and loricrin/E-cadherin protein expression. These changes were rescued by addition of exogenous cortisol (100nM). (b) QRT-PCR demonstrated decreased expression of involucrin (IVL), filaggrin (FLG) and HSD11B2, but increased HSD11B1 mRNA expression. (Data shown with SEM, n=4, *p<0.05, for IHC and IF representative figures are shown (n=3)).
Steroid treatment of psoriasis in vivo leads to restoration of the glucocorticoid biosynthesis in skin
Three patients with stable chronic plaque psoriasis were treated with medium potency topical steroid (triamcinolone acetate 0.1%) for 7 days (n=3). There was a marked decrease in epidermal thickness compared to baseline (Figure 6a) along with decreased mRNA and protein expression of inflammatory markers (hBD2, CXCL9 and CXCL10) (p<0.05). These changes were accompanied by an increase in markers of normal epidermal differentiation (loricrin, filaggrin, p<0.05 both) (Figure 6b). Furthermore, the normalization of psoriatic histology was accompanied by increased expression of HSD11B1 and HSD11B2 (p<0.01) (Figure 6b), suggesting restoration of endogenous glucocorticoid biosynthesis and homeostasis in the epidermis. Similarly, treatment of healthy skin with topical steroids for only 24 hours led to suppression of inflammatory responses and induction of genes involved in keratinocyte differentiation (Supplementary Figure 8). These data demonstrate that topical treatment with steroids leads to simultaneous suppression of inflammatory responses, normalization of epidermal differentiation, and restoration of endogenous glucocorticoid biosynthesis.
Figure 6. Topical glucocorticoids normalize epidermal differentiation and suppress inflammatory response in vivo.
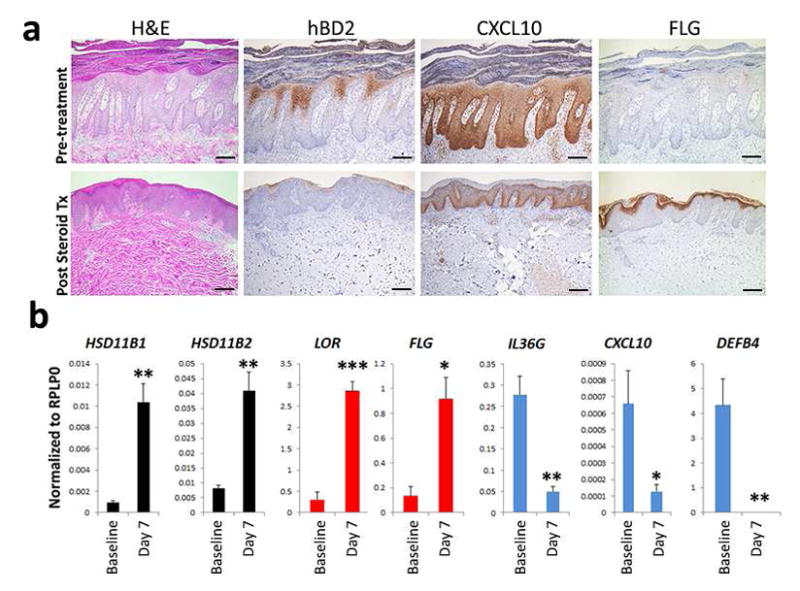
To address the role of topical glucocorticoids in vivo, patients with stable chronic plaque psoriasis were treated with medium potency topical steroid (triamcinolone acetate 0.1%) for 7 days (n=3). Compared to baseline there was marked decrease in epidermal thickness and decreased protein (a) and mRNA expression (b) of inflammatory markers, while markers of epidermal differentiation and HSD11B1/HSD11B2 mRNA expression normalized. (Data shown with SEM, n=4, *p<0.05, **p<0.01, ***p<0.001).
DISCUSSION
Psoriasis is marked by gross histologic alterations (Lowes et al., 2014) that are accompanied by pervasive transcriptomic (Jabbari et al., 2012; Li et al., 2014; Swindell et al., 2013) and proteomic (Lundberg et al., 2015; Swindell et al., 2015) changes in inflamed skin. However, global metabolic changes have not previously been addressed in psoriatic skin. Using liquid chromatography in tandem with mass spectrometry, we have identified striking metabolic shifts in psoriatic skin compared to both uninvolved and normal skin. These changes include profound deficiency of glucocorticoid hormones. Furthermore, by showing suppression of key enzymes in glucocorticoid biosynthesis, we provide evidence that this deficiency may not only be due to increased demand of glucocorticoids by the inflammatory infiltrate but also from decreased supply owing to localized suppression of endogenous glucocorticoid biosynthesis in the epidermis (Figure 2d). Notably, this explains previous observations showing impaired nuclear translocation of the glucocorticoid receptor in psoriatic skin (Man et al., 2013) (Man et al., 2013; Pang et al., 2015), and provides important insights into the importance of glucocorticoid synthesis in the epidermis (Jozic et al., 2014), and its role in inflammatory responses.
Glucocorticoids were first identified in the 1940s as potent anti-inflammatory agents (Lin and Wang, 2016). Since then, both natural and synthetic glucocorticoids have been among the most prescribed immune suppression treatments worldwide (Clark and Belvisi, 2012), and the mainstay of psoriasis treatment for over 50 years (Mrowietz and Domm, 2013). Glucocorticoids have been known to affect both proliferation and stimulate differentiation in the epidermis (Sugimoto and Endo, 1971; Sugimoto et al., 1974), and human skin has been shown to express key enzymes involved in glucocorticoid biosynthesis, and being capable of producing glucocorticoids (Dumont et al., 1992; Rogoff et al., 2001; Slominski et al., 1996; Slominski et al., 2007; Slominski et al., 2004). Our data extend these observations by demonstrating that expression of the glucocorticoid biosynthetic pathway is closely linked to epidermal differentiation (Figures 4–6), explaining why it has been difficult to detect de novo cortisol production by cultured monolayer keratinocytes (Milewich et al., 1986; Slominski et al., 2002).
Two enzymes, HSD11B1 and HSD11B2, are critical in regulating cortisol synthesis and both are expressed in skin. Whereas HSD11B1 activates cortisone to cortisol (Miller and Auchus, 2011; Slominski et al., 2004; Tiganescu et al., 2011), HSD11B2 converts cortisol to the inactive cortisone (Cirillo and Prime, 2011; Miller and Auchus, 2011; Tiganescu et al., 2011; Vukelic et al., 2011) (Figure 3). Thus, keratinocytes where HSD11B1 has been silenced produce less cortisol from cortisone than control cells (Cirillo and Prime, 2011). In contrast, silencing of HSD11B2 leads to elevation of cortisol levels (Cirillo and Prime, 2011) demonstrating that the relative levels and activities of these two enzymes determine the overall levels of glucocorticoids locally present in the skin (Cirillo and Prime, 2011; Tiganescu et al., 2011). In psoriatic skin, the mRNA expression of both HSD11B1, consistent with findings from a recent study (Terao et al., 2016), and HSD11B2 is decreased, but the ratio between these two enzymes is biased toward greater suppression of HSD11B1 and therefore less production of cortisol. This is similar to what we observe in several other skin diseases and wound healing (Supplementary file 4).
Loss of glucocorticoid receptor activity in mice has pleiotropic effects on skin and its appendages, including profound changes in epidermal differentiation (Bayo et al., 2008). Our findings using a 3D human epidermal model support the notion that glucocorticoids directly impact keratinocyte differentiation. Remarkably, many of the features in the epidermis overlap with histopathological characteristics of cutaneous psoriasis (Rittie et al., 2016). A notable difference between human psoriasis and the 3D epidermal cultures was observed when hydrocortisone supplementation was withdrawn from the culture model. Under these circumstances, the 3D epidermal tissues responded by increasing HSD11B1 and lowering HSD11B2 expression, likely in an attempt to drive up endogenous production of cortisol. As the steroid biosynthetic pathway is suppressed in psoriatic skin, this compensatory mechanism is probably not able to correct for cortisol deficiency. In addition, the glucocorticoid deficiency leads to heightened pro-inflammatory responses and increased expression of late differentiation markers in the epidermal tissue equivalents. When endogenous production of cortisol is blocked by pharmacological inhibitors, the expression of differentiation markers decreases following the pattern observed in psoriatic skin (Figure 6). These 3D skin culture data demonstrate that glucocorticoids have a critical role in epidermal biology by promoting differentiation and suppressing pro-inflammatory responses. Our ex vivo data support this notion by showing that application of topical glucocorticoids to lesional skin leads to rapid improvement with normalization of epidermal differentiation markers, decreased inflammation, and normalization of both HSD11B1 and HSD11B2 expression in order to restore endogenous glucocorticoid biosynthesis in the skin. Similarly, the application of steroid to healthy skin has similar effects many of which have been shown to have increased expression in clinically uninvolved skin of psoriatic patients (Gudjonsson et al., 2009), consistent with the elevated cortisol levels in uninvolved skin (Figure 2).
The findings presented here provide additional insights into what is the cause of the suppression of glucocorticoid biosynthesis in psoriatic skin. Elements of the hypothalamopituitary- adrenal axis (HPA) have been implicated as major regulators of glucocorticoid synthesis in skin (Slominski et al., 2007), however, we were unable to find evidence for their involvement in glucocorticoid suppression in psoriasis (Supplementary Figure 4). In keeping with the data presented here, a study looking at HPA-axis in patients with psoriatic arthritis and psoriasis failed to observe any correlation between ACTH levels and serum cortisol, or with PASI responses. Interestingly, and consistent with our data, serum cortisol levels increased as disease improved (Atzeni et al., 2008). Our data demonstrate that the expression of HSD11B1 and HSD11B2 is closely related to the differentiation state of the epidermis (Figures 4–6), and that HSD11B1 and HSD11B2 expression in stratified epidermis is suppressed by the same pro-inflammatory cytokines that are known to be key drivers of psoriasis pathogenesis (Lowes et al., 2014), including IL-17A and IL-22. These cytokines, particularly IL-22, disrupt epidermal differentiation (Sa et al., 2007). Whether the down-regulation of HSD11B1 and HSD11B2 is related to the direct effect of these pro-inflammatory cytokines or secondary to the abnormal differentiation in psoriasis (Bernard et al., 1985) remains to be investigated, but is an exciting avenue to address in future studies.
In summary, these data expand our understanding of the role of glucocorticoids both in healthy and inflamed skin. Our study illustrates a role for endogenous production of glucocorticoids in epidermal differentiation and inflammatory responses, provide important insights into the pathobiology of psoriasis with implications for other chronic inflammatory conditions commonly treated with glucocorticoids, and may lead to novel therapeutic approaches targeting mechanisms governing glucocorticoid biosynthesis.
MATERIALS AND METHODS
Patient cohort
Nine patients with moderate chronic plaque psoriasis were enrolled for this study. Two biopsies were taken under local anesthesia from each psoriatic patient and each healthy control, with one biopsy submitted for RNA-sequencing and the other matching biopsy for metabolomic profiling. For the steroid treated cohort (n=3) patients underwent a single six millimeter punch biopsy prior to initiation of treatment and after 7 days of topical steroid use (triamcinolone acetate 0.1%). Informed written consent was obtained from all patients and controls, under protocols approved by the Institutional Review Board of the University of Michigan Medical School, and Northwestern University Institutional Review Board. This study was conducted according to the Declaration of Helsinki Principles.
Serum analyses and ELISAs
Serum was obtained from 37 patients with moderate-to-severe psoriasis and 43 healthy non-psoriatic controls. These samples were obtained at evaluation of patients recruited into our psoriasis genetic cohort database (Li et al., 2014).
RNA-seq processing, QRT-PCR, statistical and bioinformatics analyses
RNA was isolated with RNeasy Plus Mini kit (Qiagen). 50 nucleotide single-end read was performed using Illumina Hi-Seq 2000 Genome Analyzer (Illumina). The reads were mapped by Cufflinks (Trapnell et al., 2010) and TopHat (Trapnell et al., 2009) and gene expression levels were expressed as fragments per kilobase of region per million mapped reads. For QRT-PCR RNA was reverse transcribed using a High Capacity cDNA Transcription kit (Applied Biosystems). qRT-PCR was performed using the 7900HT Fast Real-Time PCR system (Applied Biosystems) with Taqman primers purchased from Applied Biosystems (see supplemental methods)
Immunohistochemistry and immunofluorescence
Immunhistochemistry was performed on 5 μm thick paraffin sections from skin and epidermal rafts using antibodies (see supplemental methods)
Metabolomic profiling
Chromatography was performed on 1290 Infinity Binary LC System from Agilent together with Waters Acquity UPLC HSST3 1.8μm 2.1 × 100 mm column in connection with a Water Acquity UPLC HSS T3 1.8μm VanGuard Pre-column. Mass spectrometry was performed using Agilent Technologies 6530 Accurate-Mass Q-T of with a dual ASJ ESI ion source.
Statistics
For all experiments described, 2-tailed Student’s t tests were performed. Graphs are presented as mean ± SEM. P-values less than 0.05 were considered statistically significant. Raw data processing of metabolomic data was done using Agilent software (MassHunter Qual, and ProFinder).
Supplementary Material
Acknowledgments
The work was in part supported by the University of Michigan Babcock Endowment Fund (J.E.G., J.T.E, J.J.V), NIH awards K08-AR060802 (J.E.G.), R01-AR062110 (S.G.), R01-GM112945 (I.B.), and R01-AR069071 (J.E.G.), the A. Alfred Taubman Medical Research Institute Kenneth and Frances Eisenberg Emerging Scholar Award (J.E.G.), and Doris Duke Charitable Foundation Grant #2013106 (J.E.G), the Skin and Tissue Engineering Core of the Northwestern University SDRC, funded by the National Institute of Health (NIH) P30-AR057216-01. The work on this paper utilized Metabolomics Core Services supported by grant U24 DK097153.
Footnotes
Conflict of interest statement: The authors have declared that no conflict of interest exists.
CONFLICT OF INTEREST
The authors state no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnette C, Koetsier JL, Hoover P, Getsios S, Green KJ. In Vitro Model of the Epidermis: Connecting Protein Function to 3D Structure. Methods Enzymol. 2016;569:287–308. doi: 10.1016/bs.mie.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atzeni F, Sarzi-Puttini P, DePortu S, Cutolo M, Carrabba M, Straub RH. In etanercept-treated psoriatic arthritis patients clinical improvement correlated with an increase of serum cortisol relative to other adrenal hormones. Clin Exp Rheumatol. 2008;26:103–8. [PubMed] [Google Scholar]
- Bayo P, Sanchis A, Bravo A, Cascallana JL, Buder K, Tuckermann J, et al. Glucocorticoid receptor is required for skin barrier competence. Endocrinology. 2008;149:1377–88. doi: 10.1210/en.2007-0814. [DOI] [PubMed] [Google Scholar]
- Bernard BA, Robinson SM, Vandaele S, Mansbridge JN, Darmon M. Abnormal maturation pathway of keratinocytes in psoriatic skin. Br J Dermatol. 1985;112:647–53. doi: 10.1111/j.1365-2133.1985.tb02332.x. [DOI] [PubMed] [Google Scholar]
- Bhat BG, Hosea N, Fanjul A, Herrera J, Chapman J, Thalacker F, et al. Demonstration of proof of mechanism and pharmacokinetics and pharmacodynamic relationship with 4′-cyano-biphenyl-4-sulfonic acid (6-amino-pyridin-2-yl)-amide (PF-915275), an inhibitor of 11 -hydroxysteroid dehydrogenase type 1, in cynomolgus monkeys. J Pharmacol Exp Ther. 2008;324:299–305. doi: 10.1124/jpet.107.128280. [DOI] [PubMed] [Google Scholar]
- Boix J, Sevilla LM, Saez Z, Carceller E, Perez P. Epidermal Mineralocorticoid Receptor Plays Beneficial and Adverse Effects in Skin and Mediates Glucocorticoid Responses. J Invest Dermatol. 2016 doi: 10.1016/j.jid.2016.07.018. [DOI] [PubMed] [Google Scholar]
- Cirillo N, Prime SS. Keratinocytes synthesize and activate cortisol. J Cell Biochem. 2011;112:1499–505. doi: 10.1002/jcb.23081. [DOI] [PubMed] [Google Scholar]
- Clark AR, Belvisi MG. Maps and legends: the quest for dissociated ligands of the glucocorticoid receptor. Pharmacol Ther. 2012;134:54–67. doi: 10.1016/j.pharmthera.2011.12.004. [DOI] [PubMed] [Google Scholar]
- Dumont M, Luu-The V, Dupont E, Pelletier G, Labrie F. Characterization, expression, and immunohistochemical localization of 3 beta-hydroxysteroid dehydrogenase/delta 5-delta 4 isomerase in human skin. J Invest Dermatol. 1992;99:415–21. doi: 10.1111/1523-1747.ep12616131. [DOI] [PubMed] [Google Scholar]
- Gudjonsson JE, Ding J, Li X, Nair RP, Tejasvi T, Qin ZS, et al. Global gene expression analysis reveals evidence for decreased lipid biosynthesis and increased innate immunity in uninvolved psoriatic skin. J Invest Dermatol. 2009;129:2795–804. doi: 10.1038/jid.2009.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz S, Benner C, Spann N, Bertolino E, Lin YC, Laslo P, et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell. 2010;38:576–89. doi: 10.1016/j.molcel.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbari A, Suarez-Farinas M, Dewell S, Krueger JG. Transcriptional profiling of psoriasis using RNA-seq reveals previously unidentified differentially expressed genes. J Invest Dermatol. 2012;132:246–9. doi: 10.1038/jid.2011.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jozic I, Stojadinovic O, Kirsner RS, Tomic-Canic M. Stressing the steroids in skin: paradox or fine-tuning? J Invest Dermatol. 2014;134:2869–72. doi: 10.1038/jid.2014.363. [DOI] [PubMed] [Google Scholar]
- Li B, Tsoi LC, Swindell WR, Gudjonsson JE, Tejasvi T, Johnston A, et al. Transcriptome analysis of psoriasis in a large case-control sample: RNA-seq provides insights into disease mechanisms. J Invest Dermatol. 2014;134:1828–38. doi: 10.1038/jid.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin KT, Wang LH. New dimension of glucocorticoids in cancer treatment. Steroids. 2016 doi: 10.1016/j.steroids.2016.02.019. [DOI] [PubMed] [Google Scholar]
- Lowes MA, Suarez-Farinas M, Krueger JG. Immunology of psoriasis. Annu Rev Immunol. 2014;32:227–55. doi: 10.1146/annurev-immunol-032713-120225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg KC, Fritz Y, Johnston A, Foster AM, Baliwag J, Gudjonsson JE, et al. Proteomics of skin proteins in psoriasis: from discovery and verification in a mouse model to confirmation in humans. Mol Cell Proteomics. 2015;14:109–19. doi: 10.1074/mcp.M114.042242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man XY, Li W, Chen JQ, Zhou J, Landeck L, Zhang KH, et al. Impaired nuclear translocation of glucocorticoid receptors: novel findings from psoriatic epidermal keratinocytes. Cell Mol Life Sci. 2013;70:2205–20. doi: 10.1007/s00018-012-1255-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milewich L, Kaimal V, Shaw CB, Sontheimer Epidermal keratinocytes: a source of 5 alpha-dihydrotestosterone production in human skin. J Clin Endocrinol Metab. 1986;62:739–46. doi: 10.1210/jcem-62-4-739. [DOI] [PubMed] [Google Scholar]
- Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev. 2011;32:81–151. doi: 10.1210/er.2010-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrowietz U, Domm S. Systemic steroids in the treatment of psoriasis: what is fact, what is fiction? J Eur Acad Dermatol Venereol. 2013;27:1022–5. doi: 10.1111/j.1468-3083.2012.04656.x. [DOI] [PubMed] [Google Scholar]
- Pang X, Zhang P, Zhu S, Guo G. Expression of glucocorticoid receptor-alpha in the epidermis of patients with psoriasis vulgaris. Exp Ther Med. 2015;10:419–22. doi: 10.3892/etm.2015.2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittie L, Tejasvi T, Harms PW, Xing X, Nair RP, Gudjonsson JE, et al. Sebaceous gland atrophy in psoriasis: An explanation for psoriatic alopecia. 2016 doi: 10.1016/j.jid.2016.05.113. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogoff D, Gomez-Sanchez CE, Foecking MF, Wortsman J, Slominski A. Steroidogenesis in the human skin: 21-hydroxylation in cultured keratinocytes. J Steroid Biochem Mol Biol. 2001;78:77–81. doi: 10.1016/s0960-0760(01)00076-0. [DOI] [PubMed] [Google Scholar]
- Sa SM, Valdez PA, Wu J, Jung K, Zhong F, Hall L, et al. The effects of IL-20 subfamily cytokines on reconstituted human epidermis suggest potential roles in cutaneous innate defense and pathogenic adaptive immunity in psoriasis. J Immunol. 2007;178:2229–40. doi: 10.4049/jimmunol.178.4.2229. [DOI] [PubMed] [Google Scholar]
- Schon MP, Boehncke WH. Psoriasis. N Engl J Med. 2005;352:1899–912. doi: 10.1056/NEJMra041320. [DOI] [PubMed] [Google Scholar]
- Sevilla LM, Latorre V, Sanchis A, Perez P. Epidermal inactivation of the glucocorticoid receptor triggers skin barrier defects and cutaneous inflammation. J Invest Dermatol. 2013;133:361–70. doi: 10.1038/jid.2012.281. [DOI] [PubMed] [Google Scholar]
- Slominski A, Ermak G, Mihm M. ACTH receptor, CYP11A1, CYP17 and CYP21A2 genes are expressed in skin. J Clin Endocrinol Metab. 1996;81:2746–9. doi: 10.1210/jcem.81.7.8675607. [DOI] [PubMed] [Google Scholar]
- Slominski A, Wortsman J, Foecking MF, Shackleton C, Gomez-Sanchez C, Szczesniewski A. Gas chromatography/mass spectrometry characterization of corticosteroid metabolism in human immortalized keratinocytes. J Invest Dermatol. 2002;118:310–5. doi: 10.1046/j.0022-202x.2001.01648.x. [DOI] [PubMed] [Google Scholar]
- Slominski A, Wortsman J, Tuckey RC, Paus R. Differential expression of HPA axis homolog in the skin. Mol Cell Endocrinol. 2007;265–266:143–9. doi: 10.1016/j.mce.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A, Zjawiony J, Wortsman J, Semak I, Stewart J, Pisarchik A, et al. A novel pathway for sequential transformation of 7-dehydrocholesterol and expression of the P450scc system in mammalian skin. Eur J Biochem. 2004;271:4178–88. doi: 10.1111/j.1432-1033.2004.04356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojadinovic O, Lee B, Vouthounis C, Vukelic S, Pastar I, Blumenberg M, et al. Novel genomic effects of glucocorticoids in epidermal keratinocytes: inhibition of apoptosis, interferon-gamma pathway, and wound healing along with promotion of terminal differentiation. J Biol Chem. 2007;282:4021–34. doi: 10.1074/jbc.M606262200. [DOI] [PubMed] [Google Scholar]
- Sugimoto M, Endo H. Accelerating effect of hydrocortisone on the keratinization of chick embryonic skin growing in a chemically defined medium. J Embryol Exp Morphol. 1971;25:365–76. [PubMed] [Google Scholar]
- Sugimoto M, Tajima K, Kojima A, Endo H. Differential acceleration by hydrocortisone of the accumulation of epidermal structural proteins in the chick embryonic skin growing in a chemically defined medium. Dev Biol. 1974;39:295–307. doi: 10.1016/0012-1606(74)90241-3. [DOI] [PubMed] [Google Scholar]
- Swindell WR, Johnston A, Voorhees JJ, Elder JT, Gudjonsson JE. Dissecting the psoriasis transcriptome: inflammatory- and cytokine-driven gene expression in lesions from 163 patients. BMC Genomics. 2013;14:527. doi: 10.1186/1471-2164-14-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swindell WR, Remmer HA, Sarkar MK, Xing X, Barnes DH, Wolterink L, et al. Proteogenomic analysis of psoriasis reveals discordant and concordant changes in mRNA and protein abundance. Genome Med. 2015;7:86. doi: 10.1186/s13073-015-0208-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swindell WR, Sarkar MK, Liang Y, Xing X, Gudjonsson JE. Cross-disease transcriptomics: Unique IL-17A signaling in psoriasis lesions and an autoimmune PBMC signature. The Journal of investigative dermatology. 2016 doi: 10.1016/j.jid.2016.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terao M, Itoi S, Matsumura S, Yang L, Murota H, Katayama I. Local Glucocorticoid Activation by 11beta-Hydroxysteroid Dehydrogenase 1 in Keratinocytes: The Role in Hapten-Induced Dermatitis. Am J Pathol. 2016;186:1499–510. doi: 10.1016/j.ajpath.2016.01.014. [DOI] [PubMed] [Google Scholar]
- Tiganescu A, Walker EA, Hardy RS, Mayes AE, Stewart PM. Localization, age- and site-dependent expression, and regulation of 11beta-hydroxysteroid dehydrogenase type 1 in skin. J Invest Dermatol. 2011;131:30–6. doi: 10.1038/jid.2010.257. [DOI] [PubMed] [Google Scholar]
- Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25:1105–11. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol. 2010;28:511–5. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukelic S, Stojadinovic O, Pastar I, Rabach M, Krzyzanowska A, Lebrun E, et al. Cortisol synthesis in epidermis is induced by IL-1 and tissue injury. J Biol Chem. 2011;286:10265–75. doi: 10.1074/jbc.M110.188268. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.