Abstract
Purpose of review
Acute kidney injury (AKI) is associated with increased risk of morbidity and mortality in critically ill children and adults. Neonates remain an understudied group, although previous evidence suggests that this association holds true for them as well.
Recent findings
Attention to the issue of neonatal AKI is increasing. New studies in very low-birthweight infants, infants with congenital heart disease who undergo cardiopulmonary bypass, those who receive extracorporeal membrane oxygenation and infants with perinatal depression continue to demonstrate that AKI is common in neonates and associated with increased risk of morbidity and mortality. Additional advances in the field of neonatal AKI include adaptation of modern, categorical AKI definitions, as well as further evaluation of novel urinary biomarkers (e.g., neutrophil gelatinase-associated lipocalin) in this patient group.
Summary
AKI is an independent risk factor for poor outcomes in critically ill neonates. Our ability to improve outcomes for these patients depends on heightened awareness of this issue both at the bedside as well as in research, commitment to using standardized AKI definitions in order to pool and compare data more effectively and improvement in our diagnostic methods with better AKI biomarkers so that we can identify AKI and intervene much earlier in the disease course.
Keywords: acute kidney injury, biomarkers, epidemiology, neonates
INTRODUCTION
Acute kidney injury (AKI) is associated with mortality in critically ill children [1,2] and adults [3–7], even after controlling for medical comorbidities, severity of illness scores and patient demographics. Although data from neonatal AKI research has been sparse until recently, previous epidemiology studies have suggested that AKI in neonates is common, and those with AKI are at risk for death and long-term chronic kidney disease [8,9]. The past year has brought more evidence in this regard, with several new epidemiological studies in a number of different high-risk groups (e.g., very low birthweight and congenital heart disease). In addition, researchers are no longer relying on arbitrary, binary definitions of neonatal AKI [e.g., serum creatinine (SCr) 1.5 mg/dl] and instead are utilizing contemporary, categorical AKI frameworks such as modifications of the Acute Kidney Injury Network (AKIN) [10] staging system and the risk, injury, failure, loss and end-stage renal disease (RIFLE) classification [3,5] that allow improved diagnosis and staging of AKI by severity. Moreover, there has been exciting new work on novel urinary biomarkers in this population with ongoing optimism that these biomarkers will improve our ability to diagnose and manage neonatal AKI. The purpose of this article is, thus, to review studies published in the last year that enhance understanding of AKI epidemiology, highlight data on urine AKI biomarkers and their use in neonates and encourage ongoing attention to the issue of neonatal AKI by presenting a framework for future research.
DEFINITION OF ACUTED KIDNEY INJURY
Several important factors need to be considered with any neonatal AKI definition, especially those utilizing SCr. Neonates commonly have non-oliguric renal failure, making oliguria (urine output <0.5 ml/kg per hour) an insensitive marker of AKI. After birth, neonatal SCr reflects maternal levels. Rather than maintaining a steady state, these levels then decline at varying rates over days to weeks depending on gestational age, such that changes (or lack of change) in SCr may be difficult to interpret when evaluating for AKI. Unlike with critically ill children and adults who have SCr measured daily per routine care, there is reluctance to measure SCr frequently in neonates because of concern for blood loss with multiple blood samplings. AKI may then go unrecognized or underestimated. Relying on clinician coding of renal dysfunction may also not be sufficient, as evidenced by Walker et al. [11], who reviewed 66 526 preterm neonates (gestational age ≤30 weeks) and found coded diagnosis of ‘renal dysfunction’ in only 1.9% of the sample (n=1239) and ‘renal failure’ in 1.9% of the sample (n=1257), even though another 15.1% had documented SCr 1.3mg/dl or more and 2.5% had documented SCr 2 mg/dl or more.
Regardless of these limitations, SCr remains the most studied and widely used clinical biomarker for measuring kidney function. How SCr is used to define AKI has evolved in the adult and pediatric literature, and this last year has seen a similar shift in neonatal studies. Rather than using arbitrary, binary AKI definitions (SCr >1.5mg/dl, urine output <0.5 ml/kg per hour), several new neonatal studies have utilized modifications of the AKIN staging system or the RIFLE classification. These standardized frameworks have allowed better comparisons across studies, shown consistently that even mild degrees of AKI portend poor outcomes [12*,13**, 14**,15*,16] and demonstrated worse outcomes with progressive AKI severity [14&&]. The continued use of standardized definitions in neonatal AKI research will greatly enhance our ability to pool and compare data, as currently most studies are single center with small sample sizes. Table 1 shows the definition we have proposed. Going forward, we will need to test all definitions against widely agreed upon, hard clinical endpoints (e.g., death, length of hospital stay), in order to determine which definition has the most clinical utility.
TABLE 1.
Proposed neonatal acute kidney injury classification definition
| STAGE | |
|---|---|
| 0 | No change or rise <0.3 mg/dl |
| 1 | ↑SCr 0.3 mg/dl or ↑SCr 150–200% from previous trough value |
| 2 | ↑SCr 200–300% from previous trough value |
| 3 | ↑SCr 300% from previous trough value or ↑2.5 mg/dl or receipt of dialysis |
Adapted from [13**]
INCIDENCE/EPIDEMIOLOGY
Several new studies this year in various neonatal populations show high rates of AKI and continue to illustrate the impact of AKI on outcomes in these groups.
Very low-birthweight infants
Koralkar et al. [13**] prospectively followed 229 VLBW (birth weight between 500 and 1500 g) infants from birth until 36 weeks postmenstrual age or hospital discharge. They categorized each infant according to a modified AKIN definition (Table 1). They did not include urine output in their definition, citing high rates of non-oliguric kidney injury in this population. Using this framework, 41 of 229 (18%) of the cohort developed AKI (10 in stage 1, 10 in stage 2 and 21 in stage 3). Infants with AKI were more likely to have lower birthweight, gestational age and Apgar scores, as well as higher rates of assisted ventilation and inotropic support. In the AKI group, 42% (17 of 41) died, compared with 5%(nine of 188) in the non- AKI group [hazard ratio (HR) = 9.3, 95% confidence interval (CI)= 4.1–21.0; P<0.01]. Those with AKI had higher mortality independent of multiple adjusted confounders (adjusted HR= 2.3, 95% CI= 0.9, 5.8; P= 0.06).
Infants with congenital heart disease
Incidence of AKI in infants with congenital heart disease (CHD) undergoing cardiopulmonary bypass (CPB) was examined in two studies. Blinder et al. [14**] conducted a retrospective chart review of 430 infants (<90 days, median age 7 days) with CHD.
AKI was defined using a modified AKIN definition (urine output criteria included). They documented AKI in 225 of 430 (52%) infants [133 (31%) with stage 1; 60 (14%) with stage 2 and 30 (7%) with stage 3]. Postoperative AKI of all stages was associated with longer ICU stay; AKI stages 2 and 3 were associated with increased risk of prolonged mechanical ventilation and need for postoperative inotropic therapy. The mortality rate in those with AKI was higher than in those without [27 of 225 (12%) vs. six of 205 (3%), P<0.001]. Moreover, risk of death increased with AKI severity [stage 2 odds ratio (OR) 5.1 (95% CI= 1.7–15.2), P= 0.004 and stage 3 OR 9.5 (95% CI= 2.9–30.7), P¼0.0002]. Krawczeski et al. [17**] evaluated 374 patients (including 35 neonates) with CHD for the development of postoperative AKI in their study on the use of neutrophil gelatinase-associated lipocalin (NGAL) to predict AKI after CPB. They defined AKI as an absolute increase in SCr 0.3 mg/dl or more from baseline within 48 h of surgery. Using this definition, eight of 35 (23%) had AKI by median 1 day after CPB. No differences in mortality were seen between the neonates with AKI and those without in this small neonatal subset.
Infants who receive extracorporeal membrane oxygenation
In a large retrospective cohort study using data from the Extracorporeal Life Support Organization registry, Askenazi et al. [12*] evaluated the impact of AKI and renal support therapy (RST) in infants who receive extracorporeal membrane oxygenation (ECMO) for noncardiac reasons. AKI was defined as SCr higher than 1.5 at any point in the hospitalization. Of the 7941 neonates in their cohort, 27.4% died. Non-survivors had higher rates of AKI than survivors (19 vs. 3.9%, P<0.0001), and more non-survivors received RST than survivors (39.7 vs. 16%, P<0.0001). After adjustment for numerous confounding variables, neonates with AKI had 3.2 higher odds of death than those without AKI (P<0.0001); neonates who received RST had 1.9 higher odds of death (P<0.0001) than those who did not receive RST. This data suggests that AKI (prevention, early identification) and RST (timing of initiation and duration) may be potential modifiable factors that warrant further study.
Two smaller studies also had similar findings. In a retrospective chart review of infants with congenital diaphragmatic hernia requiring ECMO, Gadepalli et al. [15*] found 48 of 68 patients (71%) had AKI by the RIFLE classification. Patients with AKI ‘failure’ (300% rise in SCr) had increased time on ECMO, decreased ventilator-free days and decreased survival (27.3 with ‘failure’ vs. 80% without AKI, P¼0.001). Shuhaiber et al. [16] also found higher rates of AKI in non-survivors vs. survivors (80 vs. 40%, P= 0.03) in a small sample of patients (n= 20, 75% neonates) requiring more than one ECMO run after congenital heart surgery.
Infants with perinatal depression
Kaur et al. [18] evaluated 36 neonates who were 34 or more weeks of gestation with 1 min Apgar score higher than seven. AKI developed in 9.1% (one of 11) with moderate asphyxia and in 56% (12 of 25) infants with severe asphyxia. AKI persisted in 16.6% neonates at 96 h of life.
BIOMARKERS
As discussed previously, SCr-based AKI definitions have multiple shortcomings, especially in the neonatal population. Of the most important, SCr estimates glomerular function, not damage, and takes days to rise after an injury has occurred. Thus, the focus has been to find better AKI biomarkers to diagnose this process earlier in the course, allow the development of interventions and ultimately improve outcomes. Urinary biomarkers show great promise in this regard. The last year has seen more data on the use of AKI biomarkers in children with CHD, and additional data in premature infants has emerged.
Urine acute kidney injury biomarkers in neonates with congenital heart disease undergoing cardiopulmonary bypass
Children with CHD undergoing CPB represent an ideal population for studying AKI because the timing of injury is known, and they do not have underlying chronic renal disease. Data on serum and urine NGAL [17**], urine liver fatty acid-binding protein [19], serum and urine cystatin C [20,21], urine interleukin 18 (IL-18) [22], urine aprotinin [23], urine netrin-1 [24] and others has been published in this cohort.
As mentioned above, Krawczeski et al. [17**] evaluated the use of NGAL as an early marker of AKI in children with CHD undergoing CPB. In their subgroup of 35 neonates (>37 weeks gestational age), both plasma and urine NGAL were significant predictors of the development of AKI, rising within 2 h after CPB [area under the curves (AUC) for both >0.88, all P<0.05]. Urine NGAL thresholds were higher in the neonatal group vs. non-neonates (185 vs. 48 ng/ml). In this small neonatal sample, NGAL did not correlate with longer duration of AKI or hospital stay as it did in the non-neonatal group.
Urine acute kidney injury biomarkers in premature infants
Premature infants are born with immature renal tubules, as renal development normally continues until 34 weeks post-conception. Baseline evaluation of urine NGAL in premature infants shows that urine biomarkers are inversely related to both gestational age and birthweight [25]. Askenazi et al. [26*] found similar changes in urine NGAL and other candidate urine AKI biomarkers, noting that kidney injury molecule-1 (KIM-1), cystatin C (CysC), b-2 microglobulin and osteopontin (OPN), but not IL-18, are affected by gestational age.
To determine if candidate AKI biomarkers can predict a rise in SCr and mortality in premature infants, Askenazi et al. [27**] conducted two separate case–control studies. Compared with non-AKI patients (n= 21), those with AKI (n= 9; modified AKIN definition – Table 1) had higher maximum NGAL [odds ratio= 1.2 (1.0, 1.6), P<0.01; receiver operator characteristics (ROC) AUC= 0.80] and higher maximum OPN [OR= 3.2 (1.5, 9.9); P<0.01; ROC AUC= 0.83] values. Compared with survivors (n¼100), non-survivors (n= 23) had higher maximum urine KIM-1 [OR= 1.1 (1.0, 1.2); P<0.02; ROC AUC= 0.64] and OPN [OR= 1.8 (1.2, 2.7); P<0.001; ROC AUC= 0.78]. Combination of biomarkers improved predictability for both AKI and mortality. Controlling for gestational age and birthweight did not affect results.
These studies suggest that urine biomarkers can predict AKI and mortality in premature infants. Controlling for gestational age will be imperative when evaluating urine biomarkers in premature infants going forward.
THERAPIES FOR ACUTE KIDNEY INJURY IN NEONATES
Unfortunately, despite the known impact of AKI on patient outcomes, there is little data on its prevention or treatment. Currently, only RSTs are approved, although there is little data regarding their application in this population. Two recent studies explore potential AKI treatments; however, further studies on these and other therapies will be needed before they can be used to intervene in the natural course of AKI.
In 2008, Ricci et al. [28] evaluated 60 neonates undergoing cardiac surgery with CPB and found that low-dose fenoldopam, a selective dopamine-1 receptor, did not improve urine output, fluid balance or AKI in neonates. In 2011, however, the same group showed that urinary NGAL and Cys C values were significantly reduced at the end of surgery in infants who received high-dose fenoldopam compared with those who received placebo (P= 0.025 and 0.039, respectively) [29]. Hobbs et al. [30] used rasburicase, a recombinant urate oxidase, for management of hyperuricemia in seven infants with AKI (SCr >1.5 mg/dl) and hyperuricemia (serum uric acid >8 mg/dl). Within 24 h, serum uric acid decreased from 13.6±4.5 to 0.9±0.6mg/dl (P<0.05), SCr decreased from 3.2±2.0 to 2.0±1.2 mg/dl (P<0.05), and urine output increased from 2.4±1.2 to 5.9±1.8 ml/kg per hour (P<0.05).
FUTURE RESEARCH FRAMEWORK
In 2007, the AKIN [31] proposed a model to guide further research in the field of AKI (Fig. 1). Arrows represent potential for either worsening or improvement at each step of the AKI pathway. At each step, there is also risk for the development of complications. At each arrow, there is opportunity for hypotheses to be generated and tested. Here, we provide a framework for future research in neonatal AKI that we hope will encourage ongoing attention to this issue.
Unique populations of neonates are at risk for AKI, including premature infants, those with CHD who undergo CPB and those who suffer perinatal depression. In each of these groups, there are demographic and clinical factors that put them at risk for developing AKI (arrow 1). What are the risk factors? Can risk category stratification help guide the care of these infants?
At-risk individuals may suffer kidney injury (arrow 2a) that may then lead to decreased glomerular filtration rate (GFR) (arrow 2c) and ultimately renal failure (arrow 2d). Alternatively, the decrease in GFR may be temporary (arrow 2b) without subsequent kidney damage (prerenal azotemia). Will novel urinary biomarkers help distinguish true kidney ‘injury’ from prerenal azotemia? Can ‘injury’ biomarkers predict a rise in SCr? Can they predict hard clinical endpoints such as mortality or guide preventive or therapeutic interventions?
Infants at risk for AKI can develop complications inherent to the underlying process (arrow 3) that will affect outcomes (arrow 5a), such as the inflammatory response that occurs after CPB. To what extent does the kidney ameliorate or exacerbate this response?
Animal models of nephrotoxic and ischemia/reperfusion kidney injury show that AKI is a multisystem disease, with crosstalk between the kidneys and the heart, liver, brain and lung. Complications (arrows 4a, 4b and 4c) that arise after AKI in neonates have not been explored. What are these complications? What are the effects of AKI on other organ systems?
Studies in neonates, adults and children show that outcomes after AKI are poor. However, the reasons why AKI leads to poor outcomes need further elucidation. Renal failure causes complications (arrow 4c), which then could lead to death (arrow 5c). For example, fluid overload has been shown to be an important, modifiable complication in pediatric and adult critically ill patients. Alternatively, renal failure itself can lead to death (arrow 5b). What are the complications? Can they be prevented or treated? If so, how? Can RST improve outcomes? If so, which modality? When should we intervene?
FIGURE 1.
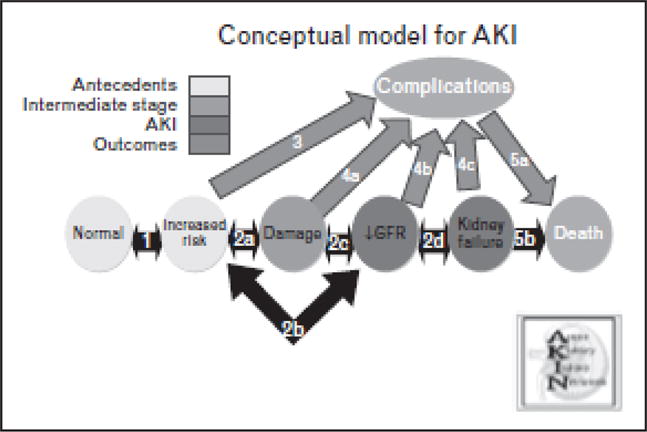
Conceptual model for acute kidney injury research. Adapted from [31].
AKI — acute kidney injury; GFR — glomerular filtration rate
CONCLUSION
The field of neonatal AKI research has grown over the last year. Researchers are adapting categorical definitions of AKI, biomarkers are being tested and neonatal AKI epidemiology is better understood. However, there is still a tremendous amount of work to be done. With this intensification of research, there is hope that we will be able to decrease morbidity and mortality associated with AKI in this population.
KEY POINTS.
Acute kidney injury (AKI) is common in critically ill neonates and associated with increased risk of morbidity and mortality.
Use of standardized definitions will enhance recognition of AKI at the bedside and facilitate cross-study comparison of limited data.
Novel urinary biomarkers show promise in their ability to predict AKI in the neonatal population.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, been highlighted as:
*of special interest
**of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue.
- 1.Akcan-Arikan A, Zappitelli M, Loftis LL, et al. Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int. 2007;71:1028–1035. doi: 10.1038/sj.ki.5002231. [DOI] [PubMed] [Google Scholar]
- 2.Zappitelli M, Parikh CR, Akcan-Arikan A, et al. Ascertainment and epidemiology of acute kidney injury varies with definition interpretation. Clin J Am Soc Nephrol. 2008;3:948–954. doi: 10.2215/CJN.05431207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ricci Z, Cruz D, Ronco C. The RIFLE criteria and mortality in acute kidney injury: a systematic review. Kidney Int. 2008;73:538–546. doi: 10.1038/sj.ki.5002743. [DOI] [PubMed] [Google Scholar]
- 4.Cuhaci B. More data on epidemiology and outcome of acute kidney injury with AKIN criteria: benefits of standardized definitions, AKIN and RIFLE classifications. Crit Care Med. 2009;37:2659–2661. doi: 10.1097/CCM.0b013e3181ad76c2. [DOI] [PubMed] [Google Scholar]
- 5.Uchino S. Outcome prediction for patients with acute kidney injury. Nephron Clin Pract. 2008;109:c217–c223. doi: 10.1159/000142931. [DOI] [PubMed] [Google Scholar]
- 6.Macedo E, Castro I, Yu L, et al. Impact of mild acute kidney injury (AKI) on outcome after open repair of aortic aneurysms. Ren Fail. 2008;30:287–296. doi: 10.1080/08860220701857522. [DOI] [PubMed] [Google Scholar]
- 7.Bagshaw SM, George C, Bellomo R. Changes in the incidence and outcome for early acute kidney injury in a cohort of Australian intensive care units. Crit Care. 2007;11:R68. doi: 10.1186/cc5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Askenazi DJ, Griffin R, McGwin G, et al. Acute kidney injury is independently associated with mortality in very low birthweight infants: a matched case control analysis. Pediatr Nephrol. 2009;24:991–997. doi: 10.1007/s00467-009-1133-x. [DOI] [PubMed] [Google Scholar]
- 9.Andreoli SP. Acute renal failure in the newborn. Semin Perinatol. 2004;28:112–123. doi: 10.1053/j.semperi.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Mehta RL, Kellum JA, Shah SV, et al. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walker MW, Clark RH, Spitzer AR. Elevation in plasma creatinine and renal failure in premature neonates without major anomalies: terminology, occurrence and factors associated with increased risk. J Perinatol. 2011;31:199–205. doi: 10.1038/jp.2010.82. [DOI] [PubMed] [Google Scholar]
- 12*.Askenazi DJ, Ambalavanan N, Hamilton K, et al. Acute kidney injury and renal replacement therapy independently predict mortality in neonatal and pediatric noncardiac patients on extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2011;12:e1–e6. doi: 10.1097/PCC.0b013e3181d8e348. Large, retrospective cohort study of AKI in critically ill patients on ECMO, with neonatal data analyzed separately from pediatric data. Large sample size allowed authors to control for confounding variables and demonstrate that AKI and renal replacement therapy are independent risk factors for mortality. [DOI] [PubMed] [Google Scholar]
- 13**.Koralkar R, Ambalavanan N, Levitan EB, et al. Acute kidney injury reduces survival in very low birth weight infants. Pediatr Res. 2011;69:354–358. doi: 10.1203/PDR.0b013e31820b95ca. Largest prospective epidemiologic study of AKI in neonates. Utilized AKIN definition and classified AKI based on severity. Demonstrated association between AKI and outcomes even after controlling for other confounders. [DOI] [PubMed] [Google Scholar]
- 14**.Blinder JJ, Goldstein SL, Lee VV, et al. Congenital heart surgery in infants: effects of acute kidney injury on outcomes. J Thorac Cardiovasc Surg. 2011 doi: 10.1016/j.jtcvs.2011.06.021. [Epub ahead of print] First study to utilize the AKIN classification to stage infant AKI outcomes following cardiopulmonary bypass. Supports previous data that shows increased risk of morbidity and mortality with even small changes in SCr as well as progressively worse outcomes with greater AKI severity. [DOI] [PubMed] [Google Scholar]
- 15*.Gadepalli SK, Selewski DT, Drongowski RA, Mychaliska GB. Acute kidney injury in congenital diaphragmatic hernia requiring extracorporeal life support: an insidious problem. J Pediatr Surg. 2011;46:630–635. doi: 10.1016/j.jpedsurg.2010.11.031. Retrospective chart review that demonstrates an increased risk of mortality with more severe AKI (RIFLE ‘failure’). First study to use categorical definition AKI in patients with congenital diaphragmatic hernia on ECMO. [DOI] [PubMed] [Google Scholar]
- 16.Shuhaiber J, Thiagarajan RR, Laussen PC, et al. Survival of children requiring repeat extracorporeal membrane oxygenation after congenital heart surgery. Ann Thorac Surg. 2011;91:1949–1955. doi: 10.1016/j.athoracsur.2011.01.078. [DOI] [PubMed] [Google Scholar]
- 17**.Krawczeski CD, Woo JG, Wang Y, et al. Neutrophil gelatinase-associated lipocalin concentrations predict development of acute kidney injury in neonates and children after cardiopulmonary bypass. J Pediatr. 2011;158:1009–1015. e1. doi: 10.1016/j.jpeds.2010.12.057. The study demonstrates the ability of urinary NGAL to predict AKI within hours after injury (CPB) in neonates and provides important information regarding optimal cutoff values for both plasma and urine NGAL. It illustrates that thresholds for neonatal urinary biomarkers are likely different from those in older children due to immature renal tubules. [DOI] [PubMed] [Google Scholar]
- 18.Kaur S, Jain S, Saha A, et al. Evaluation of glomerular and tubular renal function in neonates with birth asphyxia. Ann Trop Paediatr. 2011;31:129–134. doi: 10.1179/146532811X12925735813922. [DOI] [PubMed] [Google Scholar]
- 19.Portilla D, Dent C, Sugaya T, et al. Liver fatty acid-binding protein as a biomarker of acute kidney injury after cardiac surgery. Kidney Int. 2008;73:465–472. doi: 10.1038/sj.ki.5002721. [DOI] [PubMed] [Google Scholar]
- 20.Zappitelli M, Krawczeski CD, Devarajan P, et al. Early postoperative serum cystatin C predicts severe acute kidney injury following pediatric cardiac surgery. Kidney Int. 2011;80:655–662. doi: 10.1038/ki.2011.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hall IE, Koyner JL, Doshi MD, et al. Urine cystatin C as a biomarker of proximal tubular function immediately after kidney transplantation. Am J Nephrol. 2011;33:407–413. doi: 10.1159/000326753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parikh CR, Devarajan P, Zappitelli M, et al. Postoperative biomarkers predict acute kidney injury and poor outcomes after pediatric cardiac surgery. J Am Soc Nephrol. 2011;22:1737–1747. doi: 10.1681/ASN.2010111163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nguyen MT, Dent CL, Ross GF, et al. Urinary aprotinin as a predictor of acute kidney injury after cardiac surgery in children receiving aprotinin therapy. Pediatr Nephrol. 2008;23:1317–1326. doi: 10.1007/s00467-008-0827-9. [DOI] [PubMed] [Google Scholar]
- 24.Ramesh G, Krawczeski CD, Woo JG, et al. Urinary netrin-1 is an early predictive biomarker of acute kidney injury after cardiac surgery. Clin J Am Soc Nephrol. 2010;5:395–401. doi: 10.2215/CJN.05140709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lavery AP, Meinzen-Derr JK, Anderson E, et al. Urinary NGAL in premature infants. Pediatr Res. 2008;64:423–428. doi: 10.1203/PDR.0b013e318181b3b2. [DOI] [PubMed] [Google Scholar]
- 26*.Askenazi D, Koralkar R, Levitan EB, et al. Baseline values of candidate urine acute kidney injury (AKI) biomarkers vary by gestational age in premature infants. Pediatric Research. 2011;70:302–306. doi: 10.1203/PDR.0b013e3182275164. The study contributes important new data on baseline values of several candidate urinary AKI biomarkers in premature infants of varying gestational ages. It highlights the importance of controlling for gestational age in future studies of these biomarkers in infants with variations in baseline levels likely due to immaturity of renal tubules. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27**.Askenazi DJ, Montesanti A, Hunley H, et al. Urine biomarkers predict acute kidney injury and mortality in very low birth weight infants. J Pediatr. 2011;159:907–912.e1. doi: 10.1016/j.jpeds.2011.05.045. The first study to evaluate the ability of novel urinary biomarkers to predict AKI in this high-risk group. It evaluated six urinary biomarkers individually and in combination. First study to evaluate the use of osteopontin as a predictor of AKI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ricci Z, Stazi GV, Di Chiara L, et al. Fenoldopam in newborn patients undergoing cardiopulmonary bypass: controlled clinical trial. Interact Cardiovasc Thorac Surg. 2008;7:1049–1053. doi: 10.1510/icvts.2008.185025. [DOI] [PubMed] [Google Scholar]
- 29.Ricci Z, Luciano R, Favia I, et al. High-dose fenoldopam reduces postoperative neutrophil gelatinase-associated lipocaline and cystatin C levels in pediatric cardiac surgery. Crit Care. 2011;15:R160. doi: 10.1186/cc10295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hobbs DJ, Steinke JM, Chung JY, et al. Rasburicase improves hyperuricemia in infants with acute kidney injury. Pediatr Nephrol. 2010;25:305–309. doi: 10.1007/s00467-009-1352-1. [DOI] [PubMed] [Google Scholar]
- 31.Murray PT, Devarajan P, Levey AS, et al. A framework and key research questions in AKI diagnosis and staging. Clin J Am Soc Nephrol. 2008;3:864–868. doi: 10.2215/CJN.04851107. [DOI] [PubMed] [Google Scholar]


