Abstract
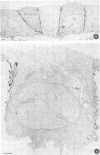
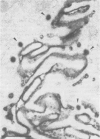
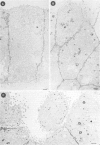
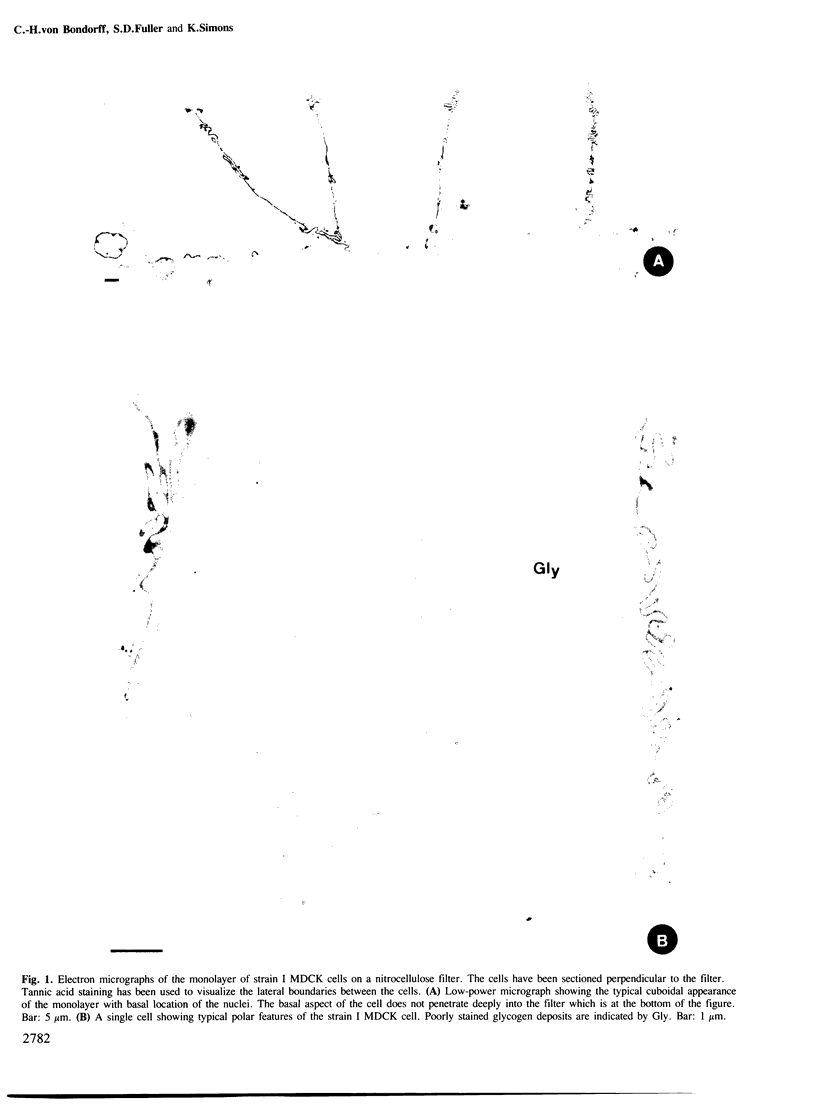
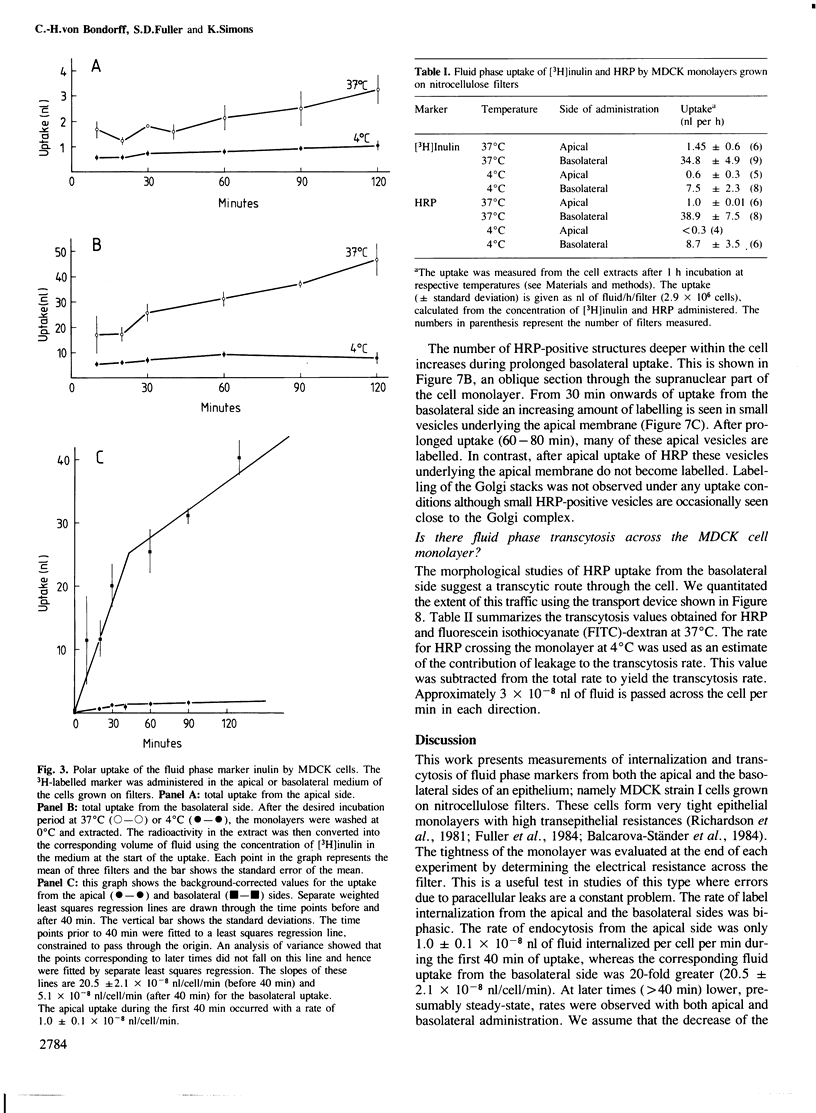
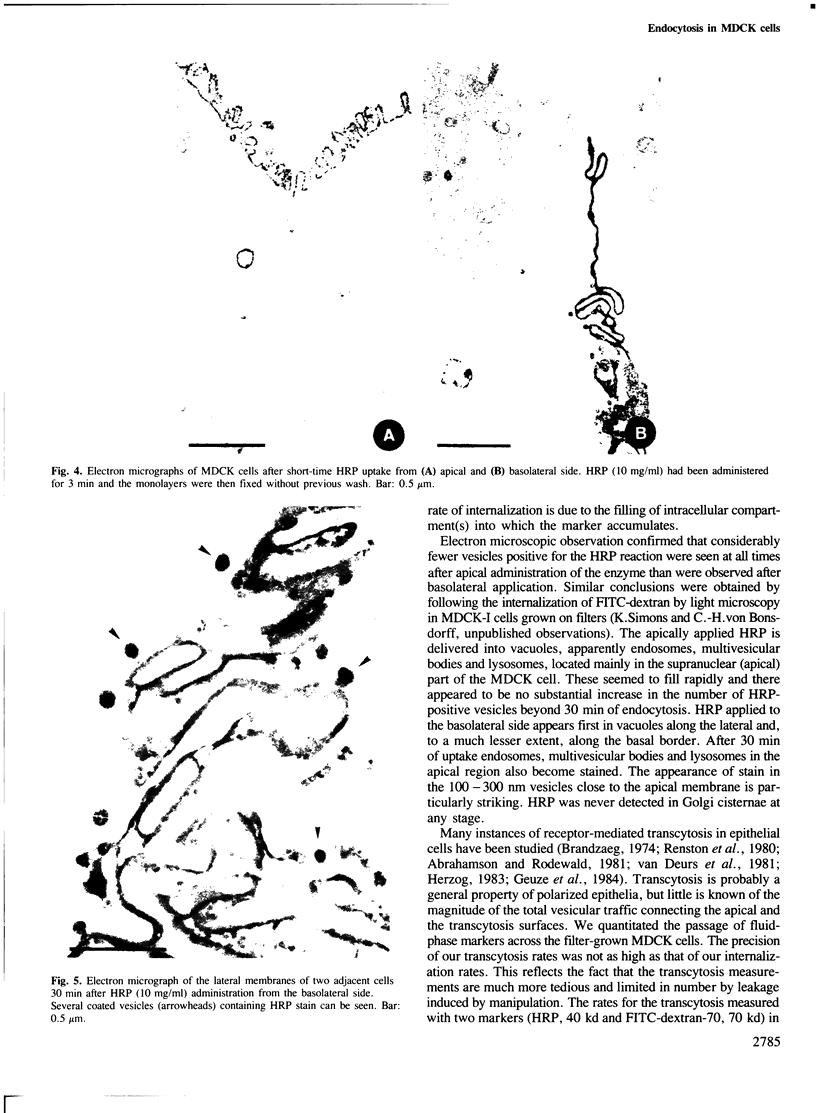
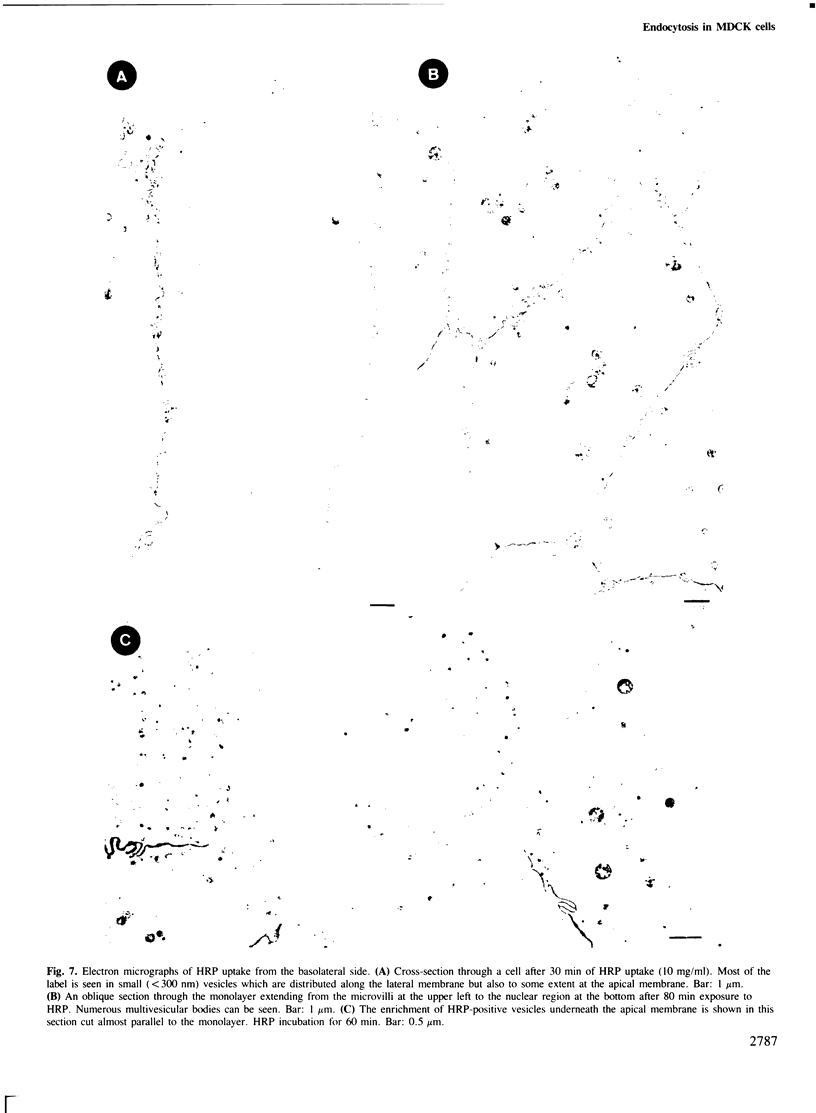
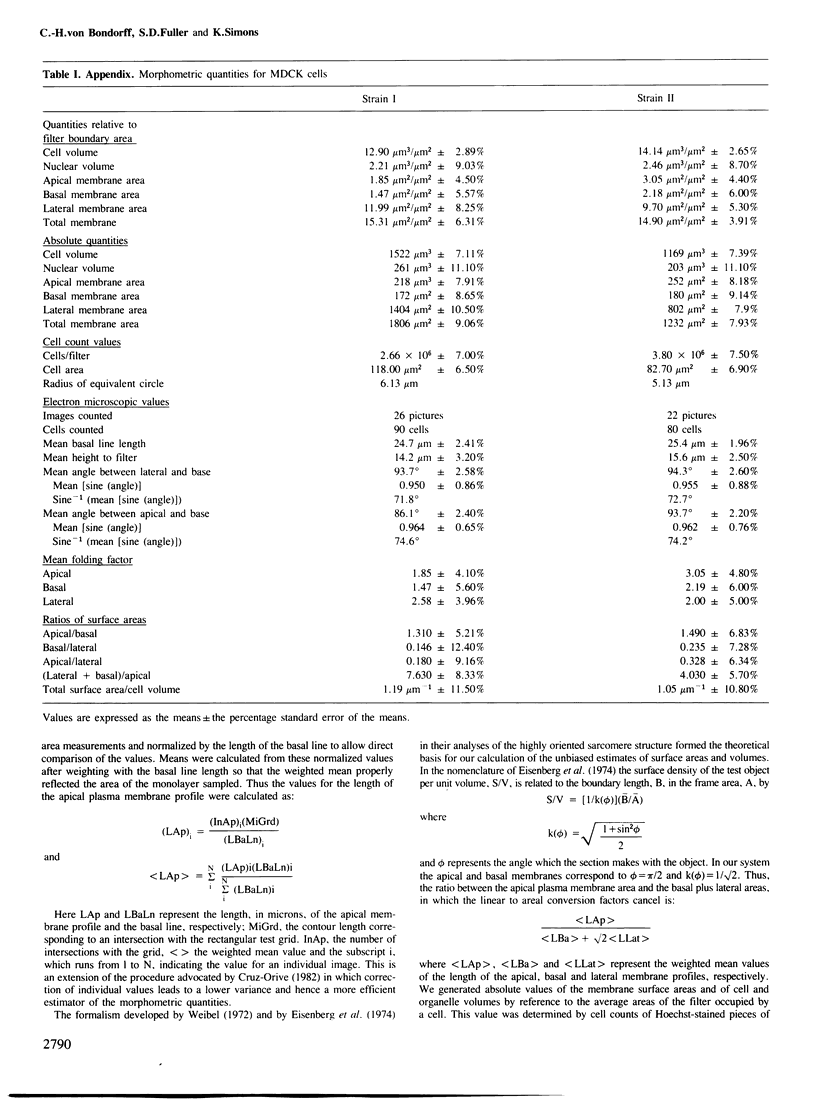
Madin-Darby canine kidney (MDCK) cells (strain I) grown on 0.45 micron pore size nitrocellulose filters formed monolayers which were highly polarized and had high transepithelial electrical resistance (greater than 3000 ohm X cm2). Morphometric analysis showed that the area of the basolateral surface domain was 7.6 times larger than that of the apical. The uptake of fluid-phase markers [3H]inulin and horseradish peroxidase (HRP) was studied from the apical and the basal side of the monolayer. Uptake of [3H]inulin was biphasic and the rate during the first 40 min corresponded to a fluid phase uptake of 20.5 X 10(-8) nl/min per cell from the basolateral side, and 1.0 X 10(-8) nl/min per cell from the apical side. Electron micrographs of the monolayers after HRP uptake showed that the marker was rapidly delivered into endosome-like vesicles and into multivesicular bodies. No labelling of the Golgi complex could be observed during 2 h of uptake. Evidence was obtained for the transport of fluid phase markers across the cell. HRP and fluorescein isothiocyanate-dextran crossed the monolayers in either direction at a rate corresponding to approximately 3 X 10(-8) nl of fluid/min/cell. Adding the transcytosis rate to the rate of fluid accumulation into the cell yielded a total basolateral endocytic rate which was 6-fold greater than the apical rate. When the uptake rates were normalized for membrane area the apical and basolateral endocytic rates were about equal per unit cell surface area.
Full text
PDF


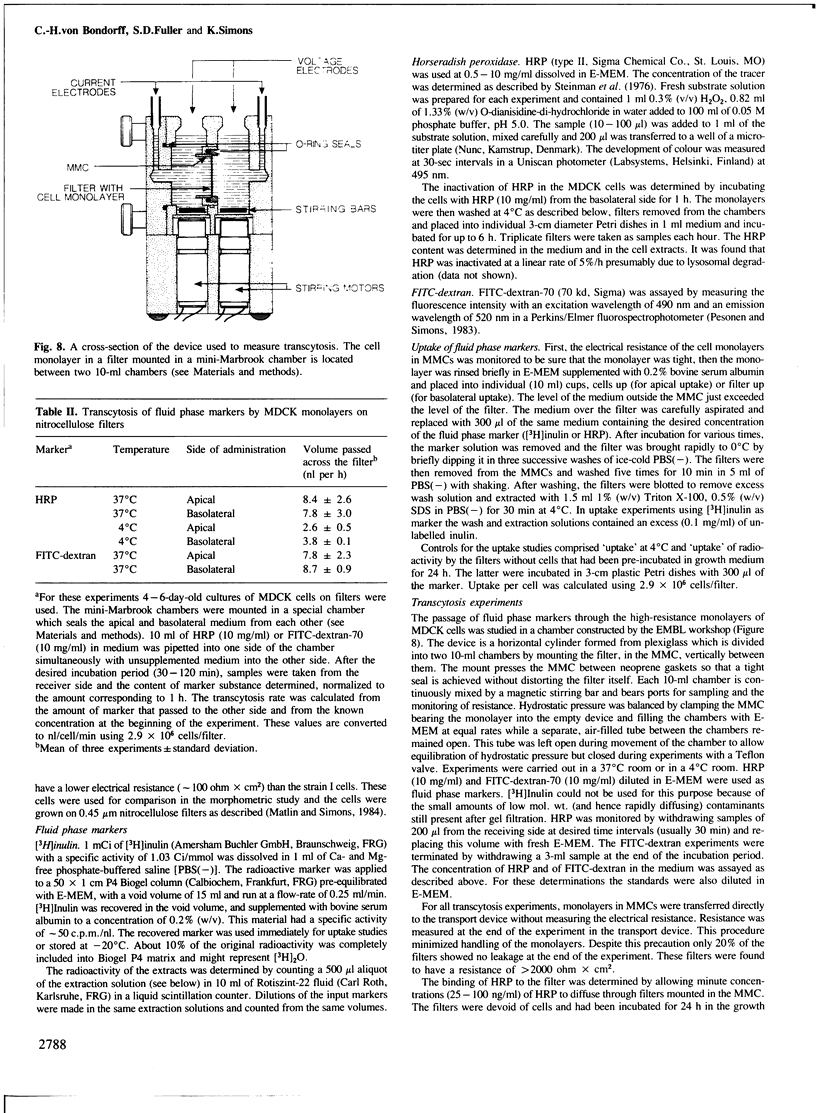



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahamson D. R., Rodewald R. Evidence for the sorting of endocytic vesicle contents during the receptor-mediated transport of IgG across the newborn rat intestine. J Cell Biol. 1981 Oct;91(1):270–280. doi: 10.1083/jcb.91.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balcarova-Ständer J., Pfeiffer S. E., Fuller S. D., Simons K. Development of cell surface polarity in the epithelial Madin-Darby canine kidney (MDCK) cell line. EMBO J. 1984 Nov;3(11):2687–2694. doi: 10.1002/j.1460-2075.1984.tb02194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besterman J. M., Airhart J. A., Woodworth R. C., Low R. B. Exocytosis of pinocytosed fluid in cultured cells: kinetic evidence for rapid turnover and compartmentation. J Cell Biol. 1981 Dec;91(3 Pt 1):716–727. doi: 10.1083/jcb.91.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandtzaeg P. Mucosal and glandular distribution of immunoglobulin components: differential localization of free and bound SC in secretory epithelial cells. J Immunol. 1974 Apr;112(4):1553–1559. [PubMed] [Google Scholar]
- Christensen E. I. Rapid membrane recycling in renal proximal tubule cells. Eur J Cell Biol. 1982 Nov;29(1):43–49. [PubMed] [Google Scholar]
- Eisenberg B. R., Kuda A. M., Peter J. B. Stereological analysis of mammalian skeletal muscle. I. Soleus muscle of the adult guinea pig. J Cell Biol. 1974 Mar;60(3):732–754. doi: 10.1083/jcb.60.3.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller S., von Bonsdorff C. H., Simons K. Vesicular stomatitis virus infects and matures only through the basolateral surface of the polarized epithelial cell line, MDCK. Cell. 1984 Aug;38(1):65–77. doi: 10.1016/0092-8674(84)90527-0. [DOI] [PubMed] [Google Scholar]
- Geuze H. J., Slot J. W., Strous G. J., Peppard J., von Figura K., Hasilik A., Schwartz A. L. Intracellular receptor sorting during endocytosis: comparative immunoelectron microscopy of multiple receptors in rat liver. Cell. 1984 May;37(1):195–204. doi: 10.1016/0092-8674(84)90315-5. [DOI] [PubMed] [Google Scholar]
- Gonnella P. A., Neutra M. R. Membrane-bound and fluid-phase macromolecules enter separate prelysosomal compartments in absorptive cells of suckling rat ileum. J Cell Biol. 1984 Sep;99(3):909–917. doi: 10.1083/jcb.99.3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Griffiths G., Warren G., Quinn P., Mathieu-Costello O., Hoppeler H. Density of newly synthesized plasma membrane proteins in intracellular membranes. I. Stereological studies. J Cell Biol. 1984 Jun;98(6):2133–2141. doi: 10.1083/jcb.98.6.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G., Warren G., Quinn P., Mathieu-Costello O., Hoppeler H. Density of newly synthesized plasma membrane proteins in intracellular membranes. I. Stereological studies. J Cell Biol. 1984 Jun;98(6):2133–2141. doi: 10.1083/jcb.98.6.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzlinger D. A., Easton T. G., Ojakian G. K. The MDCK epithelial cell line expresses a cell surface antigen of the kidney distal tubule. J Cell Biol. 1982 May;93(2):269–277. doi: 10.1083/jcb.93.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog V. Pathways of endocytosis in thyroid follicle cells. Int Rev Cytol. 1984;91:107–139. doi: 10.1016/s0074-7696(08)61315-7. [DOI] [PubMed] [Google Scholar]
- Herzog V. Transcytosis in thyroid follicle cells. J Cell Biol. 1983 Sep;97(3):607–617. doi: 10.1083/jcb.97.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louvard D. Apical membrane aminopeptidase appears at site of cell-cell contact in cultured kidney epithelial cells. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4132–4136. doi: 10.1073/pnas.77.7.4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M., Helenius A. Adsorptive endocytosis of Semliki Forest virus. J Mol Biol. 1980 Sep 25;142(3):439–454. doi: 10.1016/0022-2836(80)90281-8. [DOI] [PubMed] [Google Scholar]
- Matlin K. S., Simons K. Sorting of an apical plasma membrane glycoprotein occurs before it reaches the cell surface in cultured epithelial cells. J Cell Biol. 1984 Dec;99(6):2131–2139. doi: 10.1083/jcb.99.6.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matlin K., Bainton D. F., Pesonen M., Louvard D., Genty N., Simons K. Transepithelial transport of a viral membrane glycoprotein implanted into the apical plasma membrane of Madin-Darby canine kidney cells. I. Morphological evidence. J Cell Biol. 1983 Sep;97(3):627–637. doi: 10.1083/jcb.97.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura S., Nakada H., Sawamura T., Tashiro Y. Distribution of an asialoglycoprotein receptor on rat hepatocyte cell surface. J Cell Biol. 1982 Dec;95(3):864–875. doi: 10.1083/jcb.95.3.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver C. Endocytic pathways at the lateral and basal cell surfaces of exocrine acinar cells. J Cell Biol. 1982 Oct;95(1):154–161. doi: 10.1083/jcb.95.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver C., Hand A. R. Uptake and fate of luminally administered horseradish peroxidase in resting and isoproterenol-stimulated rat parotid acinar cells. J Cell Biol. 1978 Jan;76(1):207–229. doi: 10.1083/jcb.76.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesonen M., Ansorge W., Simons K. Transcytosis of the G protein of vesicular stomatitis virus after implantation into the apical plasma membrane of Madin-Darby canine kidney cells. I. Involvement of endosomes and lysosomes. J Cell Biol. 1984 Sep;99(3):796–782. doi: 10.1083/jcb.99.3.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesonen M., Bravo R., Simons K. Transcytosis of the G protein of vesicular stomatitis virus after implantation into the apical membrane of Madin-Darby canine kidney cells. II. Involvement of the Golgi complex. J Cell Biol. 1984 Sep;99(3):803–809. doi: 10.1083/jcb.99.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesonen M., Simons K. Transepithelial transport of a viral membrane glycoprotein implanted into the apical plasma membrane of Madin-Darby canine kidney cells. II. Immunological quantitation. J Cell Biol. 1983 Sep;97(3):638–643. doi: 10.1083/jcb.97.3.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renston R. H., Maloney D. G., Jones A. L., Hradek G. T., Wong K. Y., Goldfine I. D. Bile secretory apparatus: evidence for a vesicular transport mechanism for proteins in the rat, using horseradish peroxidase and [125I]insulin. Gastroenterology. 1980 Jun;78(6):1373–1388. [PubMed] [Google Scholar]
- Richardson J. C., Scalera V., Simmons N. L. Identification of two strains of MDCK cells which resemble separate nephron tubule segments. Biochim Biophys Acta. 1981 Feb 18;673(1):26–36. [PubMed] [Google Scholar]
- Richardson J. C., Scalera V., Simmons N. L. Identification of two strains of MDCK cells which resemble separate nephron tubule segments. Biochim Biophys Acta. 1981 Feb 18;673(1):26–36. [PubMed] [Google Scholar]
- Steinman R. M., Brodie S. E., Cohn Z. A. Membrane flow during pinocytosis. A stereologic analysis. J Cell Biol. 1976 Mar;68(3):665–687. doi: 10.1083/jcb.68.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Mellman I. S., Muller W. A., Cohn Z. A. Endocytosis and the recycling of plasma membrane. J Cell Biol. 1983 Jan;96(1):1–27. doi: 10.1083/jcb.96.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentich J. D. Morphological similarities between the dog kidney cell line MDCK and the mammalian cortical collecting tubule. Ann N Y Acad Sci. 1981;372:384–405. doi: 10.1111/j.1749-6632.1981.tb15490.x. [DOI] [PubMed] [Google Scholar]
- Wall D. A., Maack T. Endocytic uptake, transport, and catabolism of proteins by epithelial cells. Am J Physiol. 1985 Jan;248(1 Pt 1):C12–C20. doi: 10.1152/ajpcell.1985.248.1.C12. [DOI] [PubMed] [Google Scholar]
- Weibel E. R. A stereological method for estimating volume and surface of sarcoplasmic reticulum. J Microsc. 1972 Apr;95(2):229–242. doi: 10.1111/j.1365-2818.1972.tb03722.x. [DOI] [PubMed] [Google Scholar]
- Welling L. W., Evan A. P., Welling D. J., Gattone V. H., 3rd Morphometric comparison of rabbit cortical connecting tubules and collecting ducts. Kidney Int. 1983 Feb;23(2):358–367. doi: 10.1038/ki.1983.27. [DOI] [PubMed] [Google Scholar]
- Welling L. W., Evan A. P., Welling D. J. Shape of cells and extracellular channels in rabbit cortical collecting ducts. Kidney Int. 1981 Aug;20(2):211–222. doi: 10.1038/ki.1981.123. [DOI] [PubMed] [Google Scholar]
- Welling L. W., Welling D. J., Hill J. J. Shape of cells and intercellular channels in rabbit thick ascending limb of Henle. Kidney Int. 1978 Feb;13(2):144–151. doi: 10.1038/ki.1978.21. [DOI] [PubMed] [Google Scholar]
- Welling L. W., Welling D. J. Shape of epithelial cells and intercellular channels in the rabbit proximal nephron. Kidney Int. 1976 May;9(5):385–394. doi: 10.1038/ki.1976.48. [DOI] [PubMed] [Google Scholar]
- Welling L. W., Welling D. J. Surface areas of brush border and lateral cell walls in the rabbit proximal nephron. Kidney Int. 1975 Dec;8(6):343–348. doi: 10.1038/ki.1975.125. [DOI] [PubMed] [Google Scholar]