Abstract
Bifunctional polynuclear platinum compounds represent a novel class of metal-based antitumor drugs which are currently undergoing preclinical development. A typical agent is [{trans-PtCl(NH3)2}2H2N(CH2)4NH2]Cl2 (1,1/t,t), which coordinates to bases in DNA and forms various types of covalent crosslinks. It also forms a 1,2-d(GpG) intrastrand adduct, the equivalent of the major DNA lesion of ‘classical’ cisplatin. In the present study differential scanning calorimetry and spectroscopic techniques were employed to characterize the influence of this crosslink on the thermal stability and energetics of 20 bp DNA duplexes site-specifically modified by 1,1/t,t. Thermal denaturation data revealed that the crosslink of 1,1/t,t reduced thermal and thermodynamical stability of the duplex noticeably more than that of ‘classical’ cisplatin. The energetic consequences of the intrastrand crosslink at the d(GG) site are discussed in relation to the structural distortions induced by this adduct in DNA and to its recognition and binding by HMG domain proteins. It has been suggested that the results of the present work are consistent with different DNA binding modes of cisplatin and polynuclear bifunctional DNA-binding drugs, which might be relevant to their distinct biological effectiveness.
INTRODUCTION
Cisplatin is a clinically very important anticancer metal-based drug which coordinates to DNA and distorts its double-helical conformation (1–5). The anticancer effect of cisplatin is associated with the capability of these distortions to terminate DNA polymerase (6) and to induce apoptosis (7). In addition, these distortions attract various damaged-DNA-binding proteins [for instance those containing a high mobility group (HMG) domain] and the binding of these proteins has been postulated to mediate the antitumor properties of cisplatin (8,9).
Administration of cisplatin is associated with some limitations, such as inherent or acquired resistance and side effects. A search for new platinum anticancer drugs is driven by the hypothesis that platinum compounds which bind to DNA in a fundamentally different manner to that of cisplatin will have altered biological properties, including the spectrum and intensity of antitumor activity. In agreement with this argument a new class of anticancer platinum compounds has been designed based on polynuclear platinum complexes (10). A typical example of one of these compounds is the bifunctional dinuclear agent [{trans-PtCl(NH3)2}2H2N(CH2)4NH2]Cl2 (1,1/t,t) (Fig. 1; 11). It forms various types of DNA crosslinks capable of terminating DNA replication (12,13). Interestingly, it also forms minor 1,2-d(GpG) intrastrand adducts (14) which are major DNA lesions of cisplatin (15,16). We demonstrated in our recent work that the 1,2-d(GpG) intrastrand crosslink of 1,1/t,t distorts DNA in a different way than ‘classical’ cisplatin (14). The major difference consists of different DNA bending. The 1,2-d(GpG) intrastrand adduct of 1,1/t,t results in a flexible non-directional bend not recognized by HMG domain proteins. This is in fundamental contrast to the same type of lesion formed by cisplatin, which produces a rigid, directed bend into the major groove of DNA (17–19) which is attracted by HMG domain proteins (20).
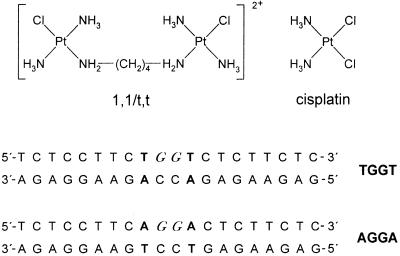
Figure 1.
Structures of platinum complexes and the sequences of the synthetic 20 bp oligodeoxyribonucleotide duplexes used in the present study with their abbreviations. The top and bottom strands in the pair of oligonucleotides are designated top and bottom, respectively, in the text. Two central and adjacent G residues in italics in the top strand of each duplex indicate the location of the intrastrand crosslink after modification of the oligonucleotide by 1,1/t,t complex or cisplatin in the way described in Materials and Methods. The base pairs flanking the two central G·C base pairs of each duplex are depicted in bold.
Structural information about the 1,2-d(GpG) intrastrand crosslink formed in double-helical DNA by 1,1/t,t is available (14), but little is known about its effect on thermodynamic stability. It has been shown (21,22) that the stability of the duplex is a parameter affecting protein recognition and binding. The energetic consequences of the site-specific 1,2-d(GpG) intrastrand crosslink of cisplatin have already been determined (23,24). In the present work the energetic consequences of the same type of adduct formed by 1,1/t,t have been elucidated by differential scanning calorimetry (DSC) and compared with those of cisplatin. In addition, to assess the influence of the flanking bases on the impact of a platinum intrastrand crosslink the adduct was placed between either two T·A or two A·T base pairs (the sequences of the two duplexes were identical except for the base pairs flanking the crosslink). These two sequences were chosen because their variation in the case of the 1,2-d(GpG) intrastrand crosslink of cisplatin revealed pronounced differences as regards the impact of this adduct on the thermodynamic properties of duplex DNA (24). Thus, differences between the observed properties of duplexes can be attributed to differences induced, on the one hand, by the same type of crosslink formed by two different platinum compounds and, on the other, by the flanking bases. The differences are discussed in terms of different structural perturbations induced by the 1,2-d(GpG) intrastrand crosslinks of 1,1/t,t and ‘classical’ cisplatin and their different recognition by HMG domain proteins.
MATERIALS AND METHODS
Chemicals
1,1/t,t (Fig. 1) was prepared as described previously (25). The synthetic oligodeoxyribonucleotides (Fig. 1) were purchased from IDT (Coralville, IA) and purified as described previously (26). In the present work the molar concentrations of the single-stranded oligonucleotides are related to the single-stranded molecules 20 nt long. Molar extinction coefficients for the single-stranded oligonucleotides (related to the strands 20 nt long) were determined by phosphate analysis (23). The following extinction coefficients at 260 nm and 25°C (in dm3/cm.mol strand) were obtained: 164 000 and 181 000 for the upper and lower strands of the unmodified TGGT duplex, respectively; 193 000 and 166 000 for the upper strand of the TGGT containing 1,2-d(GpG) intrastrand crosslink of 1,1/t,t and cisplatin, respectively; 170 000 and 213 000 for the upper and lower strands of the unmodified AGGA duplex, respectively; 177 000 and 176 000 for the upper strand of AGGA containing the 1,2-d(GpG) intrastrand crosslink of 1,1/t,t and cisplatin, respectively. Isothermal mixing experiments (23) using unmodified or crosslinked top strands of the TGGT or AGGT duplexes and their complementary bottom strands revealed 1:1 stoichiometries, a ratio consistent with duplex formation. In the present work the molar concentrations of the oligonucleotide duplexes are related to the double-stranded molecules 20 bp long.
Platination of oligonucleotides
The single-stranded oligodeoxyribonucleotides (the top strand of the duplex TGGT or AGGA) at a concentration of 90 µM were reacted in stoichiometric amounts with either 1,1/t,t or cisplatin for 24 h at 37°C in 10 mM NaClO4 (26). The platinated oligonucleotides were repurified by ion exchange fast protein liquid chromatography (FPLC). It was verified by platinum flameless atomic absorption spectrophotometry (FAAS) and by measurement of the optical density that the modified oligonucleotides contained two platinum atoms. It was also verified using DMS footprinting of platinum on DNA (27,28) that in the platinated top strands of the duplexes the N7 position of both G residues was not accessible for reaction with DMS. The unmodified or platinated top strands were allowed to anneal with non-platinated complementary strands (the bottom strand of TGGT or AGGA) in 10 mM sodium cacodylate pH 7.2, 100 mM NaCl, 10 mM MgCl2 and 0.1 mM EDTA. The annealing involved heating the mixture of the complementary oligonucleotides to 95°C for 20 min followed by slow cooling to 25°C at a rate of 30°C/h. The duplex samples were further equilibrated at 25°C for 20 min and vacuum degassed prior to use. FPLC purification and FAAS measurements were carried out on a Pharmacia Biotech FPLC system with a MonoQ HR 5/5 column and a Unicam 939 AA spectrometer equipped with a graphite furnace, respectively. Other details can be found in the literature (6,14,23,24,26–28).
Differential scanning calorimetry
Excess heat capacity (ΔCp,xs) versus temperature profiles for the thermally induced transitions of TGGT and AGGA duplexes unmodified or containing a unique 1,1/t,t- or cisplatin-induced 1,2-d(GpG) intrastrand crosslink were measured using a VP-DSC Calorimeter (Microcal, Northampton, MA). In the DSC experiments the concentrations of the TGGT and AGGA duplexes were 3.5 and 12.5 µM, the heating rate was 60°C/h and the maximum temperature was 95°C. After reaching the maximum temperature the samples were cooled at the same rate to the starting temperature of 25°C. In the present paper ΔCp,xs is defined as excess heat capacity, which is baseline subtracted and concentration normalized (29). The reference scans were subtracted from the sample scans to obtain ΔCp,xs versus temperature profiles. Enthalpies (ΔH) and entropies (ΔS) of duplex formation were calculated from the areas under the experimental ΔCp,xs versus T and derived ΔCp,xs/T versus T curves, respectively, using ORIGIN v.5.0 software (Microcal). The free energy of duplex formation at 37°C (ΔG37) was calculated using the standard thermodynamic relationship given in 1 and the corresponding ΔH and ΔS values:
ΔG37 = ΔH – (310.15)ΔS 1
The duplexes TGGT and AGGA at concentrations of 3.5 and 12.5 µM, respectively, were dissolved in buffer containing 10 mM sodium cacodylate pH 7.2, 100 mM NaCl, 10 mM MgCl2 and 0.1 mM EDTA. The formation of 1:1 complexes between the top and bottom strands of TGGT and AGGA unmodified or containing the crosslink was verified by recording UV absorbance mixing curves at 25°C (23). It was also verified in the same way as described in a previous paper (23) that the melting transitions of both the platinated and unmodified duplexes were fully reversible.
UV absorption spectrophotometry
UV absorbance measurements were conducted on a Beckman DU-7400 spectrophotometer equipped with a thermoelectrically controlled cell holder and quartz cells with a path length of 1 cm. Absorbance versus temperature profiles were measured at 260 nm. The temperature was raised using a linear heating rate of 1.0°C/min. For each optically detected transition the melting temperature (Tm) was determined as previously described (26). The solutions of TGGT and AGGA duplexes ranged from 0.2 to 3.7 and 0.2 to 13 µM in duplex, respectively, and contained 10 mM sodium cacodylate pH 7.2, 100 mM NaCl, 10 mM MgCl2 and 0.1 mM EDTA.
Circular dichroism (CD) spectrophotometry
CD spectra were recorded using Jasco J-720 spectropolarimeter equipped with a thermoelectrically controlled cell holder. The cell path length was 1 cm. Isothermal CD spectra were recorded from 220 to 320 nm in 1 nm increments with an averaging time of 5 s. The DNA concentration was 6 µM in duplex and buffer conditions were 10 mM sodium cacodylate pH 7.2, 100 mM NaCl, 10 mM MgCl2 and 0.1 mM EDTA.
RESULTS
DSC
A calorimetric technique was employed to characterize the influence of the 1,2-d(GpG) intrastrand crosslink of 1,1/t,t on the thermal stability and energetics of site-specifically platinated 20 bp DNA duplexes. Such thermodynamic data can reveal how the platinum adduct influences duplex stability, a property that has been shown to play a significant role in the mechanism of antitumor activity of platinum drugs. Recently, calorimetric and spectroscopic techniques were employed to characterize the influence of intrastrand and interstrand crosslinks on the thermal stability and energetics of short oligodeoxyribonucleotide duplexes site-specifically modified by cisplatin (23,24,30) and its mononuclear analogs containing enantiomeric amine ligands, such as cis-[PtCl2(RR-DAB)] and cis-[PtCl2(SS-DAB)] (DAB, 2,3-diaminobutane) (31). We expanded these studies on 20 bp duplexes containing a unique 1,2-d(GpG) site-specific intrastrand adduct of the dinuclear platinum complex 1,1/t,t formed in duplexes TGGT and AGGA (Fig. 1).
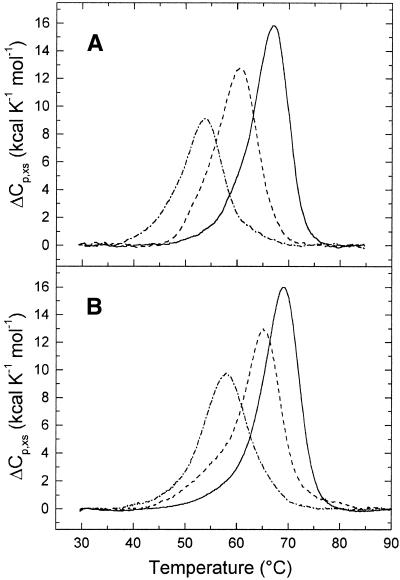
DSC melting profiles (ΔCp,xs versus T) for the parent, unmodified 20 bp duplexes TGGT and AGGA (solid curves) and the same duplexes containing a single 1,2-d(GpG) intrastrand crosslink of 1,1/t,t are shown in Figure 2. For comparative purposes the oligodeoxyribonucleotide duplex TGGT was used, which had a nucleotide sequence identical to that used in a previous study (23) in which thermal stability and energetics of duplex TGGT containing the same cisplatin crosslink were investigated. Figure 2 indicates that crosslink formation by 1,1/t,t reduced the thermal stability of both duplexes TGGT and AGGA, by 13 and 11°C, respectively. Denaturation (heating) and renaturation (cooling) curves for the unmodified and platinated duplexes were superimposable, which is consistent with the reversibility of this melting equilibrium. Thus, meaningful thermodynamic data from our calorimetric and spectrophotometric measurements described below could be obtained. In addition, the pre- and post-test baselines coincided for both unmodified and platinated duplexes, which suggests no differential heat capacity change due to the presence of the crosslink.
Figure 2.
DSC thermograms for the TGGT (A) and AGGA (B) duplexes unmodified (full lines) and containing a 1,2-d(GpG) intrastrand crosslink of 1,1/t,t (dotted and dashed lines) or cisplatin (dashed lines). The concentrations of the TGGT and AGGT duplexes were 3.5 and 13 µM, respectively, and the buffer conditions were 10 mM sodium cacodylate pH 7.2, 100 mM NaCl, 10 mM MgCl2 and 0.1 mM EDTA.
DSC melting curves were analyzed as described in Materials and Methods to obtain the results listed in Table 1. Inspection of these thermodynamic parameters reveals that crosslink formation in duplexes TGGT and AGGA by 1,1/t,t resulted in a large decrease in the change in enthalpy of duplex formation by 48 and 33 kcal/mol, respectively. In other words, the intrastrand crosslink of 1,1/t,t enthalpically destabilizes the duplex relative to its unmodified counterpart. In addition, crosslink formation by 1,1/t,t resulted in a substantial increase in the entropy of duplexes TGGT and AGGA of 131 and 84 cal/K.mol (TΔΔS = 41 and 26 kcal/mol at 37°C), respectively. In other words, the intrastrand crosslink of 1,1/t,t increases entropy of the platinated duplexes and in this way entropically stabilizes the duplex. Thus, the 48 or 33 kcal/mol enthalpic destabilization of the TGGT and AGGA duplexes due to the crosslink of 1,1/t,t is partially, but not completely, compensated by the entropic crosslink-induced stabilization of the duplex of 41 and 26 kcal/mol at 37°C, respectively. The net result of these enthalpic and entropic effects is that 1,2-d(GpG) intrastrand crosslink formation in duplexes TGGT and AGGA by 1,1/t,t at 37°C induces a decrease in duplex thermodynamic stability (ΔΔG37) of 7.4 or 6.3 kcal/mol, respectively, with this destabilization being enthalpic in origin. Importantly, the effects of the crosslink of 1,1/t,t on duplex transition enthalpy and entropy were only slightly more pronounced in the case of the TGGT duplex in comparison with the AGGA duplex.
Table 1. Calorimetrically derived thermodynamic parameters for formation of the 20 bp duplexes unmodified or containing a single site-specific 1,1/t,t- or cisplatin-induced 1,2-d(GpG) intrastrand crosslinka.
| Duplex |
Tmaxb (°C) |
ΔHc (kcal/mol duplex) |
ΔΔHd (kcal/mol duplex) |
ΔSc (cal/K.mol duplex) |
ΔΔSd (cal/K.mol duplex) |
ΔG37c (kcal/mol duplex) |
ΔΔG37d (kcal/mol
duplex) |
| TGGT | 67.1 ± 0.2) | –151 ± 4 | –446 ± 11 | –12.7 ± 0.8 | |||
| TGGT–1,1/t,t | 54.0 ± 0.2 | –103 ± 3 | +48 ± 7 | –315 ± 9 | +131 ± 20 | –5.3 ± 0.5 | +7.4 ± 1.3 |
| TGGT–cisPt | 60.7 ± 0.2 | –135 ± 3 | +16 ± 6 | –404 ± 10 | +42 ± 21 | –9.7 ± 0.7 | +3.0 ± 1.5 |
| AGGA | 69.1 ± 0.2 | –152 ± 4 | –445 ± 12 | –14.0 ± 0.7 | |||
| AGGA–1,1/t,t | 57.9 ± 0.2 | –119 ± 4 | +33 ± 8 | –359 ± 12 | +84 ± 24 | –7.7 ± 0.5 | +6.3 ± 1.2 |
| AGGA–cisPt | 65.1 ± 0.2 | –149 ± 3 | +3 ± 7 | –443 ± 9 | +2 ± 21 | –11.6 ± 0.7 | +2.4 ± 1.4 |
aThe ΔH and ΔS values are averages derived from three independent experiments, with the indicated errors corresponding to the average deviations from the mean.
bTmax denotes the temperature corresponding to the maximum in the DSC melting profiles shown in Figure 2.
cΔH, ΔS and ΔG37 denote, respectively, the enthalpy, entropy and free energy (at 37°C) of duplex formation.
d(ΔΔH, ΔΔS, ΔΔG37) = (ΔH, ΔS, ΔG37)platinated – (ΔH, ΔS, ΔG37)unmodified.
Absorption spectrophotometry
The UV thermal denaturation profiles for unmodified duplexes TGGT and AGGA and the same duplexes containing a single, site-specific intrastrand crosslink of 1,1/t,t were obtained at concentrations of 0.2–3.7 and 0.2–13 µM in duplex, respectively (not shown). The Tm values of the unmodified and platinated duplexes were dependent on the duplex concentration. For instance, increasing the concentration of duplex TGGT from 0.2 to 3.7 µM increased the Tm of the unmodified and platinated duplex from 61.5 to 66.2°C and 46.6 to 53.1°C, respectively. This observation indicates that melting of the unmodified and platinated duplexes took place with molecularities greater than one. A van’t Hoff analysis of these UV melting data could also be used to thermodynamically characterize the thermal transitions exhibited by the duplexes investigated in the present work. This approach can yield model-dependent thermodynamic parameters, in contrast to model-independent data obtained directly by calorimetry.
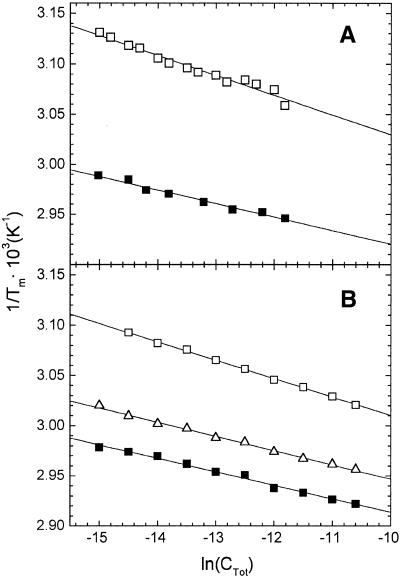
The van’t Hoff analysis was performed as described in earlier reports by plotting the reciprocal of the melting temperature 1/Tm versus the logarithm of the total strand concentration ln(CTot). Such plots for the unmodified and platinated duplexes studied in the present work are shown in Figure 3. As also shown earlier (23,24), for bimolecular associations of two non-self-complementary strands, such as those investigated in the present work, van’t Hoff enthalpies (ΔHvH) and entropies (ΔSvH) of duplex formation can be derived from the slopes and y-intercepts of these lines, respectively, using the relationship:
Figure 3.
van’t Hoff plots of 1/Tm versus ln(CTot) depicting the dependence on the total strand concentration (CTot) of the melting temperatures (Tm) for the duplex to single strand transition of the TGGT (A) and AGGA (B) duplexes. Full squares, unmodified duplexes; open squares, duplex containing a site-specific 1,1/t,t-induced 1,2-d(GpG) intrastrand crosslink; open triangles, duplex containing a site-specific cisplatin-induced 1,2-d(GpG) intrastrand crosslink. Each 1/Tm value is an average derived from three melting profiles acquired at 260 nm.
1/Tm = (R/ΔHvH) lnCTot + [(ΔSvH – 1.39R)/ΔHvH] 2
where R is the gas constant. Thus, a plot of 1/Tm versus ln(CTot) should be linear and have a slope R/ΔHvH and a y-intercept of (ΔSvH – 1.39R)/ΔHvH. The linear plot of 1/Tm versus ln(CTot) was observed for all duplexes studied in the present work, with a slope of R/ΔHvH. The ΔHvH values determined in this way are listed in Table 2, along with the corresponding ΔHcal values measured calorimetrically. The van’t Hoff analysis assumes an all-or-none, two-state process of duplex formation, with no significant thermodynamic contributions from intermediate states. The reasonable equality of the spectrophotometrical van’t Hoff and calorimetrically determined enthalpies (Table 2) confirms the validity of this assumption for both unmodified and platinated duplexes. This result also demonstrates that the 1,2-d(GpG) intrastrand crosslink of 1,1/t,t does not alter the ability of the duplex to propagate the interactions required for cooperative melting. A similar behavior was also observed for the 1,2-d(GpG) intrastrand crosslink of cisplatin and several other DNA lesions (23,24).
Table 2. Calorimetric and van’t Hoff enthalpies and entropies for formation of the 20 bp duplexes unmodified or containing a single site-specific 1,1/t,t- or cisplatin-induced 1,2-d(GpG) intrastrand crosslink.
| Duplex |
ΔHcala (kcal/mol duplex) |
ΔHvHb (kcal/mol duplex) |
ΔHvH/ΔHcal |
ΔScala (cal/K.mol
duplex) |
ΔSvHb (cal/K.mol
duplex) |
| TGGT | –151 ± 4 | –151 ± 5 | 1.00 | –446 ± 11 | –418 ± 21 |
| TGGT–1,1/t,t | –103 ± 3 | –101 ± 5 | 0.98 | –315 ± 9 | –284 ± 20 |
| TGGT–cisPt | –135 ± 3 | –136 ± 6c | 1.01 | –404 ± 10 | –384 ± 35c |
| AGGA | –152 ± 4 | –148 ± 7 | 0.97 | –445 ± 12 | –409 ± 19 |
| AGGA–1,1/t,t | –119 ± 4 | –109 ± 6 | 0.92 | –359 ± 12 | –306 ± 19 |
| AGGA–cisPt | –149 ± 3 | –140 ± 6 | 0.94 | –443 ± 9 | –389 ± 17 |
aCalorimetric enthalpies and entropies (ΔHcal and ΔScal) were derived from DSC experiments as described in the text.
bvan’t Hoff enthalpies and entropies (ΔHvH and ΔSvH) were derived from the concentration dependence of optically derived Tm values as described in the text.
cTaken from Poklar et al. (23).
The interpretation of our data described below is also based on the assumption that all thermodynamic parameters for formation of unmodified and platinated duplexes are ascribed to differences in the initial duplex states. This implies that the final single-stranded states should be thermodynamically equivalent at the elevated temperatures at which they are formed. This assumption has been verified in earlier reports by recording identical CD spectra for samples of non-platinated and platinated duplexes which were heated to high temperatures (23,24). Therefore, we also recorded CD spectra of samples of the unmodified and platinated duplexes TGGT and AGGA heated to 95°C (not shown) in the present work. The spectra recorded at 95°C were identical for each pair of unmodified and platinated sample. Thus, validity of the assumption that the final single-stranded states of the unmodified and platinated duplexes were thermodynamically equivalent at elevated temperatures was also verified for the duplexes investigated in the present work.
DISCUSSION
Calorimetric and spectroscopic techniques were used to demonstrate that formation of a 1,1/t,t intrastrand crosslink reduces the thermal stability of the host duplexes TGGT and AGGA by 13 and 11°C. The net result of enthalpic and entropic changes is that crosslink formation induces a decrease in thermodynamic stability of duplexes TGGT and AGGA (ΔΔGCal at 37°C) of 7.4 or 6.3 kcal/mol, respectively, with this destabilization being enthalpic in origin.
We tried to rationalize this destabilizing effect in terms of crosslink-induced structural perturbations in the host duplex. Conformational changes in DNA induced by a single, site-specific 1,2-d(GpG) intrastrand crosslink of 1,1/t,t have already been investigated by studying the effect of this crosslink on the reactivity of OsO4 and diethyl pyrocarbonate (DEPC) towards duplex TGGT (14). OsO4 and DEPC are complementary chemical probes of DNA conformation capable of revealing the location of A·T base pairs the secondary structure of which has been perturbed by the crosslink. Importantly, these chemical probes do not represent measures of base pair disruption as they can proceed even if base pairing is maintained (32). Formation of adducts by these probes requires out-of-plane attack by the electrophile so that they are sterically hindered by stacking of neighboring base pairs. Thus, OsO4 and DEPC are essentially probes of base stacking (32). These chemical probes reacted with 2 bp 5′ to the adduct and 1 bp 3′ to the adduct, which indicates that the 1,2-d(GpG) intrastrand crosslink resulted in distortions associated with disruption of the stacking interactions between the 5 bp at the d(GG) site involved in the crosslink (14). Such a conformational alteration should be enthalpically unfavorable, which is consistent with the observations of the present work. The enthalpic cost that results from disruption of the four relevant nearest neighbor stacking interactions, namely CT/GA, TG/AC, GG/CC and GT/CA, in the TGGT duplex yields a ΔΔH upper limit value of 31 kcal/mol (33). Our experimental calorimetric data (see Table 1) reflect a crosslink-induced ΔΔHcal value of 48 ± 7 kcal/mol. A further decrease in transition enthalpy could be caused by a change in hydration of the DNA molecule; the aliphatic linker chain of 1,1/t,t involved in the crosslink could decrease hydration of the sugar–phosphate backbone around its binding site to DNA. Another possible factor contributing to the large crosslink-induced enthalpic effects observed in the present work is suggested to be associated with the observation that the 1,2-d(GpG) intrastrand crosslink of 1,1/t,t results in a non-directional bend also increasing flexibility of the duplex. However, prediction of the energetic consequences of such conformational changes is difficult because of the limited current knowledge of thermodynamic consequences of distortions and transitions in DNA duplexes.
Our thermodynamic data (Table 1) reveal that a 1,2-d(GpG) intrastrand crosslink of 1,1/t,t enthalpically destabilizes the host duplexes TGGT and AGGA far more (∼3 times) than the same adduct of cisplatin (Table 1; 23). A possible explanation for this differential crosslink effect is that enthalpy lost owing to crosslink-induced disruption of stacking interactions is compensated for in the case of the crosslink formed by cisplatin by enthalpy gained by other features of conformational alterations that cannot occur when the crosslink of 1,1/t,t is formed. One of these structural features is DNA bending. Formation of the 1,2-d(GpG) intrastrand crosslink of cisplatin results in a rigid bend of the host DNA in the direction of the major groove (17–19). A crude estimate of its contribution to stabilization of the global duplex structure was ∼6.4 kcal (23). On the other hand, formation of the 1,2-d(GpG) intrastrand crosslink of 1,1/t,t results in only a flexible non-directional bend that may not contribute to stabilization of the duplex (14). Since the current structural data on the 1,2-d(GpG) intrastrand crosslink of 1,1/t,t are limited, we are not yet able to test the proposal rigorously. Nevertheless, the calorimetric and spectrophotometric results of the present work suggest that the extent and thermodynamic origins of 1,2-d(GpG) crosslink-induced duplex destabilization are dependent on the type of the bifunctional platinum compound forming this adduct.
As noted above, intrastrand crosslinks formed in double-helical DNA by cisplatin between neighboring purine residues attract various damaged-DNA-binding proteins, including those containing a HMG domain, and binding of these proteins to these adducts has been postulated to mediate the antitumor properties of cisplatin. It was shown recently (24) that binding of HMG domain proteins to this intrastrand crosslink is modulated by the nature of the bases that flank the adduct and that this modulatory effect correlates with the impact of the flanking base sequence on crosslink-induced energetic destabilization of the host duplex. For instance, flanking thymine bases resulted in higher destabilization of the host 15 bp duplex than flanking adenine bases (24) (the same effect, although less pronounced, was also observed in the present work for the host 20 bp duplexes; Table 1) and, simultaneously, relative to adenines the presence of flanking thymine bases considerably reduced the affinity of the cisplatin–DNA adduct for HMG domain proteins (34). In the present work we have also analyzed a duplex in which the bases flanking intrastrand crosslinks of both 1,1/t,t and cisplatin were thymines (20 bp TGGT duplex), but energetic destabilization of this host duplex by this type of intrastrand crosslink was affected by the nature of the bifunctional platinum compound forming this adduct. The crosslink of 1,1/t,t resulted in considerably higher destabilization of the 20 bp host duplexes TGGT (ΔΔG37 was 7.4 kcal/mol duplex) than did that of cisplatin (ΔΔG37 was only 3.0 kcal/mol duplex) (Table 1) and, simultaneously, in contrast to cisplatin, the cross link of 1,1/t,t was not recognized by HMG domain protein (28).
The affinity of HMG domain proteins for 1,2-d(GpG) intrastrand crosslinks of platinum compounds seems to be associated with the bending induced by this adduct (20). As noted above, the bent conformation induced by the crosslink of 1,1/t,t may be less energetically and structurally predisposed towards the conformation favored by HMG domains in comparison with that induced by the crosslink of cisplatin. Several factors contribute to the specificities with which the HMG domain proteins bind platinated duplexes. One such factor could be an enhanced local flexibility at the site of the adduct (35,36). The enhanced flexibility was revealed to correlate with a lowering of the affinity of damaged-DNA-binding proteins for DNA adducts of platinum (35,36) and we suggest that it might also be a factor contributing to the enhanced crosslink-induced destabilization of the host duplex.
In conclusion, the results of the present work further support the idea of different DNA binding modes of cisplatin and novel polynuclear platinum antitumor drugs and thus may also help explain their distinct biological effectiveness when compared with that of ‘classical’ cisplatin.
Acknowledgments
ACKNOWLEDGEMENTS
This work was supported by the Grant Agency of the Czech Republic (grant no. 305/99/0695) and the Grant Agency of the Academy of Sciences of the Czech Republic (grant nos A5004101 and S5004009). C.H. was supported by a doctoral fellowship from the Faculty of Sciences, Masaryk University, Brno.
References
- 1.Rosenberg B. (1999) Platinum complexes for the treatment of cancer: why the search goes on? In Lippert,B. (ed.), Cisplatin. Chemistry and Biochemistry of a Leading Anticancer Drug. Wiley-VCH, Zürich, Switzerland, pp. 3–30.
- 2.O’Dwyer P.J., Stevenson,J.P. and Johnson,S.W. (1999) Clinical status of cisplatin, carboplatin and other platinum-based antitumor drugs. In Lippert,B. (ed.), Cisplatin. Chemistry and Biochemistry of a Leading Anticancer Drug. Wiley-VCH, Zürich, Switzerland, pp. 31–72.
- 3.Reedijk J. (1996) Improved understanding in platinum antitumour chemistry. Chem. Commun., 801–806. [Google Scholar]
- 4.Lepre C.A. and Lippard,S.J. (1990) Interaction of platinum antitumor compounds with DNA. Nucleic Acids Mol. Biol., 4, 9–38. [Google Scholar]
- 5.Johnson N.P., Butour,J.-L., Villani,G., Wimmer,F.L., Defais,M., Pierson,V. and Brabec,V. (1989) Metal antitumor compounds: the mechanism of action of platinum complexes. Prog. Clin. Biochem. Med., 10, 1–24. [Google Scholar]
- 6.Comess K.M., Burstyn,J.N., Essigmann,J.M. and Lippard,S.J. (1992) Replication inhibition and translesion synthesis on templates containing site-specifically placed cis-diamminedichloroplatinum(II) DNA adducts. Biochemistry, 31, 3975–3990. [DOI] [PubMed] [Google Scholar]
- 7.Eastman A. (1999) The mechanism of action of cisplatin: from adducts to apoptosis. In Lippert,B. (ed.), Cisplatin. Chemistry and Biochemistry of a Leading Anticancer Drug. Wiley-VCH, Zürich, Switzerland, pp. 111–134.
- 8.Jamieson E.R. and Lippard,S.J. (1999) Structure, recognition and processing of cisplatin-DNA adducts. Chem. Rev., 99, 2467–2498. [DOI] [PubMed] [Google Scholar]
- 9.Zamble D.B. and Lippard,S.J. (1999) The response of cellular proteins to cisplatin-damaged DNA. In Lippert,B. (ed.), Cisplatin. Chemistry and Biochemistry of a Leading Anticancer Drug. Wiley-VCH, Zürich, Switzerland, pp. 73–110.
- 10.Farrell N. (2000) Polynuclear charged platinum compounds as a new class of anticancer agents—toward a new paradigm. In Farrell,N.P. and Kelland,L.R. (eds), Platinum-based Drugs in Cancer Therapy. Humana Press, Totowa, NJ, pp. 321–338.
- 11.Farrell N., Qu,Y., Bierbach,U., Valsecchi,M. and Menta,E. (1999) Structure-activity relationship within di- and trinuclear platinum phase I clinical agents. In Lippert,B. (ed.), Cisplatin. Chemistry and Biochemistry of a Leading Anticancer Drug. Wiley-VCH, Zurich, Switzerland, pp. 479–496.
- 12.Farrell N., Appleton,T.G., Qu,Y., Roberts,J.D., Fontes,A.P.S., Skov,K.A., Wu,P. and Zou,Y. (1995) Effects of geometric isomerism and ligand substitution in bifunctional dinuclear platinum complexes on binding properties and conformational changes in DNA. Biochemistry, 34, 15480–15486. [DOI] [PubMed] [Google Scholar]
- 13.Zaludova R., Zakovska,A., Kasparkova,J., Balcarova,Z., Kleinwachter,V., Vrana,O., Farrell,N. and Brabec,V. (1997) DNA interactions of bifunctional dinuclear platinum(II) antitumor agents. Eur. J. Biochem., 246, 508–517. [DOI] [PubMed] [Google Scholar]
- 14.Kasparkova J., Mellish,K.J., Qu,Y., Brabec,V. and Farrell,N. (1996) Site-specific d(GpG) intrastrand cross-links formed by dinuclear platinum complexes. Bending and NMR studies. Biochemistry, 35, 16705–16713. [DOI] [PubMed] [Google Scholar]
- 15.Fichtinger-Schepman A.M.J., Van der Veer,J.L., Den Hartog,J.H.J., Lohman,P.H.M. and Reedijk,J. (1985) Adducts of the antitumor drug cis-diamminedichloroplatinum(II) with DNA: formation, identification and quantitation. Biochemistry, 24, 707–713. [DOI] [PubMed] [Google Scholar]
- 16.Eastman A. (1987) The formation, isolation and characterization of DNA adducts produced by anticancer platinum complexes. Pharmacol. Ther., 34, 155–166. [DOI] [PubMed] [Google Scholar]
- 17.Bellon S.F. and Lippard,S.J. (1990) Bending studies of DNA site-specifically modified by cisplatin, trans-diamminedichloroplatinum(II) and cis-Pt(NH3)2(N3-cytosine)Cl+. Biophys. Chem., 35, 179–188. [DOI] [PubMed] [Google Scholar]
- 18.Takahara P.M., Frederick,C.A. and Lippard,S.J. (1996) Crystal structure of the anticancer drug cisplatin bound to duplex DNA. J. Am. Chem. Soc., 118, 12309–12321. [Google Scholar]
- 19.Gelasco A. and Lippard,S.J. (1998) NMR solution structure of a DNA dodecamer duplex containing a cis-diammineplatinum(II) adduct d(GpG) intrastrand cross-link, the major adduct of the anticancer drug cisplatin. Biochemistry, 37, 9230–9239. [DOI] [PubMed] [Google Scholar]
- 20.Ohndorf U.M., Rould,M.A., He,Q., Pabo,C.O. and Lippard,S.J. (1999) Basis for recognition of cisplatin-modified DNA by high-mobility-group proteins. Nature, 399, 708–712. [DOI] [PubMed] [Google Scholar]
- 21.Pilch D.S., Plum,G.E. and Breslauer,K.J. (1995) The thermodynamics of DNA structures that contain lesions or guanine tetrads. Curr. Opin. Struct. Biol., 5, 334–342. [DOI] [PubMed] [Google Scholar]
- 22.Plum G.E., Grollman,A.P., Johnson,F. and Breslauer,K.J. (1995) Influence of the oxidatively damaged adduct 8-oxodeoxyguanosine on the conformation, energetics and thermodynamic stability of a DNA duplex. Biochemistry, 34, 16148–16160. [DOI] [PubMed] [Google Scholar]
- 23.Poklar N., Pilch,D.S., Lippard,S.J., Redding,E.A., Dunham,S.U. and Breslauer,K.J. (1996) Influence of cisplatin intrastrand crosslinking on the conformation, thermal stability and energetics of a 20-mer DNA duplex. Proc. Natl Acad. Sci. USA, 93, 7606–7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pilch D.S., Dunham,S.U., Jamieson,E.R., Lippard,S.J. and Breslauer,K.J. (2000) DNA sequence context modulates the impact of a cisplatin 1,2-d(GpG) intrastrand cross-link and the conformational and thermodynamic properties of duplex DNA. J. Mol. Biol., 296, 803–812. [DOI] [PubMed] [Google Scholar]
- 25.Farrell N., Qu,Y., Feng,L. and Van Houten,B. (1990) Comparison of chemical reactivity, cytotoxicity, interstrand cross-linking and DNA sequence specificity of bis(platinum) complexes containing monodentate or bidentate coordination spheres with their monomeric analogues. Biochemistry, 29, 9522–9531. [DOI] [PubMed] [Google Scholar]
- 26.Brabec V., Reedijk,J. and Leng,M. (1992) Sequence-dependent distortions induced in DNA by monofunctional platinum(II) binding. Biochemistry, 31, 12397–12402. [DOI] [PubMed] [Google Scholar]
- 27.Brabec V. and Leng,M. (1993) DNA interstrand cross-links of trans-diamminedichloroplatinum(II) are preferentially formed between guanine and complementary cytosine residues. Proc. Natl Acad. Sci. USA, 90, 5345–5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kasparkova J., Farrell,N. and Brabec,V. (2000) Sequence specificity, conformation and recognition by HMG1 protein of major DNA interstrand cross-links of antitumor dinuclear platinum complexes. J. Biol. Chem., 275, 15789–15798. [DOI] [PubMed] [Google Scholar]
- 29.Haynie D.T. (1998) Quantitative analysis of differential scanning calorimetric data. In Ladbury,J.E. and Chowdhry,B.Z. (eds), Biocalorimetry. Applications of Calorimetry in the Biological Sciences. John Wiley & Sons, Chichester, UK, pp. 183–205.
- 30.Hofr C. and Brabec,V. (2001) Thermal and thermodynamic properties of duplex DNA containing site-specific interstrand cross-link of antitumor cisplatin or its clinically ineffective trans isomer. J. Biol. Chem., 276, 9655–9661. [DOI] [PubMed] [Google Scholar]
- 31.Malina J., Hofr,C., Maresca,L., Natile,G. and Brabec,V. (2000) DNA interactions of antitumor cisplatin analogs containing enantiomeric amine ligands. Biophys. J., 78, 2008–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bailly C., Gentle,D., Hamy,F., Purcell,M. and Waring,M.J. (1994) Localized chemical reactivity in DNA associated with the sequence-specific bisintercalation of echinomycin. Biochem. J., 300, 165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Breslauer K.J., Frank,R., Blocker,H. and Marky,L.A. (1986) Predicting DNA duplex stability from the base sequence. Proc. Natl Acad. Sci. USA, 83, 3746–3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dunham S.U. and Lippard,S.J. (1997) DNA sequence context and protein composition modulate HMG-domain protein recognition of ciplatin-modified DNA. Biochemistry, 36, 11428–11436. [DOI] [PubMed] [Google Scholar]
- 35.Kasparkova J. and Brabec,V. (1995) Recognition of DNA interstrand cross-links of cis-diamminedichloroplatinum(II) and its trans isomer by DNA-binding proteins. Biochemistry, 34, 12379–12387. [DOI] [PubMed] [Google Scholar]
- 36.Brabec V. (2000) Chemistry and structural biology of 1,2-interstrand adducts of cisplatin. In Kelland,L.R. and Farrell,N.P. (eds), Platinum-based Drugs in Cancer Therapy. Humana Press, Totowa, NJ, pp. 37–61.