Abstract
A number of endocrinopathies have been described after hematopoietic cell transplantation (HCT), but data are limited in patients with Fanconi anemia (FA). We report several endocrine-based disorders in a cohort of 44 patients with FA after HCT compared with both 74 patients who received HCT for hematologic malignancies and with 275 healthy controls. Endocrinopathies assessed included hypothyroidism, hypogonadism, short stature, dyslipidemia, insulin resistance, abnormalities in body composition, and bone health. Most (86%) patients with FA had at least 1 endocrinopathy, with 11% having 3 or more. Hypothyroidism was seen in 57%, hypogonadism in 27%, short stature in 50%, and reduced total body and lumbar spine bone mineral density (BMD) (height adjusted Z-score < −1) in 57% and 21%, respectively. Vitamin D deficiency was seen in 71%. Short stature was associated with younger age at HCT and gonadal failure was associated with older age at HCT. Insulin resistance was associated with increased percent fat mass and increased android/gynoid ratio by dual energy X-ray absorptiometry. Hypothyroidism, short stature, and reduced total body BMD were more prevalent in patients with FA compared with patients with hematologic malignancies. We recommend an assessment before transplantation and close follow-up afterwards to ensure proper clinical management. Future studies should continue to explore the impact of HCT on endocrinopathies in FA patients.
Keywords: Fanconi anemia, Hematopoietic cell, transplantation, Endocrine, Hypothyroidism, Bone mineral density, Insulin resistance
INTRODUCTION
Outcomes after hematopoietic cell transplantation (HCT) for patients with Fanconi anemia (FA) have improved significantly in recent years [1, 2]. Survival after alternative-donor HCT with a fludarabine-containing regimen in patients with FA without prior opportunistic infections or transfusions is 94% at 5 years [3]. Therefore, long-term complications need to be investigated, particularly in this population with an increased rate of endocrinopathies at baseline [4]. Data on endocrinopathies after HCT in patients with FA are limited, with most reports combining late effects among patients with a variety of malignant and nonmalignant diseases [5–11].
Our primary aim was to comprehensively evaluate the endocrine, bone health, and metabolic profile of patients with FA who underwent allogeneic HCT using a uniform total body irradiation—(TBI) containing conditioning regimen. Our secondary aims were to evaluate for associations of these endocrine deficits with patient characteristics and to compare these outcomes to both a healthy sibling control group (healthy control) and with patients who received HCT for a hematologic malignancy (cancer HCT). We hypothesized that in patients with FA, the following would be observed: (1) insulin resistance would be associated with increased central adiposity, increased percent fat mass, and increased triglycerides; and (2) the prevalence of endocrinopathies would be higher in patients who underwent HCT for FA compared with those who underwent HCT for hematologic malignancies because of an increased risk of endocrinopathies in patients with FA at baseline.
MATERIALS AND METHODS
The institutional review boards at the University of Minnesota and the Fred Hutchinson Cancer Research Center/Seattle Children’s Hospital approved the study. All patients and/or guardians signed institutional review board—approved informed consent for HCT and data collection in accordance with the Declaration of Helsinki. Studies were registered at http:www.clinicaltrials.gov under NCT00352976 and NCT00920842.
Study Participants
FA HCT
Our case population included 44 patients with FA who underwent allogeneic donor HCT for severe marrow failure at less than 35 years of age between 2006and 2013 at the University of Minnesota with at least 1year of follow-up. All were treated on the same protocol, with a single dose of TBI 300 cGy with thymic shielding, cyclophosphamide of 10 mg/kg for 4 days, fludarabine of 35 mg/m2 for 4 days, with or without antithymocyte globulin and methylprednisolone with mycophenolate mofetil and cyclosporine or sirolimus as graft-versus-host disease (GVHD) prophylaxis as previously reported [3]. Donor grafts were T cell depleted related (n = 3) and unrelated (n = 27) donor marrow, unrelated donor single cord blood (n = 12), and unrelated donor double cord blood (n = 2).
Cancer HCT
This control group comprised 74 patients who received myeloablative HCT for hematologic malignancies at the Fred Hutchinson Cancer Center and the University of Minnesota between 1975 and 2008, as previously reported [12]. Diseases included acute myeloid leukemia (n = 32), acute lymphoblastic leukemia (n = 20), myelodysplastic syndrome (n = 12), chronic myelogenous leukemia (n = 7), non-Hodgkin lymphoma (n = 2), and juvenile myelomonocytic leukemia (n = 1). Sixty patients received chemotherapy and TBI (median, 1320; range, 750 to 1575 cGy) and 14 received chemotherapy alone. Donor grafts were T cell replete related (n = 40) and unrelated (n = 17) donor marrow, related (n = 1) and unrelated (n = 13) donor cord blood, and related(n = 2)and unrelated(n = 1) donor peripheral blood stem cells.
Healthy control
This control group consisted of 275 healthy siblings of patients with cancer [12, 13]. Controls were excluded if they had a history of malignancy, previous HCT, or known chronic illness, such as hypothyroidism.
Study Procedures
Clinical and laboratory data were systematically and prospectively collected annually after HCT. All data were obtained from patients with FA; all data except metabolic parameters and vitamin D levels were obtained from both control groups. Endocrinologists performed Tanner staging. For females, separate breast and pubic hair staging were performed and the higher of these was recorded as the patient’s Tanner stage. For males, only pubic hair Tanner stage was recorded since males with FA can have small genitalia. Chemiluminescent immunoassays were used to measure 25-hydroxyvitamin D, estradiol, thyroid stimulating hormone (TSH), follicle stimulating hormone (FSH), and luteinizing hormone levels. Free thyroxine was measured by competitive immunoassay and total testosterone by liquid chromatography/tandem mass spectrometry. Dual energy X-ray absorptiometry (DXA) scans were used to measure bone mineral density (BMD). DXA measures included Z-scores for total body BMD (TBMD) and including head and posterior anterior lumbar spine BMD (LBMD) at L1 to L4. BMD Z-scores were adjusted for sex and age, using reference data based on healthy ambulatory subjects from the general population who were free from chronic diseases affecting bone and not taking bone-altering medications. BMD Z-scores were also height-adjusted at the total body and lumbar spine (TBMDHAZ and LBMDHAZ respectively), using a method described by Zemel et al. [14]. The android to gynoid ratio (A/G) ratio and percent fat mass were measured by DXA, as previously described [15]. To better characterize adiposity beyond body mass index (BMI), percent fat mass was used to organize patients into normal, moderate, and elevated categories by age and sex, as previously defined [16]. Glycemic abnormalities and measures of insulin resistance were evaluated by oral glucose tolerance testing in the FA HCT group. After an overnight fast, 1.75 g/kg (maximum of 75 grams) of oral glucose was ingested, and glucose and insulin were measured by chemiluminescent immunoassay at 0, 30, 60, 90, and 120 minutes. The Matsuda index was used to calculate insulin resistance and was deemed abnormal if < 4.5 [17–19]. Hypothyroidism was defined by treatment with thyroid hormone replacement at the time of evaluation, free T4 < .7 ng/dL, or TSH > 5.0 mU/L before 2014 and > 4.0 mU/L starting in July of 2014, because of a change in the normative reference data. Hypogonadism was defined by treatment with estrogen or FSH > 40IU/L in a female > 10 years of age. In a male, hypogonadism was defined as treatment with testosterone, FSH > 18 IU/L, or luteinizing hormone > 10 and low testosterone in a male > 11 years of age. Gonadal hormone levels were obtained in all females > 10 years and males > 11 years old. Short stature was defined as a height Z-score of less than -2. Vitamin D deficiency was defined as a 25-hydroxyvitamin D level of less than 30 ug/L Reduced BMD was defined as a height-adjusted Z-score < −1.
Statistical Analysis
Descriptive statistics were expressed as frequencies and percent or mean/median ± standard deviation (SD), and range, as appropriate. For data involving healthy controls or repeated measures, P values were generated from generalized estimating equations with robust standard errors in the form of linear (continuous variable), logistic (binary variable), or multinomial regression (categorical variable). Otherwise, P values were calculated from the 2-sample t-test or the Chi-square test. If the overall test for 3-group comparison showed significant, pairwise comparison was done. Matsuda index was transformed in natural log, and its association with the A/G ratio was examined using linear mixed model. All analyses were done using the SAS system (v. 9.3; SAS Institute, Cary, NC). P values were 2-sided with < .05 considered statistically significant. Bonferroni adjustment was applied for multiple comparisons.
RESULTS
Characteristics of Study Participants
There was no difference in gender distribution among the 3 groups (Table 1). There was no difference in age at HCT for the FA HCT and cancer HCT groups. The cancer HCT group had longer follow-up and were, thus, older and more sexually mature at the latest evaluation. Both FA HCT patients and cancer HCT patients were shorter than healthy controls (P < .001). A significantly higher proportion of cancer HCT patients developed acute GVHD (57% versus 11%, P < .001) and chronic GVHD (35% versus 5%, P = .002), compared with the patients with FA.
Table 1.
Characteristics of Study Participants
| Characteristic | A FA HCT n = 44 |
B Cancer HCT n = 74 |
C Healthy Control n = 275 |
Pairwise P Values
|
||
|---|---|---|---|---|---|---|
| A versus B | A versus C | B versus C | ||||
| Sex, male | 24 (54.6) | 46 (62.2) | 150 (54.6) | NS | NS | NS |
| Age at HCT, mean (SD) range, yr | 10.7 (7.2) 3.3–34.3 | 10.5 (6.0) .6–22.6 | NS | |||
| Years since HCT, mean (SD) range | 3.1 (1.9) 1.0–8.1 | 11.1 (5.9) 2.7–26.9 | <.001 | |||
| Age at last study, mean (SD) range, yr | 13.8 (7.1) 5.9–36.3 | 21.6 (6.3) 11.0–36.5 | 15.6 (4.7) 9.0–36.4 | <.001 | NS | <.001 |
| Height Z-score, mean (SD), range | −1.8 (1.1) −4.7–.3 | −.6 (1.1) −3.1–1.9 | .4 (1.0) −1.8–3.1 | <.001 | <.001 | <.001 |
| Tanner stage, median (SD), range | 1.0 (1.4) 1–5 | 5.0 (1.3) 1–5 | 4.0 (1.4) 1–5 | <.001 | <.001 | <.001 |
NS indicates not statistically significant.
Items in bold are statistically significant.
P value < .0167 is considered statistically significant after multiple comparison adjustment using Bonferroni method.
Endocrinopathies in FA HCT Patients
Endocrinopathies were observed in 86% of patients with FA and included hypothyroidism (57%), hypogonadism (27%), and short stature (50%). In addition, vitamin D deficiency was seen in 71% (Table 2). Short stature was associated with younger age at transplantation (P = .01) and hypogonadism with older age at transplantation (P = .02). Although patients with FA who did not receive growth hormone had a decrease in height Z-score from −.91 before transplantation to −1.53 at last evaluation, patients with FA who received growth hormone had an increase in height Z-score from −2.76 to −2.47. However, there was no statistically significant difference in the 2 groups (P = .11). GVHD and FA complementation groups were not associated with endocrinopathies. Only 1 FA patient received androgen therapy before HCT. Growth hormone therapy was used in 10 patients (23%) from the FA HCT group (3 before HCT and 7 after HCT) and 8 patients (11%) from the cancer HCT group after HCT.
Table 2.
FA HCT: Endocrinopathies in Relation to Patient Characteristics
| Characteristic | Hypothyroidism n = 44 | Hypogonadism n = 30 | Short Stature n = 44 | Vitamin D Deficiency n = 42 |
|---|---|---|---|---|
| Age at transplantation, yr | ||||
| <10 | 17 of 29 (58.6) | 1 of 15 (6.7) | 18 of 29 (62.1) | 21 of 28 (75) |
| 10–16 | 5 of 8 (62.5) | 3 of 8 (37.5) | 4 of 8 (50) | 6 of 8 (75) |
| >16 | 3 of 7 (42.9) | 4 of 7 (57.1) | 0 of 7 (0) | 3 of 6 (50) |
| P value | .47 | .02 | .01 | .44 |
| Sex | ||||
| Male | 13 of 24 (54.2) | 2 of 15 (13.3) | 13 of 24 (54.2) | 16 of 23 (69.6) |
| Female | 12 of 20 (60) | 6 of 15 (40) | 9 of 20 (45) | 14 of 19 (73.7) |
| P value | >.99 | .21 | .76 | .99 |
| GVHD | ||||
| Yes | 4 of 6 (66.6) | 4 of 6 (66.7) | 3 of 6 (50) | 3 of 5 (60) |
| No | 21 of 38 (55.3) | 4 of 24 (16.7) | 19 of 38 (50) | 27 of 37 (73) |
| P value | .55 | .30 | >.99 | .60 |
| Complementation group | ||||
| A | 20 of 31 (64.5) | 5 of 23 (2.17) | 16 of 31 (51.6) | 23 of 29 (79.3) |
| C | 3 of 7 (42.9) | 0 of 3 (0) | 3 of 7 (42.9) | 4 of 7 (57.1) |
| Other | 2 of 6 (33.3) | 3 of 4 (76) | 3 of 6 (50) | 3 of 6 (50) |
| P value | .74 | .06 | .77 | .82 |
GVHD is acute and/or chronic.
FA complementation groups included FANCA (31), FANCC (7), FANCG (2), FANCF (1), and unknown (3).
Metabolic Profile of FA HCT Patients
Sex, age, complementation group, and high-density lipoprotein levels were not risk factors for insulin resistance (Table 3). All 5 patients who were obese at time of assessment had insulin resistance. In addition, 9 of 25 patients with normal BMI (36%) had insulin resistance and 2 of 4 (50%) of overweight patients had insulin resistance. Elevated percent fat mass and an increased A/G ratio were associated with insulin resistance. There was a clear inverse relationship between A/G ratio and Matsuda index (Figure 1), reflecting an increased incidence of insulin resistance with increased A/G ratio. Although only 5 patients were obese by BMI, 12 patients had elevated percent fat mass by DXA and 15 demonstrated abdominal obesity with an elevated A/G ratio.
Table 3.
Metabolic Profile of FA HCT Patients* by Matsuda Index
| Characteristic | Variables | Matsuda Index
|
P Value | |
|---|---|---|---|---|
| < 4.5 (n = 16) | ≥ 4.5 (n = 20) | |||
| Sex, n (%) | Male | 7 (43.75) | 11 (55) | .92 |
| Female | 9 (56.25) | 9 (45) | ||
| Age at study, mean (SD), yr | 16.76 (10.02) | 12.75 (8.33) | .75 | |
| Complementation group, n (%) | A | 14 (87.50) | 14 (70) | .19 |
| C | 2 (12.50) | 2 (10) | ||
| Others | 0 (0) | 4 (20) | ||
| BMI percentile | Normal | 9 of 16 (56.25) | 16 of 18 (88.89) | .08 |
| Overweight (>85th) | 2 of 16 (12.50) | 2 of 18 (11.11) | ||
| Obese (>95th) | 5 of 16 (31.25) | 0 of 18 (0) | ||
| Percent fat mass | Normal | 2 of 12 (16.67) | 6 of 16 (37.50) | .03 |
| Moderate | 1 of 12 (8.33) | 7 of 16 (43.75) | ||
| Elevated | 9 of 12 (75) | 3 of 16 (18.75) | ||
| A/G ratio | <.7 | 0 of 11 (0) | 3 of 15 (20) | .05 |
| .7–.85 | 2 of 11 (18.18) | 6 of 15 (40) | ||
| >.85 | 9 of 11 (81.82) | 6 of 15 (40) | ||
| HDL | < 40 | 4 of 16 (25) | 5 of 20 (25) | .91 |
| ≥ 40 | 12 of 16 (75) | 15 of 20 (75) | ||
| Triglycerides | >150 | 5 of 16 (31.25) | 3 of 20 (15) | .30 |
| ≤ 150 | 11 of 16 (68.75) | 17 of 20 (85) | ||
| Height-adjusted BMD z-score | N | 12 | 15 | |
| LBMD, mean (SD) | −.84 (.86) | −1.46 (.80) | .32 | |
| TBMD, mean (SD) | .25 (1.29) | −.66 (1.12) | .10 | |
HDL indicates high-density lipoprotein.
Thirty-six observations were contributed from 25 patients.
Figure 1.
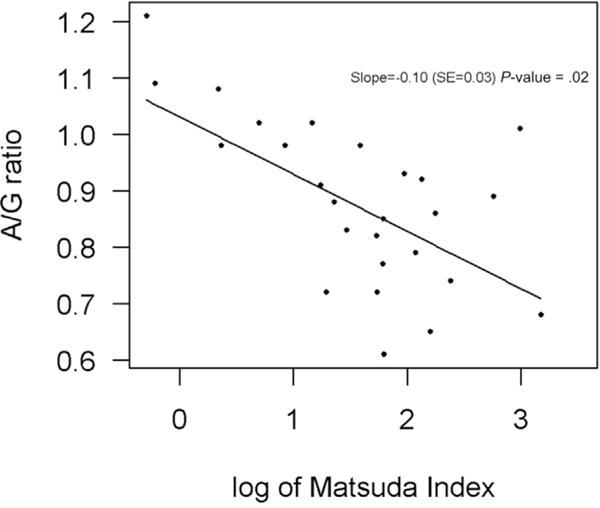
Inverse relationship between Matsuda index (<4.5 indicates insulin resistance) and A/G ratio (ratio of measurement at waist to hip by DXA scan) in patients with Fanconi anemia after hematopoietic cell transplantation.
Prevalence of Endocrinopathies and Reduced BMD in Comparison to Control Groups
As seen in Table 4, the prevalence of hypothyroidism in FA HCT patients was significantly higher than for cancer HCT patients (57% versus 30%, P = .004). FA HCT patients were shorter than those in the cancer HCT and healthy control groups (P < .001). There was no difference in insulin-like growth factor 1 or insulin-like growth factor binding protein 3 SD scores (P > .12). Height-adjusted TBMD scores, but not LBMD Z-scores, were significantly lower in FA HCT patients than in cancer HCT patients and healthy controls (P < .001). In patients with FA, TBMD and LBMD (height adjusted Z-score < −2) were low in 43% and 0%, respectively. In contrast to our FA HCT group, there was no association of short stature (P = .44) or hypogonadism (P = .89) with age in our cancer HCT group.
Table 4.
Prevalence of Endocrinopathies and Reduced BMD in Comparison to Control Groups
| Diagnosis | A FA HCT n = 44 |
B Cancer HCT n = 74 |
C Healthy Control n = 275 |
Pairwise P Values
|
||
|---|---|---|---|---|---|---|
| A versus B | A versus C | B versus C | ||||
| Hypothyroidism | 25 of 44 (56.8) | 22 of 74 (29.7) | 1 of 275 (.4) | .0042 | <.001 | <.001 |
| Hypogonadism | 8 of 30 (26.7) | 25 of 64 (39.1) | NS | |||
| Short stature | 22 of 44 (50) | 8 of 74 (10.8) | 0 of 275 (0) | <.001 | <.001 | <.001 |
| TBMDHAZ < −1 | 24 of 42 (57.1) | 14 of 73 (19.2) | 32 of 269 (11.9) | <.001 | <.001 | NS |
| LBMDHAZ < −1 | 9 of 43 (20.9) | 14 of 72 (19.4) | 41 of 273 (15.0) | NS | NS | NS |
BMDHAZ Z-score indicates adjustment for height-for-age Z-score (HAZ) using the approach of Zemel et al. [14]asmeasured at the total body (TBMDHAZ) and L1 to L4 lumbar spine (LBMDHAZ).
DISCUSSION
This study is the largest comprehensive report of the endocrine, bone health, and metabolic profile of patients with FA who have undergone allogeneic HCT. We found that hypothyroidism, short stature, and reduced TBMD were common in patients with FA. Younger age at HCT has been identified as a risk factor for thyroid dysfunction after HCT [20]. However, age had no influence on occurrence of hypothyroidism in our FA HCT group. This may reflect a high rate of hypothyroidism at baseline in patients with FA, as incidence has ranged from 36% to 65% in mixed cohorts of patients with FA who had and who had not undergone transplantation [21–23]. Younger FA HCT patients were found to be of short stature much more often than older patients. Although patients with FA are known to be short at baseline, we found that younger age at HCT was associated with an increased prevalence of short stature. This may be because younger age at HCT is a risk factor for short stature in patients with FA as has been seen in other HCT survivors [24]. This may be a consequence of growth hormone deficiency after HCT in younger children, which could impede the normal pubertal growth spurt [25].
Our results demonstrate that patients with FA are at increased risk for insulin resistance after HCT. Abdominal fat mass has been shown to be strongly associated with cardiovascular risk factors in adults [26]. In pediatric patients, an increased A/G ratio was found to be associated with insulin resistance [27, 28]. Patients with FA after HCT appear to represent a particularly vulnerable group, as they may be susceptible to deleterious effects of reactive oxygen species (ROS) on insulin signaling [29]. Recent data in the mouse model showed that high levels of ROS are seen in FANC-A and FANC-C knockout mice. These high ROS have been mechanistically linked to critical events in impaired insulin signaling. Further, administration of antioxidants led to improvement in oral glucose tolerance testing [30]. TNF-α can also drive ROS, so patients with FA may be more susceptible to insulin resistance as cytokine levels rise after HCT [31]. Although obesity is a risk factor for insulin resistance, other parameters observed from DXA scans, such as A/G ratio and percent fat mass, can also help predict insulin resistance, even in patients with normal weight and/or BMI. Other modalities to gauge sarcopenic obesity may be utilized as well, from simple measurements of abdominal circumference to more comprehensive evaluations of body composition with air displacement plethysmogaphy [32]. We advocate close monitoring of these parameters in all patients with FA and particularly in those with increased percent fat mass and/or abdominal obesity.
Relative to published data, 3 studies to date have focused on patients with FA, but they have consisted of a combination of patients who had and had not undergone transplantation. The studies had an aggregate of 28 patients who underwent HCT [21–23]. Rose et al. [21] reported the endocrine phenotype of 61 children who did not undergo transplantation with FA: hypothyroidism was seen in 60%, hypogonadism in 0%, short stature in 61%, and low BMD (Z score < −2 SD) in 5%. Of the 17 pediatric HCT recipients, 65% had hypothyroidism, 25% had hypogonadism, 56% had short stature, and none had low BMD. Wajnrajch et al. [22] evaluated 54 patients; of these, only 2 had received HCT. For the entire cohort, 36% had hypothyroidism and 57% had short stature. Giri et al. [23] evaluated 45 patients with FA, 9 of whom underwent HCT: 37% had hypothyroidism, 50% of males had hypogonadism and 69% females had premature ovarian failure. Other observed endocrinopathies after HCT include lower BMD [33] and insulin resistance [34] but data in patients with FA after HCT remain limited.
We found that patients with FA after HCT were at higher risk for endocrinopathies and suboptimal BMD compared with patients with hematologic malignancies after HCT. We would expect a cohort of patients with more GVHD and, thus, increased glucocorticoid exposure to have reduced BMD [35] and an increased prevalence of hypothyroidism [36]. Even though our cancer HCT group had significantly higher rates of GVHD compared with our FA HCT group, the prevalence of hypothyroidism and reduced TBMDHAZ was higher in our FA HCT group. The higher risk of endocrinopathies in patients with FA at baseline is likely a strong contributor to the increased incidence of endocrinopathies seen in our patient population.
This study has some limitations. The length of follow-up for our FA HCT group was relatively short and may underestimate the longer-term impact of HCT on endocrine-based disorders. Even with longer follow-up and more GVHD in the cancer HCT group, our FA HCT group had a higher prevalence of endocrinopathies. Another limitation is the lack of pre-transplantation baseline data that prohibits the ability to appropriately evaluate the impact of transplantation on these endocrine-based disorders. An ideal control group in this study would have been patients with FA who had not undergone transplantation, but there were not enough of these patients with comprehensive endocrine data for evaluation.
In summary, our study highlights the importance of monitoring bone health, endocrine, and metabolic complications after transplantation in the FA population. Patients with FA will benefit from a baseline evaluation before transplantation, which will allow us to more directly study the impact of HCT. There are some potential strategies to prevent endocrinopathies after HCT. One group administered TSH-suppressive doses of levothyroxine before irradiation for lymphoma [37]. The concept of thyroid shielding is another attractive option for patients undergoing transplantation for nonmalignant conditions such as FA. Preventing abdominal adiposity by further analysis of additional risk factors involved and targeting dietary intervention and exercise may help decrease risks for insulin resistance. This may facilitate identification of modifiable risk factors and randomized controlled studies of preventative therapies. The impact of insulin resistance on development of diabetes mellitus and cardiovascular complications warrants further study. Baseline pre-HCT evaluations in patients with FA and close long-term follow-up will lead to a better understanding of the impact of HCT in this susceptible patient population.
Acknowledgments
The authors thank the nurses, nurse coordinators, nurse practitioners, physician assistants, social workers, ancillary and support staff, and physicians who cared for these patients and their families.
Financial disclosure statement: This study was supported by Kidz1stFund.
Footnotes
Conflict of interest statement: The authors declare no conflict of interest.
Authorship statement: J.L.B. designed the research study, gathered and analyzed the data, and wrote the paper. M.L.M., A.P., and B.N. participated in research design and data analysis. A.P., K.S.B., J.S., J.E.W., M.L.M., and J.L.B. contributed patient data. L.Z. and T.E.D. performed statistical analyses. All authors critically read and revised the manuscript.
References
- 1.Bhatia S, Francisco L, Carter A, et al. Late mortality after allogeneic hematopoietic cell transplantation and functional status of long-term survivors: report from the Bone Marrow Transplant Survivor Study. Blood. 2007;110:3784–3792. doi: 10.1182/blood-2007-03-082933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Remberger M, Ackefors M, Berglund S, et al. Improved survival after allogeneic hematopoietic stem cell transplantation in recent years. A single-center study. Biol Blood Marrow Transplant. 2011;17:1688–1697. doi: 10.1016/j.bbmt.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 3.MacMillan ML, DeFor TE, Young JH, et al. Alternative donor hematopoietic cell transplantation for Fanconi anemia. Blood. 2015;125:3798–3804. doi: 10.1182/blood-2015-02-626002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petryk A, Kanakatti Shankar R, Giri N, et al. Endocrine disorders in Fanconi anemia: recommendations for screening and treatment. J Clin Endocrinol Metab. 2015;100:803–811. doi: 10.1210/jc.2014-4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishiguro H, Yasuda Y, Tomita Y, et al. Long-Term follow-up of thyroid function in patients who received bone marrow transplantation during childhood and adolescence. J Clin Endocrinol Metab. 2004;89:5981–5986. doi: 10.1210/jc.2004-0836. [DOI] [PubMed] [Google Scholar]
- 6.Dvorak CC, Gracia CR, Sanders JE, et al. NCI, NHLBI/PBMTC first international conference on late effects after pediatric hematopoietic cell transplantation: endocrine challenges-thyroid dysfunction, growth impairment, bone health, & reproductive risks. Biol Blood Marrow Transplant. 2011;17:1725–1738. doi: 10.1016/j.bbmt.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mostoufi-Moab S, Ginsberg JP, Bunin N, et al. Bone density and structure in long-term survivors of pediatric allogeneic hematopoietic stem cell transplantation. J Bone Miner Res. 2012;27:760–769. doi: 10.1002/jbmr.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nysom K, Holm K, Michaelsen KF, et al. Bone massafter allogeneic BMT for childhood leukaemia or lymphoma. Bone Marrow Transplant. 2000;25:191–196. doi: 10.1038/sj.bmt.1702131. [DOI] [PubMed] [Google Scholar]
- 9.Baker KS, Chow E, Goodman P, et al. Adverse impact of hematopoietic cell transplantation (HCT) on body composition and insulin resistance (IR) is associated with increased cardiovascular risk [Abstract] Biol Blood Marrow Transplant. 2011;17:S174–S175. [Google Scholar]
- 10.Mostoufi-Moab S, Magland J, Isaacoff E, et al. Adverse fat depots and marrow adiposity are associated with skeletal deficits and insulin resistance in long-term survivors of pediatric hematopoietic stem cell transplantation. J Bone Miner Res. 2015;30:1657–1666. doi: 10.1002/jbmr.2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dvorak C, Wright N, Wong W, et al. Safety of hematopoietic stem cell transplantation in children less than three years of age. Pediatr Hematol Oncol. 2008;25:705–722. doi: 10.1080/08880010802243524. [DOI] [PubMed] [Google Scholar]
- 12.Petryk A, Polgreen LE, Zhang L, et al. Bone mineral deficits in recipients of hematopoietic cell transplantation: the impact of young age at transplant. Bone Marrow Transplant. 2013;49:258–263. doi: 10.1038/bmt.2013.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polgreen L, Petryk A, Dietz A, et al. Modifiable risk factors associated with bone deficits in childhood cancer survivors. BMC Pediatrics. 2012;12:40. doi: 10.1186/1471-2431-12-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zemel BS, Leonard MB, Kelly A, et al. Height adjustment in assessing dual energy x-ray absorptiometry measurements of bone mass and density in children. J Clin Endocrinol Metab. 2010;95:1265–1273. doi: 10.1210/jc.2009-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petak S, Barbu CG, Yu EW, et al. The official positions of the International Society for Clinical Densitometry: body composition analysis reporting. J Clin Densitom. 2013;16:508–519. doi: 10.1016/j.jocd.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 16.Freedman DS, Wang J, Thornton JC, et al. Classification of body fatness by body mass index-for-age categories among children. Arch Pediatr Adolesc Med. 2009;163:805–811. doi: 10.1001/archpediatrics.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsuda M, DeFronzo R. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 18.Brar PC, Koren D, Gallagher PR, et al. Comparison of oral and intravenous glucose tolerance test derived sensitivity and secretory indices in obese adolescents. Clin Pediatr (Phila) 2013;52:247–253. doi: 10.1177/0009922812472251. [DOI] [PubMed] [Google Scholar]
- 19.Yeckel CW, Weiss R, Dziura J, et al. Validation of insulin sensitivity indices from oral glucose tolerance test parameters in obese children and adolescents. J Clin Endocrinol Metab. 2004;89:1096–1101. doi: 10.1210/jc.2003-031503. [DOI] [PubMed] [Google Scholar]
- 20.Sanders J, Hoffmeister P, Woolfrey A, et al. Thyroid function following hematopoietic cell transplantation in children: 30 years’ experience. Blood. 2008;113:306–308. doi: 10.1182/blood-2008-08-173005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rose S, Myers K, Rutter M, et al. Endocrine phenotype of children and adults with Fanconi anemia. Pediatr Blood Cancer. 2012;59:690–696. doi: 10.1002/pbc.24095. [DOI] [PubMed] [Google Scholar]
- 22.Wajnrajch MP, Gertner JM, Huma Z, et al. Evaluation of growth and hormonal status in patients referred to the International Fanconi Anemia Registry. Pediatrics. 2001;107:744–754. doi: 10.1542/peds.107.4.744. [DOI] [PubMed] [Google Scholar]
- 23.Giri N, Batista DL, Alter BP, Stratakis CA. Endocrine abnormalities in patients with Fanconi anemia. J Clin Endocrinol Metab. 2007;92:2624–2631. doi: 10.1210/jc.2007-0135. [DOI] [PubMed] [Google Scholar]
- 24.Sanders JE. Growth and development after hematopoietic cell transplant in children. Bone Marrow Transplant. 2008;41:223–227. doi: 10.1038/sj.bmt.1705875. [DOI] [PubMed] [Google Scholar]
- 25.Forlenza GP, Polgreen LE, Miller BS, et al. Growth hormone treatment of patients with Fanconi anemia after hematopoietic cell transplantation. Pediatr Blood Cancer. 2014;61:1142–1143. doi: 10.1002/pbc.24910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wiklund P, Toss F, Weinehall L, et al. Abdominal and gynoid fat mass are associated with cardiovascular risk factors in men and women. J Clin Endocrinol Metab. 2008;93:4360–4366. doi: 10.1210/jc.2008-0804. [DOI] [PubMed] [Google Scholar]
- 27.Samsell L, Regier M, Walton C, Cottrell L. Importance of android/gynoid fat ratio in predicting metabolic and cardiovascular disease risk in normal weight as well as overweight and obese children. J Obes. 2014;2014:846578. doi: 10.1155/2014/846578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aucouturier J, Meyer M, Thivel D, et al. Effect of android to gynoid fat ratio on insulin resistance in obese youth. Arch Pediatr Adolesc Med. 2009;163:826–831. doi: 10.1001/archpediatrics.2009.148. [DOI] [PubMed] [Google Scholar]
- 29.Kumari U, Ya Jun W, Huat Bay B, Lyakhovich A. Evidence of mitochondrial dysfunction and impaired ROS detoxifying machinery in Fanconi anemia cells. Oncogene. 2014;33:165–172. doi: 10.1038/onc.2012.583. [DOI] [PubMed] [Google Scholar]
- 30.Li J, Sipple J, Maynard S, et al. Fanconi anemia links reactive oxygen species to insulin resistance and obesity. Antioxid Redox Signal. 2012;17:1083–1098. doi: 10.1089/ars.2011.4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao HL, Sun AN, Han Y, et al. Clinical significance of TNF-alpha, IL-1beta and IFN-gamma levels at early phase after allogeneic hematopoietic stem cell transplantation. J Exp Hematol. 2009;17:1321–1325. [PubMed] [Google Scholar]
- 32.Lowry DW, Tomiyama AJ. Air displacement plethysmography versus dual-energy x-ray absorptiometry in underweight, normal-weight, and overweight/obese individuals. PLoS One. 2015;10:e0115086. doi: 10.1371/journal.pone.0115086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petryk A, Polgreen LE, Barnum JL, et al. Bone mineral density in children with Fanconi anemia after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2015;21:894–899. doi: 10.1016/j.bbmt.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Polgreen LE, Thomas W, MacMillan ML, et al. First phase insulin release and glucose tolerance in children with Fanconi anemia after hematopoietic cell transplantation. Pediatr Blood Cancer. 2009;53:191–196. doi: 10.1002/pbc.22043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petryk A, Bergemann TL, Polga KM, et al. Prospective study of changes in bone mineral density and turnover in children after hematopoietic cell transplantation. J Clin Endocrinol Metab. 2006;91:899–905. doi: 10.1210/jc.2005-1927. [DOI] [PubMed] [Google Scholar]
- 36.Haugen BR. Drugs that suppress TSH or cause central hypothyroidism. Best Pract Res Clin Endocrinol Metab. 2009;23:793–800. doi: 10.1016/j.beem.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Massimino M, Gandola L, Pignoli E, et al. TSH suppression as a possible means of protection against hypothyroidism after irradiation for childhood Hodgkins lymphoma. Pediatr Blood Cancer. 2011;57:166–168. doi: 10.1002/pbc.22915. [DOI] [PubMed] [Google Scholar]


