Abstract
Aim
Thymectomy is the main treatment for thymoma and patients with myasthenia gravis (MG). The traditional approach is through a median sternotomy, but, recently, thymectomy through minimally invasive approaches is increasingly performed. Our purpose is an analysis and discussion of the clinical presentation, the diagnostic procedures and the surgical technique. We also consider post-operative complications and results, over a period of 5 years (May 2011–June 2016), in thymic masses admitted in our Thoracic Surgery Unit.
Methods
We analyzed 8 patients who underwent surgical treatment for thymic masses over a period of 5 years. 6 patients (75%) had thymoma, 2 patients (25%) had thymic carcinomas. 2 patients with thymoma (33%) had myasthenia gravis. We performed a complete surgical resection with median sternotomy as standard approach.
Results
One patient (12%) died in the postoperative period. The histological study revealed 6 (75%) thymoma and 2 (25%) thymic carcinomas. Post-operative morbidity occurred in 2 patients (25%) and were: pneumonia in 1 case (12%), atrial fibrillation and pleural effusion in 2 patients (25%). One patient with thymoma type A recurred at skeletal muscle 2-years after surgery.
Conclusions
Thymic malignancies are rare tumors. Surgical resection is the main treatment, but a multimodal approach is useful for many patients. Radical thymectomy is completed removing all the soft tissue in the anterior mediastinum between the two phrenic nerves and this is the most important factor in controlling myasthenia and influencing survival in patients with thymoma. Open (median sternotomy) approach has been the standard approach for thymectomy for the better visualization of the anatomical structures. Actually, video-assisted thoracoscopic surgery (VATS) thymectomy and robotic video-assisted thoracoscopic (R-VATS) approach versus open surgery has an equal if not superior oncological efficacy, better perioperative complications and survival outcomes.
Keywords: Thymoma, Thymectomy, Myasthenia gravis, Median sternotomy, Video-assisted thoracoscopic surgery (VATS), Robotic video-assisted thoracoscopic (R-VATS)
1. Introduction
The work has been reported in line with the SCARE criteria [52]
Thymoma is the most frequent primary mediastinal neoplasm in adults [1], usually benign and slow-growing. The most frequent metastatic localizations are to the pleura, pericardium and/or diaphragm [1]. Therefore, complete surgical resection is the main treatment. Median sternotomy has been long considered the best for resection approaches [2], [3]. Actually, minimally invasive methods have emerged over recent decades including video-assisted thoracoscopic (VATS) and robotic video-assisted thoracoscopic (R-VATS) approaches [4], [5], [6], [7], [8]. Thymic malignancies are uncommon tumors. The most important histological patterns in mediastinal neoplasm are: thymomas, thymic carcinomas (TC) and neuroendocrine thymic tumors (NETT). Thymoma is most frequent between 35 and 70 years of age and has a gender distribution slightly more common in older women [9]. Patients with myasthenia gravis are generally younger, with a broad peak between 30 and 60 years. Thymomas have an indolent growth pattern and may be confused with a benign growth. Autoimmune diseases, such as myasthenia gravis, systemic lupus erythematosus (SLE), rheumatoid arthritis, thyroiditis, present a linkage in the aetiology of thymoma. Thymoma could be asymptomatic (30%) and discovered incidentally including chest surgery for unrelated reasons. About 30% of patients with thymomas have myasthenia gravis [10], but only 21% of patients with myasthenia have thymomas [11]. Thymomas are primary tumors of thymic epithelial cells, generally benign, instead of thymic carcinomas considered malignant with a worse prognosis [12], [13]. Thymic epithelial tumors are classified according to World Health Organization (WHO), considering both histological and morphological features [14]. The Masaoka staging, introduced in 1981, is the most widely used one and it is based on clinical description of local extension of the neoplasm [15], [16]. Another classification is the Tumor-node-metastasis (TNM) staging. In general, approximately 40% thymomas present at Stage I, 25% each at Stages II and III, 10% at Stage IVa and 1%–2% at Stage IVb. Invasion into mediastinal tissue (Stages II, III) is present in 50% of thymomas, with pleural invasion being most common followed by pulmonary and pericardial invasion. Approximately 30% of these cases have involvement of innominate vein or superior vena cava and 20% have phrenic nerve involvement. Direct extension is also seen into aorta and pulmonary artery (11%) and chest wall (8%) [17]. Most of thymic tumors have non-malignant appearing thymic epithelial cells mixed with variable proportions of lymphocytes. Despite his lack of malignant cytological features and their indolent behavior, thymomas have invasive and metastatic potential and should not be considered benign [18], [19]. Computed tomography (CT) is the standard diagnostic and preoperative staging modality for thymoma. Thymomas typically appear as rounded or oval masses in early stages. Presence of irregular margins, multiple calcifications and areas of low attenuation are suggestive of invasion [20]. Preservation of fat planes between the thymoma and the adjacent structures indicate a noninvasive tumor while their absence is suspicious for invasion. The presence of smooth or lobulated contour, homogenous enhancement, absence of pericardial or pleural effusion or calcification in the tumor are indirect signs of a thymoma or a well-differentiated thymic carcinoma [21].
2. Materials and methods
Over a period of five years, May 2011–June 2016, in the Thoracic Surgery Unit of “S. Giovanni di Dio e Ruggi D’Aragona” Hospital of University of Salerno, were observed and treated 8 patients with thymic masses (4 men and 4 women with a mean age of 55 years – range 25–72 years). Six patients (75%) had thymoma, 2 patients (25%) had thymic carcinomas. Two patients with thymoma (33%) had myasthenia gravis. The symptoms of 8 patients is summarized in Table 1. Thymoma could be asymptomatic (3 cases in our study) and discovered incidentally including chest surgery for unrelated reasons. About 40% of patients have local symptoms. They usually include chest pain (3 patients in our observations), cough (4 cases) and shortness of breath (2 cases) from compression of the airways or from the neuromuscular effects of myasthenia gravis. Less commonly superior vena cava syndrome (1 case) and weight loss occurs (2 cases) in rapidly growing tumors. When clinically manifest myasthenia gravis present with ptosis (2 cases), double vision (2 cases) and generalized fatigue (2 cases), particularly worse late during the day. One patient showed fever and night sweats making it difficult to differentiate from lymphoma.
Table 1.
Patients’ symptoms on presentation (patients may have more than one clinical feature).
| Numbers | Percentage% | |
|---|---|---|
| Patients | 8 | |
| Chest pain | 3 | 37 |
| Cough | 4 | 50 |
| Shortness of breath | 2 | 25 |
| Superior Vena Cava syndrome | 1 | 12 |
| Weight loss | 2 | 25 |
| Ptosis | 2 | 25 |
| Double vision | 2 | 25 |
| Generalized fatigue | 2 | 25 |
| Fever | 1 | 12 |
| Night sweats | 1 | 12 |
| Asymptomatic | 3 | 37 |
The preoperative diagnosis was made by anamnesis, clinically examination, thoracic X-ray examination, total body CT scan exploration, flessible bronchoscopy. For histopathologic diagnosis we performed Fine needle aspiration (FNA) in 4 cases (only 2 resulted suspected for thymomas) and needle biopsy or open biopsy (anterior parasternal mediastinotomy, thoracoscopy) in 2 cases. Two cases presented with myiastenia gravis symptoms and had no histopathologic examination.
Six patients (75%) had thymoma, 2 patients (25%) had thymic carcinomas. Two of 6 patients with hymoma had myastenia gravis.
En-bloc resection of the entire thymus gland and surrounding areolar tissue was performed with median sternotomy as standard approach. Radical thymectomy was obtained by removing all the soft tissue in the anterior mediastinum between the two phrenic nerves. These surgical interventions were performed under general anesthesia with tracheal intubation. Patients were positioned in the supine position with the arms either tucked in at their sides or slightly extended at the side. All patients were ventilated with a single-lumen endotracheal tube. Either a complete (6 cases in our study) or a partial sternotomy (2 cases in our observation) was used and the thymus and thymic pathology were resected en bloc.
A patient with a thymoma type A recurred at skeletal muscle 2-years after surgery. Therefore, we performed a radical surgery resection of metastases before adjuvant treatment [26].
3. Results
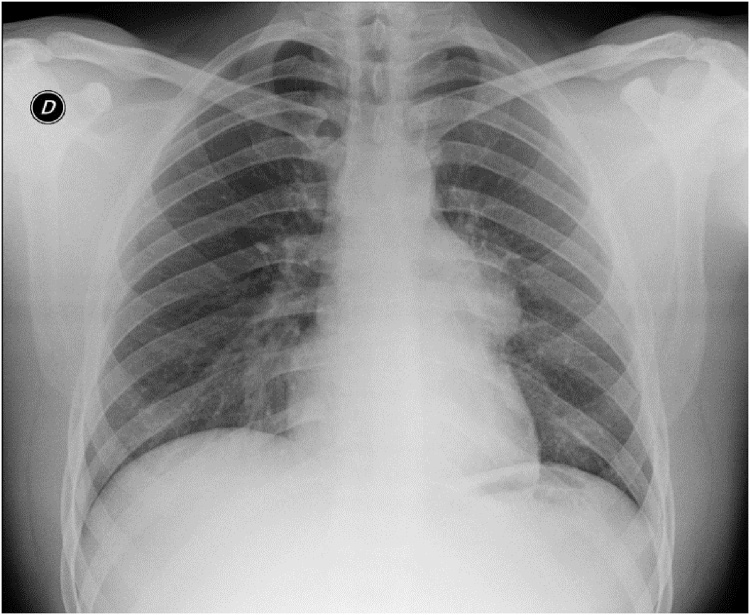
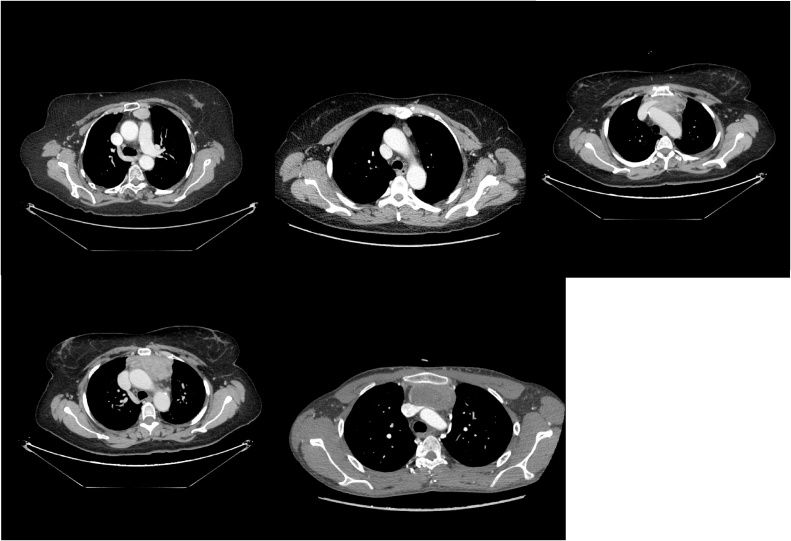
The standard thoracic X-ray revealed an enlargement of the mediastinal opacity (Fig. 1). Computed tomography (CT) is the standard diagnostic and preoperative staging modality in order to define preoperative treatment. CT, performed in all patients, has always consented to evaluate size, location and relationships of enlarged thymic mass. (Figs. 2, 3, 4, 5, 6 ). Intravenous contrast is essential in determining vascular anatomy and its relationship to the tumor. CT is important in predicting whether a tumor is easily resectable or not, and which structures may need to also be removed. CT is also useful for detecting recurrence after a previous resection. Histological examination revealed a thymoma in six patients (75%), 2 with Masaoka I stage (33%) and 4 with Masaoka II stage (66%). A thymic carcinomas was revealed in 2 patients (25%). Two of 6 patients with thymoma had myastenia gravis. One patient (12%) died within 30 days after surgery for respiratory failure. Complications are reported in Table 2 and were: pneumonia in 1 case (12%), atrial fibrillation and pleural effusion in 2 patients (25%). The hospitalization was, on average, 9.8 days, (5.4–19.0) with an intensive care unit (ICU) stay of 1.5 days. The duration of chest drainage and volume drained were 4.4 days, and 745.5 mL respectively. All patients still survive at 1, 2, and 5 years. One recurrence was observed at skeletal muscle 2-years after surgery in one patient with thymoma. The metastases were completely resected.
Fig. 1.
Standard thoracic X-ray: enlargement of the mediastinal opacity.
Figs. 2–6.
Thoracic sections of the CT scan showing different type of thymic masses and its relationship with surrounding structures.
Table 2.
Complications after surgical tratment.
| Sternotomy | |
|---|---|
| N. cases | 8 |
|
9.8 days |
|
2 (25%) |
|
1 (12%) |
|
1 (12%) |
|
2 (25%) |
|
2 (25%) |
4. Discussion
Thymoma, although is a rare disease, represents the most common primary mediastinal neoplasm in adults. Thymectomy is an important component in the treatment of early stage thymoma and anterior mediastinal tumors while stage and radical resection are considered as very important predictors of survival. Presence of myasthenia gravis is not an independent prognostic factor but may play a role in early detection of thymic tumors [22]. All thymomas are malignant tumors [23]. They are rare and usually have an indolent growth pattern rendering them to be confused with a benign growth. About one third are associated with myasthenia gravis and their resection significantly improves the symptoms of myasthenia gravis. Thymoma could be asymptomatic. About 40% of patients have local manifestations due to the compression of mediastinal structures (chest pain, cough and dyspnea) or to the neuromuscular effects of myasthenia gravis. Less commonly, superior vena cava syndrome and weight loss occurs in rapidly growing tumors [24]. About 40% of thymomas present with systemic syndromes (myasthenia gravis, pure red cell aplasias (PRCA), parathyroid adenoma, and hypogammaglobulinemia). About 20% of patients with myasthenia gravis presents thymomas [25].
In general, approximately 40% thymomas present at Stage I (2 cases in our study), 25% each at Stages II (4 cases in our study) and III, 10% at Stage IVa and 1%–2% at Stage IVb. Invasion into mediastinal tissue (Stages II, III) is present in 50% of thymomas, with pleural invasion being most common followed by pulmonary and pericardial invasion. Approximately 30% of these cases have involvement of innominate vein (1 case of our study) or superior vena cava and 20% have phrenic nerve involvement. The majority of thymic tumors have non-malignant appearing thymic epithelial cells mixed with variable proportions of lymphocytes. Despite his lack of malignant cytological features and their indolent behavior, thymomas have invasive and metastatic potential and should not be considered benign. Thoracic CT scan was used for detecting the presence and the extent of thymic mass and its relation to adjacent structures in the chest. A correlation between CT findings and histopathological staging system have been proposed; Stage I: intact capsule with clear space between tumor and fat tissues; stage II: blurred capsule with mediastinal fat of pleural involvement; stage III: pericardial, large blood vessel or lung tissue invasion; stage IV: pericardial, distant pleural or lymph node involvement or distant metastasis. In our series, CT of the chest was routinely performed for diagnosis and for the decision on which approach type of surgical intervention was mostly suited. The standard thoracic X-ray revealed an enlargement of the mediastinal opacity and, in some cases, a tracheal compression with lateral tracheal deviation (2 cases, 25%). Nuclear Magnetic Risonance (NMR) is useful to better define the relationship of continuity between the mediastinal component and with the vascular structures. Fine needle aspiration (FNA) was performed in 4 cases (only 2 resulted suspected for thymomas) and needle biopsy or open biopsy (anterior parasternal mediastinotomy, thoracoscopy) in 2 cases. Two cases presented with myiastenia gravis symptoms and had no histopathologic examination.
Tracheobroncoscopy should be performed in presence of clinical signs of an upper airway obstruction (wheezing and stridor), dyspnea, dysphonia and tracheal profile modifications. It becomes necessary for suspected malignancy of the thymic mass to detect any irregularities in tracheal mucosa or neoplastic infiltration of the wall, and in cases of an important tracheal compression.
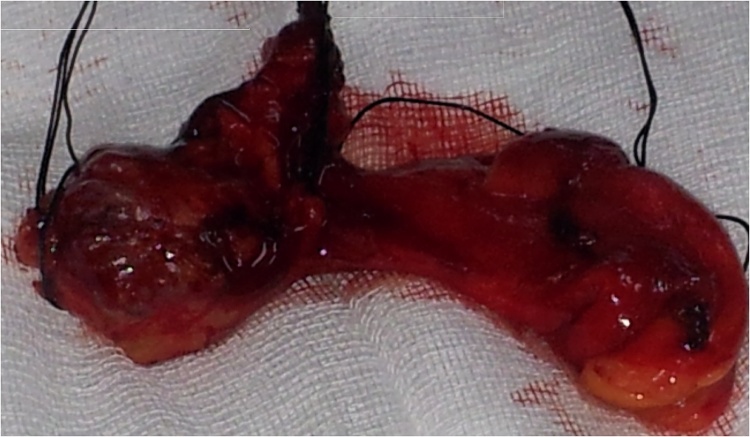
Surgical resection is the mainstay of treatment, but a multimodal approach is useful for many patients. En-bloc resection of the entire thymus gland and surrounding areolar tissue in the pre-vascular plane of the anterior mediastinum between the two phrenic nerves is the most important factor in controlling myasthenia and influencing survival in patients with thymoma. Open (median sternotomy) approach offers better visualization of the thymic tissue and has been the standard approach for thymectomy. We performed a complete surgical resection with median sternotomy because 6 cases were thymomas diagnosed before 2014, while two thymic carcinomas measured over 5 cm and one of them involved the innominate vein. En-bloc resection of the entire thymus gland and surrounding areolar tissue is performed in most centers today. A complete resection has been observed as one of major prognostic factor in the treatment of thymomas of every stage. The thymus is completely excised including all 4 lobes (Fig. 7). All pericardial fat should be removed except a small amount around the phrenic nerves. Any adherent structure is should be resected en bloc.
Fig. 7.
Surgical specimen after en-bloc resection of the entire thymus gland and surrounding areolar tissue.
While median sternotomy has long been the accepted standard approach, minimally invasive methods have emerged over recent decades including transcervical, video-assisted thoracoscopic (VATS), and robotic video-assisted thoracoscopic (R-VATS) approaches [2], [27], [28], [29], [30], [31], [32], [33], [34]. VATS approaches include bilateral and unilateral VATS (either left or right). According to some authors, the left side is safer with a more suitable dissection of all the soft fatty tissue around the peri-cardiophrenic angle because the superior vena cava lies outside the surgical field.
The right side is preferred by other surgeons because they clearly identified and used the superior vena cava as a landmark to dissect around the innominate veins. In the bilateral VATS approach, some authors report a more suitable complete excision for a better visualization of anatomical structures. Several studies have demonstrated the superiority of the VATS approach over sternotomy such as a smaller incisions with better cosmesis and less trauma to the chest wall, a decreased post-operative stay, a complete remission and no difference in outcome as compared to the open approach allowing an early adjuvant chemo-radiation treatment in advanced cases. However, most of the authors use the VATS approach when the tumor diameter is less than 5 cm or 6 cm, not involving the innominate vein, with little or no evidence of invasion or compression of major mediastinal structures [32], [35], [36], [37], [38], [39], [40], [41], [42]. Furthermore, VATS is considered a better approach for less intra-operative blood loss and early removal of chest drains. Regarding to complications, pneumonia was observed more frequently in the open approach (4.1% vs.1.9%), while in VATS is reported a higher rate of phrenic nerve injury (6.7% vs. 0%). Therefore, in the surgical treatment of thymomas, VATS thymectomy is considered as a progressively suitable and efficacious alternative to open surgery. The da Vinci system robotic video-assisted thoracoscopic approach (R-VATS) is another minimally invasive surgical approach to thymectomy with potentially less morbidity [43], [44], [45], [46], [47], [48], [49], [50], [51]. This robotic approach has outcomes comparable to conventional video-assisted techniques. Robotic assisted mini-invasive technique may lead to a safer and effective strategies for radical thymic resection, by a better visualization and instrument control in comparison with non-robotic mini-invasive technique approaches.
Conflicts of interest
All authors have no conflict of interests.
Funding
All authors have no source of funding.
Ethical approval
Ethical approval was requested and obtained from the “Azienda Ospedaliera Universitaria”San Giovanni di Dio e Ruggi d'Aragona” ethical committee.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contribution
Vincenzo Di Crescenzo: Participated substantially in conception, design, and execution of the study and in the analysis and interpretation of data; also participated substantially in the drafting and editing of the manuscript.
Filomena Napolitano: Participated substantially in conception, design, and execution of the study and in the analysis and interpretation of data.
Claudio Panico: Participated substantially in conception, design, and execution of the study and in the analysis and interpretation of data.
Rosa Maria Di Crescenzo: Participated substantially in conception, design, and execution of the study and in the analysis and interpretation of data.
Pio Zeppa: Participated substantially in conception, design, and execution of the study and in the analysis and interpretation of data.
Alessandro Vatrella: Participated substantially in conception, design, and execution of the study and in the analysis and interpretation of data.
Paolo Laperuta: Participated substantially in conception, design, and execution of the study and in the analysis and interpretation of data; also participated substantially in the drafting and editing of the manuscript.
Guarantor
GUARANTOR: Vincenzo Di Crescenzo.
Authors’ information
VDC: Associate Professor of Thoracic Surgery at University of Salerno.
FN: Assistant Surgeon of Thoracic Surgery at Azienda Ospedaliera Universtaria, San Giovanni di Dio e Ruggi d’Aragona, Salerno.
CP: Assistant Surgeon of Thoracic Surgery at Azienda Ospedaliera Universtaria, San Giovanni di Dio e Ruggi d’Aragona, Salerno.
RMDC: Resident Doctor of Pathology at Federico II University of Naples.
PZ: Associate Professor of Pathology at University of Salerno.
AV: Associate Professor of Section of Respiratory Diseases, University of Salerno
PL: Assistant Surgeon of Thoracic Surgery at Azienda Ospedaliera Universtaria, San Giovanni di Dio e Ruggi d’Aragona, Salerno.
Contributor Information
Vincenzo Giuseppe Di Crescenzo, Email: vdicrescenzo@unisa.it.
Filomena Napolitano, Email: milena.napolitano@yahoo.it.
Claudio Panico, Email: panico@sangiovannieruggi.it.
Rosa Maria Di Crescenzo, Email: rose.dicrescenzo@libero.it.
Pio Zeppa, Email: pzeppa@unisa.it.
Alessandro Vatrella, Email: avatrella@unisa.it.
Paolo Laperuta, Email: laper@libero.it.
References
- 1.Davenport E., Malthaner R.A. The role of surgery in the management of thymoma: a systematic review. Ann. Thorac. Surg. 2008;86:673–684. doi: 10.1016/j.athoracsur.2008.03.055. [DOI] [PubMed] [Google Scholar]
- 2.Zahid I., Sharif S., Routledge T. Video-assisted thoracoscopic surgery or transsternal thymectomy in the treatment of myasthenia gravis? Interact. Cardiovasc. Thorac. Surg. 2011;12:40–46. doi: 10.1510/icvts.2010.251041. [DOI] [PubMed] [Google Scholar]
- 3.Kondo K. Therapy for thymic epithelial tumors. Gen. Thorac. Cardiovasc. Surg. 2014;62:468–474. doi: 10.1007/s11748-014-0420-z. [DOI] [PubMed] [Google Scholar]
- 4.Jaretzki A., 3rd Thymectomy for myasthenia gravis: analysis of controversies patient management. Neurologist. 2003;9:77–92. doi: 10.1097/01.nrl.0000051446.03160.2e. [DOI] [PubMed] [Google Scholar]
- 5.Cooper J.D., Al-Jilaihawa A.N., Pearson F.G. An improved technique to facilitate transcervical thymectomy for myasthenia gravis. Ann. Thorac. Surg. 1988;45:242–247. doi: 10.1016/s0003-4975(10)62457-5. [DOI] [PubMed] [Google Scholar]
- 6.Keating C.P., Kong Y.X., Tay V. VATS thymectomy for nonthymomatous myasthenia gravis: standardized outcome assessment using the myasthenia gravis foundation of America clinical classification. Innovations (Phila) 2011;6:104–109. doi: 10.1097/IMI.0b013e3182165cdb. [DOI] [PubMed] [Google Scholar]
- 7.Rea F., Marulli G., Bortolotti L. Experience with the da Vinci robotic system for thymectomy in patients with myasthenia gravis: report of 33 cases. Ann. Thorac. Surg. 2006;81:455–459. doi: 10.1016/j.athoracsur.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 8.Keijzers M., de Baets M., Hochstenbag M. Robotic thymectomy in patients with myasthenia gravis: neurological and surgical outcomes. Eur. J. Cardiothorac. Surg. 2015;48:40–45. doi: 10.1093/ejcts/ezu352. [DOI] [PubMed] [Google Scholar]
- 9.Detterbeck F.C. Evaluation and treatment of stage I and II thymoma. J. Thorac. Oncol. 2010;5:318–322. doi: 10.1097/JTO.0b013e3181f20dab. [DOI] [PubMed] [Google Scholar]
- 10.Pennathur A., Qureshi I., Schuchert M.J. Comparison of surgical techniques for early-stage thymoma: feasibility of minimally invasive thymectomy and comparison with open resection. J. Thorac. Cardiovasc. Surg. 2011;141:694–701. doi: 10.1016/j.jtcvs.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Mao Z.F., Mo X.A., Qin C. Incidence of thymoma in myasthenia gravis: a systematic review. J. Clin. Neurol. 2012;8:161–169. doi: 10.3988/jcn.2012.8.3.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levine G.D., Rosai J. Thymic hyperplasia and neoplasia: a review of current concepts. Hum. Pathol. 1978:495–515. doi: 10.1016/s0046-8177(78)80131-2. [DOI] [PubMed] [Google Scholar]
- 13.Mayer R., Beham-Schmid C., Groell R. Radiotherapy for invasive thymoma and thymic carcinoma. Clinicopathological review. Strahlenther. Onkol. 1999;175:271–278. doi: 10.1007/BF02743578. [DOI] [PubMed] [Google Scholar]
- 14.Detterbeck F.C. Clinical value of the WHO classification system of thymoma. Ann. Thorac. Surg. 2006;81:2328–2334. doi: 10.1016/j.athoracsur.2005.11.067. [DOI] [PubMed] [Google Scholar]
- 15.Sellke F.W., del Nido P.J., Swanson S.J. 8th ed. Elsevier; Philadelphia: 2010. Sabiston and Spencer’s Surgery of the Chest; p. 2520. [Google Scholar]
- 16.Masaoka A., Monden Y., Nakahara K. Follow-up study of thymomas with special reference to their clinical stages. Cancer. 1981;48:2485–2492. doi: 10.1002/1097-0142(19811201)48:11<2485::aid-cncr2820481123>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 17.Detterbeck F.C., Parsons A.M. Management of stage I and II thymoma. Thorac. Surg. Clin. 2011;21:59–67. doi: 10.1016/j.thorsurg.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Verley J.M., Hollmann K.H. Thymoma. A comparative study of clinical stages, histologic features, and survival in 200 cases. Cancer. 1985;55:1074–1086. doi: 10.1002/1097-0142(19850301)55:5<1074::aid-cncr2820550524>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 19.Lewis J.E., Wick M.R., Scheithauer B.W., Bernatz P.E., Taylor W.F. Thymoma, a clinicopathologic review. Cancer. 1987;60:2727–2743. doi: 10.1002/1097-0142(19871201)60:11<2727::aid-cncr2820601125>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 20.Qu Y.J., Liu G.B., Shi H.S., Liao M.Y., Yang G.F., Tian Z.X. Preoperative CT findings of thymoma are correlated with postoperative Masaoka clinical stage. Acad. Radiol. 2013;20:66–72. doi: 10.1016/j.acra.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 21.Tomiyama N.T., Johkoh T., Mihara N., Honda O., Kozuka T., Koyama M. Using the World Health Organization Classification of thymic epithelial neoplasms to describe CT findings. AJR. 2002;179:881–886. doi: 10.2214/ajr.179.4.1790881. [DOI] [PubMed] [Google Scholar]
- 22.Detterbeck F., Youssef S., Ruffini E., Okumura M. A review of prognostic factors in thymic malignancies. J. Thorac. Oncol. 2011;6:S1698–S1704. doi: 10.1097/JTO.0b013e31821e7b12. [DOI] [PubMed] [Google Scholar]
- 23.Detterbeck F., Parsons A. Thymic tumors: a review of current diagnosis, classification, and treatment. In: Patterson J., Lerut J.D., Luketich T.W., Rice F.G., editors. Thoracic and Esophageal Surgery. 3rd ed. Elsevier; Philadelphia: 2008. pp. 1589–1614. [Google Scholar]
- 24.Detterbeck F.C., Zeeshan A. Thymoma: current diagnosis and treatment. Chin. Med. J. (Engl) 2013;126:2186–2191. (Review) [PubMed] [Google Scholar]
- 25.Di Crescenzo V., Laperuta P., Garzi A., Napolitano F., Cascone A., Vatrella A. Small cell lung cancer associated with solitary fibrous tumors of the pleura: a case study and literature review. Int. J. Surg. 2014;12(Suppl 1):S19–21. doi: 10.1016/j.ijsu.2014.05.032. [DOI] [PubMed] [Google Scholar]
- 26.Souadjian J.V., Enriquez P., Silverstein M.N., Pépin J.M. The spectrum of diseases associated with thymoma: coincidence or syndrome? Arch. Intern. Med. 1974;134:374–379. [PubMed] [Google Scholar]
- 27.Laperuta P., Napolitano F., Garzi A., Amato B., Vatrella A., Di Crescenzo V. Extrathoracic recurrence of type A thymoma. Int. J. Surg. 2014;12(Suppl 1):S16–S18. doi: 10.1016/j.ijsu.2014.05.043. [DOI] [PubMed] [Google Scholar]
- 28.Raza A., Woo E. Video-assisted thoracoscopic surgery versus sternotomy in thymectomy for thymoma and myasthenia gravis. Ann. Cardiothorac. Surg. 2016;5(1):33–37. doi: 10.3978/j.issn.2225-319X.2015.10.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hess N.R., Sarkaria I.S., Pennathur A., Levy R.M., Christie N.A., Luketich J.D. Minimally invasive versus open thymectomy: a systematic review of surgical techniques, patient demographics, and perioperative outcomes. Ann Cardiothorac Surg. 2016;5(1):1–9. doi: 10.3978/j.issn.2225-319X.2016.01.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Crescenzo V., Laperuta P., Napolitano F., Carlomagno C., Danzi M., Amato B., Garzi A., Vitale M. Unusual case of exacerbation of sub-acute descending necrotizing mediastinitis. BMC Surg. 2013;13(Suppl 2):S31. doi: 10.1186/1471-2482-13-S2-S31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rowse P.G., Roden A.C., Corl F.M., Allen M.S., Cassivi S.D., Nichols F.C., Shen K.R., Wigle D.A., Blackmon S.H. Minimally invasive thymectomy: the Mayo Clinic experience. Ann. Cardiothorac. Surg. 2015;4(November (6)):519–526. doi: 10.3978/j.issn.2225-319X.2015.07.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caleo A., Vigliar E., Vitale M., Di Crescenzo V., Cinelli M., Carlomagno C., Garzi A., Zeppa P. Cytological diagnosis of thyroid nodules in Hashimoto thyroiditis in elderly patients. BMC Surg. 2013;13(Suppl 2):S41. doi: 10.1186/1471-2482-13-S2-S41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guerra A., Carrano M., Angrisani E., Puzziello A., Izzo G., Di Crescenzo V., Vatrella A., Vitale M. Detection of RAS mutation by pyrosequencing in thyroid cytology samples. Int. J. Surg. 2014:91–94. doi: 10.1016/j.ijsu.2014.05.045. [DOI] [PubMed] [Google Scholar]
- 34.Fiorelli A., Mazzella A., Cascone R., Caronia F.P., Arrigo E., Santini M. Bilateral thoracoscopic extended thymectomy versus sternotomy. Asian Cardiovasc Thorac. Ann. 2016;24(July (6)):555–561. doi: 10.1177/0218492316647215. [DOI] [PubMed] [Google Scholar]
- 35.Guerra A., Di Crescenzo V., Garzi A., Cinelli M., Carlomagno C., Pepe S., Zeppa P., Tonacchera M., Vitale M. Diagnostic utility of BRAFV600E mutation testing in thyroid nodules in elderly patients. BMC Surg. 2013;13(Suppl 2):S37. doi: 10.1186/1471-2482-13-S2-S37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guerra A., Di Crescenzo V., Garzi A., Cinelli M., Carlomagno C., Tonacchera M., Zeppa P., Vitale M. Genetic mutations in the treatment of anaplastic thyroid cancer: a systematic review. BMC Surg. 2013;13(Suppl 2):S44. doi: 10.1186/1471-2482-13-S2-S44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laperuta P., Napolitano F., Vatrella A., Di Crescenzo R.M., Cortese A., Di Crescenzo V. Post-pneumonectomy broncho-pleural fistula successfully closed by open-window thoracostomy associated with V.A.C therapy. Int. J. Surg. 2014;12(Suppl 2):S17–19. doi: 10.1016/j.ijsu.2014.08.390. [DOI] [PubMed] [Google Scholar]
- 38.Baldi A., Mottolese M., Vincenzi B., Campioni M., Mellone P., Di Marino M., Di Crescenzo V.G., Visca P., Menegozzo S., Spugnini E.P., Citro G., Ceribelli A., Mirri A., Chien J., Shridhar V., Ehrmann M., Santini M., Facciolo F. The serine protease HtrA1 is a novel prognostic factor for human mesothelioma. Pharmacogenomics. 2008;9(August (8)):1069–1077. doi: 10.2217/14622416.9.8.1069. [DOI] [PubMed] [Google Scholar]
- 39.Santini M., Fiorelli A., Messina G., Laperuta P., Mazzella A., Accardo M. Use of the LigaSure device and the Stapler for closure of the small bowel: a comparative ex vivo study. Surg. Today. 2013;43(7):787–793. doi: 10.1007/s00595-012-0336-0. [DOI] [PubMed] [Google Scholar]
- 40.Santini M., Fiorello A., Vicidomini G., Di Crescenzo V.G., Laperuta P. Role of diffusing capacity in predicting complications after lung resection for cancer. Thorac. Cardiovasc. Surg. 2007;55(September (6)):391–394. doi: 10.1055/s-2007-965326. [DOI] [PubMed] [Google Scholar]
- 41.Di Crescenzo V., Laperuta P., Napolitano F., Carlomagno C., Garzi A., Vitale M. Pulmonary sequestration presented as massive left hemothorax and associated with primary lung sarcoma. BMC Surg. 2013:1–3. doi: 10.1186/1471-2482-13-S2-S34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fiorelli A., Morgillo F., Fasano M., Vicidomini G., Di Crescenzo V.G., Di Domenico M., Accardo M., Santini M. The value of matrix metalloproteinase 9 and vascular endothelial growth factor receptor 1 pathway in diagnosing indeterminate pleural. Interact. Cardiovasc. Thorac. Surg. 2012:1–7. doi: 10.1093/icvts/ivs466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Di Crescenzo V., Laperuta P., Napolitano F., Carlomagno C., Garzi A., Vitale M. An unusual case of primary choriocarcinoma of the lung. BMC Surg. 2013:1–3. doi: 10.1186/1471-2482-13-S2-S33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Santini M., Fiorello A., Di Crescenzo V.G., Vicidomini G., Busiello L., Laperuta P. Use of unidirectional endobronchial valves for the treatment of giant emphysematous bulla. J. Thorac. Cardiovasc. Surg. 2010;139(January (1)):224–226. doi: 10.1016/j.jtcvs.2008.05.069. [DOI] [PubMed] [Google Scholar]
- 45.Fiorelli S., Di Crescenzo G., Vicidomini S., Del Prete A., Santini M. A simple technique to facilitate dumon silicone stent placement in subglottic tracheal stenosis. Interact. Cardiovasc. Thorac. Surg. 2014;18(March (3)):390–392. doi: 10.1093/icvts/ivt504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Santini M., Fiorelli A., Vicidomini G., Laperuta P., Di Crescenzo V.G. Iatrogenic air leak successfully treated by bronchoscopic placement of unidirectional endobronchial valves. Ann. Thorac. Surg. 2010;89(June (6)):2007–2010. doi: 10.1016/j.athoracsur.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 47.Di Crescenzo V., Vitale M., Valvano L., Napolitano F., Vatrella A., Zeppa P., De Rosa G., Amato B., Laperuta P. Surgical management of cervico-mediastinal goiters: our experience and review of the literature. Int. J. Surg. 2016;28(Suppl 1 (April)):47–53. doi: 10.1016/j.ijsu.2015.12.048. [DOI] [PubMed] [Google Scholar]
- 48.Di Crescenzo V., Laperuta P., Napolitano F., Carlomagno C., Danzi M., Amato B., Garzi A., Vitale A. Migration of surgical clips through a right lobectomy stump mimicking an asthmatic syndrome. BMC Surg. 2013:1–3. doi: 10.1186/1471-2482-13-S2-S32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Santini M., Fiorello A., Di Lieto E., Di Crescenzo V.G., D’aniello G., Vicidomini G., Perrone A., Pastore V. Surgical strategies in cervico-mediastinal goiters. Minerva Chir. 2006;61:221–229. [PubMed] [Google Scholar]
- 50.Conzo G., Avenia N., Bellastella G., Candela G., de Bellis A., Esposito K., Pasquali D., Polistena A., Santini L., Sinisi A.A. The role of surgery in the current management of differentiated thyroid cancer. Endocrine. 2014;47:380–388. doi: 10.1007/s12020-014-0251-9. [DOI] [PubMed] [Google Scholar]
- 51.Conzo G., Perna A.F., Savica V., Palazzo A., DellaPietra C., Ingrosso D., Satta E., Capasso G., Santini L., Docimo G. Impact of parathyroidectomy on cardiovascular outcomes and serviva in chronic hemodialysis patients with secondary hyperparathyroidism. A retrospective study of 50 cases prior to calcimimetics era. BMC Surg. 2013;13(Suppl 2):1–8. doi: 10.1186/1471-2482-13-S2-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Agha R.A., Fowler A.J., Saetta A., Barai I., Rajmohan S., Orgill D.P., the SCARE Group The SCARE Statement: consensus-based surgical case report guidelines. Int. J. Surg. 2016;34:180–186. doi: 10.1016/j.ijsu.2016.08.014. [DOI] [PubMed] [Google Scholar]