Abstract
Introduction
In the CAMERA population-based MRI study, migraineurs below the age of 50 had decreased T2-values indicative of increased iron deposition in several deep brain nuclei. Longer migraine history was associated with lower T2-values, suggesting an association between migraine attacks and iron accumulation. In the present nine-year follow-up study of the CAMERA cohort we re-measured the T2-values in deep brain nuclei to assess the evolution over time.
Methods
Baseline and follow-up T2-values measured in several basal ganglia of 128 participants (38 control, 90 migraine) were analyzed using quantitative T2 measurements and multivariate regression analysis.
Results
T2-values of most deep brain nuclei were increased - instead of an expected further decrease when only age related iron accumulation would have played a role - compared to baseline (both among controls and migraineurs) and were not different in both groups. In migraineurs, no differences were found by gender, migraine severity or subtype.
Conclusion
This study did not provide supportive data for migraine related increased iron accumulation in deep brain nuclei, but neither is able to reject such hypotheses. Increased T2-values probably point at microstructural tissue changes, that counter-acted earlier accumulated iron effects. We hypothesize that with aging migraine-induced iron-related brain changes are obscured by age-related other tissue changes.
Keywords: Migraine, T2-value, iron, brain, CAMERA
Introduction
In normal aging, iron accumulates throughout the brain, particularly in the basal ganglia. Iron deposits are visible as diffuse hypo-intense changes in deep brain structures on T2-weighted and T2*-weighted MR images.(1, 2) Specific neurodegenerative diseases (eg. pantothenate kinase associated neurodegeneration, but also Parkinson’s and Huntington’s disease) are associated with increased iron accumulation in specific brain regions. In migraineurs, lower T2-values were also found in deep brain nuclei and the periaqueductal gray matter.(3–6) In the population-based CAMERA MRI-study, we reported evidence of increased iron accumulation in the putamen, globus pallidus, and nucleus ruber of migraineurs. (5) An inverse relationship with attack frequency and a migraine history was found, suggestive of a causal relation between recurring attacks and accumulation of iron. Because of the cross-sectional design of the study at that time, we could not verify this hypothesis.
To assess whether iron accumulation is indeed associated with recurring migraine attacks, we measured T2 values in the CAMERA-2 study which is a prospective, nine-year follow-up of the original CAMERA cohort.(7)
Methods
Study population
In the CAMERA-study we had measured brain iron in 213 participants (138 migraineurs and 75 matched controls). Clinical characteristics have been described elsewhere.(5) In short, participants (mean age 49; 69% female) from the general population were identified as migraineurs or non-migraine controls according to the International Headache Society criteria.(8) Both groups had comparable cardiovascular risk profiles. None of the patients or controls showed clinical signs of basal ganglia dysfunction at a standard physical and neurological examination.
Magnetic Resonance Imaging
To guarantee methodological comparability with the original CAMERA study nine-years earlier, we used the same scanner and scan protocol.(5) Whole brain MR images were acquired on a 1.5T scanner in Maastricht (ACS-NT; Philips Medical Systems, Best, The Netherlands). Images were acquired with 48 contiguous 3-mm axial slices (field of view 22 cm; matrix, 190–205 × 256). Pulse sequences included a combined proton density and T2-weighted fast spin-echo (repetition time/echo time, 3000/27–120) and fluid-attenuated inversion-recovery (FLAIR; repetition time/echo time/inversion time 8000/100/2000).
Quantitative T2 measurements were carried out in exactly the same way during baseline and follow-up, by applying the same post-processing steps as described before.(5) In short, the observer who was blinded for diagnosis and patient characteristics, measured signal intensities (SIs) of six regions of interest, bilaterally. Regions of interest were the same as in the baseline study: putamen, putamen posterior, nucleus caudatus, globus pallidus, substantia nigra (pars reticularis and pars compacta), and nucleus ruber. T2 values were estimated using the expression T2 = (TE2 − TE1)/[ln (S1/S2)], where S1 and S2 are the measured signal intensities in the early-echo (= proton density, TE1=27 ms) and late-echo (= T2, TE2=120 ms) respectively.
The protocol was approved by the local Medical Ethics Committees and all participants gave written informed consent.
Statistics
To test for any differences in the distributions and means of measured characteristics among the study groups, χ2 tests and unpaired t tests were used. Linear regression analyses (controlled for age) were used to test for differences in the measured T2 values. Statistical tests were not corrected for comparison of multiple nuclei. T2 values of deep nuclei are greatly influenced by non-iron related changes after about 50 years of age. Since baseline analyses had only shown T2 differences between migraineurs and controls younger than 50 years old, we analyzed the follow-up results also in stratified subgroups younger and older than 50 years.
Results
Study population
In the follow-up CAMERA MRI study, we measured T2 values in 128 of the 213 baseline participants (60%; 38 controls, 90 migraineurs; Table 1). Clinical characteristics of migraineurs and controls were similar (all p>0.05; Table 1). Participant characteristics were not significantly different between participants (n=128) and non-participants (n=85) (all clinical characteristics p>0.05). Only data from participants with follow-up MRI available were included in analyses. Reasons for non-participation in follow-up were: lack of interest (n=33), non-neurological co-morbidity (n=20), participation in CAMERA-2, but no rescan (n=22), unable to establish contact before the end of inclusion (n=4), exclusion (n=5), and deceased (n=1). Mean baseline T2-values of non-participants were significantly lower than of participants but this effect was similar for migraineurs and controls. Clinical characteristics of the non-participants at the time of follow-up was not available.
Table 1.
Characteristics of study participants
| Characteristic | Control (n=38) | Migraine (n=90) |
|---|---|---|
| Age, mean (SE), yrs | 55 (1.3) | 58 (0.8) |
| Female | 68% | 67% |
| Body mass index, mean (SE) | 26 (0.6) | 26 (0.5) |
| Hypertension (self-reported, doctor-diagnosed) | 29% | 34% |
| Diabetes (self-reported) | 2.6% | 7.8% |
| Smoking, ever | 68% | 70% |
| Smoking, pack-years, mean (SE) | 25 (3.5) | 17 (2.0) |
| Duration migraine history, yrs (SE) | ||
| Until baseline study | - | 23.7 (1.2) |
| Until follow-up study | - | 30.1 (1.4) |
| Estimated total number of migraine attacks (SE) | ||
| Until baseline study | - | 358 (40) |
| Until follow-up study | - | 508 (61) |
Differences between controls and migraineurs were not significant (p>0.05)
T2-values
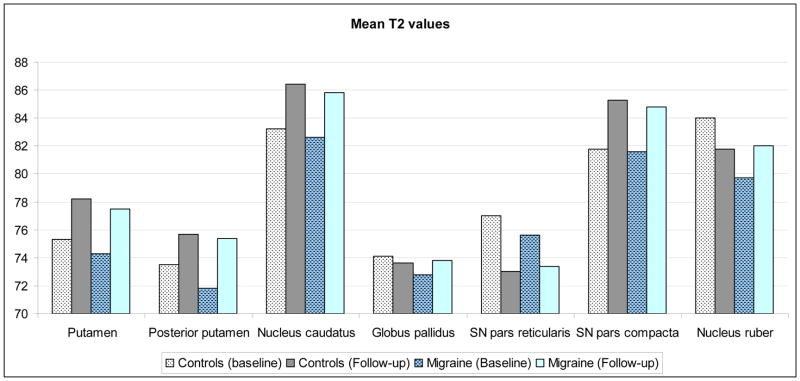
Mean T2-values of the putamen, posterior putamen, nucleus caudatus, and substantia nigra pars compacta were increased compared to baseline in both control and migraine groups (p<0.001; Figure 1; Table 2). T2-values of the substantia nigra pars reticularis showed a non-significant decrease in controls (95% CI [−8.0 – 0.0];p=0.05) and migraineurs (95% CI [−4.6 – 0.3];p=0.08). T2-values of the globus pallidus remained the same over time for both groups (p>0.1). The nucleus ruber showed a different pattern: in controls mean T2-value decreased over time whereas it increased in migraineurs, resulting in a similar T2-value at follow-up for migraineurs and controls. Areas of the brain that were evaluated by T2-values measurement did not demonstrate focal T2 hyperintense lesions or infarcts.
Figure 1.
T2 values (ms) in controls and migraineurs
Table 2.
Difference between baseline and follow-up T2-values (ms) in migraineurs and controls
| Putamen | Putamen Posterior | Nucleus Caudatus | Globus Pallidus | Substantia Nigra (pars reticularis) | Substantia Nigra (pars compacta) | Nucleus Ruber | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CO | MIG | CO | MIG | CO | MIG | CO | MIG | CO | MIG | CO | MIG | CO | MIG | |
| T2-value Baseline Follow-up | 75.3 | 74.3 | 73.5 | 71.8 | 83.2 | 82.5 | 74.1 | 72.9 | 77.0 | 75.5 | 81.8 | 81.6 | 84.0 | 79.7 |
| 78.2 | 77.5 | 75.7 | 75.4 | 86.4 | 85.8 | 73.6 | 73.8 | 73.0 | 73.4 | 85.3 | 84.8 | 81.8 | 82.0 | |
| [95% CI];p-valueBaseline vs. follow-up (adjusted for age) | [2.0–3.8] | [2.3–4.1] | [1.1–3.4] | [2.3–4.8] | [2.1–4.3] | [2.4–4.1] | [−2.1–2.0] | [−0.3–2.0] | [−8.0–0.0] | [−4.6–0.3] | [1.8–5.3] | [2.0–4.4] | [−6.7–2.4] | [0.0–4.6] |
| < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | 0.6 | 0.1 | 0.05 | 0.08 | < 0.001 | < 0.001 | 0.3 | 0.05 | |
CO=controls; MIG=migraineurs
Thus, the changes over the years resulted in similar basal ganglia T2-values at follow-up in migraineurs and controls. Cross sectional analyses showed no differences between groups at follow-up (Table 3). No differences were found between controls and subgroups of migraine with and without aura, nor between men and women (p>0.05). Likewise, no differences were found when analyzing only the group younger than 50 years at baseline (Table 4). Baseline T2-values of followed-up migraine participants were still lower than baseline T2-values of follow-up control participants for putamen (95% CI [−2.0 – −0.1];p=0.02), posterior putamen (95% CI [−3.1 – −0.7];p=0.02), and nucleus ruber (95% CI [−10.0 – −1.3];p=0.01)
Table 3.
Changes of T2 values (SD, in ms) after 9 years follow-up
| Change of T2 value in: | Control (n=38) | Migraine (n=90) | 95% CI | p-value (age adjusted) |
|---|---|---|---|---|
| Putamen | 2.9 (1.6) | 3.2 (1.9) | −0.6 – 0.8 | 0.8 |
| Putamen posterior | 2.2 (3.0) | 3.6 (3.9) | −0.2 – 2.7 | 0.09 |
| Nucleus caudatus | 3.2 (2.1) | 3.2 (2.2) | −0.9 – 0.7 | 0.7 |
| Globus pallidus | −0.4 (4.9) | 0.9 (4.5) | −0.6 – 3.0 | 0.2 |
| Substantia nigra pars reticularis | −4.0 (12.2) | −2.0 (10.5) | −2.0 – 6.6 | 0.3 |
| Substantia nigra pars compacta | 3.5 (5.5) | 3.2 (4.6) | −2.5 – 1.3 | 0.5 |
| Nucleus ruber | −2.3 (13.7) | 2.3 (10.8) | 0.9 – 10.0 | 0.02 |
Negative numbers indicate a decrease in T2-value between baseline and follow-up
Table 4.
T2 values (SD, in ms) after 9 years follow-up
| Participants who were younger than 50 yrs at baseline | ||||||
|---|---|---|---|---|---|---|
| Mean T2 value of: | Control (n=38) | Migraine (n=90) | [95% CI];p-value (age adjusted) | Control (n=29) | Migraine (n=45) | [95% CI];p-value (age adjusted) |
| Putamen | 78.2 (2.2) | 77.5 (3.5) | [−2.2–0.2];0.1 | 77.7 (2.0) | 77.0 (2.8) | [−2.0–0.5];0.2 |
| Putamen posterior | 75.7 (2.5) | 75.4 (5.2) | [−2.5–1.0];0.4 | 75.4 (2.4) | 74.5 (3.8) | [−2.4–0.7];0.3 |
| Nucleus caudatus | 86.4 (2.4) | 85.8 (3.0) | [−1.7–0.5];0.3 | 86.5 (2.3) | 85.9 (2.7) | [−1.9–0.6];0.3 |
| Globus pallidus | 73.6 (3.0) | 73.8 (3.5) | [−1.5–1.1];0.8 | 73.5 (2.3) | 73.3 (2.4) | [−1.3–1.0];0.8 |
| Substantia nigra pars reticularis | 73.0 (3.3) | 73.4 (4.2) | [−1.3–1.8];0.7 | 73.2 (3.6) | 73.1 (4.4) | [−2.0–1.9];0.9 |
| Substantia nigra pars compacta | 85.3 (2.8) | 84.8 (4.0) | [−2.2–0.5];0.2 | 85.4 (2.9) | 84.0 (4.1) | [−3.2–0.3];0.1 |
| Nucleus ruber | 81.8 (4.0) | 82.0 (4.0) | [−1.9–1.0];0.5 | 80.8 (3.6) | 81.0 (3.2) | [−1.3–1.8];0.8 |
T2-values and migraine severity
Neither T2-values at follow up nor the change in T2-values over the years correlated with the estimated number of migraine attacks suffered between baseline and follow up (p-values between 0.1–0.9). Migraine activity (defined as having had at least one attack during follow-up) was also not related to (change in) T2-values. Finally, migraine duration (years of migraine) was not associated with T2-values at follow up.
Discussion
In contrast to the CAMERA-1 study, we failed to find differences in basal ganglia T2-values between migraineurs and controls in more-or-less the same study population nine years later. This was true for both the whole population and those under age 50.
We originally had hypothesized that iron accumulation was due either to disruptive iron homeostasis in dysfunctioning neurons or to repeated activation and hyperemia of nuclei associated with pain processing during migraine attacks. Although the current negative follow-up data do not support these hypotheses, they also not necessarily reject them.
In general, in the absence of focal visible lesions, increases in T2-values can be histologically explained by cellular and axonal loss, presence of gliosis, or microinfarction.(9–11) As once accumulated iron does not disappear from the brain, such T2-increasing changes in parenchymal microstructure probably have counter-acted and overcome T2-lowering effects of earlier accumulated iron(12, 13) The disappearance of the earlier differences between migraineurs and controls might further suggest that diffuse T2-increasing changes over time are more progressive in migraineurs. Although the current data cannot prove this hypothesis, such observation would be in line with the reported progression of diffuse T2 hyperintensities in the brainstem in migraineurs.(7) A technological explanation seems unlikely, since we used the same scanner, scanning protocol and post-processing steps.
The nucleus ruber showed a pattern, different from the other nuclei: mean T2-value in the control group decreased, whereas mean T2-value in the migraine group increased over the years. The reason for this is unknown. The migraineurs of the current cohort did not show visible hyperintense lesions in the nucleus ruber, which could have been a plausible cause for the increase in T2-value. Possibly, a migraine-specific process leading to increases in T2-signal plays a role, as the nucleus ruber is known to be involved in nociception. (14) This interesting finding calls for further research on the role of the nucleus ruber in migraine.
Mean age of the cohort was 57 years at follow up which makes measurement of T2-values more susceptible to other age related effects rather than iron related factors. However, both study groups (migraineurs and controls) aged by the same amount of years.
A potential limitation of the present study is that not all participants at baseline also participated at follow-up, limiting the statistical power of the present study and potentially introducing selection bias. Although the clinical characteristics of participants and non-participants were similar, T2-values at baseline were lower in the migraine and control non-participants, probably resulting in an overall higher T2-value among the participant group at follow-up. It has to be noted that T2-weighted images are less sensitive to iron accumulation compared to T2*-weighted images. Unfortunately, T2*-weighted images were not part of the baseline and follow-up scanning protocol.
Major advantages of the present study are the population-based design with detailed clinical information allowing for sub-group analyses, and the large number of unbiased MR measurements at baseline and follow-up for which, importantly, we used exactly the same protocols and procedures to ensure technical comparability over time.
To explain the disappearance over time of the difference in T2 values between migraineurs and controls, we hypothesize that age-related signal increases have counteracted the iron-related signal decreases. The current findings, therefore, are not necessarily in conflict with previous findings.
Key Findings.
Baseline CAMERA study reported evidence of increased iron accumulation (i.e. lower T2-values) in deep brain nuclei among migraineurs of the general population compared to non-migraineurs
Current nine year follow-up study showed an increase of T2-values of most deep brain nuclei instead of an expected further decrease
T2-values are known to be influenced by other (non-iron) related brain tissue changes with aging
We hypothesize that with aging migraine-induced iron-related brain changes are obscured by age-related other tissue changes
Footnotes
I.H. Palm-Meinders, H. Koppen, L.J Launer, M.A van Buchem, and M.C. Kruit report no disclosures relevant to the manuscript. G.M. Terwindt reports independent support from NWO, European Community, the Dutch Heart Foundation, and the Dutch Brain Foundation. report no disclosures relevant to the manuscript. M.D. Ferrari has, in the past 5 years, received grants and consultancy or industry support from Medtronic and independent support from the European Community, NWO, NIH, and the Dutch Heart Foundation.
References
- 1.Drayer B, Burger P, Darwin R, Riederer S, Herfkens R, Johnson GA. MRI of brain iron. AJR AmJRoentgenol. 1986;147:103–10. doi: 10.2214/ajr.147.1.103. [DOI] [PubMed] [Google Scholar]
- 2.Haacke EM, Cheng NY, House MJ, Liu Q, Neelavalli J, Ogg RJ, et al. Imaging iron stores in the brain using magnetic resonance imaging. Magn ResonImaging. 2005;23:1–25. doi: 10.1016/j.mri.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Tepper SJ, Lowe MJ, Beall E, Phillips MD, Liu K, Stillman MJ, et al. Iron Deposition in Pain-Regulatory Nuclei in Episodic Migraine and Chronic Daily Headache by MRI. Headache. 2011 doi: 10.1111/j.1526-4610.2011.02056.x. [DOI] [PubMed] [Google Scholar]
- 4.Welch KM. Iron in the migraine brain; a resilient hypothesis. Cephalalgia. 2008 doi: 10.1111/j.1468-2982.2008.01725.x. [DOI] [PubMed] [Google Scholar]
- 5.Kruit MC, Launer LJ, Overbosch J, van Buchem MA, Ferrari MD. Iron accumulation in deep brain nuclei in migraine: a population-based magnetic resonance imaging study. Cephalalgia. 2009;29:351–9. doi: 10.1111/j.1468-2982.2008.01723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Welch KM, Nagesh V, Aurora SK, Gelman N. Periaqueductal gray matter dysfunction in migraine: cause or the burden of illness? Headache. 2001;41:629–37. doi: 10.1046/j.1526-4610.2001.041007629.x. [DOI] [PubMed] [Google Scholar]
- 7.Palm-Meinders IH, Koppen H, Terwindt GM, Launer LJ, Konishi J, Moonen JM, et al. Structural brain changes in migraine. Jama. 2012;308:1889–97. doi: 10.1001/jama.2012.14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Society HCSotIH. The International Classification of Headache Disorders: 2nd edition. Cephalalgia. 2004;24(Suppl 1):9–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 9.Aquino D, Bizzi A, Grisoli M, Garavaglia B, Bruzzone MG, Nardocci N, et al. Age-related iron deposition in the basal ganglia: quantitative analysis in healthy subjects. Radiology. 2009;252:165–72. doi: 10.1148/radiol.2522081399. [DOI] [PubMed] [Google Scholar]
- 10.Bartzokis G, Beckson M, Hance DB, Marx P, Foster JA, Marder SR. MR evaluation of age-related increase of brain iron in young adult and older normal males. Magn ResonImaging. 1997;15:29–35. doi: 10.1016/s0730-725x(96)00234-2. [DOI] [PubMed] [Google Scholar]
- 11.Schenker C, Meier D, Wichmann W, Boesiger P, Valavanis A. Age distribution and iron dependency of the T2 relaxation time in the globus pallidus and putamen. Neuroradiology. 1993;35:119–24. doi: 10.1007/BF00593967. [DOI] [PubMed] [Google Scholar]
- 12.Cherubini A, Peran P, Caltagirone C, Sabatini U, Spalletta G. Aging of subcortical nuclei: microstructural, mineralization and atrophy modifications measured in vivo using MRI. Neuroimage. 2009;48:29–36. doi: 10.1016/j.neuroimage.2009.06.035. [DOI] [PubMed] [Google Scholar]
- 13.Peran P, Cherubini A, Luccichenti G, Hagberg G, Demonet JF, Rascol O, et al. Volume and iron content in basal ganglia and thalamus. HumBrain Mapp. 2009;30:2667–75. doi: 10.1002/hbm.20698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaggi AS, Singh N. Role of different brain areas in peripheral nerve injury-induced neuropathic pain. Brain Res. 2011;1381:187–201. doi: 10.1016/j.brainres.2011.01.002. [DOI] [PubMed] [Google Scholar]