Abstract
Ovarian aging is characterized by a decline in both the total number and overall quality of oocytes, the latter of which has been experimentally tied to mitochondrial dysfunction. Clinical studies in the late 1990s demonstrated that transfer of cytoplasm aspirated from eggs of young female donors into eggs of infertile women at the time of intracytoplasmic sperm injection improved pregnancy success rates. However, donor mitochondria were identified in offspring, and the United States Food and Drug Administration raised questions about delivery of foreign genetic material into human eggs at the time of fertilization. Accordingly, heterologous cytoplasmic transfer, while promising, was in effect shut down as a clinical protocol. The recent discovery of adult oogonial (oocyte-generating) stem cells in mice, and subsequently in women, has since re-opened the prospects of delivering a rich source of pristine and patient-matched germline mitochondria to boost egg health and embryonic developmental potential without the need for young donor eggs to obtain cytoplasm. Herein we overview the science behind this new protocol, which has been patented and termed autologous germline mitochondrial energy transfer, and its use to date in clinical studies for improving pregnancy success in women with a prior history of assisted reproduction failure.
Keywords: germline stem cell, oogonial stem cell, mitochondria, ooplasmic transfer, oocyte, in vitro fertilization
For decades, it was widely believed by the scientific and medical communities that human females are provided with a set number of oocytes, contained within follicles, around the time of birth.1 In turn, progressive depletion of this pool to the point of near exhaustion during adult life drives ovarian aging.2 In addition to an irreversible decline in the total number of oocytes present in the ovaries as women grow older, a second fundamental feature of ovarian aging—namely, the deterioration of egg quality2,3—also has its roots in the belief that there is a nonrenewing oocyte population that, because of the passage of time, eventually becomes functionally compromised during a period in life often referred to “advanced maternal age.” Although this latter feature of ovarian aging is the focal point of our discussions, we note that accumulating evidence from multiple laboratories around the world now collectively disputes that mammalian females are incapable of new oocyte generation during adult life.4–18
At a most basic level, poor quality eggs are defined as having an extremely low potential to be fertilized or, if fertilized, to develop normally into healthy embryos capable of completing implantation and gestation leading to live births (Fig. 1). The reasons why a given egg is, or becomes, unable to fulfill its prime directive are diverse, ranging from meiotic progression abnormalities and chromosomal imbalance to mitochondrial dysfunction and low bioenergetic potential.19–22 While differences in opinion on root causes may exist, there is uniform consensus that maternal aging triggers or amplifies events that ultimately reduce egg quality. As a consequence, considerable experimental effort has been expended to identify interventions that might mitigate or even reverse the negative effects of aging on oocytes, with the hope of restoring more “youthful” characteristics to eggs of women who present with fertility issues. As one example, past work with mice has shown that chronic administration of antioxidants during adolescent and adult life minimizes the increased frequency of chromosomal abnormalities normally seen in eggs with advancing age.23 Likewise, dietary caloric restriction in mice during adulthood prevents meiotic spindle abnormalities, chromosomal errors, mitochondrial disorganization, and decreased bioenergetic potential in eggs of females at later ages that would otherwise be associated with pending reproductive failure,24 thereby extending reproductive lifespan.25 Other studies have shown that the beneficial effects of dietary caloric restriction on oocyte quality parameters and fertility with age can be replicated by treating adult mice with coenzyme-Q10 or with resveratrol.26,27
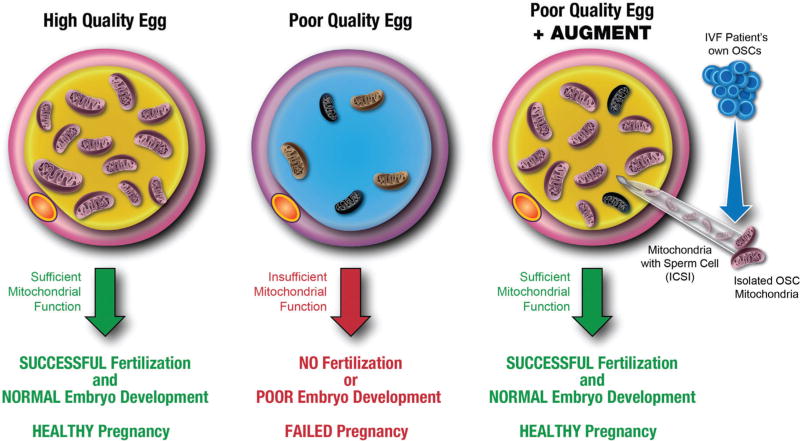
Fig. 1.
Conceptual depiction of AUGMENT in human assisted reproduction. In high-quality eggs, mitochondria are sufficient in number, function, and quality to provide the energy required for successful fertilization and normal preimplantation embryonic development, resulting in a healthy pregnancy (left panel). With age, the functional integrity of the mitochondrial pool in women’s eggs decreases, leading to poor egg quality that results in reduced rates of fertilization, compromised embryonic development, and ultimately failed pregnancy (center panel). By providing a source of germline mitochondria from an IVF patient’s own OSCs, AUGMENT introduces a proprietary amount of autologous egg precursor cell-derived mitochondria into the egg along with the sperm at the time of ICSI. This bolus of pristine mitochondria provides an otherwise compromised egg with sufficient energetic potential for successful fertilization and subsequent embryonic development, restoring the natural potential to achieve a healthy pregnancy (right panel). AUGMENT, autologous germline mitochondrial energy transfer; ICSI, intracytoplasmic sperm injection; OSCs, oogonial stem cells.
Although these experiments with mice currently have no direct practical application in women seeking fertility care, a common theme is that all of this work can be tied to alterations in mitochondrial dynamics or function. As such, it is reasonable to posit that this critical energy-producing organelle serves as a principal nexus point for diverse stimuli to feed into a common pathway of intracellular decisions that ultimately dictate overall egg quality.21,22,28–30 In keeping with this, prior studies with mouse and human oocytes have shown that impaired mitochondrial function and lower ATP levels are tightly associated with meiotic spindle abnormalities, embryonic developmental arrest, and failed conception.24,31,32 Other work has shown that fertilization success and embryonic developmental competency positively correlate with mitochondrial DNA (mtDNA) copy numbers such that a minimal threshold of mtDNA content per egg must apparently be met to ensure optimal embryonic development after fertilization.33
These types of experimental observations, coupled with results from clinical studies conducted in the 1990s on the potential benefit of nonautologous cytoplasmic transfer to assisted reproduction outcomes in women with a history of repeated in vitro fertilization (IVF) failure,34–39 provided us several years ago with a solid foundation on which to pursue mitochondrial-based approaches for improving overall egg health and, consequently, pregnancy success rates. By combining these efforts with the unique power of a newly discovered population of adult oocyte-producing stem cells in ovaries of women,9 the concept of autologous germline mitochondrial energy transfer (AUGMENT) was conceived40,41 (Fig. 1), experimentally developed and then clinically validated for launch in several fertility centers around the world. Three different groups have reported their initial clinical experiences with AUGMENT thus far. In the sections to follow, we provide a detailed overview of the science behind this new protocol, and the outcomes of its use to date to combat one of the most troubling aspects of ovarian aging—the deterioration of egg quality.
Ovarian Aging, Mitochondrial Function, and Egg Quality
In women, the ovaries are among the first organs to fail with advancing age. In addition to a reduced overall number of oocyte-containing follicles, infertility associated with advancing maternal age is often attributed poor egg quality. Mitochondria provide cellular energy in the form of ATP, which in oocytes is essential for successful meiotic spindle assembly, proper segregation of chromosomes, maturation, fertilization, and preimplantation embryogenesis.21,28,30 Additionally, mitochondria participate in several other cellular processes, including ion fluxes and management of reduction-oxidation status, which are central to fertilization and development.28 Accordingly, mitochondrial deficits or dysfunction can severely compromise the developmental potential of oocytes, and the embryos they produce, on many levels. Data drawn from analysis of both animal models and clinical IVF outcomes reveal that a spectrum of mitochondrial defects can accrue in oocytes concomitant with advancing maternal age. These include a reduction in total mitochondrial numbers and mitochondrial membrane potential—the latter of which reflects oxidative phosphorylation status, diminished bioenergetic capacity, aberrant mitochondrial localization and aggregation, suboptimal mtDNA content, and elevated mtDNA mutational load.42–47
As, however, a single mitochondrion can contain anywhere from 1 to 10 mtDNA molecules, caution needs to be exercised when assessing the relationship of mitochondrial numbers to mtDNA content, and the potential role of each variable as an independent biomarker, and perhaps determinant, of egg and embryo quality. For example, in studies of mouse and hamster oocytes, total mitochondrial numbers decline with age, and this occurs concomitant with reduced ATP levels and mtDNA content per oocyte.47 Studies of human oocytes that failed to fertilize showed that eggs retrieved from women older than 40 years exhibited reduced mtDNA content when compared with eggs obtained from women younger than 40 years.46 These observations agree with, and support, several earlier studies that concluded successful fertilization and developmental potential in mouse and human eggs is dependent on a minimum threshold of mtDNA content being present at the time of sperm penetration.33,42–44 In mice, this lower threshold has been estimated at 40,000 to 50,000 mtDNA copies,33 whereas in humans the minimum mtDNA content is not yet known. However, lower than average mtDNA copy numbers in human oocytes has been tied to failure to fertilize and ovarian insufficiency.42–44 Conversely, blastomere volume and rate of embryonic cleavage are positively correlated with mtDNA content.46
But, when it comes to mtDNA copy number, it appears that too much of a good thing can actually be a harbinger of inherent problems in the mitochondrial population in those human embryos that will ultimately fail implantation. In comprehensive clinical studies conducted by Fragouli and colleagues49 of human cleavage-stage embryos (n = 39; all of which were characterized as chromosomally normal by array comparative genomic hybridization and underwent uterine transfer) and blastocysts (n = 340, 123 of which were characterized as aneuploid; 131 of the remaining 217 blastocysts characterized as chromosomally normal underwent uterine transfer), blastomeres collected from embryos generated by women up to 37 years of age were found to contain higher levels of mtDNA when compared with blastomeres of embryos generated by women 38 years of age and older. While these findings are in keeping with prior observations of an inverse relationship between maternal age and mtDNA content in oocytes,46–48 a very different situation emerged when blastocysts were subjected to similar analyses. Independent of chromosomal status, blastocysts generated by women between 38 and 42 years of age contained significantly higher levels of mtDNA when compared with blastocysts generated by women 37 years of age and younger.49 Interestingly, across all age groups, mtDNA content was higher in those blastocysts characterized as chromosomally abnormal, indicating that maternal age and aneuploidy are independent factors tied to alterations in mtDNA content in human embryos.49
The most striking aspect of this study,49 however, was the retrospective identification of a threshold amount of mtDNA (0.003) in blastocysts that, if exceeded, was consistently associated with implantation failure independent of blastocyst morphology, patient age, or IVF clinic involved in the study. In turn, every clinical pregnancy obtained resulted from transfer of blastocysts with mtDNA contents less than the 0.003 threshold. So, how can this all be reconciled? The answer may be found by tracing the origin of the increase in mtDNA content in blastocysts that are apparently destined to fail. To this end, Fragouli and colleagues further observed no differences in the amount of mtDNA in blastomeres of human cleavage stage embryos that subsequently implanted after uterine transfer versus those that failed to implant.49 Since mitochondrial replication is believed to cease upon fertilization of the egg and not reactivate until the blastocyst stage of embryogenesis is reached,33,50,51 the lack of a difference in mtDNA content in blastomeres of embryos that ultimately implant versus those that fail to implant indicates that the elevation in mtDNA copy number over the 0.003 threshold in blastocysts that will fail to implant must arise as a consequence of excessive mitochondrial replication just prior to implantation. This, as it turns out, may be a key observation in understanding why procedures such as heterologous cytoplasmic transfer conducted in the late 1990s and, more recently, AUGMENT enhance pregnancy success in human IVF programs.
Boosting Egg Health through Cytoplasmic Transfer
A positive influence of donor ooplasm on preimplantation embryonic development was first reported in the early 1980s from a study in which mouse oocytes that exhibit embryonic arrest in vitro were “rescued” from this developmental block by injection of cytoplasm collected from oocytes of a different strain that undergo normal embryonic development after fertilization in vitro.52 Fifteen years later, Cohen and colleagues announced the birth of a child conceived from an infertility patient’s egg that had received a small bolus of donor oocyte cytoplasm (ooplasm) at the time of fertilization by intracytoplasmic sperm injection (ICSI).34 This patient, along with 26 other women who consented to this new clinical procedure termed cytoplasmic or ooplasmic transfer, had a history of repeated IVF failure. Despite the extremely poor prognosis for any of these patients to become pregnant through yet another IVF cycle, 30 attempts of ooplasmic transfer performed with these 27 women yielded a remarkable 13 live births.34,35,39 Although one of the babies delivered exhibited an abnormal karyotype (45, XO) diagnosed as pervasive development disorder at 18 months of age, 16 other babies were delivered healthy without any genetic anomalies. Similarly, encouraging pregnancy results were obtained from several other fertility centers using either donor eggs or tripronucleate zygotes as a source of cytoplasm for transfer into eggs of women undergoing ICSI for idiopathic infertility or repeated implantation failure.36–38
However, interest in expanding the clinical use of cytoplasmic transfer diminished quickly for two principal reasons. The first is that children conceived using this procedure were subsequently shown to possess mitochondria derived from both the biological mother and the cytoplasmic donor,53,54 which raised several ethical, legal, and potential long-term health questions surrounding mitochondrial heteroplasmy. The second is regulatory in nature. Based on the fact that mitochondria contain their own DNA distinct from the nuclear genome pool, the United States Food and Drug Administration (FDA) concluded in 2001 that cytoplasmic transfer, as practiced at that time using donor eggs or zygotes, involved the introduction of foreign genetic material into human eggs that were subsequently fertilized to produce embryos for the purpose of reproduction. As a consequence, the FDA required that any further use of heterologous cytoplasmic transfer in human assisted reproduction would be contingent on review and testing under Investigational New Drug (IND) guidelines.55 This decision, in effect, shut down cytoplasmic transfer as a clinical protocol in IVF programs.
To potentially circumvent issues surrounding the introduction of donor mitochondria isolated from eggs or tripronucleate zygotes of one woman into the eggs of another (viz., genetically unmatched) woman, one option would be to replace the donor mitochondria-containing cytoplasm with autologous mitochondria-containing cytoplasm isolated from somatic cells of the same woman seeking the IVF-based treatments for infertility. However, mtDNA in somatic cells is subject to progressive mutations with age,56 and thus transfer of autologous somatic cell mitochondria into eggs at fertilization could lead to incorporation, and subsequent replication, of “inferior” or damaged mitochondrial genomes in the resultant embryos and offspring. Interestingly, there is a single preliminary report from 2004 of autologous cytoplasmic transfer in human assisted reproduction, which used cumulus-granulosa cells collected during follicular aspirations for egg retrieval as the source of cytoplasm delivered along with the sperm by ICSI. While promising initial results were described in this abstract,57 a full publication containing the actual clinical data on pregnancy outcomes and live birth rates is still lacking more than a decade later.
Despite the abrupt halt in the use of cytoplasmic transfer for improving pregnancy and live birth success rates in human assisted reproduction after the FDA ruling, the technology sparked several interesting scientific questions that were subsequently pursued. One of the most critical pertained to identification of the actual “factor” present in the donor ooplasm that boosted fertilization success rates and embryonic developmental potential in recipient eggs.58 To this end, independent studies of murine and porcine oocytes demonstrated, in eggs of either species, that the beneficial effects of ooplasmic transfer on IVF outcomes could be reproduced entirely by microinjection of isolated mitochondria.59–61 Although these investigations do not rule out the existence of other egg health-promoting “factors” in ooplasm, the bolus of donor mitochondria delivered into recipient eggs by cytoplasmic transfer can alone explain the clinical outcomes reported by Cohen and others.34–39 Moreover, in retrospect, these outcomes also align well with a now widespread recognition of the fundamental importance of mitochondrial function and bioenergetic capacity to successful fertilization and preimplantation embryogenesis (see section “Ovarian Aging, Mitochondrial Function, and Egg Quality”).
In addition to providing mechanistic insights into how cytoplasmic transfer may benefit eggs that would otherwise be developmentally compromised, other experiments that followed the initial clinical reports of cytoplasmic transfer in human assisted reproduction shed light on a different question surrounding the use of donor eggs or tripronucleate embryos as a source of cytoplasm for injection—the impact, if any, of mitochondrial heteroplasmy on offspring development and long-term health. The first of these, published in 2007, reported that neutral mitochondrial heteroplasmy in mice was associated with significant elevations in blood pressure early in adulthood, as well as a progressive increase in overall body mass and total fat mass with advancing age, compared with non-heteroplasmic control animals.62 Additionally, Sharpley and colleagues demonstrated that C57BL/6J mice carrying an admixture of NZB and 129S6 mtDNAs (NZB-129S6 mitochondrial heteroplasmy) exhibited adult-onset reductions in physical activity and respiratory exchange ratios, abnormalities in fear and stress responses, and cognitive impairments in spatial learning and memory when compared with their NZB or 129S6 homoplasmic mtDNA counterparts.63 These experimental data indicate that technologies such as heterologous cytoplasmic transfer could have significant long-term health consequences associated with as-yet undefined genetic or epigenetic disturbances resulting from the presence of two different mitochondrial genomes in the same organism or individual.
Adult Stem Cells, Aging, and Human Oogenesis
Over a century ago, Alexander Maximow published his initial discovery of the existence of adult stem cells in bone marrow dedicated to the production of mature blood cells.64 Scientists have since identified adult stem cell populations that participate in tissue repair and homeostasis in essentially every major physiological system.65 For many organs, such as the lungs, brain, and heart, initial publications on the existence of tissue-specific adult stem cells were met with intense skepticism, as it was previously thought that, with rare exception, tissue development from less differentiated cellular precursors ended early in chronological life, ceasing entirely by adulthood. However, a substantial body of evidence now supports that stem cell populations residing in most, if not all, tissues, including bone marrow, skin, gastrointestinal tract, blood, lungs, skeletal muscle, cardiac muscle, brain, testes, and ovaries, play fundamental roles in adult organ function and perhaps aging-related organ failure.
Adult stem cells are characterized by their ability to self-renew and differentiate, with committed progeny progressing through a lineage-specific differentiation program to acquire a desired functional property.66,67 To avoid abnormal tissue growth, adult stem cells are found in relatively low abundance and are generally maintained in a quiescent state until demand for their activity, usually via extrinsic factors emanating from their highly specialized microenvironments or “niches,” triggers symmetric or asymmetric divisions.68 Interestingly, aging-related dysfunction of adult stem cells is thought to be driven almost entirely by changes in either the composition of their niches69–71 or systemic factors that directly modulate stem cell function.72–75 In turn, many types of adult stem cells present in aged tissues can functionally reactivate following transplantation into a “young” environment or can rejuvenate failing tissue once a “young” niche is reestablished.72,75,76
The first report of the existence of a self-renewing population of oocyte-generating precursor cells, termed female germline stem cells or oogonial stem cells (OSCs), was from a study published in 2004 using mice as a model.4 Multiple lines of evidence were provided which were collectively disputed the concept of a fixed pool of oocytes being endowed at birth. Five years later, OSCs were isolated from mouse ovaries, expanded, and characterized ex vivo.5 Importantly, intragonadal transplantation-based assays clearly demonstrated the capacity of OSCs to generate fertilization-competent eggs that give rise to viable offspring.5 It is worth noting that this latter approach has been universally accepted as the gold standard for germline stem cell identity confirmation in males for more than 20 years.77,78 This work was followed by a report in 2012 that not only independently verified the functional ability of OSCs purified from adult mouse ovaries to differentiate into fertilization-competent eggs and viable embryos but also extended this line of investigation to include purification and characterization of a comparable population of oocyte-forming stem cells from ovarian cortical tissue of reproductive age women.9
Like their murine counterparts, human OSCs possess a unique gene expression profile consistent with a primitive germ cell identity, and these cells can be established in culture for ex vivo propagation and studies of in vitro oocyte formation.9 Although in vivo fate-tracing studies to document the functionality of OSCs, such as those conducted with mice5,9 and subsequently with rats,14 are not feasible to pursue with human OSCs, a system was devised in which human OSCs engineered to express green fluorescent protein (GFP) were injected into small pieces of adult human ovarian cortical tissue and then either grafted subcutaneously into immunocompromised mouse hosts9 or cultured ex vivo79 as a means to monitor oocyte and follicle formation. Under both conditions, GFP-expressing human OSCs are fully capable of generating GFP-positive immature oocytes enclosed within defined granulosa cell layers as follicles. Significantly, these findings represent not only the first evidence that human OSCs can directly support new oocyte formation but also that adult human ovarian tissue remains amenable to de novo follicle formation. Others have since confirmed both the existence and the oocyte-forming properties of OSCs isolated from human ovarian tissue.18,80 The potential clinical implications of these observations, and how these findings may impact on our current understanding of ovarian aging and the menopause, are explored in more detail later (see section “Are Additional New Fertility Tools on the Horizon?”).
Development of AUGMENT
The identification of a population of adult germline stem cells devoted solely to the production of new oocytes in ovaries of reproductive-age women offers an unprecedented opportunity to test an entirely new array of fertility-enhancement technologies that could not have even been conceived of a little more than a decade ago.81 While much of this work remains experimental at this time (see section “Are Additional New Fertility Tools on the Horizon?”), the simple fact that OSCs are naturally occurring endogenous precursors of oocytes underscores a very important, and clinically useful, feature of these newly discovered cells—the mitochondria present in OSCs are the same as those found in oocytes, as the former cell type differentiates into the latter.5,9 Because of this, delivery of an IVF patient’s own OSC-derived mitochondria into her egg along with a sperm by ICSI would provide an autologous means of recapitulating the fertility-boosting benefits of heterologous cytoplasmic transfer without the drawback of having “foreign” mitochondria present in the resultant embryos and offspring. In other words, enhancing egg health by infusion of a rich source of highly energetic mitochondria from an autologous source of natural egg-producing precursor cells came into view and the concept of AUGMENT was born40,41 (Fig. 1). But, what makes OSCs so special, so unique, that these cells are the most logical, if not the only, choice for use as “mitochondrial donors” in human assisted reproduction?
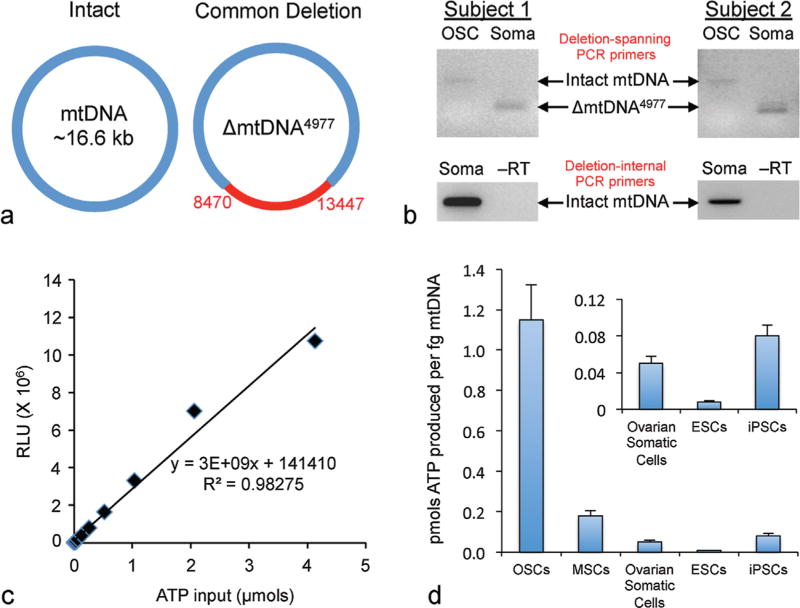
Considering that OSCs can be viewed not only as an adult stem cell population but also as natural germ cell precursors for oocyte production, these cells possess several fundamental features that fully support their desirability for use in autologous mitochondrial transfer protocols. For example, electron microscopic studies have shown that mitochondria present in human OSCs are ultrastructurally indistinguishable from mitochondria found in human eggs, whereas both populations of mitochondria differ dramatically in appearance from mitochondria present in somatic cells61 (DC Woods, PhD and JLTilly, PhD, unpublished data). Such an outcome would be expected if OSCs and oocytes were of a single-cell lineage, differing only in their degree of differentiation. In other words, OSCs and oocytes represent a cellular continuum and thus possess the same mitochondrial population. A second important feature of human OSC mitochondria is related to integrity of mtDNA, which sits in close proximity to the mitochondrial electron transport chain that produces reactive oxygen species (ROS) as a byproduct of ATP generation. This localized exposure to ROS, coupled with the circular nature of the mtDNA structure lacking a histone backbone and the absence of efficient mtDNA repair mechanisms in cells, results in a high susceptibility of mtDNA to mutational damage that accumulates with age.56 More than 150 distinct mtDNA mutations have been reported,82 with the most common in human cells resulting from a 4,977-basepair deletion (ΔmtDNA4,977, often referred to as the “common deletion”) from the mitochondrial genome (Fig. 2a).
Fig. 2.
Properties of human OSC mitochondria. (a) Schematic representation of the common deletion mutation in mtDNA (ΔmtDNA4,977). (b) In ovarian cortical tissue samples from two reproductive age women,9 PCR amplification using primers that span the common deletion (ΔmtDNA4,977) can detect two potential PCR products. The large product (>5 kb) amplifies the entire target region, and represents the intact (nonmutated) mtDNA sequence. Detection of the smaller product (<200 bp) is indicative of the mtDNA sequence spanning bases 8,470–13,447 being deleted, and thus identifies the presence of the common mutation (ΔmtDNA4,977). Note that while the common mutation deletion is observed in ovarian somatic cells, it is not detectable in patient-matched OSCs isolated in parallel to the somatic cell samples. A separate primer set designed to amplify mtDNA within the 5-kb mutation site reveals that mitochondria from ovarian somatic cells are heterogenous with respect to the mutation, as intact mtDNA can also be amplified. (c) Representative standard curve for detection of ATP generation using a bioluminescence-based assay (Roche Bioluminescence HS II Kit). (d) Mitochondria isolated from human OSCs, using the Mitochondrial Isolation Kit for Cultured Cells (Promega), are capable of generating up to sevenfold more ATP (per fg mtDNA) in the presence of 400 µm ADP than an equivalent amount of mitochondria isolated from other human cell types tested in parallel, including mesenchymal stem cells from bone marrow (MSCs), ovarian somatic cells, embryonic stem cells (ESCs), and induced pluripotent stem cells (iPSCs).
In addition to serving as a widely used marker for aging in somatic cells, the common 4,977-basepair deletion has been documented in human eggs that fail to fertilize and the incidence of ΔmtDNA4,977 increases in the oocytes of women older than 35 years.83,84 These clinical observations align well with data from animal models indicating that overall mitochondrial function, including the ability to maintain adequate production of ATP, declines in oocytes of females at advanced reproductive ages (see section “Ovarian Aging, Mitochondrial Function, and Egg Quality). So, could the reported elevation in mtDNA content in human blastocysts over a critical threshold associated with implantation failure reflect a futile attempt in embryos of older women to compensate for aging-related accumulation of dysfunctional mitochondria by making more of these organelles once the machinery for mitochondrial replication comes back online? Recent studies of human preimplantation embryos indicate that increased mtDNA mutational loads are tightly linked to parallel increases in mtDNA content, suggestive of a direct cause-effect relationship between the two events.85 However, other studies using next-generation DNA sequencing failed to identify a clear increase in mtDNA mutational load in human blastocysts exhibiting higher than average mtDNA copy numbers.49
Given these somewhat conflicting results, an answer to the question of whether in a human preimplantation embryo an aging-related accumulation in mtDNA damage actively drives mitochondrial replication as a compensatory attempt to fulfill its high energetic needs remains elusive. Nonetheless, it is probably safe to assume from the body of work discussed earlier that oocytes and embryos with greater numbers of “undamaged” mitochondria, which could serve as “clean” templates for mitochondrial replication at the blastocyst stage, would have a much greater chance of leading to a successful pregnancy than those harboring mitochondrial populations burdened by excessive mtDNA mutational loads. How, then, does all of this relate to the utility of OSCs as a source for mitochondrial transfer? A comparative assessment of the common mtDNA deletion in human subject-matched OSCs and somatic cells isolated from the same ovarian tissue sample has revealed that while somatic cells exhibit, as expected, easily detectable levels of ΔmtDNA4,977, the mutation is undetectable in OSCs analyzed in parallel (Fig. 2b). Accordingly, these cells, perhaps because of an inherently low level of metabolic activity associated with their relatively dormant stem cell state, are apparently not subject to the same degree of cumulative mtDNA damage with age as their somatic cell counterparts in adult human ovaries.
A third feature of OSC mitochondria that is highly relevant to discussions of mitochondrial transfer is their energetic potential. Through a direct comparative analysis of the in vitro ATP-forming capacity of a fixed amount of mitochondria isolated from human OSCs, ovarian somatic cells (isolated at the same time as the OSCs), human embryonic stem cells, human induced pluripotent stem cells, or human bone marrow–derived mesenchymal stem cells, OSCs emerged as the superior source of mitochondria for achieving a robust “bio-energetic boost” (Fig. 2c, d). This tremendous capacity to generate cellular energy, coupled with the lineage-matched relationship of OSCs as natural precursor cells for oocytes as well as resistance to cumulative mtDNA damage with age, collectively supports the unique and desirable properties of OSC-derived mitochondria for achieving the designed purpose of AUGMENT in human assisted reproduction.
Clinical Experience with AUGMENT to Date
As of August 2015, three international fertility centers have successfully developed and launched AUGMENT as a treatment option for women with a history of consistently poor assisted reproduction outcomes. Two of these sites—the Toronto Center for Assisted Reproductive Technologies (TCART) Fertility Partners (Toronto, Canada) and FAKIH IVF (Dubai, United Arab Emirates)—recently reported their early clinical experiences with 93 patients treated with AUGMENT who consented to participation in the OvaScience Global Registry program.86 Outcomes from 11 additional women who received the AUGMENT treatment at a third site—Gen-Art IVF (Ankara, Turkey)—were presented at the 31st Annual Meeting of the European Society of Human Reproduction and Embryology.61 Before discussing the outcomes to date, a careful evaluation of prior IVF outcome history for these patients (n = 104) is warranted to place in context the clinical effectiveness of AUGMENT in this same patient cohort.
Unlike assumptions made from bulk analysis of IVF data collected over the years from hundreds of clinical sites and under varying clinical protocols, a given patient’s prior experience(s) with assisted reproduction offers a much more unbiased view into the potential of that individual to become pregnant in a subsequent IVF attempt. For example, the prospects of a 36-year-old woman becoming pregnant who fails IVF repeatedly at a given fertility center because of poor egg quality can be quite different from a 36-year old woman who fails IVF repeatedly at the same center because she is a “poor responder.” In turn, if a clinical intervention is used to enhance responsiveness of the latter individual to hyperstimulation such that more eggs are retrieved for IVF and she becomes pregnant, it is logical to conclude that the success achieved in that cycle was tied to the intervention used to overcome her specific block to a positive assisted reproduction outcome.
With that as a preface, a total of 369 prior IVF cycles had been initiated with the 104 patients who eventually turned to AUGMENT at the three clinical sites indicated.61,86 The collective historical clinical pregnancy and live birth rates per cycle initiated without AUGMENT were 5.2% (range, 0–11%) and 1.3% (range, 0–2%), respectively, with each patient having undergone at least 2, and as many as 16, prior cycles. In this same patient population, just a single cycle of AUGMENT yielded a clinical pregnancy rate of 25.7% (range, 18–35%), with an ongoing clinical pregnancy and live birth rate of 18.1 % (range, 9–26%) per cycle initiated.61,86 Each site has reported at least one live birth—with a total of four live births including two sets of twins thus far, and the first birth through AUGMENT was achieved at TCART Fertility Partners.87
Additional key insights into the clinical effectiveness of AUGMENT can be obtained through evaluation of each site’s own clinical experience, especially when one considers that Gen-Art IVF, unlike TCART Fertility Partners and FAKIH IVF, is restricted to single embryo transfers. For Gen-Art IVF, 11 infertility patients between 27 and 46 years of age (average, 35) were offered AUGMENT, after undergoing a total of 41 prior IVF cycles (per patient average of 3.7, range of 2–7) with a 0% historical clinical pregnancy rate per cycle initiated. After a single cycle of AUGMENT, cycles in 2 of the 11 patients were cancelled and 9 were continued, ultimately producing a 27% and 33% clinical pregnancy rate per cycle initiated (3/11) and per embryo transfer (3/9), respectively. Two of the three clinical pregnancies were lost, and one was carried to term (live birth) by a 34-year-old patient diagnosed with diminished ovarian reserve who failed seven prior IVF cycles without a clinical or even a chemical pregnancy recorded.61
The results from use of AUGMENT at TCART Fertility Partners are derived from experience with 34 infertility patients between 26 and 44 years of age (average, 36) diagnosed with poor oocyte and embryo quality along with diminished ovarian reserve, ovulatory dysfunction, polycystic ovary syndrome, tubal factor, or endometriosis. This patient population had undergone a total of 71 previous IVF cycles (79 previous embryo transfers), with a prior clinical pregnancy rate of 11% per cycle initiated (8/71) or 10% per embryo transfer (8/79) and live birth rate of 1.4% per cycle initiated (1/71) or 1.3% per embryo transfer (1/79). In other words, only a single baby had been delivered after 79 embryo transfers.86 A single cycle of AUGMENT in this same patient population resulted in 26 embryo transfers and produced a clinical pregnancy rate of 35% per cycle initiated (12/34) or 46% per embryo transfer (12/26). Ongoing clinical pregnancy and live birth rates are 26% per cycle initiated (9/34) and 35% per embryo transfer (9/26), with one live birth recorded to date.86,87 Given that only a single live birth was achieved after 79 embryo transfers without AUGMENT in this patient population, the delivery of one baby and eight other ongoing pregnancies in this same cohort after only 26 embryo transfers (i.e., one-third the number of the prior cycle history) bodes well for the clinical effectiveness of AUGMENT to improve IVF pregnancy success rates in women.
This conclusion is further bolstered by clinical data from FAKIH IVF, in which 59 infertility patients (20–48 years of age; average, 37) diagnosed with poor oocyte and embryo quality along with diminished ovarian reserve, ovulatory dysfunction, or severe male factor infertility were offered AUGMENT after undergoing a total of 247 cycles of conventional IVF with a clinical pregnancy rate of 4% (9/257) and a live birth rate of just 2% (4/257). Data on rates per embryo transfer are not available due to the international nature of the patient population comprising this cohort.86 Nevertheless, after 60 cycles of AUGMENT (single cycle for 58 patients, 2 cycles for 1 patient) yielding 34 embryo transfers, the clinical pregnancy rate was 22% per cycle initiated (13/60) and 38% per embryo transfer (13/34). Even more striking, the ongoing clinical pregnancy and live birth rates to date are 18% per cycle initiated (11/60) and 32% per embryo transfer (11/34) with two live births (two sets of twins) recorded thus far.86
In evaluating the collective data from the two sites with the most clinical experience with AUGMENT (n = 93 patients), it is clear that AUGMENT increases clinical pregnancy rates per initiated cycle by threefold (TCART) to sixfold (FAKIH IVF). Perhaps even more striking is that 104 cycles of AUGMENT performed across the three international sites have already produced four live births of six babies, nearly comparable to the total of only five live births achieved in this same patient population after previously undergoing 3.5-fold more IVF attempts (369 prior cycles) without AUGMENT. Moreover, if the 17 additional clinical pregnancies still ongoing at TCART and FAKIH IVF ultimately lead to live births as well, AUGMENT will have produced an impressive 11-fold (TCART) to 18-fold (FAKIH IVF) increase in pregnancy success after IVF in a well-defined patient population with a long history of very poor assisted reproduction outcomes without AUGMENT included as a treatment. This does not even take into account the 23 “spare” embryos that have been cryopreserved for AUGMENT patients to use in future transfers to achieve pregnancy success.86
As exciting as this emerging story appears, it does not end there. In an effort to further analyze the effectiveness of AUGMENT for improving embryo quality and pregnancy outcomes, FAKIH IVF also used a direct comparative approach termed Matched Best Embryo Selection and Transfer (MBEST).86 In brief, eggs retrieved from each patient of a subset of 25 infertility patients were allocated to undergo IVF through conventional ICSI (n = 106 eggs) or through ICSI with AUGMENT (n = 171 eggs). All other parameters for embryo culture and assessment were held constant until the time of embryo selection for transfer based on standard metrics including morphological grade, kinetics of early embryonic cleavage events, and preimplantation genetic screening results. While no differences were detected in the rate of either 2-pronuclei formation or 5-day blastocyst development, embryo transfer rates were sevenfold higher in the AUGMENT treatment group (14/25) compared with the ICSI-only group (2/25) due to improvements in the selection criteria for transfer resulting from the inclusion of AUGMENT along with ICSI. This resulted in substantially higher rates of clinical and ongoing clinical pregnancies in the AUGMENT treatment group versus ICSI alone. Furthermore, when compared with the historical 0% pregnancy rate per initiated cycle in this subset of 25 infertility patients at FAKIH IVF, the 32% pregnancy rate achieved by inclusion of AUGMENT is a standalone observation in support of the clinical effectiveness of this protocol for improving outcomes of human assisted reproduction.86
Are Additional New Fertility Tools on the Horizon?
The birth of Louise Joy Brown—the world’s first IVF baby, on July 27, 1978—opened a new chapter in the field of human reproduction, a step pioneered by Robert Edwards and Patrick Steptoe.88 Although this remarkable accomplishment was at first viewed by many as immoral and unethical, the courage of Edwards and Steptoe to bring this technology forth into the realm of human application set in motion the now widespread use of assisted reproductive technologies to combat infertility around the globe. By 2013, the number of IVF babies surpassed 5 million worldwide, and the numbers continue to climb. Interestingly, however, the general process of IVF practiced today differs little from that employed by Edwards and Steptoe—unite an egg with a sperm outside of the body, and hope that a healthy embryo capable of completing implantation and gestation is produced. Of course, several improvements in the technology have been made along the way to optimize egg preparation, sperm selection, fertilization, embryo culture, and embryo selection for transfer, which have combined to now more than triple the dismally low pregnancy success rates observed during the earliest years of human IVF attempts.89 These improvements include changes in hormonal stimulation protocols for egg retrieval, in vitro oocyte maturation, gamete and embryo cryopreservation, ICSI, and genetic testing of embryos, among others. However, despite its relatively rapid evolution, IVF success rates have for the most part remained unchanged over the past few years. Is this a sign that human IVF as practiced now is reaching, or perhaps has already reached, its useful limits?
The introduction, and early clinical success, of AUGMENT indicates that pregnancy success rates in current assisted reproduction programs can actually still be improved upon, especially for those women who have a prior history of failed IVF attempts due to inferior egg and embryo quality.61,86 Time will tell if inclusion of AUGMENT during first cycle IVF can perhaps minimize the total number of cycles required for achieving a successful pregnancy. Even if so, AUGMENT requires mature eggs to be available for mitochondrial transfer and ICSI, and thus women who cannot produce eggs for whatever reason would not share in its potential benefits. Does the future hold something more for women who have lost ovarian function due to natural aging or exposure to insults such as chemotherapy?
Using methodologies similar to those described for the initial characterization of the ability of human OSCs to generate new oocytes that orchestrate primordial follicle formation in human ovarian cortical tissue,9 we are currently working toward establishment of ex vivo culture conditions that support OSC-derived oocyte growth and maturation that would allow for production of a nearly limitless supply of eggs from a self-renewing oocyte precursor cell population.79,80 Although we understand that this type of thinking pushes well past current beliefs and boundaries in reproductive medicine, realizing the enormous potential of an OSC-based ex vivo egg maturation (EVEM) strategy (OSC-EVEM) is actually only limited by a few technological hurdles, which while challenging are not in our view insurmountable. In short, OSC-EVEM can be broken down into three sequential steps, each one of which alone has a published precedent for success. The first is the use of purified OSCs, either reaggregated with dispersed ovarian somatic cells or injected into adult ovarian cortical tissue, to generate new oocytes contained within primordial-stage follicles ex vivo.9,11,18 The second is to connect this established technology to ovarian cortical strip culture systems that facilitate activation and growth of primordial follicles to more advanced stages of follicle development.90–92 Finally, to complete platform development we then need to connect this series of steps to validated protocols for in vitro maturation (IVM) of oocytes contained within cumulus-granulosa cell complexes aspirated from small antral follicles.93,94 From that point forward, resultant eggs would be subjected to conventional IVF and embryo culture protocols to obtain blastocysts for genetic screening and, ultimately, transfer.
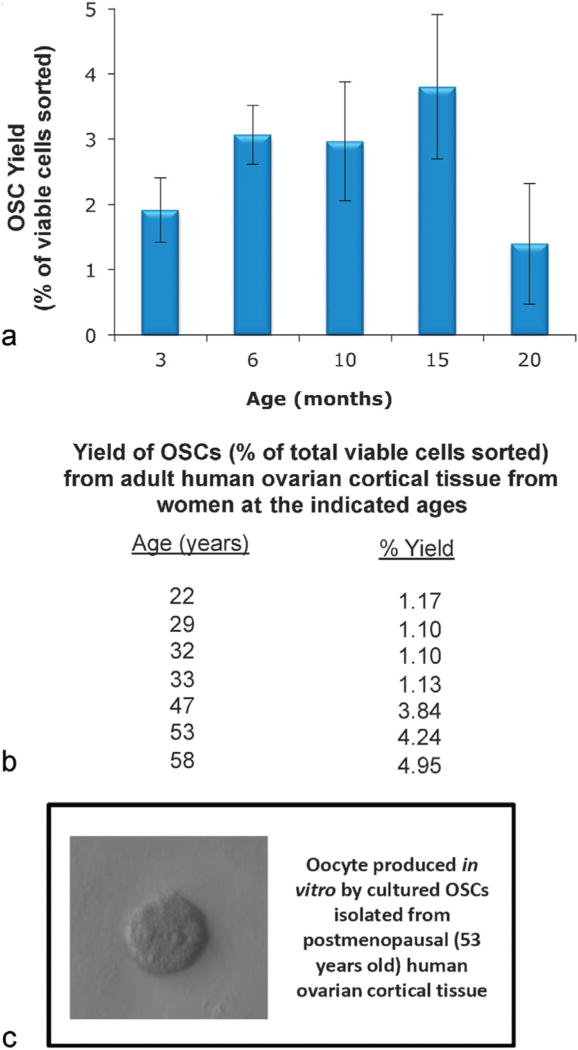
Although all of the parts are available, the production of eggs from OSC-derived oocytes will involve validation of a series of culture conditions that, at present, remain much more poorly defined for nonhuman primates and humans than for research animal models.92 Nonetheless, the existing technological framework for achieving OSC-EVEM is straightforward, and it will undoubtedly be propelled ahead by continuing advances in tissue engineering that enable routine generation of fertilization-competent eggs from primordial follicles ex vivo.95 If such a technology is eventually realized, can women incapable of producing eggs through conventional hyperstimulation protocols use OSC-EVEM for fertility restoration? In mice, OSCs persist in the ovaries well past the time of age-related ovarian failure (Fig. 3a), and heterochronic transplantation experiments have demonstrated that OSCs present in the aged ovarian tissue resume oogenesis after grafting the tissue into young adult female mice.96 Parallel studies have shown that OSCs similarly persist in human ovarian cortical tissue past the time of menopause (Fig. 3b). Furthermore, once isolated and established in culture, OSCs from “aged” human ovaries, like OSCs from ovaries of reproductive age women,9,11 resume spontaneous formation of immature oocytes in vitro (Fig. 3c). Based on these observations, and the established ability of purified OSCs to differentiate into fully competent eggs once returned to an appropriate ovarian microenvironment,5,9,14 the current limits of human fertile lifespan may indeed be amenable to extension.
Fig. 3.
Presence of OSCs in aged mouse and human ovaries. (a) Yield of OSCs (as a percent of the total viable cells sorted; see White et al9 and Woods and Tilly11 for methodological details) from ovaries of C57BL/6 female mice throughout reproductive life into advanced ages (graph represents results from analysis of a minimum of four pooled ovaries for each OSC sort at each age, with each age point replicated a minimum of three times using different mice for each replicate; mean ± SEM). (b) Yield of OSCs (as a percent of total viable cells sorted) from ovaries of women during reproductive life and through peri- and postmenopausal years (see White et al9 and Woods and Tilly11 for methodological details). (c) Example of an immature oocyte formed spontaneously in cultures of OSCs isolated from ovarian cortical tissue of a woman at 53 years of age.
In addition to generating eggs through OSC-EVEM, the innate ability of OSCs to spontaneously generate immature oocytes in defined feeder cell-free cultures9,11 is of potential value for a different reason. Following routine passage of pure OSC cultures (mouse or human), a subset of OSCs initiates differentiation into oocytes.9 Without proper instruction from their normal somatic cell partners (i.e., granulosa cells), however, these oocytes will not go on to form fertilization-competent eggs. Nonetheless, the rate of oocyte formation is very consistent between passage and across cell lines. Thus, by seeding a defined number of OSCs into culture wells subsequently exposed to different experimental conditions, the rate of oocyte formation over time can be monitored as an “oogenic” readout. For example, treatment of cultured OSCs with bone morphogenetic protein 4 (BMP4) results in activation of the SMAD signaling cascade, which then triggers upregulation of key meiotic events, including induction of Stimulated by retinoic acid gene 8 (Stra8), followed by an increase in oocyte formation.13 Such high-throughput screening of factors and cues that can drive oogenesis in OSC cultures offers a powerful new tool for development of biological agents, new chemical entities, and devices designed to replenish the ovarian reserve in women of advanced maternal age or with primary ovarian insufficiency.79
Acknowledgments
A Method to Extend Research in Time (MERIT) Award from the National Institute on Aging (NIH R37-AG012279), and a grant from the Glenn Foundation for Medical Research, supported work conducted by the authors discussed herein. We thank Michael Cooper (Cooper Graphics, www.cooper247.com) for outstanding graphic arts assistance in preparation of Figure 1. We also thank Yasushi Yakai, Osamu Ishihara, and Hiroyuki Seki (Saitama Medical Center, Saitama University, Saitama, Japan) for their invaluable contributions to our past and ongoing IRB-approved studies of human OSCs.
Footnotes
Competing Financial Interests
Dori C. Woods declares interest in intellectual property described in U.S. Patent 8,642,329 and U.S. Patent 8,647,869, and is a recipient of a corporate-sponsored research award from OvaScience, Inc. (Waltham, MA). Jonathan L Tilly discloses interest in intellectual property described in U.S. Patent 7,195,775, U.S. Patent 7,850,984, U. S. Patent 7,955,846, U.S. Patent 8,642,329, U.S. Patent 8,647,869, and U.S. Patent 8,652,840, and is a scientific cofounder and a current member of the Scientific Advisory Board of OvaScience, Inc.
During the final preparation of this article, additional key studies of central importance to verifying the ability of isolated mouse OSCs to generate fertilization competent eggs and viable offspring,97 and to identifying the existence of OSCs in adult baboon ovaries,98 were published; additionally, a third clinical site reporting its experience with AUGMENT in human assisted reproduction was published.99
References
- 1.Zuckerman S. The number of oocytes in the mature ovary. Recent Prog Horm Res. 1951;6:63–108. [Google Scholar]
- 2.Navot D, Bergh PA, Williams MA, et al. Poor oocyte quality rather than implantation failure as a cause of age-related decline in female fertility. Lancet. 1991;337(8754):1375–1377. doi: 10.1016/0140-6736(91)93060-m. [DOI] [PubMed] [Google Scholar]
- 3.Broekmans FJ, Soules MR, Fauser BC. Ovarian aging: mechanisms and clinical consequences. Endocr Rev. 2009;30(5):465–493. doi: 10.1210/er.2009-0006. [DOI] [PubMed] [Google Scholar]
- 4.Johnson J, Canning J, Kaneko T, Pru JK, Tilly JL. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature. 2004;428(6979):145–150. doi: 10.1038/nature02316. [DOI] [PubMed] [Google Scholar]
- 5.Zou K, Yuan Z, Yang Z, et al. Production of offspring from a germline stem cell line derived from neonatal ovaries. Nat Cell Biol. 2009;11(5):631–636. doi: 10.1038/ncb1869. [DOI] [PubMed] [Google Scholar]
- 6.Pacchiarotti J, Maki C, Ramos T, et al. Differentiation potential of germ line stem cells derived from the postnatal mouse ovary. Differentiation. 2010;79(3):159–170. doi: 10.1016/j.diff.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Yang Z, Yang Y, et al. Production of transgenic mice by random recombination of targeted genes in female germline stem cells. J Mol Cell Biol. 2011;3(2):132–141. doi: 10.1093/jmcb/mjq043. [DOI] [PubMed] [Google Scholar]
- 8.Zou K, Hou L, Sun K, Xie W, Wu J. Improved efficiency of female germline stem cell purification using fragilis-based magnetic bead sorting. Stem Cells Dev. 2011;20(12):2197–2204. doi: 10.1089/scd.2011.0091. [DOI] [PubMed] [Google Scholar]
- 9.White YAR, Woods DC, Takai Y, Ishihara O, Seki H, Tilly JL. Oocyte formation by mitotically active germ cells purified from ovaries of reproductive-age women. Nat Med. 2012;18(3):413–421. doi: 10.1038/nm.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woods DC, Tilly JL. An evolutionary perspective on adult female germline stem cell function from flies to humans. Semin Reprod Med. 2013;31(1):24–32. doi: 10.1055/s-0032-1331794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woods DC, Tilly JL. Isolation, characterization and propagation of mitotically active germ cells from adult mouse and human ovaries. Nat Protoc. 2013;8(5):966–988. doi: 10.1038/nprot.2013.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imudia AN, Wang N, Tanaka Y, White YA, Woods DC, Tilly JL. Comparative gene expression profiling of adult mouse ovary-derived oogonial stem cells supports a distinct cellular identity. Fertil Steril. 2013;100(5):1451–1458. doi: 10.1016/j.fertnstert.2013.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park ES, Woods DC, Tilly JL. Bone morphogenetic protein 4 promotes mammalian oogonial stem cell differentiation via Smad1/5/8 signaling. Fertil Steril. 2013;100(5):1468–1475. doi: 10.1016/j.fertnstert.2013.07.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou L, Wang L, Kang JX, et al. Production of fat-1 transgenic rats using a post-natal female germline stem cell line. Mol Hum Reprod. 2014;20(3):271–281. doi: 10.1093/molehr/gat081. [DOI] [PubMed] [Google Scholar]
- 15.Xie W, Wang H, Wu J. Similar morphological and molecular signatures shared by female and male germline stem cells. Sci Rep. 2014;4:5580. doi: 10.1038/srep05580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park ES, Tilly JL. Use of DEAD-box polypeptide-4 (Ddx4) gene promoter-driven fluorescent reporter mice to identify mitotically active germ cells in post-natal mouse ovaries. Mol Hum Reprod. 2015;21(1):58–65. doi: 10.1093/molehr/gau071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khosravi-Farsani S, Amidi F, Habibi Roudkenar M, Sobhani A. Isolation and enrichment of mouse female germ line stem cells. Cell J. 2015;16(4):406–415. doi: 10.22074/cellj.2015.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grieve KM, McLaughlin M, Dunlop CE, Telfer EE, Anderson RA. The controversial existence and functional potential of oogonial stem cells. Maturitas. 2015 doi: 10.1016/j.maturitas.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 19.Hassold T, Chiu D. Maternal age-specific rates of numerical chromosome abnormalities with special reference to trisomy. Hum Genet. 1985;70(1):11–17. doi: 10.1007/BF00389450. [DOI] [PubMed] [Google Scholar]
- 20.Battaglia DE, Goodwin P, Klein NA, Soules MR. Influence of maternal age on meiotic spindle assembly in oocytes from naturally cycling women. Hum Reprod. 1996;11(10):2217–2222. doi: 10.1093/oxfordjournals.humrep.a019080. [DOI] [PubMed] [Google Scholar]
- 21.Van Blerkom J. Mitochondrial function in the human oocyte and embryo and their role in developmental competence. Mitochondrion. 2011;11(5):797–813. doi: 10.1016/j.mito.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 22.Tilly JL, Sinclair DA. Germline energetics, aging, and female infertility. Cell Metab. 2013;17(6):838–850. doi: 10.1016/j.cmet.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tarín JJ, Pérez-Albalá S, Cano A. Oral antioxidants counteract the negative effects of female aging on oocyte quantity and quality in the mouse. Mol Reprod Dev. 2002;61(3):385–397. doi: 10.1002/mrd.10041. [DOI] [PubMed] [Google Scholar]
- 24.Selesniemi K, Lee HJ, Muhlhauser A, Tilly JL. Prevention of maternal aging-associated oocyte aneuploidy and meiotic spindle defects in mice by dietary and genetic strategies. Proc Natl Acad Sci USA. 2011;108(30):12319–12324. doi: 10.1073/pnas.1018793108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Selesniemi K, Lee HJ, Tilly JL. Moderate caloric restriction initiated in rodents during adulthood sustains function of the female reproductive axis into advanced chronological age. Aging Cell. 2008;7(5):622–629. doi: 10.1111/j.1474-9726.2008.00409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ben-Meir A, Burstein E, Borrego-Alvarez A, et al. Coenzyme Q10 restores oocyte mitochondrial function and fertility during reproductive aging. Aging Cell. 2015;14(5):887–895. doi: 10.1111/acel.12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu M, Yin Y, Ye X, et al. Resveratrol protects against age-associated infertility in mice. Hum Reprod. 2013;28(3):707–717. doi: 10.1093/humrep/des437. [DOI] [PubMed] [Google Scholar]
- 28.Dumollard R, Duchen M, Carroll J. The role of mitochondrial function in the oocyte and embryo. Curr Top Dev Biol. 2007;77:21–49. doi: 10.1016/S0070-2153(06)77002-8. [DOI] [PubMed] [Google Scholar]
- 29.Bentov Y, Esfandiari N, Burstein E, Casper RF. The use of mitochondrial nutrients to improve the outcome of infertility treatment in older patients. Fertil Steril. 2010;93(1):272–275. doi: 10.1016/j.fertnstert.2009.07.988. [DOI] [PubMed] [Google Scholar]
- 30.Bentov Y, Yavorska T, Esfandiari N, Jurisicova A, Casper RF. The contribution of mitochondrial function to reproductive aging. J Assist Reprod Genet. 2011;28(9):773–783. doi: 10.1007/s10815-011-9588-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Blerkom J, Davis PW, Lee J. ATP content of human oocytes and developmental potential and outcome after in-vitro fertilization and embryo transfer. Hum Reprod. 1995;10(2):415–424. doi: 10.1093/oxfordjournals.humrep.a135954. [DOI] [PubMed] [Google Scholar]
- 32.Thouas GA, Trounson AO, Wolvetang EJ, Jones GM. Mitochondrial dysfunction in mouse oocytes results in preimplantation embryo arrest in vitro. Biol Reprod. 2004;71(6):1936–1942. doi: 10.1095/biolreprod.104.033589. [DOI] [PubMed] [Google Scholar]
- 33.Wai T, Ao A, Zhang X, Cyr D, Dufort D, Shoubridge EA. The role of mitochondrial DNA copy number in mammalian fertility. Biol Reprod. 2010;83(1):52–62. doi: 10.1095/biolreprod.109.080887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cohen J, Scott R, Schimmel T, Levron J, Willadsen S. Birth of infant after transfer of anucleate donor oocyte cytoplasm into recipient eggs. Lancet. 1997;350(9072):186–187. doi: 10.1016/S0140-6736(05)62353-7. [DOI] [PubMed] [Google Scholar]
- 35.Cohen J, Scott R, Alikani M, et al. Ooplasmic transfer in mature human oocytes. Mol Hum Reprod. 1998;4(3):269–280. doi: 10.1093/molehr/4.3.269. [DOI] [PubMed] [Google Scholar]
- 36.Lanzendorf SE, Mayer JF, Toner J, Oehninger S, Saffan DS, Muasher S. Pregnancy following transfer of ooplasm from cryopreserved-thawed donor oocytes into recipient oocytes. Fertil Steril. 1999;71(3):575–577. doi: 10.1016/s0015-0282(98)00504-4. [DOI] [PubMed] [Google Scholar]
- 37.Huang CC, Cheng TC, Chang HH, et al. Birth after the injection of sperm and the cytoplasm of tripronucleate zygotes into meta-phase II oocytes in patients with repeated implantation failure after assisted fertilization procedures. Fertil Steril. 1999;72(4):702–706. doi: 10.1016/s0015-0282(99)00309-x. [DOI] [PubMed] [Google Scholar]
- 38.Dale B, Wilding M, Botta G, et al. Pregnancy after cytoplasmic transfer in a couple suffering from idiopathic infertility: case report. Hum Reprod. 2001;16(7):1469–1472. doi: 10.1093/humrep/16.7.1469. [DOI] [PubMed] [Google Scholar]
- 39.Barritt J, Willadsen S, Brenner C, Cohen J. Cytoplasmic transfer in assisted reproduction. Hum Reprod Update. 2001;7(4):428–435. doi: 10.1093/humupd/7.4.428. [DOI] [PubMed] [Google Scholar]
- 40.Tilly JL, Woods DC. Compositions and Methods for Autologous Germline Mitochondrial Energy Transfer. 8,642,329. United States Patent and Trademark Office; United States Patent Number. 2014
- 41.Tilly JL, Woods DC. Compositions and Methods for Autologous Germline Mitochondrial Energy Transfer. 8,647,869. United States Patent and Trademark Office; United States Patent Number. 2014
- 42.Reynier P, May-Panloup P, Chrétien MF, et al. Mitochondrial DNA content affects the fertilizability of human oocytes. Mol Hum Reprod. 2001;7(5):425–429. doi: 10.1093/molehr/7.5.425. [DOI] [PubMed] [Google Scholar]
- 43.May-Panloup P, Chrétien MF, Jacques C, Vasseur C, Malthièry Y, Reynier P. Low oocyte mitochondrial DNA content in ovarian insufficiency. Hum Reprod. 2005;20(3):593–597. doi: 10.1093/humrep/deh667. [DOI] [PubMed] [Google Scholar]
- 44.Santos TA, El Shourbagy S, St John JC. Mitochondrial content reflects oocyte variability and fertilization outcome. Fertil Steril. 2006;85(3):584–591. doi: 10.1016/j.fertnstert.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 45.Duran HE, Simsek-Duran F, Oehninger SC, Jones HW, Jr, Castora FJ. The association of reproductive senescence with mitochondrial quantity, function, and DNA integrity in human oocytes at different stages of maturation. Fertil Steril. 2011;96(2):384–388. doi: 10.1016/j.fertnstert.2011.05.056. [DOI] [PubMed] [Google Scholar]
- 46.Murakoshi Y, Sueoka K, Takahashi K, et al. Embryo developmental capability and pregnancy outcome are related to the mitochondrial DNA copy number and ooplasmic volume. J Assist Reprod Genet. 2013;30(10):1367–1375. doi: 10.1007/s10815-013-0062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simsek-Duran F, Li F, Ford W, Swanson RJ, Jones HW, Jr, Castora FJ. Age-associated metabolic and morphologic changes in mitochondria of individual mouse and hamster oocytes. PLoS One. 2013;8(5):e64955. doi: 10.1371/journal.pone.0064955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kushnir VA, Ludaway T, Russ RB, Fields EJ, Koczor C, Lewis W. Reproductive aging is associated with decreased mitochondrial abundance and altered structure in murine oocytes. J Assist Reprod Genet. 2012;29(7):637–642. doi: 10.1007/s10815-012-9771-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fragouli E, Spath K, Alfarawati S, et al. Altered levels of mitochondrial DNA are associated with female age, aneuploidy, and provide an independent measure of embryonic implantation potential. PLoS Genet. 2015;11(6):e1005241. doi: 10.1371/journal.pgen.1005241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Larsson NG, Wang J, Wilhelmsson H, et al. Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. Nat Genet. 1998;18(3):231–236. doi: 10.1038/ng0398-231. [DOI] [PubMed] [Google Scholar]
- 51.St John JC, Facucho-Oliveira J, Jiang Y, Kelly R, Salah R. Mitochondrial DNA transmission, replication and inheritance: a journey from the gamete through the embryo and into offspring and embryonic stem cells. Hum Reprod Update. 2010;16(5):488–509. doi: 10.1093/humupd/dmq002. [DOI] [PubMed] [Google Scholar]
- 52.Muggleton-Harris A, Whittingham DG, Wilson L. Cytoplasmic control of preimplantation development in vitro in the mouse. Nature. 1982;299(5882):460–462. doi: 10.1038/299460a0. [DOI] [PubMed] [Google Scholar]
- 53.Barritt JA, Brenner CA, Malter HE, Cohen J. Mitochondria in human offspring derived from ooplasmic transplantation. Hum Reprod. 2001;16(3):513–516. doi: 10.1093/humrep/16.3.513. [DOI] [PubMed] [Google Scholar]
- 54.Brenner CA, Barritt JA, Willadsen S, Cohen J. Mitochondrial DNA heteroplasmy after human ooplasmic transplantation. Fertil Steril. 2000;74(3):573–578. doi: 10.1016/s0015-0282(00)00681-6. [DOI] [PubMed] [Google Scholar]
- 55.Zoon KC. Human cells used in therapy involving the transfer of genetic material by means other than the union of gamete nuclei. United States FDA; 2001. Available at: http://www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/ucm105852.htm. [Google Scholar]
- 56.Bratic A, Larsson NG. The role of mitochondria in aging. J Clin Invest. 2013;123(3):951–957. doi: 10.1172/JCI64125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tzeng CR, Hsieh RH, Au HK, Yen YH, Chang SJ, Cheng YF. Mitochondria transfer (MIT) into oocyte from autologous cumulus granulosa cells (cGCs) Fertil Steril. 2004;82(Suppl 2):S53. [Google Scholar]
- 58.Harvey AJ, Gibson TC, Quebedeaux TM, Brenner CA. Impact of assisted reproductive technologies: a mitochondrial perspective of cytoplasmic transplantation. Curr Top Dev Biol. 2007;77:229–249. doi: 10.1016/S0070-2153(06)77009-0. [DOI] [PubMed] [Google Scholar]
- 59.El Shourbagy SH, Spikings EC, Freitas M, St John JC. Mitochondria directly influence fertilisation outcome in the pig. Reproduction. 2006;131(2):233–245. doi: 10.1530/rep.1.00551. [DOI] [PubMed] [Google Scholar]
- 60.Yi YC, Chen MJ, Ho JYP, Guu HF, Ho ES. Mitochondria transfer can enhance the murine embryo development. J Assist Reprod Genet. 2007;24(10):445–449. doi: 10.1007/s10815-007-9161-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.31st Annual Meeting of the European Society of Human Reproduction and Embryology (ESHRE) Lisbon, Portugal: 2015. Proceedings from the Workshop on Experts in Egg Health Advancing Fertility Care. Available at: http://www.ovascience.com/files/ESHRE_Symposium_2015_Final_For_Website_Posting_FINAL-2.pdf. [Google Scholar]
- 62.Acton BM, Lai I, Shang X, Jurisicova A, Casper RF. Neutral mitochondrial heteroplasmy alters physiological function in mice. Biol Reprod. 2007;77(3):569–576. doi: 10.1095/biolreprod.107.060806. [DOI] [PubMed] [Google Scholar]
- 63.Sharpley MS, Marciniak C, Eckel-Mahan K, et al. Heteroplasmy of mouse mtDNA is genetically unstable and results in altered behavior and cognition. Cell. 2012;151(2):333–343. doi: 10.1016/j.cell.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Maximow A. The lymphocyte as a stem cell common to different blood elements in embryonic development and during the post-fetal life of mammals. Folia Haematol (Frankf) 1909;8:125–134. [Google Scholar]
- 65.Mimeault M, Batra SK. Recent progress on tissue-resident adult stem cell biology and their therapeutic implications. Stem Cell Rev. 2008;4(1):27–49. doi: 10.1007/s12015-008-9008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Orford KW, Scadden DT. Deconstructing stem cell self-renewal: genetic insights into cell-cycle regulation. Nat Rev Genet. 2008;9(2):115–128. doi: 10.1038/nrg2269. [DOI] [PubMed] [Google Scholar]
- 67.Jung Y, Brack AS. Cellular mechanisms of somatic stem cell aging. CurrTop Dev Biol. 2014;107:405–438. doi: 10.1016/B978-0-12-416022-4.00014-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 2010;327(5965):542–545. doi: 10.1126/science.1180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boyle M, Wong C, Rocha M, Jones DL. Decline in self-renewal factors contributes to aging of the stem cell niche in the Drosophila testis. Cell Stem Cell. 2007;1(4):470–478. doi: 10.1016/j.stem.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 70.Pan L, Chen S, Weng C, et al. Stem cell aging is controlled both intrinsically and extrinsically in the Drosophila ovary. Cell Stem Cell. 2007;1(4):458–469. doi: 10.1016/j.stem.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 71.Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132(4):598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433(7027):760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 73.Brack AS, Conboy MJ, Roy S, et al. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317(5839):807–810. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- 74.Villeda SA, Luo J, Mosher KI, et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477(7362):90–94. doi: 10.1038/nature10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ruckh JM, Zhao JW, Shadrach JL, et al. Rejuvenation of regeneration in the aging central nervous system. Cell Stem Cell. 2012;10(1):96–103. doi: 10.1016/j.stem.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ryu BY, Orwig KE, Oatley JM, Avarbock MR, Brinster RL. Effects of aging and niche microenvironment on spermatogonial stem cell self-renewal. Stem Cells. 2006;24(6):1505–1511. doi: 10.1634/stemcells.2005-0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brinster RL, Zimmermann JW. Spermatogenesis following male germ-cell transplantation. Proc Natl Acad Sci USA. 1994;91(24):11298–11302. doi: 10.1073/pnas.91.24.11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brinster RL, Avarbock MR. Germline transmission of donor haplo-type following spermatogonial transplantation. Proc Natl Acad Sci USA. 1994;91(24):11303–11307. doi: 10.1073/pnas.91.24.11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Woods DC, Tilly JL. Germline stem cells in adult mammalian ovaries. In: Sanders S, editor. Ten Critical Topics in Reproductive Medicine. Washington, DC: Science/AAAS; 2013. pp. 10–12. [Google Scholar]
- 80.Silvestris E, D’Oronzo S, Cafforio P, DAmato G, Loverro G. Perspective in infertility: the ovarian stem cells. J Ovarian Res. 2015;8:55. doi: 10.1186/s13048-015-0184-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Woods DC, Tilly JL. The next (re)generation of human ovarian biology and female fertility: is current science tomorrow’s practice? Fertil Steril. 2012;98(1):3–10. doi: 10.1016/j.fertnstert.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yesodi V, Yaron Y, Lessing JB, Amit A, Ben-Yosef D. The mitochondrial DNA mutation (ΔmtDNA5286) in human oocytes: correlation with age and IVF outcome. J Assist Reprod Genet. 2002;19(2):60–66. doi: 10.1023/A:1014439529813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Keefe DL, Niven-Fairchild T, Powell S, Buradagunta S. Mitochondrial deoxyribonucleic acid deletions in oocytes and reproductive aging in women. Fertil Steril. 1995;64(3):577–583. [PubMed] [Google Scholar]
- 84.Chan CC, Liu VW, Lau EY, Yeung WS, Ng EH, Ho PC. Mitochondrial DNA content and 4977 bp deletion in unfertilized oocytes. Mol Hum Reprod. 2005;11(12):843–846. doi: 10.1093/molehr/gah243. [DOI] [PubMed] [Google Scholar]
- 85.Monnot S, Samuels DC, Hesters L, et al. Mutation dependence of the mitochondrial DNA copy number in the first stages of human embryogenesis. Hum Mol Genet. 2013;22(9):1867–1872. doi: 10.1093/hmg/ddt040. [DOI] [PubMed] [Google Scholar]
- 86.Fakih MH, El Shmoury M, Szeptycki J, et al. The AUGMENTSM treatment: physician reported outcomes of the initial global patient experience. JFIV Reprod Med Genet. 2015;3:154. [Google Scholar]
- 87.Park A. The incredible, surprising, controversial new way to make a baby. Time. 2015;185(18):42–45. [PubMed] [Google Scholar]
- 88.Steptoe PC, Edwards RG. Birth after the reimplantation of a human embryo. Lancet. 1978;2(8085):366. doi: 10.1016/s0140-6736(78)92957-4. [DOI] [PubMed] [Google Scholar]
- 89.Steptoe PC, Edwards RG, Walters DE. Observations on 767 clinical pregnancies and 500 births after human in-vitro fertilization. Hum Reprod. 1986;1(2):89–94. doi: 10.1093/oxfordjournals.humrep.a136366. [DOI] [PubMed] [Google Scholar]
- 90.Telfer EE, McLaughlin M, Ding C, Thong KJ. A two-step serum-free culture system supports development of human oocytes from primordial follicles in the presence of activin. Hum Reprod. 2008;23(5):1151–1158. doi: 10.1093/humrep/den070. [DOI] [PubMed] [Google Scholar]
- 91.Telfer EE, McLaughlin M. In vitro development of ovarian follicles. Semin Reprod Med. 2011;29(1):15–23. doi: 10.1055/s-0030-1268700. [DOI] [PubMed] [Google Scholar]
- 92.Telfer EE, Zelinski MB. Ovarian follicle culture: advances and challenges for human and nonhuman primates. Fertil Steril. 2013;99(6):1523–1533. doi: 10.1016/j.fertnstert.2013.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cha KY, Chian RC. Maturation in vitro of immature human oocytes for clinical use. Hum Reprod Update. 1998;4(2):103–120. doi: 10.1093/humupd/4.2.103. [DOI] [PubMed] [Google Scholar]
- 94.Chang EM, Song HS, Lee DR, Lee WS, Yoon TK. In vitro maturation of human oocytes: Its role in infertility treatment and new possibilities. Clin Exp Reprod Med. 2014;41(2):41–46. doi: 10.5653/cerm.2014.41.2.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shea LD, Woodruff TK, Shikanov A. Bioengineering the ovarian follicle microenvironment. Annu Rev Biomed Eng. 2014;16:29–52. doi: 10.1146/annurev-bioeng-071813-105131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Niikura Y, Niikura T, Tilly JL. Aged mouse ovaries possess rare premeiotic germ cells that can generate oocytes following transplantation into a young host environment. Aging (Albany, NY Online) 2009;1(12):971–978. doi: 10.18632/aging.100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xiong J, Lu Z, Wu M, et al. Intraovarian transplantation of female germline stem cells rescues ovarian function in chemotherapy-injured ovaries. PLoS One. 2015;10(10):e0139824. doi: 10.1371/journal.pone.0139824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Woods DC, Tilly JL. Reply to human and mouse ovaries lack DDX4-expressing functional oogonial stem cells. Nat Med. 2015;21(10):1118–1121. doi: 10.1038/nm.3775. [DOI] [PubMed] [Google Scholar]
- 99.Oktay K, Baltaci V, Sonmezer M, et al. Oogonial precursor cell derived autologous mitochondria injection (AMI) to improve outcomes in women with multiple IVF failures due to low oocyte quality: a clinical translation. Reprod Sci. 2015;22(12):1612–1617. doi: 10.1177/1933719115612137. [DOI] [PubMed] [Google Scholar]