Abstract
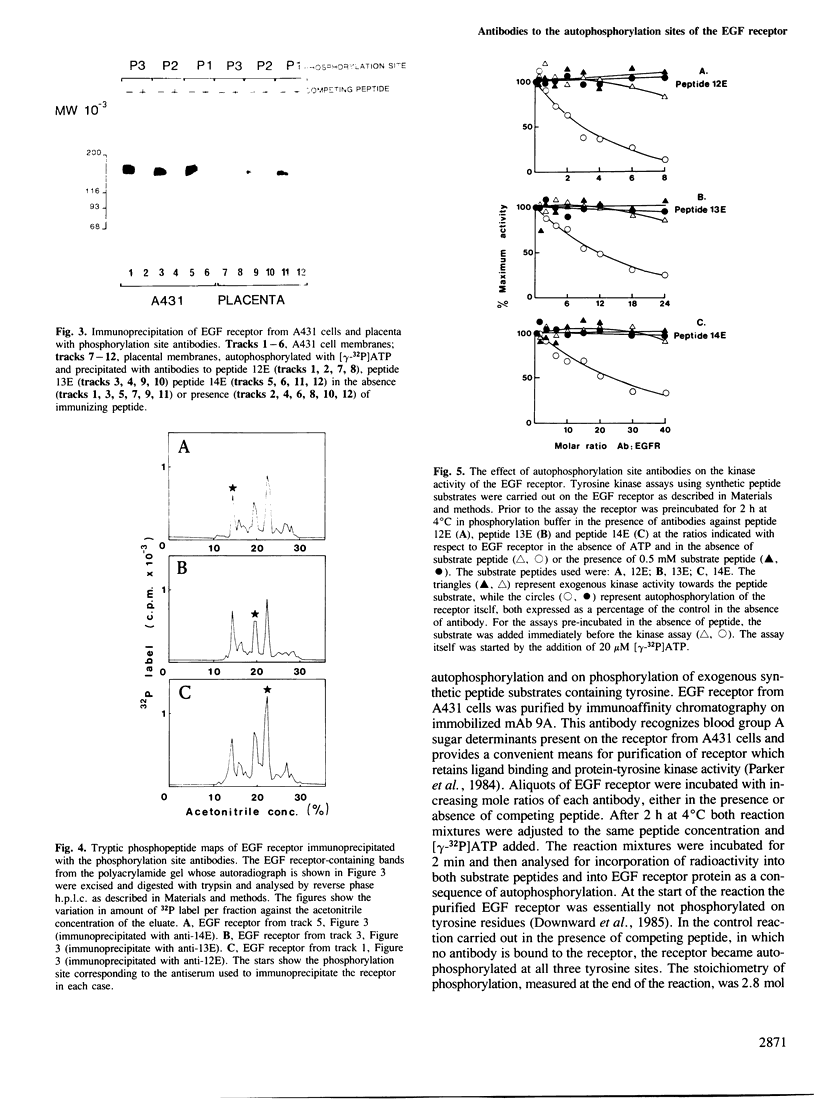
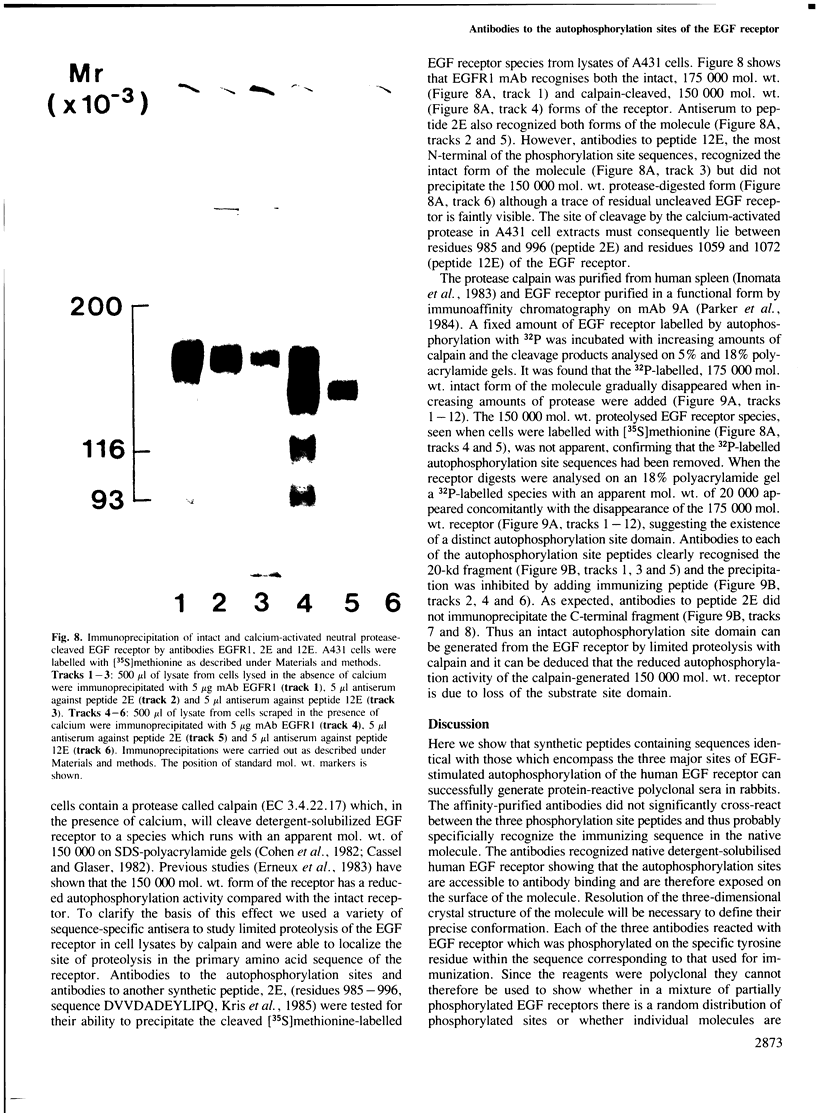
Antisera were prepared against three synthetic peptides with amino acid sequences identical to those surrounding the three major autophosphorylation sites of the epidermal growth factor (EGF) receptor. The affinity-purified antibodies reacted strongly in an enzyme-linked immunosorbent assay against the immunizing peptide but showed little cross-reaction with the other two phosphorylation site peptides. EGF receptors labelled by autophosphorylation could be specifically precipitated by each of the phosphorylation site antibodies. The antibodies recognised EGF receptors labelled at each of the autophosphorylation sites, indicating that they could bind to the immunizing sequences irrespective of their states of phosphorylation. The antibodies were able to inhibit EGF receptor autophosphorylation without affecting EGF-stimulated tyrosine kinase activity towards exogenous peptide substrates, suggesting that the kinase and autophosphorylation sites were in distinct domains. Immunofluorescent staining of A431 cells showed that the autophosphorylation site sequences resided inside the cell. The autophosphorylation sites were shown to be within a domain of 20 000 mol. wt. which could be cleaved from the receptor through limited proteolysis by the calcium-dependent protease, calpain. The position of cleavage of the EGF receptor by the protease was mapped to lie between residues 996 and 1059. These results are discussed in the context of a model for the structure and function of the human EGF receptor.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson E. D., Rees A. R. Epidermal growth factor receptors. Mol Cell Biochem. 1981 Feb 11;34(3):129–152. doi: 10.1007/BF02359619. [DOI] [PubMed] [Google Scholar]
- Basu M., Biswas R., Das M. 42,000-molecular weight EGF receptor has protein kinase activity. Nature. 1984 Oct 4;311(5985):477–480. doi: 10.1038/311477a0. [DOI] [PubMed] [Google Scholar]
- Brown D. J., Gordon J. A. The stimulation of pp60v-src kinase activity by vanadate in intact cells accompanies a new phosphorylation state of the enzyme. J Biol Chem. 1984 Aug 10;259(15):9580–9586. [PubMed] [Google Scholar]
- Carpenter G., Cohen S. Epidermal growth factor. Annu Rev Biochem. 1979;48:193–216. doi: 10.1146/annurev.bi.48.070179.001205. [DOI] [PubMed] [Google Scholar]
- Cassel D., Glaser L. Proteolytic cleavage of epidermal growth factor receptor. A Ca2+-dependent, sulfhydryl-sensitive proteolytic system in A431 cells. J Biol Chem. 1982 Aug 25;257(16):9845–9848. [PubMed] [Google Scholar]
- Chinkers M., Brugge J. S. Characterization of structural domains of the human epidermal growth factor receptor obtained by partial proteolysis. J Biol Chem. 1984 Sep 25;259(18):11534–11542. [PubMed] [Google Scholar]
- Cooper J. A., Bowen-Pope D. F., Raines E., Ross R., Hunter T. Similar effects of platelet-derived growth factor and epidermal growth factor on the phosphorylation of tyrosine in cellular proteins. Cell. 1982 Nov;31(1):263–273. doi: 10.1016/0092-8674(82)90426-3. [DOI] [PubMed] [Google Scholar]
- Decker S. J. Phosphorylation of the erbB gene product from an avian erythroblastosis virus-transformed chick fibroblast cell line. J Biol Chem. 1985 Feb 25;260(4):2003–2006. [PubMed] [Google Scholar]
- Downward J., Parker P., Waterfield M. D. Autophosphorylation sites on the epidermal growth factor receptor. Nature. 1984 Oct 4;311(5985):483–485. doi: 10.1038/311483a0. [DOI] [PubMed] [Google Scholar]
- Downward J., Yarden Y., Mayes E., Scrace G., Totty N., Stockwell P., Ullrich A., Schlessinger J., Waterfield M. D. Close similarity of epidermal growth factor receptor and v-erb-B oncogene protein sequences. Nature. 1984 Feb 9;307(5951):521–527. doi: 10.1038/307521a0. [DOI] [PubMed] [Google Scholar]
- Ebina Y., Ellis L., Jarnagin K., Edery M., Graf L., Clauser E., Ou J. H., Masiarz F., Kan Y. W., Goldfine I. D. The human insulin receptor cDNA: the structural basis for hormone-activated transmembrane signalling. Cell. 1985 Apr;40(4):747–758. doi: 10.1016/0092-8674(85)90334-4. [DOI] [PubMed] [Google Scholar]
- Erneux C., Cohen S., Garbers D. L. The kinetics of tyrosine phosphorylation by the purified epidermal growth factor receptor kinase of A-431 cells. J Biol Chem. 1983 Apr 10;258(7):4137–4142. [PubMed] [Google Scholar]
- Gentry L. E., Rohrschneider L. R., Casnellie J. E., Krebs E. G. Antibodies to a defined region of pp60src neutralize the tyrosine-specific kinase activity. J Biol Chem. 1983 Sep 25;258(18):11219–11228. [PubMed] [Google Scholar]
- Gilmore T., DeClue J. E., Martin G. S. Protein phosphorylation at tyrosine is induced by the v-erbB gene product in vivo and in vitro. Cell. 1985 Mar;40(3):609–618. doi: 10.1016/0092-8674(85)90209-0. [DOI] [PubMed] [Google Scholar]
- Gullick W. J., Downward D. J., Marsden J. J., Waterfield M. D. A radioimmunoassay for human epidermal growth factor receptor. Anal Biochem. 1984 Aug 15;141(1):253–261. doi: 10.1016/0003-2697(84)90454-8. [DOI] [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Protein-tyrosine kinases. Annu Rev Biochem. 1985;54:897–930. doi: 10.1146/annurev.bi.54.070185.004341. [DOI] [PubMed] [Google Scholar]
- Hunter T. The epidermal growth factor receptor gene and its product. Nature. 1984 Oct 4;311(5985):414–416. doi: 10.1038/311414a0. [DOI] [PubMed] [Google Scholar]
- Inomata M., Hayashi M., Nakamura M., Imahori K., Kawashima S. Purification and characterization of a calcium-activated neutral protease from rabbit skeletal muscle which requires calcium ions of microM order concentration. J Biochem. 1983 Jan;93(1):291–294. doi: 10.1093/oxfordjournals.jbchem.a134166. [DOI] [PubMed] [Google Scholar]
- Kris R. M., Lax I., Gullick W., Waterfield M. D., Ullrich A., Fridkin M., Schlessinger J. Antibodies against a synthetic peptide as a probe for the kinase activity of the avian EGF receptor and v-erbB protein. Cell. 1985 Mar;40(3):619–625. doi: 10.1016/0092-8674(85)90210-7. [DOI] [PubMed] [Google Scholar]
- Parker P. J., Young S., Gullick W. J., Mayes E. L., Bennett P., Waterfield M. D. Monoclonal antibodies against the human epidermal growth factor receptor from A431 cells. Isolation, characterization, and use in the purification of active epidermal growth factor receptor. J Biol Chem. 1984 Aug 10;259(15):9906–9912. [PubMed] [Google Scholar]
- Petruzzelli L., Herrera R., Rosen O. M. Insulin receptor is an insulin-dependent tyrosine protein kinase: copurification of insulin-binding activity and protein kinase activity to homogeneity from human placenta. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3327–3331. doi: 10.1073/pnas.81.11.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purchio A. F., Wells S. K., Collett M. S. Increase in the phosphotransferase specific activity of purified Rous sarcoma virus pp60v-src protein after incubation with ATP plus Mg2+. Mol Cell Biol. 1983 Sep;3(9):1589–1597. doi: 10.1128/mcb.3.9.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen O. M., Herrera R., Olowe Y., Petruzzelli L. M., Cobb M. H. Phosphorylation activates the insulin receptor tyrosine protein kinase. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3237–3240. doi: 10.1073/pnas.80.11.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T., Kikuchi T., Yumoto N., Yoshimura N., Murachi T. Comparative specificity and kinetic studies on porcine calpain I and calpain II with naturally occurring peptides and synthetic fluorogenic substrates. J Biol Chem. 1984 Oct 25;259(20):12489–12494. [PubMed] [Google Scholar]
- Sen S., Houghten R. A., Sherr C. J., Sen A. Antibodies of predetermined specificity detect two retroviral oncogene products and inhibit their kinase activities. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1246–1250. doi: 10.1073/pnas.80.5.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder M. A., Bishop J. M., Colby W. W., Levinson A. D. Phosphorylation of tyrosine-416 is not required for the transforming properties and kinase activity of pp60v-src. Cell. 1983 Mar;32(3):891–901. doi: 10.1016/0092-8674(83)90074-0. [DOI] [PubMed] [Google Scholar]
- Tamura T., Bauer H., Birr C., Pipkorn R. Antibodies against synthetic peptides as a tool for functional analysis of the transforming protein pp60src. Cell. 1983 Sep;34(2):587–596. doi: 10.1016/0092-8674(83)90391-4. [DOI] [PubMed] [Google Scholar]
- Thom D., Powell A. J., Lloyd C. W., Rees D. A. Rapid isolation of plasma membranes in high yield from cultured fibroblasts. Biochem J. 1977 Nov 15;168(2):187–194. doi: 10.1042/bj1680187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Bell J. R., Chen E. Y., Herrera R., Petruzzelli L. M., Dull T. J., Gray A., Coussens L., Liao Y. C., Tsubokawa M. Human insulin receptor and its relationship to the tyrosine kinase family of oncogenes. 1985 Feb 28-Mar 6Nature. 313(6005):756–761. doi: 10.1038/313756a0. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Coussens L., Hayflick J. S., Dull T. J., Gray A., Tam A. W., Lee J., Yarden Y., Libermann T. A., Schlessinger J. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. 1984 May 31-Jun 6Nature. 309(5967):418–425. doi: 10.1038/309418a0. [DOI] [PubMed] [Google Scholar]
- Wang J. Y., Baltimore D. Localization of tyrosine kinase-coding region in v-abl oncogene by the expression of v-abl-encoded proteins in bacteria. J Biol Chem. 1985 Jan 10;260(1):64–71. [PubMed] [Google Scholar]
- Waterfield M. D., Mayes E. L., Stroobant P., Bennet P. L., Young S., Goodfellow P. N., Banting G. S., Ozanne B. A monoclonal antibody to the human epidermal growth factor receptor. J Cell Biochem. 1982;20(2):149–161. doi: 10.1002/jcb.240200207. [DOI] [PubMed] [Google Scholar]
- Weinmaster G., Zoller M. J., Smith M., Hinze E., Pawson T. Mutagenesis of Fujinami sarcoma virus: evidence that tyrosine phosphorylation of P130gag-fps modulates its biological activity. Cell. 1984 Jun;37(2):559–568. doi: 10.1016/0092-8674(84)90386-6. [DOI] [PubMed] [Google Scholar]







