Abstract
The concept that oogenesis continues into reproductive life has been well established in nonmammalian species. Recent studies of mice and women indicate that oocyte formation is also not, as traditionally believed, restricted to the fetal or perinatal periods. Analogous to de novo oocyte formation in flies and fish, newly formed oocytes in adult mammalian ovaries arise from germline stem cells (GSCs) or, more specifically, oogonial stem cells (OSCs). Studies of mice have confirmed that isolated OSCs, once delivered back into adult ovaries, are capable of generating fully functional eggs that fertilize to produce healthy embryos and offspring. Parallel studies of OSCs recently purified from ovaries of reproductive-age women indicate that these cells closely resemble their mouse ovary–derived counterparts, although the fertilization competency of oocytes generated by human OSCs awaits clarification. Despite the ability of OSCs to produce new oocytes during adulthood, oogenesis will still ultimately cease with age, contributing to ovarian failure. The causal mechanisms behind these events in mammals are unknown, but studies of flies have revealed that GSC niche dysfunction plays a critical role in age-related oogenic failure. Such insights derived from evaluation of nonmammalian species, in which postnatal oogenesis has been studied in depth, may aid in development of new strategies to alleviate ovarian failure and infertility in mammals.
Keywords: germline stem cell, oogonial stem cell, oogenesis, oocyte, ovary, human
A rapidly expanding body of work indicates that active formation of oocytes and follicles in ovaries of adult females during the reproductive period is a fundamental biological process that has been conserved through evolution.1–11 In species such as flies and fish, where maternal investment is limited and a single female is capable of generating hundreds to thousands of offspring in a single lifespan, it is logical to assume and has been well documented that oogenesis continues during adulthood.1–4 By comparison, in animals that produce a comparatively limited number of offspring, including mammals, conventional theory has been that females of these species are born with a nonrenewable population of oocytes that declines in number throughout the reproductive period until the oocyte pool is eventually exhausted.12 In humans, these events drive the menopausal transition. Although it has been hypothesized that selection pressure (or a lack thereof in later years) may have played a role in the evolution of an abrupt decline in reproductive function with age in mammals, another theory is that ovarian failure occurs as a result of senescence based on both lifespan and a species-specific time frame for infant dependency.13
Irrespective of why it happens, ovarian failure with age in mammals should not, however, be taken at face value as de facto evidence of an absence of oogonial stem cells (OSCs) or oogenesis in adult ovaries. In fact, even in female flies where active renewal of oocytes during adulthood is uncontested, female germline stem cell (GSC) function becomes compromised to the point of failure well before the female fly dies of advanced age.2 This does not change the fact that during the prime reproductive period in female Drosophila, fresh eggs are constantly produced from to maximize the opportunity for reproductive success.1 This example of evolutionary pressure to ensure the presence of fresh gametes for reproduction is not only conserved in male flies, which actively produce new sperm during adulthood through male GSCs (also referred to as spermatogonial stem cells, or SSCs), but in adult males of all animal species studied to date including humans.14–17 After all, what possible advantage would be gained for mammalian males to diverge during evolution and start generating all the sperm they will ever have during the perinatal period rather than keep to the course of producing fresh sperm on a daily basis? In females of long-lived mammalian species, such as humans and elephants that can reproduce into the fifth and seventh decades of life, respectively, the dogma of a nonrenewing pool of oocytes being endowed at birth would equate to eggs fertilized toward the end of reproductive life being decades old.12 From both evolutionary and practical perspectives, it is unclear why, if not entirely illogical, that mammalian females would have developed such a divergent reproductive strategy from females of other so-called less evolved taxa and males of their own species.
In this regard, recent studies from multiple independent research groups have collectively begun to paint a much different picture of postnatal oocyte dynamics in mammals that includes oogenesis as a critical feature, thus aligning more closely with a basic reproductive tenet established early in evolution for both sexes.4–11,18 Specifically, several groups have succeeded in isolating OSCs from neonatal and adult mouse ovaries, and they have established these cells successfully in long-term cultures for characterization.8,10,11 Importantly, two groups have extended their in vitro observations to mapping the in vivo fate of mouse OSCs once transplanted back into adult ovary tissue. These latter studies consistently demonstrate that OSCs generate fully functional eggs that fertilize to produce healthy embryos and offspring.8,11 Importantly, using cell isolation and xenograft-based transplantation studies, we have recently extended the outcomes of these mouse studies to human females during reproductive life.11,19 Together, these data build a strong case that adult mammalian females are in fact capable of oogenesis and de novo follicle formation, not unlike their nonmammalian counterparts.
Oogenesis in Adult Flies
Although female GSC function has been reported for insect species other than flies,20 a simple but highly organized anatomical structure and relatively short lifespan, combined with ease of genetic manipulation, have made Drosophila one of the most studied and best defined animal models for the analysis of adult oogenesis and GSC aging.1,2,21–23 Drosophila ovaries contain both female GSCs and somatic follicle stem cells (FSCs) that together continually produce new oocyte-containing follicles during the adult reproductive period.24 Drosophila ovaries are structurally well defined, with each gonad consisting of approximately 18 ovarioles that serve as the functional follicle-producing units of the adult fly ovary. Spatially, follicle generation begins at the anterior pole of the ovariole in a specialized structure termed the germarium, which contains both GSCs and FSCs in their respective niches. In each germarium, two to three GSCs are present and attached to somatic cap cells by adherens junctions.25 Initiation of follicle formation occurs when a GSC undergoes an asymmetric division, giving rise to two daughter cells, a GSC and a cystoblast. Whereas the newly formed GSC remains in contact with the cap cells and undergoes quiescence for the remainder of the cycle, the cystoblast and its subsequent daughter cells, which are surrounded by a thin layer of somatic escort cells, undergo four successive mitoses. This process results in the formation of 16 germline cysts that are linked by ring canals interconnected by a spectrin-based cytoplasmic matrix called the fusome.26–28 Of the 16 cells in these germline cysts, only one differentiates into an oocyte; the remaining 15 cells become nurse cells. The exact mechanisms responsible for determining which germ cell in a cyst will differentiate into an oocyte are unknown; however, it has been hypothesized that the germ cell containing the most fusome material will ultimately develop into the oocyte.29 Whatever the case, follicles are formed when the cystoblasts shed the escort layer and acquire a somatic follicle cell layer derived from the FSCs. The newly formed follicles will continue to grow and eventually bud from the germarium.
Interestingly, female fecundity declines with age in Drosophila despite the presence of GSCs and active oogenesis during adulthood. Even more striking is the observation that both the rate of oocyte production per day as well as egg quality progressively diminish with age, with the decline in peak egg production beginning 3 to 9 days after eclosion and continuing through adulthood to the point of failure.2,22,30 It has been suggested that the decrease in oogenesis occurs due to a decline in germ cell division, although it is not known if this postulated replicative senescence of GSCs is cell autonomous or results from changes in extrinsic factors, such as a lack of supportive cues from niche cells.31,32 Although both events may play a role, experimental evidence clearly indicates that ovarian niches in aging female flies no longer provide adequate support for stem cell growth and differentiation. For example, during follicle formation, the cap cells play a central role in suppressing the differentiation of GSCs into cytoblasts through the secretion of bone morphogenetic protein (BMP)-like factors, such as decapentaplegic (Dpp). These factors act on adjacent GSCs to promote self-renewal and inhibit differentiation through suppression of bag of marbles (bam) gene expression. Consequently, following a GSC mitotic division, the daughter cell in direct contact with the cap cells remains a GSC, whereas the daughter cell one cell length away from the cap cells no longer receives the inhibitory signal, expresses Bam, and differentiates into a cytoblast. Thus, proximity of a GSC to its niche cap cells is a critical determinant of germ cell fate. This proximity is maintained by cell adhesion via E-cadherin.25 As niche cells age, both Dpp signaling initiated from the cap cells as well as E-cadherin–mediated adhesion in the junctions decrease, leading to a loss of GSC activity. This age-dependent decline can be rescued experimentally by overexpressing either BMP-like ligands in cap cells or E-cadherin in GSCs,23 indicating a contributory loss of function in both niche cells and GSCs in aged Drosophila ovaries. Should these types of mechanisms be conserved in vertebrate species, insights into female GSC function obtained from genetic studies of flies may provide a basic framework on which new strategies to alleviate age-associated ovarian failure in mammals could be built.
Given the importance of cell adhesion and spatial (niche) proximity to GSC-supported oogenesis in Drosophila, development of culture systems to test these types of signaling strategies in vitro will be far more involved than simply adding or subtracting growth factors and measuring a given outcome. For example, in vitro analysis of BMP-like ligand signaling in Drosophila S2 cells, a hemacyte-derived cell line, has demonstrated that BMP-like factors cooperate with Dally, a coreceptor for BMP-like ligands, to signal in-trans from neighboring cells in vitro.33 In the fly ovary, Dally is normally expressed in cap cells, and it is essential for GSC maintenance.34 When so-called signal-receiving S2 cells are transfected to express Mad, which is the signaling target for Dpp, and then co-cultured with a set of so-called signal-sending S2 cells transfected to express both Dally and Dpp, expression of Dally greatly enhances Mad signaling in the signal-receiving cells.33 These data demonstrate how proximity to the niche not only provides female GSCs with secreted signals required for the suppression of differentiation, but that cell-to-cell contact provides an additional layer of regulatory control over GSC division and differentiation. In aged female flies, these critical cell-to-cell contacts are lost, leading to impaired GSC division and eventually a cessation of oogenesis.2,22
Adult Oogenesis from Flies to Fish
Although comparably much less is known about the function of female GSCs in vertebrate species, OSCs have been identified through both histologic and functional assays in several teleost fish species.3,4,35–43 Most notably, OSCs have recently been shown to generate new oocytes in the ovaries of adult medaka,3,4 in a manner very similar to what has been described in female Drosophila.1 Using medaka genetically modified to express nanos2-driven enhanced green fluorescent protein (nos2p-EGFP) to track germ cells or sox9b-driven EGFP (sox9bp-EGFP) to track somatic cells, Nakamura and colleagues traced the division and differentiation of OSCs and somatic follicle cells throughout the process of oocyte formation in adult ovaries.3 To confirm that OSCs give rise to fertilizable oocytes, lineage tracing was also performed using a dual reporter system, in which heat shock leads to transient expression of EGFP in nanos2-expressing OSCs followed by stable expression of EGFP driven by the 3′-untranslated region of olvas, the medaka homolog of the Drosophila germline-specific gene vasa. Analysis of EGFP-expression after heat shock revealed a progressive pattern of oocyte differentiation in adult ovaries, in which oogenesis begins within the germinal cradles composed of pockets of OSCs nested within interstitially connected sox9b-expressing somatic cells. The OSCs divide and form mitotic cyst cells, some of which enter meiosis and ultimately form fertilizable oocytes.3 Structurally akin to the germarium in adult ovaries of Drosophila, the medaka model for oogenesis is the first to functionally demonstrate that the concept of reproductive potential being reliant on new oocytes derived from female GSCs during adulthood is conserved from invertebrates to vertebrates.4 Indeed, based on their observations, Nakamura and colleagues concluded that “these similarities might reflect a fundamental process governing oogenesis across animal species.”3
Although the importance of OSCs to adult egg formation and reproduction is now clear in medaka, and likely to be experimentally extended to additional teleost species, how the somatic environment influences OSC division and differentiation remains to be determined in vertebrates. However, the somatic environment must play a critical role in oogenesis in fish species because the regenerative capacity of the entire organ has been well documented. For example, experiments to determine whether or not ovariectomy could serve to sterilize Chinese grass carp revealed that the remaining ovarian tissue was capable of regeneration.44 Similarly, ovariectomy is followed by ovarian regeneration in rainbow trout.45 Recently, using a zebrafish transgenic model for selective oocyte ablation during adulthood, White and colleagues demonstrated that targeted elimination of oocytes leads to a rapid degeneration of the ovary, although the ovarian remnants still contained oocyte precursor cells and somatic cells.46 When the animals were allowed to recover, not only did oocytes regenerate and repopulate the ovary, but the somatic cells differentiated into follicular cells and continued to participate in follicle formation.46 Whether or not the activity of OSCs in vertebrate species is, like Drosophila, tightly regulated by a somatic niche remains to be determined. Nevertheless, it is clear that there is, at minimum, some degree of synchronicity between OSCs and their adjacent somatic cell environment that is required for oogenesis and folliculogenesis during adult life in vertebrates.
Moving Up through Evolution: Postnatal Oogenesis in Mice
The possible existence and function of OSCs within adult ovaries of mammalian species were topics of considerable debate among many scientists throughout the first half of the 20th century. In 1945, a comprehensive review of the literature both for and against the idea of continued oogenesis in adult mammals by Everett revealed that much of this debate actually reflected a difference in personal views by “two or more competent investigators who have studied the same form” over how comparable data sets should be interpreted.47 In addition, studies of nonmammalian species, such as castration experiments in adult chickens that documented ovarian regeneration followed by even greater rates of egg production than in noncastrate controls,48 were largely ignored by those who favored the idea of a nonrenewable oocyte pool in mammals. A significant turning point in this debate occurred in 1951, with a publication from Zuckerman that essentially set the dogma for the next 50 or so years that the mammalian oocyte pool is fixed at birth with no prospects of renewal.12 Interestingly, no evidence was provided by Zuckerman in this publication, actually proving that postnatal ovaries in mammals are incapable of oogenesis. Instead, the entire premise of his conclusion, as he acknowledged many years later,49 was rooted simply in his own personal beliefs that no data available at the time were inconsistent with a finite oocyte reserve best set forth during the perinatal period. This conclusion was accepted with little question or resistance from the field, despite the fact that experimental evidence arguing against Zuckerman’s conclusions in 1951 not only existed in past studies from others but continued to be published over the following several years.50–57
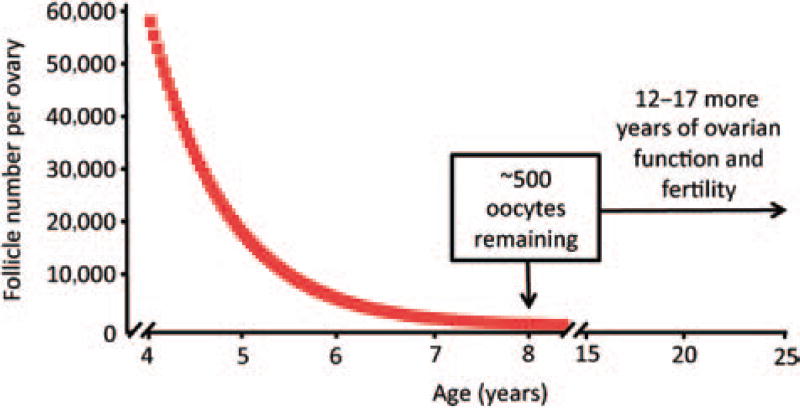
For example, in an elegant investigation of follicular dynamics in adult female rhesus monkeys reported in 1956, Vermande-Van Eck studied three key variables necessary for accurate mathematical modeling of how follicle numbers change over time: the total number of oocyte-containing follicles, the incidence of follicle loss via atresia, and the rate of clearance of atretic follicles.56 In female monkeys at or just after sexual maturity, the total reserve of follicles was estimated at close to 60,000 per ovary. The incidence of atresia in the same ovarian samples was determined to be ~4.5%, with clearance of atretic immature follicles—assessed by synchronous activation of atresia with low-level radio-therapy—occurring over a span of no more than 2 weeks.56 If one plots these three variables in a decay curve, the resultant prediction is that >90% of the total ovarian reserve of oocytes present at puberty will be lost within 2 years and <1% will remain by 8 years of age (Fig. 1). Because rhesus monkeys reach puberty ~4 years of age and exhibit ovarian function until at least 20 to 25 years of age,58 the model of oocyte loss just laid out is completely at odds with what actually occurs, unless the rate of oocyte loss through follicular atresia during adulthood is partially counterbalanced by input of new oocyte-containing follicles into the reserve.
Figure 1.
Mathematical decay curve depicting the rate of oocyte loss from ovaries of young adult rhesus monkeys. Past work from Vermande-Van Eck published in 1956 reported that female rhesus monkeys at or just after sexual maturation (~4 years of age) possess ~58,000 oocyte-containing follicles per ovary, with atresia occurring at an incidence of 4.5% and clearance of atretic follicles completed within 14 days.56 Plotting these three variables in a mathematical decay curve, in the absence of any new oocyte input, predicts that >90% of the oocytes present at puberty will be eliminated by the time these females reach 6 years of age and <500 oocytes will remain at 8 years of age. Such an outcome is highly discordant with the fact that female rhesus monkeys exhibit ovarian function and natural fertility at ≤20 years of age. The most logical explanation for this observation is that the loss of oocytes through follicular atresia is partly offset by de novo oogenesis and folliculogenesis during adulthood, to allow maintenance of ovarian function through 20 to 25 years of age.
Studies such as these failed to get the widespread recognition they deserved and ultimately became buried in a huge volume of historical literature on the topic of oocyte and follicle dynamics in mammals as the decades passed. Fortunately, such work was brought to our attention shortly following the publication of our paper in 2004, which concluded from studies of mice that oogenesis does not cease during the perinatal period and OSCs are important contributors to maintenance of the postnatal follicle reserve.5 Among many approaches used to arrive at this conclusion, assessment of changes in healthy follicle numbers over time versus direct counts of atretic follicles and their clearance rates produced the same mathematical dilemma arrived at in 1956 by Vermande-Van Eck in her studies of adult primate ovaries.56 The rate of atresia far exceeded the actual net decline in the healthy follicle pool as female mice transitioned through the period of sexual maturation.5 After seeing this new report nearly 50 years after her pioneering work with monkeys, Vermande-Van Eck shared her findings with us and urged us to keep moving forward in an effort to hopefully one day convince the “OB/GYN community that oogenesis must also occur in the human female” (Fig. 2).
Figure 2.
Personal correspondence from Gertrude Vermande (Van Eck) following publication of our paper in 2004, which challenged a principal dogma in the field of reproduction by concluding that oogonial stem cells exist in adult mouse ovaries and actively contribute to oocyte and follicular renewal.5 This example serves as a reminder of the importance of historical observations to the interpretation of more contemporary studies.
Not surprisingly, this challenge to the validity of traditional beliefs that female mammals are incapable of oocyte production during postnatal life had a polarizing effect on the field of reproductive biology, with many scientists expressing skepticism if not outright disbelief.18,59–67 Nevertheless, additional reports questioning the dogma soon followed, including an independent study published in 2009 demonstrating that OSCs could be isolated from neonatal and adult mouse ovaries, propagated in vitro for months, and then transplanted into ovaries of chemotherapy-conditioned adult female mice for functional testing.8 In addition to participating in de novo oogenesis and follicle formation in vivo, oocytes derived from the transplanted OSCs, which were engineered to express green fluorescent protein (GFP) for cell tracking, matured normally, ovulated, and fertilized in natural mating trials to produce viable healthy offspring.8 In effect, this study not only independently confirmed what had been previously concluded—mammalian ovaries possess a rare population of premeiotic germline cells that can support oogenesis and folliculogenesis during adulthood5—but also quickly silenced much of the skepticism surrounding 5 years of prior work on OSCs and postnatal oogenesis in mammals by others. Importantly, additional independent confirmation that OSCs exist in, and can be purified from, postnatal mouse ovaries came the following year. Using a transgenic mouse line with germ cell–specific expression of GFP, Pacchiarotti and colleagues purified OSCs and established the cells in vitro for long-term propagation.10 In addition to confirming that these cells can be stably expanded for months, this study also reported that cultured mouse OSCs spontaneously generate what appear to be oocytes in vitro. Interestingly, aggregation of oocytes derived from OSCs in vitro with granulosa cells obtained from neonatal mouse ovaries resulted in the formation of primordial follicle-like structures that closely resembled follicles that were newly formed from endogenous oocytes recombined with granulosa cells in parallel.10
Building on this expanding body of evidence supporting the existence of OSCs in mice, White and colleagues reported the development and validation of a fluorescence-activated cell sorting (FACS)-based approach for purification of OSCs from adult mouse ovaries.11 The cells obtained were homogenous in size, morphology, and expression of classic markers of primitive germ cells including Prdm1 (Blimp1), Dppa3 (Stella), Ifitm3 (Fragilis), and Tert (catalytic subunit of telomerase). After in vitro establishment, the isolated OSCs actively divided for months while maintaining uniform expression of primitive germline markers. In addition, and consistent with prior observations,10 oocytes were spontaneously formed from mouse OSCs maintained in culture, as deduced by morphology, expression of oocyte-specific markers, and progression through meiosis to generate 1n (haploid) cells.11 For functionality testing, mouse OSCs were transduced with a GFP expression vector for cell tracking and then injected into ovaries of young adult female mice. In contrast to prior studies in which chemotherapy-conditioned female mice were used to demonstrate that intraovarian injection of mouse GFP-expressing OSCs generate eggs that fertilize and produce viable offspring,8 White and colleagues did not damage the ovaries with cytotoxic drugs prior to OSC transplantation. Assessment of these mice several months later revealed that GFP-positive oocytes were still being generated, and superovulation of the transplanted females yielded green metaphase II eggs that fertilized to produce hatching blastocysts.11 Hence with three different research groups now independently reporting the successful isolation of OSCs from mouse ovaries over the past few years using three different strategies,8,10,11 there should no longer be lingering doubts in the field over their existence. However, the in vivo significance of OSCs as active contributors to function of adult mammalian ovaries under physiologic conditions remains to be established.
The Final Step: Purification of OSCs from Women
Armed with an extensively validated FACS-based protocol for isolation of OSCs from adult mouse ovarian tissue, White and colleagues recently reported the successful isolation of OSCs from the ovaries of reproductive-age women using this same method.11 Through parallel comparative studies to adult mouse ovary-derived OSCs, the human equivalent cells were thoroughly characterized with respect to the presence of a primitive germline gene expression signature, capacity to undergo stable ex-vivo expansion in defined cultures for months, in-vitro oogenic potential and, through GFP-based tracking protocols, to generate new oocytes that become enveloped within granulosa cells as primordial follicles in adult human ovarian cortical tissue xenografted in mice as an in vivo model of human oogenesis.11 For the latter experiments, oocytes formed from human GFP-expressing OSCs injected into adult human ovarian cortex were indistinguishable from existing primordial oocytes in terms of size, morphology, and the spatially correct expression of oocyte markers including YBX2 (also referred to as MSY2), which is restricted to germ cells at the diplotene stage of meiosis.68,69
Even with this remarkable parallelism to every characteristic feature of mouse OSCs tested, one critical question remains unanswered regarding human OSCs. Do these cells, like their counterparts purified from adult mouse ovaries,8,11 generate oocytes that are fully competent to complete maturation and successfully fertilize? Although relatively straight-forward to evaluate in female mice, addressing this question with human OSCs presents several hurdles, both methodological and ethical, with the latter obvious in any situation involving fertilization of human eggs for experimental purposes. The actual generation of metaphase II human eggs from OSCs is likely to be even more complicated because there are currently no reports of the successful formation of human eggs entirely ex vivo, even if preformed oocytes contained in early stage (primordial and primary) follicles are used as starting material. Nonetheless, encouraging progress has been made over the past several years in the development of in vitro human follicle culture systems that have produced mature antral follicles from primordial follicles through a multistep process.70,71 If such methods are ultimately shown to yield mature human eggs in vitro, combining the now proven ability of human OSCs to form oocytes contained in primordial follicles in adult human ovarian cortex with culture strategies that utilize human primordial follicles as the starting material for the generation of metaphase II eggs ex vivo should be feasible.19
Conclusions and Future Considerations
Less than a decade ago, essentially everyone involved in the field of reproductive biology and medicine, including ourselves, worked under the belief that mammalian females lose the capacity for oocyte renewal during the perinatal period, leading to the endowment of a fixed bank account of oocytes at birth. Through serendipitous observations that arose from our efforts to better define the contribution of oocyte death (apoptosis) to postnatal follicular dynamics, we uncovered a surprising discordance in the net decline in healthy oocyte numbers per ovary as neonatal mice transitioned into adulthood versus a much higher rate of oocyte loss if one actually measures the incidence of follicular atresia over this same time period.5 Unbeknownst to us at the time, a similar discordance was reported nearly 50 years earlier, which demonstrated in adult rhesus monkeys that mathematical modeling of postpubertal oocyte depletion with new oocyte input not included as a variable predicted >90% loss in ~2 years, when in fact rhesus ovaries actually retain normal function for ≤20 years.56 Despite the compelling nature of these arguments, skepticism over postnatal oogenesis in mammals remained high until OSCs were eventually purified from mouse ovaries by three different groups between 2009 and 2012, with the latest study simultaneously reporting the successful isolation of the equivalent cells from adult human ovaries.8,10,11
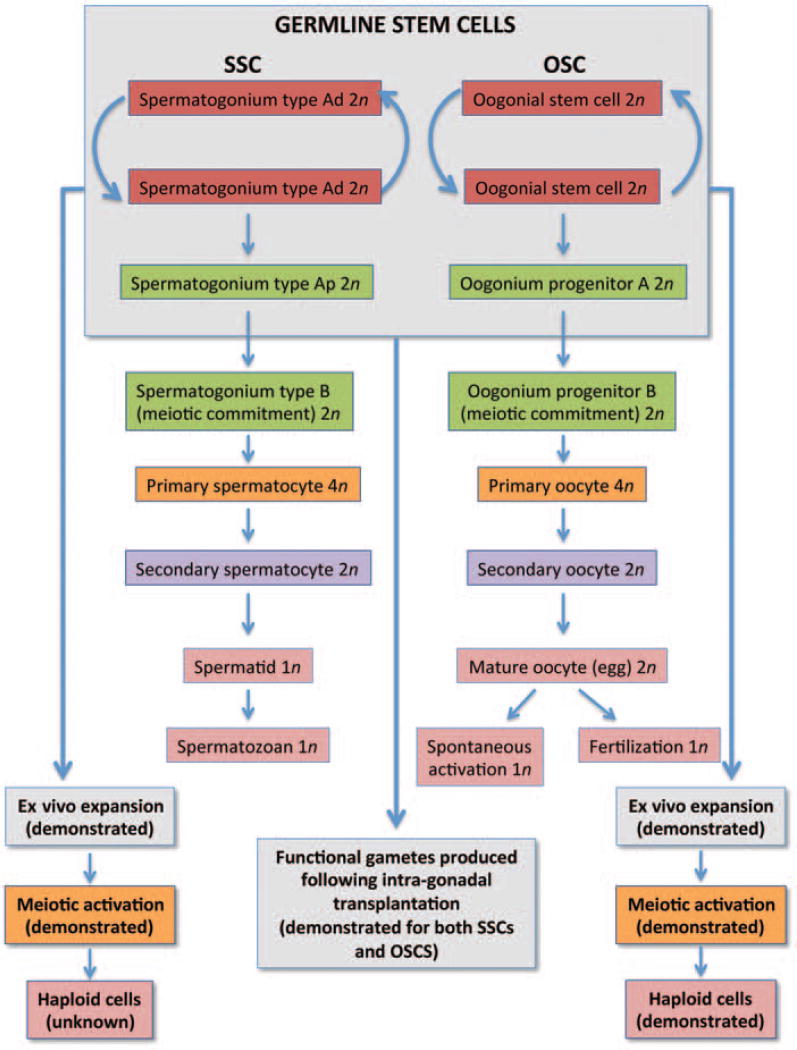
If one considers the approaches used to document the identity of SSCs in males over the past 4 decades, comparable outcomes have already been achieved with mammalian OSCs in just a fifth the time, even with progress periodically stalled due to considerable resistance from the field. Two main properties of SSCs isolated from adult testis tissue are the ability of these cells to undergo stable ex vivo expansion (mitotic activity) and to generate fertilization-competent sperm (functional potential) once transplanted back into adult testes. Both of these experimental end points have now been accomplished with mammalian OSCs, allowing a strong parallel to be drawn between the two cell types (Fig. 3). It is also noteworthy that despite many years of work by numerous laboratories, successful in vitro propagation of SSCs collected from adult human testes, which have been identified since the late 1970s,14 has only very recently been achieved.72,73 Moreover, the formation of haploid cells in cultures of SSCs from any species has not yet been reported. Both of these end points have already been accomplished for cultured mouse and human OSCs (Fig. 3). And although the progression of meiosis in oocytes generated in vitro by OSCs is in all likelihood aberrant due to the fact that these cells are lacking critical cues normally received from their intimately associated granulosa cells in vivo, this new germ cell culture system nonetheless offers a defined model for in-depth exploration of human female gametogenesis.11 In addition, results obtained from future studies using this human female germ cell culture system will likely be more physiologically relevant than observations made from differentiating cultures of human embryonic stem cells or induced pluripotent stem cells.74–76
Figure 3.
Comparison of mammalian gametogenesis and germline stem cell function in the gonads of adult males and adult females. In most if not all studies, spermatogonial stem cells (SSCs) are isolated as a mix of type A dark (Ad) and type A pale (Ap) spermatogonia. Once isolated, SSCs can be stably expanded ex vivo, although the in vitro generation of haploid (1n) cells from cultured SSCs of any species has not yet been reported. Functionality of SSCs is classically assessed through intratesticular transplantation protocols, in which transplanted SSCs carrying a traceable marker gene repopulate chemotherapy-damaged seminiferous tubules to produce fertilization-competent spermatozoa with the donor cell haplotype. In females, oogonial stem cells (OSCs) isolated from adult mouse and human ovaries can also be stably expanded ex vivo, although little is currently known of the intermediate stages of germ cells formed as OSCs undergo expansion and ultimately differentiation through meiosis. Accordingly, possible OSC progenitors A and B have been depicted by drawing parallels to SSC biology. In addition to evidence of stable ex vivo expansion, the generation of haploid germ cells in cultures of mouse and human OSCs has also been reported. In studies comparable with those used to test SSC functionality, transplantation of OSCs carrying green fluorescent protein (GFP) as a traceable marker gene into adult wild-type mouse ovaries, with or without prior chemotherapy conditioning, results in the formation of GFP-positive oocytes that mature, ovulate, and fertilize to produce viable embryos and offspring.
Finally, the discovery that cells comparable with those used by other organisms, such as flies and fish, for the generation of new eggs during reproductive life also exist in the ovaries of mammals is important but still only an early step. Much more work is needed to understand in what ways these cells contribute to the function, and perhaps age-related failure, of the ovaries, as well as to define the hormones and signaling pathways used by mammalian OSCs to control their mitotic activity and oogenic differentiation potential.19,77 On a more fundamental level, information provided by studies of GSCs in model organisms such as Drosophila may provide important clues into evolutionarily conserved processes that will facilitate future studies of mammalian OSCs. For example, as mentioned earlier, ovarian stem cell niche deterioration is a critical facet of Drosophila GSC failure with age. Importantly, the decline in Drosophila GSC activity in aging females manifests not only as an abrupt drop in egg production rates but also as a dramatic reduction in the quality of the limited number of eggs that are still being produced.2 Hence the widespread belief that oocytes of women at advanced reproductive ages become functionally compromised simply as a result of decades of accumulated damage may require rethinking. More specifically, if basic processes that control egg formation in Drosophila during adulthood are indeed conserved in mammalian females, the progressive decline in oocyte competency known to occur in women as they age may not reflect old eggs per se. Rather, these observations may be due to a progressive breakdown in quality control associated with an oocyte production pipeline driven by OSCs that, in aging ovaries, fail to receive appropriate guidance signals from their microenvironment necessary for proper differentiation. Whether such a hypothesis will ultimately impact on our understanding of human ovarian aging is at present unclear; however, the testing of such a hypothesis, like many others aimed at understanding the biology of mammalian OSCs, should be encouraged by the scientific community rather than bear the brunt of dogmatic criticism before the needed experiments have even been started.78
Acknowledgments
Work conducted by the authors discussed herein was supported by a Method to Extend Research in Time (MERIT) Award from the National Institute on Aging (NIH R37-AG012279 to Jonathan Tilly), a Ruth L. Kirschstein National Research Service Award (NIH F32-AG034809 to Dori Woods), the Henry and Vivian Rosenberg Philanthropic Fund, the Sea Breeze Foundation, and Vincent Memorial Hospital Research Funds.
Footnotes
Competing Financial Interests
Dori Woods declares interest as a scientific consultant for OvaScience, Inc. (Cambridge, MA). Jonathan Tilly declares interest in intellectual property described in U.S. Patent 7,955,846 and is a cofounder of OvaScience, Inc.
References
- 1.Kirilly D, Xie T. The Drosophila ovary: an active stem cell community. Cell Res. 2007;17(1):15–25. doi: 10.1038/sj.cr.7310123. [DOI] [PubMed] [Google Scholar]
- 2.Zhao R, Xuan Y, Li X, Xi R. Age-related changes of germline stem cell activity, niche signaling activity and egg production in Drosophila. Aging Cell. 2008;7(3):344–354. doi: 10.1111/j.1474-9726.2008.00379.x. [DOI] [PubMed] [Google Scholar]
- 3.Nakamura S, Kobayashi K, Nishimura T, Higashijima S, Tanaka M. Identification of germline stem cells in the ovary of the teleost medaka. Science. 2010;328(5985):1561–1563. doi: 10.1126/science.1185473. [DOI] [PubMed] [Google Scholar]
- 4.Nakamura S, Kobayashi K, Nishimura T, Tanaka M. Ovarian germline stem cells in the teleost fish, medaka (Oryzias latipes) Int J Biol Sci. 2011;7(4):403–409. doi: 10.7150/ijbs.7.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson J, Canning J, Kaneko T, Pru JK, Tilly JL. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature. 2004;428(6979):145–150. doi: 10.1038/nature02316. [DOI] [PubMed] [Google Scholar]
- 6.Johnson J, Bagley J, Skaznik-Wikiel M, et al. Oocyte generation in adult mammalian ovaries by putative germ cells in bone marrow and peripheral blood. Cell. 2005;122(2):303–315. doi: 10.1016/j.cell.2005.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niikura Y, Niikura T, Tilly JL. Aged mouse ovaries possess rare premeiotic germ cells that can generate oocytes following transplantation into a young host environment. Aging (Albany, NY Online) 2009;1(12):971–978. doi: 10.18632/aging.100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zou K, Yuan Z, Yang Z, et al. Production of offspring from a germline stem cell line derived from neonatal ovaries. Nat Cell Biol. 2009;11(5):631–636. doi: 10.1038/ncb1869. [DOI] [PubMed] [Google Scholar]
- 9.Wang N, Tilly JL. Epigenetic status determines germ cell meiotic commitment in embryonic and postnatal mammalian gonads. Cell Cycle. 2010;9(2):339–349. doi: 10.4161/cc.9.2.10447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pacchiarotti J, Maki C, Ramos T, et al. Differentiation potential of germ line stem cells derived from the postnatal mouse ovary. Differentiation. 2010;79(3):159–170. doi: 10.1016/j.diff.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 11.White YAR, Woods DC, Takai Y, Ishihara O, Seki H, Tilly JL. Oocyte formation by mitotically active germ cells purified from ovaries of reproductive-age women. Nat Med. 2012;18(3):413–421. doi: 10.1038/nm.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zuckerman S. The number of oocytes in the mature ovary. Recent Prog Horm Res. 1951;6:63–108. [Google Scholar]
- 13.Packer C, Tatar M, Collins A. Reproductive cessation in female mammals. Nature. 1998;392(6678):807–811. doi: 10.1038/33910. [DOI] [PubMed] [Google Scholar]
- 14.Schulze C. Morphological characteristics of the spermatogonial stem cells in man. Cell Tissue Res. 1979;198(2):191–199. doi: 10.1007/BF00232003. [DOI] [PubMed] [Google Scholar]
- 15.Lin H. The stem-cell niche theory: lessons from flies. Nat Rev Genet. 2002;3(12):931–940. doi: 10.1038/nrg952. [DOI] [PubMed] [Google Scholar]
- 16.Brinster RL. Male germline stem cells: from mice to men. Science. 2007;316(5823):404–405. doi: 10.1126/science.1137741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Cuevas M, Matunis EL. The stem cell niche: lessons from the Drosophila testis. Development. 2011;138(14):2861–2869. doi: 10.1242/dev.056242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tilly JL, Niikura Y, Rueda BR. The current status of evidence for and against postnatal oogenesis in mammals: a case of ovarian optimism versus pessimism? Biol Reprod. 2009;80(1):2–12. doi: 10.1095/biolreprod.108.069088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woods DC, Tilly JL. The next (re)generation of human ovarian biology and female fertility: is current science tomorrow’s practice? Fertil Steril. 2012;98(1):3–10. doi: 10.1016/j.fertnstert.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tworzydlo W, Kloc M, Bilinski SM. Female germline stem cell niches of earwigs are structurally simple and different from those of Drosophila melanogaster. J Morphol. 2010;271(5):634–640. doi: 10.1002/jmor.10824. [DOI] [PubMed] [Google Scholar]
- 21.Wong MD, Jin Z, Xie T. Molecular mechanisms of germline stem cell regulation. Annu Rev Genet. 2005;39:173–195. doi: 10.1146/annurev.genet.39.073003.105855. [DOI] [PubMed] [Google Scholar]
- 22.Waskar M, Li Y, Tower J. Stem cell aging in the Drosophila ovary. AGE. 2005;27:201–212. doi: 10.1007/s11357-005-2914-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pan L, Chen S, Weng C, et al. Stem cell aging is controlled both intrinsically and extrinsically in the Drosophila ovary. Cell Stem Cell. 2007;1:470–478. doi: 10.1016/j.stem.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 24.Nystul T, Spradling A. Regulation of epithelial stem cell replacement and follicle formation in the Drosophila ovary. Genetics. 2010;184(2):503–515. doi: 10.1534/genetics.109.109538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song X, Zhu CH, Doan C, Xie T. Germline stem cells anchored by adherens junctions in the Drosophila ovary niches. Science. 2002;296(5574):1855–1857. doi: 10.1126/science.1069871. [DOI] [PubMed] [Google Scholar]
- 26.Spradling AC. Germline cysts: communes that work. Cell. 1993;72(5):649–651. doi: 10.1016/0092-8674(93)90393-5. [DOI] [PubMed] [Google Scholar]
- 27.Lin H, Yue L, Spradling AC. The Drosophila fusome, a germline-specific organelle, contains membrane skeletal proteins and functions in cyst formation. Development. 1994;120(4):947–956. doi: 10.1242/dev.120.4.947. [DOI] [PubMed] [Google Scholar]
- 28.de Cuevas M, Lee JK, Spradling AC. α-spectrin is required for germline cell division and differentiation in the Drosophila ovary. Development. 1996;122(12):3959–3968. doi: 10.1242/dev.122.12.3959. [DOI] [PubMed] [Google Scholar]
- 29.Lin H, Spradling AC. Fusome asymmetry and oocyte determination in Drosophila. Dev Genet. 1995;16(1):6–12. doi: 10.1002/dvg.1020160104. [DOI] [PubMed] [Google Scholar]
- 30.David J, Cohet Y, Foluillet P. The variability between individuals as a measure of senescence: a study of the number of eggs laid and the percentage of hatched eggs in the case of Drosophila melanogaster. Exp Gerontol. 1975;10(1):17–25. doi: 10.1016/0531-5565(75)90011-x. [DOI] [PubMed] [Google Scholar]
- 31.Xie T, Spradling AC. A niche maintaining germline stem cells in the Drosophila ovary. Science. 2000;290(5490):328–330. doi: 10.1126/science.290.5490.328. [DOI] [PubMed] [Google Scholar]
- 32.Song X, Call GB, Kirilly D, Xie T. Notch signaling controls germline stem cell niche formation in the Drosophila ovary. Development. 2007;134(6):1071–1080. doi: 10.1242/dev.003392. [DOI] [PubMed] [Google Scholar]
- 33.Dejima K, Kanai MI, Akiyama T, Levings DC, Nakato H. Novel contact-dependent bone morphogenetic protein (BMP) signaling mediated by heparan sulfate proteoglycans. J Biol Chem. 2011;286(19):17103–17111. doi: 10.1074/jbc.M110.208082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hayashi Y, Kobayashi S, Nakato H. Drosophila glypicans regulate the germline stem cell niche. J Cell Biol. 2009;187(4):473–480. doi: 10.1083/jcb.200904118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wallace RA, Selman K. Ultrastructural aspects of oogenesis and oocyte growth in fish and amphibians. J Electron Microsc Tech. 1990;16(3):175–201. doi: 10.1002/jemt.1060160302. [DOI] [PubMed] [Google Scholar]
- 36.Selman K, Wallace R, Sarka A, Qi X. Stages of oocyte development in the zebrafish, Brachydanio rerio. J Morphol. 1993;218:203–224. doi: 10.1002/jmor.1052180209. [DOI] [PubMed] [Google Scholar]
- 37.Grier H. Ovarian germinal epithelium and folliculogenesis in the common snook, Centropomus undecimalis (Teleostei: centropomidae) J Morphol. 2000;243(3):265–281. doi: 10.1002/(SICI)1097-4687(200003)243:3<265::AID-JMOR4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 38.Lo Nostro F, Grier H, Andreone L, Guerrero GA. Involvement of the gonadal germinal epithelium during sex reversal and seasonal testicular cycling in the protogynous swamp eel, Synbranchus marmoratus Bloch 1795 (Teleostei, Synbranchidae) J Morphol. 2003;257(1):107–126. doi: 10.1002/jmor.10105. [DOI] [PubMed] [Google Scholar]
- 39.Abascal FJ, Medina A. Ultrastructure of oogenesis in the bluefin tuna, Thunnus thynnus. J Morphol. 2005;264(2):149–160. doi: 10.1002/jmor.10325. [DOI] [PubMed] [Google Scholar]
- 40.Draper BW, McCallum CM, Moens CB. nanos1 is required to maintain oocyte production in adult zebrafish. Dev Biol. 2007;305(2):589–598. doi: 10.1016/j.ydbio.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grier HJ, Uribe MC, Parenti LR. Germinal epithelium, folliculogenesis, and postovulatory follicles in ovaries of rainbow trout, Oncorhynchus mykiss (Walbaum, 1792) (Teleostei, protacanthopterygii, salmoniformes) J Morphol. 2007;268(4):293–310. doi: 10.1002/jmor.10518. [DOI] [PubMed] [Google Scholar]
- 42.Leu DH, Draper BW. The ziwi promoter drives germline-specific gene expression in zebrafish. Dev Dyn. 2010;239(10):2714–2721. doi: 10.1002/dvdy.22404. [DOI] [PubMed] [Google Scholar]
- 43.Yoshizaki G, Ichikawa M, Hayashi M, et al. Sexual plasticity of ovarian germ cells in rainbow trout. Development. 2010;137(8):1227–1230. doi: 10.1242/dev.044982. [DOI] [PubMed] [Google Scholar]
- 44.Underwood JL, Hestand RS, III, Thompson BZ. Gonad regeneration in grass carp following bilateral gonadectomy. Prog Fish-Cult. 1986;48:54–56. [Google Scholar]
- 45.Kersten CA, Krisfalusi M, Parsons JE, Cloud JG. Gonadal regeneration in masculinized female or steroid-treated rainbow trout (Oncorhynchus mykiss) J Exp Zool. 2001;290(4):396–401. doi: 10.1002/jez.1080. [DOI] [PubMed] [Google Scholar]
- 46.White YAR, Woods DC, Wood AW. A transgenic zebrafish model of targeted oocyte ablation and de novo oogenesis. Dev Dyn. 2011;240(8):1929–1937. doi: 10.1002/dvdy.22695. [DOI] [PubMed] [Google Scholar]
- 47.Everett NB. The present status of the germ-cell problem in vertebrates. Biol Rev Camb Philos Soc. 1945;20:40–45. [Google Scholar]
- 48.Pearl R, Schoppe WF. Studies on the physiology of reproduction in the domestic fowl. J Exp Zool. 1921;34:101–118. [Google Scholar]
- 49.Zuckerman S. The Frontiers of Public and Private Science. New York, NY: Taplinger; 1971. Beyond the Ivory Tower; pp. 22–34. [Google Scholar]
- 50.Arai H. On the postnatal development of the ovary (albino rat), with especial reference to the number of ova. Am J Anat. 1920;27:405–462. [Google Scholar]
- 51.Allen E. Ovogenesis during sexual maturity. Am J Anat. 1923;31:439–470. [Google Scholar]
- 52.Davenport CB. Regeneration of ovaries in mice. J Exp Zool. 1925;42:1–12. [Google Scholar]
- 53.Butcher EO. The origin of definitive ova in the white rat (Mus norvegicus albinus) Anat Rec. 1927;37:13–29. [Google Scholar]
- 54.Parkes AS, Fielding U, Brambell WR. Ovarian regeneration in the mouse after complete double ovariectomy. Proc R Soc Lond Biol Sci. 1927;101:328–354. [Google Scholar]
- 55.Pansky B, Mossman HW. The regenerative capacity of the rabbit ovary. Anat Rec. 1953;116(1):19–51. doi: 10.1002/ar.1091160104. [DOI] [PubMed] [Google Scholar]
- 56.Vermande-Van Eck GJ. Neo-ovogenesis in the adult monkey; consequences of atresia of ovocytes. Anat Rec. 1956;125(2):207–224. doi: 10.1002/ar.1091250205. [DOI] [PubMed] [Google Scholar]
- 57.Artem’eva NS. Regenerative capacity of the rat ovary after compensatory hypertrophy. Bull Exp Biol Med. 1961;51:76–81. [PubMed] [Google Scholar]
- 58.Nichols SM, Bavister BD, Brenner CA, Didier PJ, Harrison RM, Kubisch HM. Ovarian senescence in the rhesus monkey (Macaca mulatta) Hum Reprod. 2005;20(1):79–83. doi: 10.1093/humrep/deh576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gosden RG. Germline stem cells in the postnatal ovary: is the ovary more like a testis? Hum Reprod Update. 2004;10(3):193–195. doi: 10.1093/humupd/dmh023. [DOI] [PubMed] [Google Scholar]
- 60.Greenfeld C, Flaws JA. Renewed debate over postnatal oogenesis in the mammalian ovary. Bioessays. 2004;26(8):829–832. doi: 10.1002/bies.20094. [DOI] [PubMed] [Google Scholar]
- 61.Hoyer PB. Can the clock be turned back on ovarian aging? Sci SAGE KE. 2004;2004(10):pe11. doi: 10.1126/sageke.2004.10.pe11. [DOI] [PubMed] [Google Scholar]
- 62.Telfer EE. Germline stem cells in the postnatal mammalian ovary: a phenomenon of prosimian primates and mice? Reprod Biol Endocrinol. 2004;2:24. doi: 10.1186/1477-7827-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Albertini DF. Micromanagement of the ovarian follicle reserve—do stem cells play into the ledger? Reproduction. 2004;127(5):513–514. doi: 10.1530/rep.1.00247. [DOI] [PubMed] [Google Scholar]
- 64.Byskov AG, Faddy MJ, Lemmen JG, Andersen CY. Eggs forever? Differentiation. 2005;73(9–10):438–446. doi: 10.1111/j.1432-0436.2005.00045.x. [DOI] [PubMed] [Google Scholar]
- 65.Skaznik-Wikiel M, Tilly JC, Lee H-J, et al. Serious doubts over “Eggs forever?”. Differentiation. 2007;75(2):93–99. doi: 10.1111/j.1432-0436.2006.00117.x. [DOI] [PubMed] [Google Scholar]
- 66.Gougeon A. Neo-oogenesis in the postnatal ovary: fantasy or reality? [in French] Gynecol Obstet Fertil. 2005;33(10):819–823. doi: 10.1016/j.gyobfe.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 67.Powell K. Going against the grain. PLoS Biol. 2007;5(12):e338. doi: 10.1371/journal.pbio.0050338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gu W, Tekur S, Reinbold R, et al. Mammalianmale and female germ cells express a germcell-specific Y-Box protein, MSY2. Biol Reprod. 1998;59(5):1266–1274. doi: 10.1095/biolreprod59.5.1266. [DOI] [PubMed] [Google Scholar]
- 69.Yang J, Medvedev S, Yu J, et al. Absence of the DNA-/RNA-binding proteinMSY2 results in male and female infertility. Proc Natl Acad Sci U S A. 2005;102(16):5755–5760. doi: 10.1073/pnas.0408718102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Telfer EE, McLaughlin M, Ding C, Thong KJ. A two-step serum-free culture system supports development of human oocytes from primordial follicles in the presence of activin. Hum Reprod. 2008;23(5):1151–1158. doi: 10.1093/humrep/den070. [DOI] [PubMed] [Google Scholar]
- 71.Telfer EE, McLaughlin M. In vitro development of ovarian follicles. Semin Reprod Med. 2011;29(1):15–23. doi: 10.1055/s-0030-1268700. [DOI] [PubMed] [Google Scholar]
- 72.Sadri-Ardekani H, Mizrak SC, van Daalen SK, et al. Propagation of human spermatogonial stem cells in vitro. JAMA. 2009;302(19):2127–2134. doi: 10.1001/jama.2009.1689. [DOI] [PubMed] [Google Scholar]
- 73.Sadri-Ardekani H, Akhondi MA, van der Veen F, Repping S, van Pelt AM. In vitro propagation of human prepubertal spermatogonial stem cells. JAMA. 2011;305(23):2416–2418. doi: 10.1001/jama.2011.791. [DOI] [PubMed] [Google Scholar]
- 74.Ko K, Schöler HR. Embryonic stem cells as a potential source of gametes. Semin Reprod Med. 2006;24(5):322–329. doi: 10.1055/s-2006-952157. [DOI] [PubMed] [Google Scholar]
- 75.Nagano MC. In vitro gamete derivation from pluripotent stem cells: progress and perspective. Biol Reprod. 2007;76(4):546–551. doi: 10.1095/biolreprod.106.058271. [DOI] [PubMed] [Google Scholar]
- 76.Nicholas CR, Chavez SL, Baker VL, Reijo Pera RA. Instructing an embryonic stem cell-derived oocyte fate: lessons from endogenous oogenesis. Endocr Rev. 2009;30(3):264–283. doi: 10.1210/er.2008-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Telfer EE, Albertini DF. The quest for human ovarian stem cells. Nat Med. 2012;18(3):353–354. doi: 10.1038/nm.2699. [DOI] [PubMed] [Google Scholar]
- 78.Woods DC, White Y, Tilly JL. Purification of oogonial stem cells from adult mouse and human ovaries: an assessment of the literature and a view toward the future. Reprod Sci. 2013;20(1):7–15. doi: 10.1177/1933719112462632. [DOI] [PMC free article] [PubMed] [Google Scholar]