Abstract
Kisspeptin, encoded by the Kiss1 gene, is required for reproduction. Humans and mice lacking kisspeptin or its receptor, Kiss1r, have impairments in reproductive physiology and fertility. In addition to being located in the hypothalamus in the anteroventral periventricular and arcuate nuclei, kisspeptin neurons are also present in several extra-hypothalamic regions, such as the medial amygdala (MeA). However, while there has been a significant focus on the reproductive roles of hypothalamic kisspeptin neurons, the regulation and function(s) of MeA and other extra-hypothalamic kisspeptin neurons have received far less attention. This review summarizes what is currently known about the regulation, development, neural projections, and potential functions of MeA kisspeptin neurons, as well as kisspeptin signaling directly within the MeA, with emphasis on data gathered from rodent models. Recent data are summarized and compared between rodent species and also between males and females. In addition, critical gaps in knowledge and important future directions are discussed.
Keywords: kisspeptin, Kiss1, Kiss1r, amygdala, GnRH, LH, reproduction, puberty
Introduction
Kisspeptin, encoded by the Kiss1 gene, is essential for reproduction. Humans and mice lacking Kiss1 or its receptor, Kiss1r, have deficits in puberty onset, reproductive hormone release, and fertility (1–4). In humans and rodents, kisspeptin treatment directly stimulates GnRH neurons to increase downstream LH and FSH secretion (5–12). Kisspeptin-synthesizing neurons are primarily located in two regions of the hypothalamus, the anteroventral periventricular (AVPV)/rostral periventricular (PeN) continuum and the arcuate nucleus (ARC) (9, 13–17). The AVPV/PeN and ARC Kiss1 populations are differentially regulated by testosterone (T) and estradiol (E2). In the AVPV/PeN, E2 increases Kiss1 levels and gonadectomy (GDX) decreases Kiss1 levels, supporting the proposed role of these neurons in mediating E2-positive feedback induction of the pre-ovulatory LH surge in females (13–15). Supporting this, Kiss1 neurons are sexually differentiated, being more numerous, and expressing higher Kiss1 mRNA levels in females (15, 18). By contrast, Kiss1 expression in the ARC increases following GDX and decreases with T or E2 treatment. Thus, ARC Kiss1 neurons are thought to participate in gonadal steroid negative feedback and the pulsatile release of GnRH secretion (13–15).
Smaller populations of Kiss1 neurons have also recently been identified in several extra-hypothalamic areas, including the medial amygdala (MeA), bed nucleus of the stria terminalis (BnST), and lateral septum (9, 19–23). However, these extra-hypothalamic neurons have not been studied extensively, and their regulation and functions are only now beginning to be determined. In particular, the MeA region, part of the limbic system, is known to have numerous behavioral and physiological functions, including (but not limited to) roles in sexual behavior and reproductive physiology (24–31). This review summarizes what is currently known about the regulation, development, and function(s) of MeA kisspeptin neurons as well as kisspeptin signaling directly within the MeA.
Identification and Regulation of Kisspeptin Neurons in the MeA
Kiss1 neurons in the MeA were first observed in male mice in 2004 (9) but were not directly studied until 2011 when Kim et al. first tested whether rodent MeA Kiss1 neurons are regulated by sex steroids (19), as occurs for hypothalamic kisspeptin neurons. The authors found that, as in the AVPV/PeN, Kiss1 levels in the MeA are strongly upregulated by sex steroids in both mice and rats. Specifically, in adult, gonadectomized (GDX) mice and rats, there are few, if any, detectable MeA Kiss1 cells, whereas exogenous treatment with T or E2 significantly increases MeA Kiss1 cell number (19) (Figure 1A). Unlike E2 treatment, DHT treatment did not increase MeA Kiss1 expression, indicating the sex steroid upregulation of MeA Kiss1 occurs through estrogen receptors (ERs) rather than androgen receptors (19). Xu and colleagues (21) similarly determined that exogenous E2 treatment in male and female rats upregulates both MeA Kiss1 mRNA and MeA kisspeptin protein expression (21).
Figure 1.
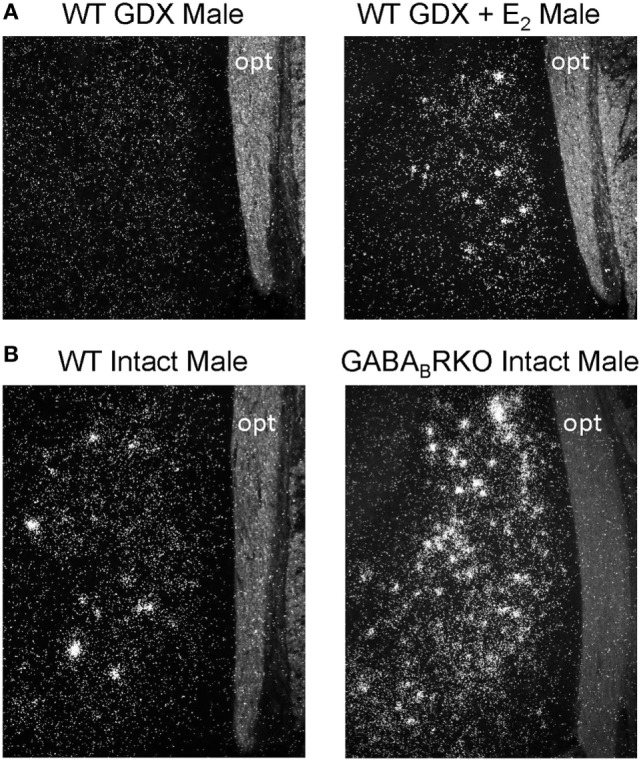
Medial amygdala (MeA) Kiss1 expression (silver grains in in situ hybridization photomicrographs) is regulated by E2 and GABA signaling. (A) Gonadectomized (GDX) mice have few, if any, Kiss1 cells in the MeA, whereas E2 treatment significantly increases MeA Kiss1 expression. (B) Gonad-intact GABABR KO mice have substantially more MeA Kiss1 than gonad-intact wild-type (WT) males, despite comparable circulating sex steroid levels. opt, optic tract.
The MeA region is known to express high levels of sex steroid receptors, including both ERα and ERβ (the latter is not highly expressed in the ARC or AVPV/PeN), but it was not initially known which ER mediates E2 stimulation of MeA Kiss1 neurons. Recent data from ERαKO mice indicate that both the hypothalamic (13, 14, 32, 33) and MeA (33) Kiss1 cells are primarily regulated via ERα. In the ARC and AVPV/PeN, E2 treatment alters Kiss1 expression in wild-type (WT) mice but not in ERαKO mice (13, 14, 32, 33). Similarly, in the MeA, E2 robustly increases Kiss1 expression in WT mice, whereas ERαKO mice given E2 failed to show comparable large increases in MeA Kiss1 levels (33). Thus, substantial E2 stimulation of MeA Kiss1 requires ERα signaling. However, E2-treated ERαKO mice did show a minor increase in MeA Kiss1 levels compared with non-E2-treated ERαKOs, indicating that another ER may partially compensate for the loss of ERα. This partial increase in Kiss1 expression in E2-treated ERαKO mice is unique to the MeA, as hypothalamic Kiss1 levels in E2-treated ERαKOs were comparable to that of non-E2-treated ERαKOs (33). By contrast, Kiss1 expression in the MeA (and hypothalamus) of ERβKO mice of both sexes mirrored that of WT mice under all hormonal conditions (13, 33). Thus, unlike ERα, ERβ is not required for E2’s regulation of either hypothalamic or MeA Kiss1.
In the MeA of gonad-intact rats and mice, Kiss1 expression is higher in males than in diestrus females (19). However, gonad-intact female rats have increased MeA Kiss1 expression during proestrus (when circulating E2 levels are highest), and relatively low levels during estrus and diestrus (19). Thus, the sex difference in MeA Kiss1 expression between gonad-intact males and females is likely due to differences in circulating sex steroid levels. Indeed, when E2 levels are equalized between males and females, the previously observed sex difference in MeA Kiss1 levels disappears, with males and females now showing comparable elevated MeA Kiss1 (19, 21) and kisspeptin (21) expression.
In addition to E2, GABA signaling via GABABR also strongly regulates MeA Kiss1 expression. In gonad-intact GABABR KO mice, MeA Kiss1 levels are drastically elevated in comparison to WT mice (22) (Figure 1B). This large increase in MeA Kiss1 expression is not due to altered sex steroid levels, as circulating T (males) or E2 (females) were similar between WT and GABABR KOs (22). Interestingly, the elevated Kiss1 levels in the MeA of GABABR KOs are typically much greater than observed for E2 stimulation of Kiss1 in this region in WT mice. This suggests that endogenous GABABR signaling is a very potent regulator of MeA Kiss1. Interestingly, this large upregulation of Kiss1 levels in GABABR KO mice was exclusive to extra-hypothalamic Kiss1 expression, as AVPV and ARC Kiss1 expression were normal in GABABR KO mice (22). Thus, GABA signaling via GABABR may normally serve to inhibit MeA Kiss1 expression, whereas GABABR signaling seems to have no major effect on hypothalamic Kiss1 levels. Whether this observed effect is due to GABABR signaling directly in MeA Kiss1 neurons remains to be determined, though MeA Kiss1 neurons do express GABABR (22).
Developmental Expression of Kisspeptin Neurons in the MeA
Although MeA Kiss1 expression is detectable in adult rodents, especially when sex steroids are elevated, MeA Kiss1 expression is not detected in prepubertal rodents, at postnatal day (PND) 14 in mice (22) or PND 19 and earlier in rats (34). Similarly, MeA Kiss1 expression was absent at PND 14 in GABABR KO mice despite being dramatically elevated in these mice in adulthood (22). These findings indicate that in juvenile and prepubertal rodents either (1) MeA Kiss1 neurons are not yet present or (2) MeA Kiss1 neurons are present but not able to express Kiss1. The latter possibility might reflect the absence of elevated circulating sex steroids before puberty, therefore precluding notable MeA Kiss1 expression at young ages.
The developmental pattern of MeA Kiss1 expression was recently examined every 5 days, from PND 15 until PND 40, in gonad-intact C57BL6 male mice, with puberty occurring around PND 35 (33). MeA Kiss1 was first detected at very low levels around PND 20–25, but did not significantly increase until PND 35, with highest expression at PND 40 (the oldest age examined). Circulating T levels in the same mice mirrored the developmental pattern of MeA Kiss1 expression, similarly increasing at PND 35 (as expected with puberty) (33). However, whether the pubertal increase in T caused or resulted from the increase in MeA Kiss1 expression is unclear. Therefore, the second study treated juvenile (PND 14) male mice with high-dose E2 for 4 days to determine if elevated E2 exposure at this prepubertal age could prematurely increase MeA Kiss1 expression (33). Indeed, juvenile E2 treatment increased MeA Kiss1 at PND 18 versus untreated controls, demonstrating that by PND 18, MeA Kiss1 neurons are present and capable of expressing notable Kiss1 if sex steroids are sufficiently elevated (33). Thus, increases in MeA Kiss1 expression around puberty are likely a response to rising circulating sex steroid levels at this time. Whether this emergence of notable MeA Kiss1 levels at puberty is functionally relevant to the pubertal process or to other physiological/behavioral processes is currently unknown.
MeA Kisspeptin Neuron Projections and Possible Functions
Understanding where MeA kisspeptin neurons project to can inform upon their potential functions. Research examining the afferent and efferent projections of MeA kisspeptin neurons only recently began, and thus, little is currently known. Using double-label immunohistochemistry, Pineda and colleagues demonstrated in male rats that some MeA kisspeptin neurons receive neural appositions from “upstream” neurons containing tyrosine hydroxylase (TH), the rate-limiting enzyme in catecholamine synthesis, and vasopressin (35). 25 and 11% of MeA kisspeptin neurons receive inputs from TH and vasopressin neurons, respectively (35). However, it is currently unknown if MeA kisspeptin neurons express either dopamine receptors or vasopressin receptors or from where in the brain the dopamine or vasopressin signaling originates. It is also unknown whether dopamine or vasopressin alters MeA Kiss1 or kisspeptin expression or neuronal activity, and if so, whether such regulation is positive or negative. Regardless, these data suggest that MeA kisspeptin neurons may have a role in participating in dopamine- or vasopressin-dependent physiology/behaviors, such as motivation and reward-seeking behaviors or social behaviors.
In rodents, the MeA has been implicated in regulating reproductive physiology because MeA lesions in rats disrupt ovarian cycles and alter pubertal timing (24–26, 31, 36–38). However, the specific cell types in the MeA that influence the reproductive axis are unknown. Given kisspeptin’s potent actions on GnRH neurons, Kiss1 neurons in the MeA are good candidates to serve as reproductive signalers from this brain region. Supporting this possibility, in female mice, MeA kisspeptin neurons send axonal projections to the preoptic area (POA), where many GnRH neurons reside (23). Moreover, injections of an AAV-DIO-YFP virus into the MeA of male KissCre-GFP mice revealed that ~15% of GnRH neurons in the POA receive close fiber appositions from MeA kisspeptin neurons (35). These anatomical data suggest that MeA kisspeptin neurons may have the potential to modulate a subset of GnRH neurons, though it should be noted that most GnRH neurons did not receive MeA kisspeptin contacts (which may be a result of technical/methodological limitations). Additional studies are therefore needed to determine if—and to what degree—MeA kisspeptin cells project to GnRH neurons.
Anterograde tracing studies in male rats recently demonstrated that the accessory olfactory bulb (AOB), but not the main olfactory bulb, projects to MeA kisspeptin neurons (35). This suggests that MeA kisspeptin neurons may also have a role in processing or responding to olfactory/pheromone cues, potentially social signals. Supporting this, selective chemogenetic activation of MeA kisspeptin neurons in male mice increased the amount of time males spent investigating estrous females (39). In that study, male KissCre-GFP mice received bilateral injections of a stimulatory viral DREADD receptor construct into the posterodorsal MeA, followed 4 weeks later with peripheral injection of clozapine-N-oxide (CNO) to selectively activate MeA kisspeptin neurons (39). Although selective DREADD activation of MeA kisspeptin neurons increased males’ investigation of females, it also increased the amount of time males spent with juvenile conspecifics (39), indicating that MeA kisspeptin neurons may modulate behavioral responses to any social odors, not just opposite-sex odors. Importantly, it remains unknown whether these induced behavioral changes are due to kisspeptin or another neuropeptide/neurotransmitter co-released from MeA kisspeptin neurons when activated. A prior study in Kiss1r KO mice showed that kisspeptin signaling is required for proper opposite-sex odor preference (40). Thus, it may be specifically kisspeptin (rather than another signaling factor) from these MeA neurons that are modulating opposite-sex and juvenile odor preference, but this still needs to be determined. Interestingly, in addition to receiving afferent projections from the AOB, MeA kisspeptin neurons also send reciprocal projections back to the AOB region, specifically the mitral and granule layers in mice and the granule layer in rats (23, 35). Mitral and granule cells are part of a reciprocal feedback circuit, with mitral cells exciting granule cells via glutamate and granule cells inhibiting mitral cells via GABA signaling (41–46). The functional relevance of such MeA kisspeptin signaling to the AOB cells remains to be determined.
Finally, Adekunbi and colleagues recently found that selective DREADD activation of MeA kisspeptin neurons reduces anxiety, with CNO-treated mice spending more time exploring the open arms in an elevated-plus maze than control mice. This suggests that MeA kisspeptin neurons may lower anxiety-related behaviors. However, these results differ from previous data showing intracerebroventricular (icv) injection of kisspeptin-13 in male rats decreased time spent in the open arms of the elevated-plus maze, suggesting that kisspeptin increased anxiety (47). These conflicting findings may reflect species differences or different methodologies (i.e., selective DREADD activation of just MeA kisspeptin neurons versus increased kisspeptin signaling throughout the brain). It is also possible that another neuropeptide/neurotransmitter released from MeA kisspeptin neurons caused the anxiolytic behavior in the DREADD study. Additional research is needed to clarify the role of MeA kisspeptin neurons in modulating anxiety.
Kisspeptin Signaling within the MeA
Thus far, this review has focused on the regulation, projections, and functions of kisspeptin neurons residing in the MeA, but several studies have also examined the role of kisspeptin signaling within the MeA. Intra-MeA injections of kisspeptin-10 in GDX + E2 (diestrus E2 levels) female rats dose dependently increased LH within an hour (48). Thus, kisspeptin acting directly within the MeA may also modulate GnRH release. Supporting this hypothesis, intra-MeA injection of a kisspeptin antagonist, peptide-234, in GDX + E2-treated females decreased LH secretion 2–4 h later (48). This suggests that endogenous kisspeptin signaling acting within the MeA is important for normal GnRH/LH secretion. Although it is possible that the kisspeptin or antagonist treatment spread outside of the MeA to other brain areas, other studies showed that icv injections of this same dose, 100 pmol, had no effect on LH levels (49, 50). Therefore, in female rats, kisspeptin may act directly within the MeA to modulate GnRH/LH release. In male rats, a similar dose of 100 pmol of kisspeptin-10 injected directly into the MeA also increased LH levels (51), with a greater LH increase after a higher dose, 1 nmol (51). Peptide-234 injected directly into the MeA of male rats did not alter LH (51), unlike in females, which may reflect a sex difference in endogenous MeA kisspeptin signaling targeting the MeA. In addition to increasing LH, intra-MeA injections of kisspeptin-10 also increased ex-copula erections in rats, which was prevented by concurrent kisspeptin antagonist treatment (51). Thus, kisspeptin signaling in the MeA of male rodents may regulate reproductive physiology and behavior.
The functional consequences of kisspeptin signaling directly within the MeA discussed above have only been examined in rats, for which there is some evidence of kisspeptin receptor, Kiss1r, in the MeA. Radiolabeled in situ hybridization found abundant expression of Kiss1r in the rat amygdala (52). However, non-radioactive ISH found no Kiss1r expression in the MeA (53), perhaps because non-radioactive ISH is less sensitive and, therefore, unable to detect low Kiss1r expression (53). Kiss1r expression has not been detected in the MeA of mice (54), indicating the functions of kisspeptin signaling acting in the MeA may be species dependent, though further studies on this issue are needed.
If kisspeptin can in fact act directly in the MeA, then where is such kisspeptin signaling coming from? It is currently unknown if any kisspeptin neuronal population, hypothalamic or extra-hypothalamic, projects to the MeA to be the potential source of kisspeptin acting in this area. One possibility could be that MeA kisspeptin neurons project locally, within the MeA, to regulate other neurons in this region, but this has not been studied. Additional research is therefore needed to determine if MeA-derived kisspeptin acts locally within the MeA or if kisspeptin action within the MeA is due to kisspeptin release from other areas.
Gaps in Knowledge and Future Directions
The MeA region is implicated in many diverse functions and behavioral processes, and deciphering the specific function(s) of kisspeptin neurons in this region is therefore not simple. Figure 2 summarizes our current understanding of the regulation and function(s) of MeA kisspeptin neurons. Although MeA kisspeptin neurons are clearly regulated by E2 and GABA signaling, via ERα and GABABR, respectively, whether this regulation occurs directly or indirectly on MeA kisspeptin neurons is unknown. Supporting a possible direct regulation, MeA kisspeptin neurons express GABABR, and ERα is heavily expressed in the MeA region (though ERα specifically in MeA kisspeptin neurons has not been determined). TH and vasopressin neurons project to MeA kisspeptin neurons; however, whether MeA kisspeptin neurons express dopamine or vasopressin receptors is unknown, as is any effect of dopamine or vasopressin on MeA kisspeptin neurons. Additional research is also needed to determine what other factors may regulate MeA kisspeptin neurons. Other than kisspeptin, it is also currently unknown what other signaling factors are produced by MeA Kiss1 neurons. This is important for knowing whether the LH or behavioral responses following DREADD activation of these neurons are due to kisspeptin or some other co-released neuropeptide/neurotransmitter.
Figure 2.
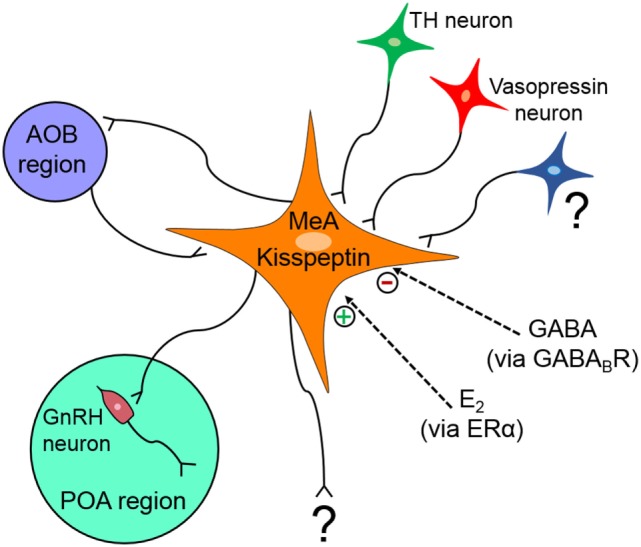
Schematic diagram summarizing what is known about the regulation and projections of medial amygdala (MeA) kisspeptin neurons. MeA kisspeptin neurons receive projections from tyrosine hydroxylase (TH) and vasopressin neurons, as well as from neurons originating in the accessory olfactory bulb (AOB). MeA kisspeptin neurons have efferent projections back to the AOB and also to some GnRH neurons in the preoptic area (POA). MeA Kiss1 neurons are upregulated by E2 via ERα and downregulated by GABA signaling via GABABR, but it is currently unknown if this E2 and GABA regulation occurs directly on MeA Kiss1 cells or indirectly via intermediary neurons. ? = unknown afferent or efferent projections of MeA kisspeptin neurons.
MeA Kiss1 expression is first detected around puberty, when gonadal steroids are also rising. Data suggest that the increase in MeA Kiss1 at this time is likely caused by the pubertal increases in gonadal sex steroids, but this requires further examination. Regardless, the presence of notable Kiss1 in the MeA only at puberty and beyond suggests that the functional relevance of kisspeptin released from the MeA is restricted to processes during sexual maturation and/or adulthood.
MeA kisspeptin neurons send axon projections to some GnRH neurons, which may indicate MeA kisspeptin neurons can modulate the reproductive axis. This is supported by LH increases after MeA Kiss1 neuron DREADD activation. Lesions of the entire MeA disrupt ovarian cycles and alter puberty, perhaps because of ablated MeA kisspeptin neurons, but this has not been studied. MeA kisspeptin neurons also form a reciprocal circuit with the AOB, and activation of MeA kisspeptin neurons increases social interactions in mice, indicating MeA kisspeptin neurons may influence social and/or sexual olfactory processing. Activation of MeA kisspeptin neurons also decreases anxiety behavior, suggesting kisspeptin or another neuropeptide/neurotransmitter released from these neurons influences anxiety. Other possible neural targets of MeA kisspeptin neurons remain to be determined and are needed to understand the functions, reproductive or otherwise, of these particular MeA neurons.
Author Contributions
Both authors researched and wrote/edited the article and designed the figures.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. The authors are supported by NSF IOS-1457226, NIH R01 HD082567, and NICHD P50 HD012303. SS also supported by F32 HD088060 and T32 HD007203.
References
- 1.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, et al. The GPR54 gene as a regulator of puberty. N Engl J Med (2003) 349(17):1614–27. 10.1056/NEJMoa035322 [DOI] [PubMed] [Google Scholar]
- 2.Topaloglu AK, Tello JA, Kotan LD, Ozbek MN, Yilmaz MB, Erdogan S, et al. Inactivating KISS1 mutation and hypogonadotropic hypogonadism. N Engl J Med (2012) 366(7):629–35. 10.1056/NEJMoa1111184 [DOI] [PubMed] [Google Scholar]
- 3.Lapatto R, Pallais JC, Zhang D, Chan YM, Mahan A, Cerrato F, et al. Kiss1-/- mice exhibit more variable hypogonadism than Gpr54-/- mice. Endocrinology (2007) 148(10):4927–36. 10.1210/en.2007-0078 [DOI] [PubMed] [Google Scholar]
- 4.de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A (2003) 100(19):10972–6. 10.1073/pnas.1834399100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dhillo WS, Chaudhri OB, Patterson M, Thompson EL, Murphy KG, Badman MK, et al. Kisspeptin-54 stimulates the hypothalamic-pituitary gonadal axis in human males. J Clin Endocrinol Metab (2005) 90(12):6609–15. 10.1210/jc.2005-1468 [DOI] [PubMed] [Google Scholar]
- 6.Navarro VM, Castellano JM, Fernandez-Fernandez R, Tovar S, Roa J, Mayen A, et al. Effects of KiSS-1 peptide, the natural ligand of GPR54, on follicle-stimulating hormone secretion in the rat. Endocrinology (2005) 146(4):1689–97. 10.1210/en.2004-1353 [DOI] [PubMed] [Google Scholar]
- 7.Navarro VM, Castellano JM, Fernandez-Fernandez R, Tovar S, Roa J, Mayen A, et al. Characterization of the potent luteinizing hormone-releasing activity of KiSS-1 peptide, the natural ligand of GPR54. Endocrinology (2005) 146(1):156–63. 10.1210/en.2004-0836 [DOI] [PubMed] [Google Scholar]
- 8.Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci U S A (2005) 102(6):2129–34. 10.1073/pnas.0409822102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, et al. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology (2004) 145(9):4073–7. 10.1210/en.2004-0431 [DOI] [PubMed] [Google Scholar]
- 10.Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, et al. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology (2004) 80(4):264–72. 10.1159/000083140 [DOI] [PubMed] [Google Scholar]
- 11.Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, et al. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci U S A (2005) 102(5):1761–6. 10.1073/pnas.0409330102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.d’Anglemont de Tassigny X, Fagg LA, Carlton MB, Colledge WH. Kisspeptin can stimulate gonadotropin-releasing hormone (GnRH) release by a direct action at GnRH nerve terminals. Endocrinology (2008) 149(8):3926–32. 10.1210/en.2007-1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology (2005) 146(9):3686–92. 10.1210/en.2005-0488 [DOI] [PubMed] [Google Scholar]
- 14.Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, et al. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology (2005) 146(7):2976–84. 10.1210/en.2005-0323 [DOI] [PubMed] [Google Scholar]
- 15.Kauffman AS, Gottsch ML, Roa J, Byquist AC, Crown A, Clifton DK, et al. Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology (2007) 148(4):1774–83. 10.1210/en.2006-1540 [DOI] [PubMed] [Google Scholar]
- 16.Franceschini I, Lomet D, Cateau M, Delsol G, Tillet Y, Caraty A. Kisspeptin immunoreactive cells of the ovine preoptic area and arcuate nucleus co-express estrogen receptor alpha. Neurosci Lett (2006) 401(3):225–30. 10.1016/j.neulet.2006.03.039 [DOI] [PubMed] [Google Scholar]
- 17.Kim W, Jessen HM, Auger AP, Terasawa E. Postmenopausal increase in KiSS-1, GPR54, and luteinizing hormone releasing hormone (LHRH-1) mRNA in the basal hypothalamus of female rhesus monkeys. Peptides (2009) 30(1):103–10. 10.1016/j.peptides.2008.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Homma T, Sakakibara M, Yamada S, Kinoshita M, Iwata K, Tomikawa J, et al. Significance of neonatal testicular sex steroids to defeminize anteroventral periventricular kisspeptin neurons and the GnRH/LH surge system in male rats. Biol Reprod (2009) 81(6):1216–25. 10.1095/biolreprod.109.078311 [DOI] [PubMed] [Google Scholar]
- 19.Kim J, Semaan SJ, Clifton DK, Steiner RA, Dhamija S, Kauffman AS. Regulation of Kiss1 expression by sex steroids in the amygdala of the rat and mouse. Endocrinology (2011) 152(5):2020–30. 10.1210/en.2010-1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cravo RM, Margatho LO, Osborne-Lawrence S, Donato J, Jr, Atkin S, Bookout AL, et al. Characterization of Kiss1 neurons using transgenic mouse models. Neuroscience (2011) 173:37–56. 10.1016/j.neuroscience.2010.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu Z, Kaga S, Mochiduki A, Tsubomizu J, Adachi S, Sakai T, et al. Immunocytochemical localization of kisspeptin neurons in the rat forebrain with special reference to sexual dimorphism and interaction with GnRH neurons. Endocr J (2012) 59(2):161–71. 10.1507/endocrj.EJ11-0193 [DOI] [PubMed] [Google Scholar]
- 22.Di Giorgio NP, Semaan SJ, Kim J, Lopez PV, Bettler B, Libertun C, et al. Impaired GABAB receptor signaling dramatically up-regulates Kiss1 expression selectively in nonhypothalamic brain regions of adult but not prepubertal mice. Endocrinology (2014) 155(3):1033–44. 10.1210/en.2013-1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeo SH, Kyle V, Morris PG, Jackman S, Sinnett-Smith LC, Schacker M, et al. Visualisation of Kiss1 neurone distribution using a Kiss1-CRE transgenic mouse. J Neuroendocrinol (2016) 28(11). 10.1111/jne.12435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beltramino C, Taleisnik S. Facilitatory and inhibitory effects of electrochemical stimulation of the amygdala on the release of luteinizing hormone. Brain Res (1978) 144(1):95–107. 10.1016/0006-8993(78)90437-7 [DOI] [PubMed] [Google Scholar]
- 25.Velasco ME, Taleisnik S. Effects of the interruption of amygdaloid and hippocampal afferents to the medial hypothalmus on gonadotrophin release. J Endocrinol (1971) 51(1):41–55. 10.1677/joe.0.0510041 [DOI] [PubMed] [Google Scholar]
- 26.Tyler JL, Gorski RA. Effects of corticomedial amydgala lesions or olfactory bulbectomy on LH responses to ovarian steroids in the female rat. Biol Reprod (1980) 22(4):927–34. 10.1095/biolreprod22.4.927 [DOI] [PubMed] [Google Scholar]
- 27.DiBenedictis BT, Ingraham KL, Baum MJ, Cherry JA. Disruption of urinary odor preference and lordosis behavior in female mice given lesions of the medial amygdala. Physiol Behav (2012) 105(2):554–9. 10.1016/j.physbeh.2011.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maras PM, Petrulis A. Chemosensory and steroid-responsive regions of the medial amygdala regulate distinct aspects of opposite-sex odor preference in male Syrian hamsters. Eur J Neurosci (2006) 24(12):3541–52. 10.1111/j.1460-9568.2006.05216.x [DOI] [PubMed] [Google Scholar]
- 29.Sano K, Nakata M, Musatov S, Morishita M, Sakamoto T, Tsukahara S, et al. Pubertal activation of estrogen receptor alpha in the medial amygdala is essential for the full expression of male social behavior in mice. Proc Natl Acad Sci U S A (2016) 113(27):7632–7. 10.1073/pnas.1524907113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fujiwara M, Nitta A, Chiba A. Regulation of sexual odor preference by sex steroids in the posterodorsal medial amygdala in female rats. Horm Behav (2016) 82:46–55. 10.1016/j.yhbeh.2016.05.007 [DOI] [PubMed] [Google Scholar]
- 31.Li XF, Hu MH, Hanley BP, Lin YS, Poston L, Lightman SL, et al. The posterodorsal medial amygdala regulates the timing of puberty onset in female rats. Endocrinology (2015) 156(10):3725–36. 10.1210/en.2015-1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gottsch ML, Navarro VM, Zhao Z, Glidewell-Kenney C, Weiss J, Jameson JL, et al. Regulation of Kiss1 and dynorphin gene expression in the murine brain by classical and nonclassical estrogen receptor pathways. J Neurosci (2009) 29(29):9390–5. 10.1523/JNEUROSCI.0763-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stephens SB, Chahal N, Munaganuru N, Parra RA, Kauffman AS. Estrogen stimulation of Kiss1 expression in the medial amygdala involves estrogen receptor-alpha but not estrogen receptor-beta. Endocrinology (2016) 157(10):4021–31. 10.1210/en.2016-1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cao J, Patisaul HB. Sex-specific expression of estrogen receptors alpha and beta and Kiss1 in the postnatal rat amygdala. J Comp Neurol (2013) 521(2):465–78. 10.1002/cne.23185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pineda R, Plaisier F, Millar RP, Ludwig M. Amygdala kisspeptin neurons: putative mediators of olfactory control of the gonadotropic axis. Neuroendocrinology (2017) 104(3):223–38. 10.1159/000445895 [DOI] [PubMed] [Google Scholar]
- 36.Docke F, Lemke M, Okrasa R. Studies on the puberty-controlling function of the mediocortical amygdala in the immature female rat. Neuroendocrinology (1976) 20(2):166–75. 10.1159/000122480 [DOI] [PubMed] [Google Scholar]
- 37.Docke F, Rohde W, Lange T, Dorner G. Evidence for direct central nervous inhibition of LH secretion during sexual maturation of female rats. Endokrinologie (1980) 75(1):1–7. [PubMed] [Google Scholar]
- 38.Docke F. Differential effects of amygdaloid and hippocampal lesions on female puberty. Neuroendocrinology (1974) 14(6):345–50. 10.1159/000122278 [DOI] [PubMed] [Google Scholar]
- 39.Adekunbi DAL, Lass G, Li XF, Colledge WH, O’Byrne K. Kisspeptin in the posterodorsal medial amygdala modulates mate preference and anxiety in male mice. Endocrine Society Annual Meeting. Orlando, FL: (2017). [Google Scholar]
- 40.Kauffman AS, Park JH, McPhie-Lalmansingh AA, Gottsch ML, Bodo C, Hohmann JG, et al. The kisspeptin receptor GPR54 is required for sexual differentiation of the brain and behavior. J Neurosci (2007) 27(33):8826–35. 10.1523/JNEUROSCI.2099-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rall W, Shepherd GM. Theoretical reconstruction of field potentials and dendrodendritic synaptic interactions in olfactory bulb. J Neurophysiol (1968) 31(6):884–915. [DOI] [PubMed] [Google Scholar]
- 42.Nicoll RA. Inhibitory mechanisms in the rabbit olfactory bulb: dendrodendritic mechanisms. Brain Res (1969) 14(1):157–72. 10.1016/0006-8993(69)90037-7 [DOI] [PubMed] [Google Scholar]
- 43.Nowycky MC, Mori K, Shepherd GM. GABAergic mechanisms of dendrodendritic synapses in isolated turtle olfactory bulb. J Neurophysiol (1981) 46(3):639–48. [DOI] [PubMed] [Google Scholar]
- 44.Jahr CE, Nicoll RA. An intracellular analysis of dendrodendritic inhibition in the turtle in vitro olfactory bulb. J Physiol (1982) 326:213–34. 10.1113/jphysiol.1982.sp014187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wellis DP, Kauer JS. GABAergic and glutamatergic synaptic input to identified granule cells in salamander olfactory bulb. J Physiol (1994) 475(3):419–30. 10.1113/jphysiol.1994.sp020082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wellis DP, Kauer JS. GABAA and glutamate receptor involvement in dendrodendritic synaptic interactions from salamander olfactory bulb. J Physiol (1993) 469:315–39. 10.1113/jphysiol.1993.sp019816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Csabafi K, Jaszberenyi M, Bagosi Z, Liptak N, Telegdy G. Effects of kisspeptin-13 on the hypothalamic-pituitary-adrenal axis, thermoregulation, anxiety and locomotor activity in rats. Behav Brain Res (2013) 241:56–61. 10.1016/j.bbr.2012.11.039 [DOI] [PubMed] [Google Scholar]
- 48.Comninos AN, Anastasovska J, Sahuri-Arisoylu M, Li X, Li S, Hu M, et al. Kisspeptin signaling in the amygdala modulates reproductive hormone secretion. Brain Struct Funct (2016) 221(4):2035–47. 10.1007/s00429-015-1024-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pheng V, Uenoyama Y, Homma T, Inamoto Y, Takase K, Yoshizawa-Kumagaye K, et al. Potencies of centrally- or peripherally-injected full-length kisspeptin or its C-terminal decapeptide on LH release in intact male rats. J Reprod Dev (2009) 55(4):378–82. 10.1262/jrd.20240 [DOI] [PubMed] [Google Scholar]
- 50.Thompson EL, Patterson M, Murphy KG, Smith KL, Dhillo WS, Todd JF, et al. Central and peripheral administration of kisspeptin-10 stimulates the hypothalamic-pituitary-gonadal axis. J Neuroendocrinol (2004) 16(10):850–8. 10.1111/j.1365-2826.2004.01240.x [DOI] [PubMed] [Google Scholar]
- 51.Gresham R, Li S, Adekunbi DA, Hu M, Li XF, O’Byrne KT. Kisspeptin in the medial amygdala and sexual behavior in male rats. Neurosci Lett (2016) 627:13–7. 10.1016/j.neulet.2016.05.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee DK, Nguyen T, O’Neill GP, Cheng R, Liu Y, Howard AD, et al. Discovery of a receptor related to the galanin receptors. FEBS Lett (1999) 446(1):103–7. 10.1016/S0014-5793(99)00009-5 [DOI] [PubMed] [Google Scholar]
- 53.Higo S, Honda S, Iijima N, Ozawa H. Mapping of kisspeptin receptor mRNA in the whole rat brain and its co-localisation with oxytocin in the paraventricular nucleus. J Neuroendocrinol (2016) 28(4). 10.1111/jne.12356 [DOI] [PubMed] [Google Scholar]
- 54.Herbison AE, de Tassigny X, Doran J, Colledge WH. Distribution and postnatal development of Gpr54 gene expression in mouse brain and gonadotropin-releasing hormone neurons. Endocrinology (2010) 151(1):312–21. 10.1210/en.2009-0552 [DOI] [PubMed] [Google Scholar]


