Abstract
Objective:
The Self-administered Leeds Assessment of Neuropathic Symptoms and Signs (S-LANSS) is a 7-item self-report scale developed to identify pain which is of predominantly neuropathic origin. The aim of this study was to develop a Malayalam version of the LANSS and to test its validity and reliability in chronic pain patients.
Methodology:
We enrolled 101 Malayalam-speaking chronic pain patients who visited the Division of Palliative Medicine, Regional Cancer Centre, Thiruvananthapuram, Kerala, India. The translated version of S- LANSS was constructed by standard means. Fifty-one neuropathic pain and fifty nociceptive pain patients were identified by an independent pain physician and were subjected to the new pain scale by a palliative care nurse who was blinded to the diagnosis. The “gold standard diagnosis” is what the physician makes after clinical examination. Its validation, sensitivity, specificity, and positive and negative predictive values were determined.
Results:
Fifty-one neuropathic pain and fifty nociceptive pain patients were subjected to the Malayalam version of S-LANSS pain scale for validity testing. The agreement by Cohen's Kappa 0.743, Chi-square test P < 0.001, sensitivity 89.58, specificity 84.91, positive predictive value 84.31, negative predictive value 90.00, accuracy by 87.13, and likelihood ratio 5.94.
Conclusion:
The Malayalam version of S-LANSS pain scale is a validated screening tool for identifying neuropathic pain in chronic pain patients in Malayalam-speaking regions.
Keywords: Leeds Assessment of Neuropathic Symptoms and Signs pain scale, linguistic validation, Malayalam language, neuropathic pain, pain assessment
INTRODUCTION
Chronic pain can be classified into neuropathic pain, nociceptive pain, and other complex pain syndromes. Bennett[1] introduced the Leeds Assessment of Neuropathic Symptoms and Signs (LANSS) pain scale which is an accepted pain scale with two parts. The first part consists of five questionnaires to find the sensory descriptors of pain and the second part consists of two questions to be filled by bedside examination. The scoring comprises a total of 24 points. LANSS pain score >12 is considered neuropathic and <12 is identified as nociceptive. This tool is designed in English. The descriptors of pain are affected by cultural and linguistic variations. This is a study to validate the LANSS pain scale in Malayalam, the mother tongue of the state of Kerala, India. The LANSS pain scale attempts to estimate the probability that neuropathic mechanisms contribute to the chronic pain experience in a given patient. The development and validation of screening tools in the form of simple questionnaires could be helpful both in daily practice and clinical research.[2] Interestingly, although they were developed in parallel in different countries and languages (English, German, Turkish, Italian, Chinees and French), most items (i.e., pain descriptors) included in these clinical tools are similar[3,4,5,6,7,8,9] Others stress that the most important feature is pain occurring in an area of abnormal or absent sensation.[10] This feeling of pain on the area of absent or abnormal sensation warrants a lot of assertions from the pain physicians to help in describing the quality and type of pain by the patient. The description of pain can vary with linguistic and cultural differences. Therefore, there is a need for validation of accepted pain scales in regional languages and find the equivalent pain scales to test the validity.[11,12,13,14]
A clinical diagnosis of neuropathic pain should only be made when the distribution of pain and the associated sensory abnormalities jointly, and in a clinical context, point to a neurological condition (Hansson and Kinnman, 1996).[15] The six sensory descriptors more frequently used by neuropathic pain patients were “electric shock,” “burning,” “cold,” “pricking,” “tingling,” and “itching.” Masson et al.[16] discriminated between diabetic neuropathy and other causes of painful diabetic legs using a combination of sensory and affective descriptors, as well as responses to the questions. Boureau et al.,[17] using French-reconstructed McGill Pain Questionnaire, demonstrated significant differences for ten sensory words and seven affective words between patients with peripheral neuropathic and nociceptive pain. This is because the successful treatment of neuropathic pain relies on its early identification, an understanding of sustaining mechanisms, and the use of alternative therapeutic approaches.[18] Wall[19] and Besson and Chaouch (1987)[20] are right to state that the nociceptive/neuropathic division is an oversimplification of complex processes. Neuropathic pain scale has been described[21] and attempted to discriminate between four diagnostic categories of neuropathic pain using single descriptors.
To date, there is no criteria to find the true gold standard in finding the neuropathic pain other than the clinical examination by the pain physicians. Naturally, it will be a tedious task for clinicians to spare much of their time in the office to work up on clinical examination of patients. This necessitates the development of pain questionnaires to use in pain clinics to screen for neuropathic pain. Hence, wherever a questionnaire is developed, it has to be compared with the clinical diagnosis by the physician who has considerable experience in identifying pain pathology. Galer and Jenson[21] studied the nociceptive and neuropathic pain alone by excluding the mixed pains and affirmed that this questionnaire cannot differentiate neuropathic pain from 25% to 75% compared to true 100% neuropathic pain.
Hans et al.[22] concluded the necessity of developing a pain scale to assist physicians in detecting neuropathic pain and later addition of adjuvant drugs in pain. Bouhassira et al.[23] developed a French questionnaire to detect neuropathic pain named Douleur Neuropathique 4 (DN4) questionnaire which was translated and validated in various languages across the world. Perez et al.[24] developed a Spanish equivalent for the French DN4 inventory to identify neuropathic pain with a Cronbach's alpha of 0.7 showing a good agreement on verbal descriptors of pain.
METHODOLOGY
Three subject experts were selected to translate the LANSS pain scale from English into Malayalam independently. These three Malayalam translates were again translated back into their English equivalent by independent three subject experts and the final Malayalam translate is identified after subjecting the Malayalam versions to the patients in the pain clinic. This is done by the pain nurse in the pain clinic. The questions that need least number of assertions, i.e., the questions that need least explanation other than the words used in the formed questions were selected. This selection of the questions in the respective domains was done by the first author and selections were ensured by the second author. The final questionnaire was constructed by picking up the words which have the least number of assertions needed and formed into the relevant individual questions in the questionnaire. The questions were constructed to protect the essence of the symptom and to illustrate the meaning of the symptom [Appendix A].
The pain physician in the clinic administers the English version of LANSS scale to the patients by his/her own capability, and those patients who satisfied the criteria for nociceptive and neuropathic pain by the original LANSS pain scale and were confirmed with his/her own clinical experience were selected to administer the newly constructed Malayalam equivalent questionnaire. This is taken as the gold standard. Here, we validate the final questionnaire against the diagnosis by the pain physician. Samples were selected by random sampling. We estimate the minimum sample size required, based on the different values of the prevalence of a disease and both sensitivity and specificity of a screening or diagnostic test (while in the meantime, the power is set to be at least 80% and the P < 0.05). Using the software nMaster developed by Christian Medical College, Vellore, Tamil Nadu, 105 patients from the pain clinic in the Regional Cancer Centre, Thiruvananthapuram, are subjected to the questionnaire after obtaining consent. And later, they were cross-examined by the pain physician for the gold standard pain diagnosis and were treated. The patients were approached by the palliative care nurses, who were blinded to the pain diagnosis by the pain physician to fill the questionnaire by asking the questions pertaining the pain diagnosis and completed the two sensory testing questions by bedside examination as mentioned in the questionnaire. One by pin prick test for hyperalgesia as advised be Mike Bennett and cotton wool test for allodynia by stroking the affected skin and the skin on the opposite side with a piece of cotton. Sensory function of the skin overlying the area of pain (the index site) was compared with that at a nonpainful control site in each patient. The control site was either a similar area in the contralateral side or a nonpainful area of adjacent skin. The sensory examination assessed the pin prick threshold (PPT) and presence of allodynia. The method used for PPT is based on that described by Chan et al.[25]
Statistical analysis
The data obtained were subjected to Cohen's Kappa test for identifying the level of agreement considering that any value <0.4 is not an appropriate question. The whole data were then subjected to Chi-square test for the significance and compared with the pain physician's diagnosis. The Malayalam version of LANSS [Appendix A] was subjected to scoring. The total scores from both the investigator and pain physician's diagnosis were compared with to evaluate the discriminant validity and reliability between raters. Item scores were also examined for the level of agreement between raters and internal consistency. Moreover, they were also subjected for sensitivity and specificity analysis.
OBSERVATION AND RESULTS
A variety of pain were analyzed ranging from cancer pain, radiation mucositis, postamputation pain, arthritis, vertebral metastasis, chronic cerebral palsy, postcoronary artery bypass grafting, multiple myeloma pain, soft tissue sarcoma, and so on [Table 1]. Gender-wise analysis shows that it a comparable study involving 57.4% of males and 42.6% of females. Patients were analyzed for the demographic variability within the groups in age <40 years - 5.9%, 40–59 years - 71.3%, and >60 years - 22.8% [Figure 1]. The frequency of distribution of patients by the gold standard diagnostic criteria was 51% (50.5%) of neuropathic pain and 50% (49.5%) of nociceptive pain [Tables 2 and 3]. Analyzing the data by 2 × 2 tables between groups, pain by the doctor's diagnosis and pain by the subjected scoring system show χ2 = 55.909, P < 0.001, and Cohen's Kappa 0.743 which is highly significant. The questions selected in Malayalam version show good agreement with the doctor's diagnosis, this is considered the gold standard. On sensitivity and specificity testing, the questionnaire proved to be 89.58% sensitive and to have 84.915% of specificity. It has a negative predictive value of 90%.
Table 1.
The distribution of causes of painful conditions amoung the patients
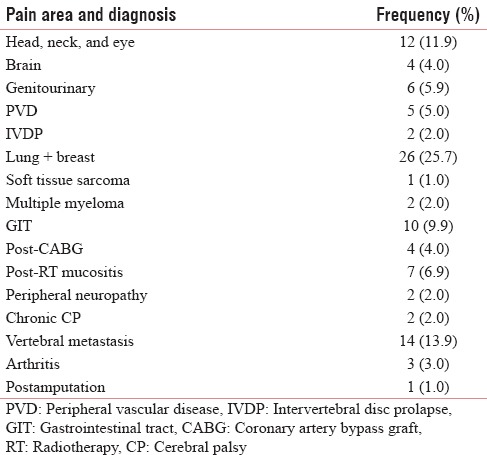
Figure 1.
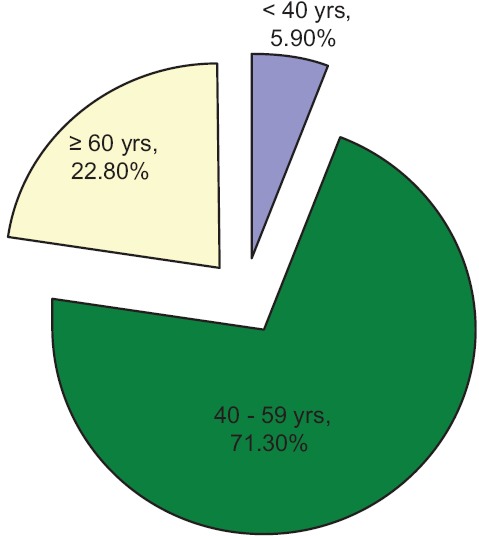
Distribution of age in the study population
Table 2.
Distribution of patients by the “gold standard” pain physician's diagnosis

Table 3.
Comparison of gold standard diagnosis and the Malayalam version of Leeds Assessment of Neuropathic Symptoms and Signs pain scale with sensitivity, specificity, positive predictive value, negative predictive value, and likelihood ratio
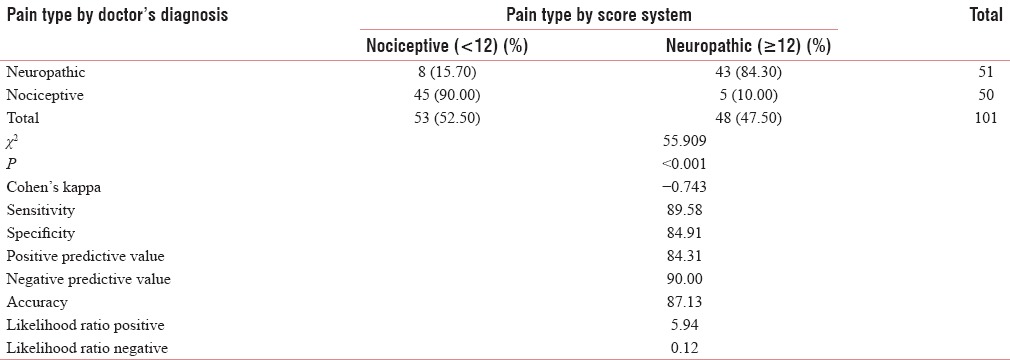
This means that the Malayalam version of LANSS pain scale can be used in clinics as a good screening test with high sensitivity and reasonable specificity. Table 4 shows the agreement on the first question, “did your pain feels like strange unpleasant sensation in your skin? Words like ‘p ricking’, ‘tingling’, ‘pins and needles’ might describe this sensation” which shows acceptable Cohen's Kappa (0.646) and a high Chi-square value (<0.001) for identifying the neuropathic pain.
Table 4.
The agreement on the first question “did your pain feels like strange unpleasant sensation in your skin? Words like ‘pricking’, ‘tingling’, ‘pins and needles’ might describe this sensation” (question 1)
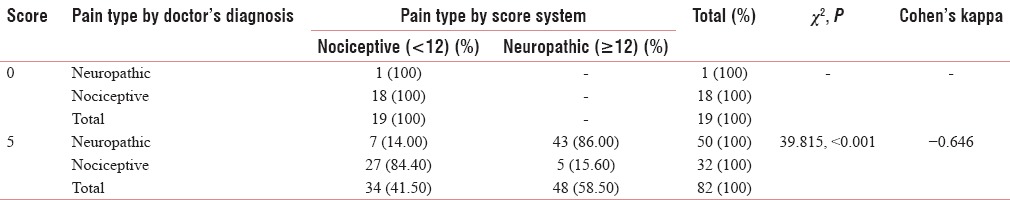
Table 5 shows the agreement on the second question, “does your pain make the affected skin abnormally sensitive to touch? Getting unpleasant sensations when lightly stroking the skin or getting pain on wearing tight clothes might describe this abnormal sensitivity,” which shows good Cohen's Kappa (0.713) for negative predictability and a significant acceptability for positive predictability. Table 6 shows the agreement on the third question “does your pain make the affected skin abnormally sensitive to touch? Getting unpleasant sensations when lightly stroking the skin or getting pain on wearing tight clothes might describe this abnormal sensitivity, hypersensitivity on the affected skin shows acceptable values of Cohen's Kappa (0.485 and 0.517) with significant Chi-square value (<0.001). Table 7 shows the agreement on the question” does your pain feel as if the skin temperature in the painful area has changed abnormally? Words like ‘hot’ and ‘burning’ scribe this sensation?” this shows very good agreement with the Malayalam variant (Cohen's Kappa 0.804, 0.491). Table 8 shows the agreement on the fifth question” does your pain feel as if the skin temperature in the painful area has changed abnormally? Words like ‘hot’ and ‘burning’ scribe this sensation? for sympathetically mediated pain like hot or burning pain shows reasonable agreement Cohen's Kappa (0.554, 0.434) with the Malayalam equivalent of LANSS pain scale. Questions 6 and 7 were bedside examinations to be completed by PPT and cotton wool test were also tested and completed the questionnaire.
Table 5.
The agreement on the question “does your pain make the skin in the painful area look different from normal? Words like ‘mottled’ or ‘more red or pink’ might describe this appearance” (question 2)
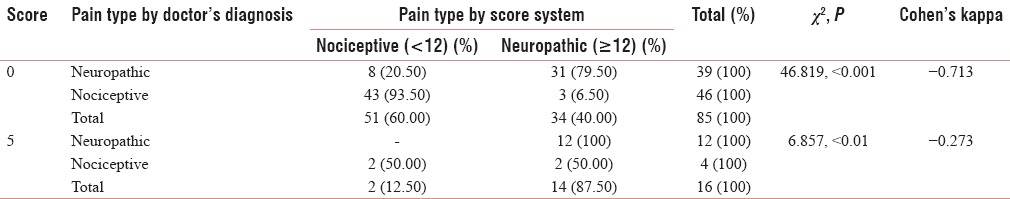
Table 6.
The agreement on the question “does your pain make the affected skin abnormally sensitive to touch? Getting unpleasant sensations when lightly stroking the skin or getting pain on wearing tight clothes might describe this abnormal sensitivity” (question 3)
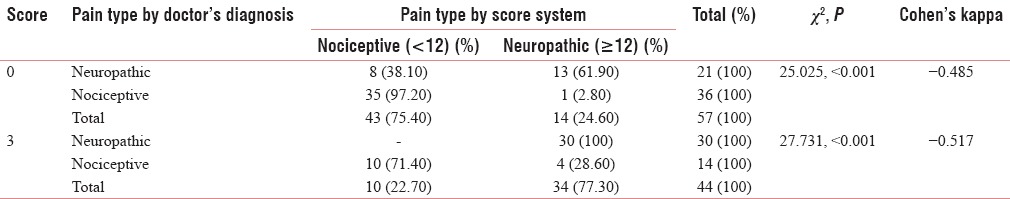
Table 7.
The agreement on the question “does your pain come on suddenly and in bursts for no apparent reason when you are still. Words like ‘electric shock’, ‘jumping and bursting’ describe this sensation?” (question 4)
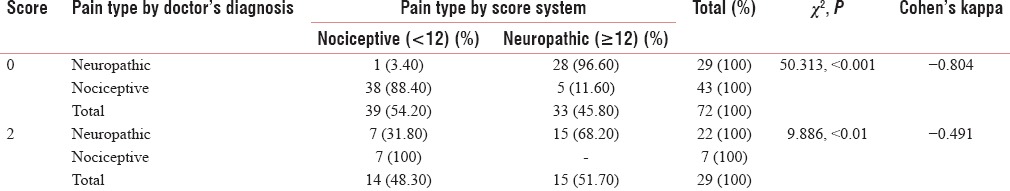
Table 8.
The agreement on the question “does your pain feel as if the skin temperature in the painful area has changed abnormally? Words like ‘hot’ and ‘burning’ scribe this sensation” (question 5)
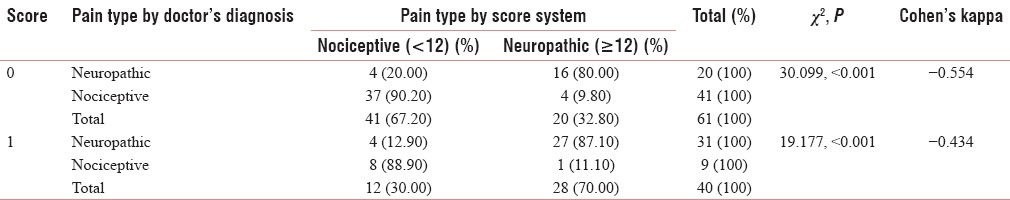
DISCUSSION
On analyzing the Malayalam version of LANSS pain scale with 101 patients against pain expert's diagnosis for the nociceptive or neuropathic pain, we got good agreement on Malayalam version for the sensory descriptors of pain with Cohen's Kappa of 0.73 (0.42–0.801), Chi-square value <0.001, sensitivity of 89.58%, specificity of 84.91%, positive predictive value of 84.31, negative predictive value of 90.00, positive likelihood ratio of 5.94, negative likelihood ratio of 0.12, and accuracy of 87.13 [Table 3].
It is comparable with other validation studies conducted in the original LANSS pain score development by Mike Bennett in 2001. In the original study by Mike Bennett published in 2001, there was good agreement between the ratings of the investigator and the clinician for LANSS score classification of pain type and individual items on the scale. Cohen's Kappa for overall classification was 0.65 (P = 0.001) and the Kappa values for scale items were between 0.6 and 0.88. When the clinical assessment was compared with the investigators’ ratings, the original LANSS pain scale was able to correctly identify 82% (33/40) of patients, representing 85% (17/20) sensitivity and 80% (16/20) specificity. A cutoff score of 12 points or more resulted in a positive predictive value of 81% (17/21) and a negative predictive value of 84% (16/19). On testing an Arabic version of LANSS pain scale by Elzahaf et al.[26] in 2012, on telephonic interview with 23 of 103 respondents in Libyan population residing in the UK, the Cohen's Kappa agreement was in the range of 0.46–1.00. In this study, Raga concluded this Arabic version as a good tool to use in identifying neuropathic pain in any pain clinic among Arabic population. This indicates that the linguistic variation on pain descriptors can vary and differ in understanding the questionnaires in a proper manner which can affect the pain diagnosis and further management of pain. In an open multicentric observational survey among 2480 respondents in Belgium population, Hans et al. in 2007[22] concluded that LANSS pain scale is effective in differentiating neuropathic pain by 90% accuracy. This LANSS pain scale can help hospitals in saving valuable time to find neuropathic pain and to decide on the addition of adjuvant drugs such as tricyclic anti-depressants, epileptics, benzodiazepines, and alpha agonists to their pain management, thereby saving insurance money. Park et al.[27] studied the Korean version of LANSS pain scale conducted among 213 pain patients and got very good agreement with verbal descriptors of pain 0.815, sensitivity of 72%, and specificity of 98%. The positive and negative predictive values were 98% and 76%, respectively. In our study, the corresponding values were 84.31% and 90.00%, respectively. Wall studied neuropathic pain in detail and says A δ and C fiber-mediated pain has to be differentiated with different tests such as PPT and heat discrimination. With the above results, it can be observed that we can use the Malayalam equivalent as comparable to the data published in the other studies. The current scale can be used in the Kerala state as a screening tool in diagnosing neuropathic pain.
CONCLUSION
The Malayalam version of LANSS pain scale can be used as a validated tool for identifying neuropathic pain in Malayalam-speaking regions, especially in Kerala, India. The limitation of the study is, in the future studies, it is better to evaluate the agreement on neuropathic pain with specific regions of pain alone and moreover to different pain diagnoses such as radiculopathy, diabetic neuropathy, phantom limb pain, and burns pain.[3,6,9,23] Further studies have to be conducted to differentiate different pain origins. It will be a major breakthrough in management and decision on adding the choice of adjuvant drugs such as antiepileptics, membrane stabilizers, and benzodiazepine if we could differentiate the extent of neuropathic and nociceptive entities in the same patient.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.

REFERENCES
- 1.Bennett M. The LANSS Pain Scale: The Leeds Assessment of Neuropathic Symptoms and Signs. Pain. 2001;92:147–57. doi: 10.1016/s0304-3959(00)00482-6. [DOI] [PubMed] [Google Scholar]
- 2.Bennett MI, Attal N, Backonja MM, Baron R, Bouhassira D, Freynhagen R, et al. Using screening tools to identify neuropathic pain. Pain. 2007;127:199–203. doi: 10.1016/j.pain.2006.10.034. [DOI] [PubMed] [Google Scholar]
- 3.Conti F, Perricone C, Reboldi G, Gawlicki M, Bartosiewicz I, Pacucci VA, et al. Validation of a disease-specific health-related quality of life measure in adult Italian patients with systemic lupus erythematosus: LupusQoL-IT. Lupus. 2014;23:743–51. doi: 10.1177/0961203314524466. [DOI] [PubMed] [Google Scholar]
- 4.Devilliers H, Amoura Z, Besancenot JF, Bonnotte B, Pasquali JL, Wahl D, et al. LupusQoL-FR is valid to assess quality of life in patients with systemic lupus erythematosus. Rheumatology (Oxford) 2012;51:1906–15. doi: 10.1093/rheumatology/kes165. [DOI] [PubMed] [Google Scholar]
- 5.Freynhagen R, Baron R, Tölle T, Stemmler E, Gockel U, Stevens M, et al. Screening of neuropathic pain components in patients with chronic back pain associated with nerve root compression: A prospective observational pilot study (MIPORT) Curr Med Res Opin. 2006;22:529–37. doi: 10.1185/030079906X89874. [DOI] [PubMed] [Google Scholar]
- 6.García-Carrasco M, Mendoza-Pinto C, Cardiel MH, Méndez-Martínez S, García-Villaseñor A, Jiménez-Hernández C, et al. Health related quality of life in Mexican women with systemic lupus erythematosus: A descriptive study using SF-36 and LupusQoL (C) Lupus. 2012;21:1219–24. doi: 10.1177/0961203312456749. [DOI] [PubMed] [Google Scholar]
- 7.Hosseini N, Bonakdar SZ, Gholamrezaei A, Fatemi A, Karimzadeh H. Evaluating the validity and reliability of Persian version of Lupus Quality of Life (LupusQol) questionnaire in Iranian patients. J Isfahan Med Sch. 2014;31:1836–47. [Google Scholar]
- 8.Koc R, Erdemoglu AK. Validity and reliability of the Turkish Self-administered Leeds Assessment of Neuropathic Symptoms and Signs (S-LANSS) questionnaire. Pain Med. 2010;11:1107–14. doi: 10.1111/j.1526-4637.2010.00837.x. [DOI] [PubMed] [Google Scholar]
- 9.Wang SL, Wu B, Leng L, Bucala R, Lu LJ. Validity of LupusQoL-China for the assessment of health related quality of life in Chinese patients with systemic lupus erythematosus. PLoS One. 2013;8:e63795. doi: 10.1371/journal.pone.0063795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glynn C. An approach to the management of the patient with deafferentation pain. Palliat Med. 1989;3:13–21. [Google Scholar]
- 11.Krause SJ, Backonja MM. Development of a neuropathic pain questionnaire. Clin J Pain. 2003;19:306–14. doi: 10.1097/00002508-200309000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Hosseini N, Bonakdar ZS, Gholamrezaei A, Mirbagher L. Linguistic Validation of the LupusQoL for the Assessment of Quality of Life in Iranian Patients with Systemic Lupus Erythematosus. Int J Rheumatol 2014. 2014:151530. doi: 10.1155/2014/151530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Portenoy R. Development and testing of a neuropathic pain screening questionnaire: ID Pain. Curr Med Res Opin. 2006;22:1555–65. doi: 10.1185/030079906X115702. [DOI] [PubMed] [Google Scholar]
- 14.Wall PD. Neuropathic pain and injured nerve: Central mechanisms. Br Med Bull. 1991;47:631–43. doi: 10.1093/oxfordjournals.bmb.a072497. [DOI] [PubMed] [Google Scholar]
- 15.Hansson P, Kinnman E. Unmasking mechanisms of peripheral pain in a clinical perspective. Pain Rev. 1996;3:272–92. [Google Scholar]
- 16.Masson EA, Hunt L, Gem JM, Boulton AJ. A novel approach to the diagnosis and assessment of symptomatic diabetic neuropathy. Pain. 1989;38:25–8. doi: 10.1016/0304-3959(89)90068-7. [DOI] [PubMed] [Google Scholar]
- 17.Boureau F, Doubrère JF, Luu M. Study of verbal description in neuropathic pain. Pain. 1990;42:145–52. doi: 10.1016/0304-3959(90)91158-F. [DOI] [PubMed] [Google Scholar]
- 18.Bennett GJ. Chronic pain due to peripheral nerve damage: An overview. In: Fields HL, Liebeskind JC, editors. Progress in Pain Research and Management. Vol. 1. Seattle, WA: IASP Press; 1994a. pp. 51–9. [Google Scholar]
- 19.Wall PD. Introduction. In: Wall PD, Melzack R, editors. Textbook of Pain. 2nd ed. Edinburgh, UK: Churchill Livingstone; 1989. pp. 1–18. [Google Scholar]
- 20.Besson JM, Chaouch A. Peripheral and spinal mechanisms of nociception. Physiol Rev. 1987;67:186. doi: 10.1152/physrev.1987.67.1.67. [DOI] [PubMed] [Google Scholar]
- 21.Galer BS, Jensen MP. Development and preliminary validation of a pain measure specific to neuropathic pain: The Neuropathic Pain Scale. Neurology. 1997;48:332–8. doi: 10.1212/wnl.48.2.332. [DOI] [PubMed] [Google Scholar]
- 22.Hans G, Masquelier E, De Cock P. The diagnosis and management of neuropathic pain in daily practice in Belgium: An observational study. BMC Public Health. 2007;7:170. doi: 10.1186/1471-2458-7-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bouhassira D, Attal N, Alchaar H, Boureau F, Brochet B, Bruxelle J, et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4) Pain. 2005;114:29–36. doi: 10.1016/j.pain.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 24.Perez C, Galvez R, Huelbes S, Insausti J, Bouhassira D, Diaz S, et al. Validity and reliability of the Spanish version of the DN4 (Douleur Neuropathique 4 questions) questionnaire for differential diagnosis of pain syndromes associated to a neuropathic or somatic component. Health Qual Life Outcomes. 2007;5:66. doi: 10.1186/1477-7525-5-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan AW, MacFarlane IA, Bowsher D, Campbell JA. Weighted needle pinprick sensory thresholds: A simple test of sensory function in diabetic peripheral neuropathy. J Neurol Neurosurg Psychiatry. 1992;55:56–9. doi: 10.1136/jnnp.55.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elzahaf RA, Tashani OA, Unsworth BA, Johnson MI. Translation and linguistic validation of the self-completed Leeds Assessment of Neuropathic Symptoms and Signs (S-LANSS) scale for use in a Libyan population. Pain Pract. 2013;13:198–205. doi: 10.1111/j.1533-2500.2012.00576.x. [DOI] [PubMed] [Google Scholar]
- 27.Park C, Lee YW, Yoon DM, Kim DW, Nam DJ, Kim DH. Cross-cultural Adaptation and Linguistic Validation of the Korean Version of the Leeds Assessment of Neuropathic Symptoms and Signs Pain Scale. J Korean Med Sci. 2015;30:1334–9. doi: 10.3346/jkms.2015.30.9.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]


