Abstract
The present study focuses on the optimization of biosurfactant (BS) production using two potential biosurfactant producer Pseudomonas stutzeri NA3 and Acinetobacter baumannii MN3 and role of enzymes in the biodegradation of crude oil. The optimal conditions for P. stutzeri NA3 and A. baumannii MN3 for biodegradation were pH of 8 and 7; temperature of 30 and 40 °C, respectively. P. stutzeri NA3 and A. baumannii MN3 produced 3.81 and 4.68 g/L of BS, respectively. Gas chromatography mass spectrometry confirmed that BS was mainly composed of fatty acids. Furthermore, the role of the degradative enzymes, alkane hydroxylase, alcohol dehydrogenase and laccase on biodegradation of crude oil are explained. Maximum biodegradation efficiency (BE) was recorded for mixed consortia (86%) followed by strain P. stutzeri NA3 (84%). Both bacterial strains were found to be vigorous biodegraders of crude oil than other biosurfactant-producing bacteria due to their enzyme production capabilities and our results suggests that the bacterial isolates can be used for effective degradation of crude oil within short time periods.
Electronic supplementary material
The online version of this article (doi:10.1007/s13205-017-0902-7) contains supplementary material, which is available to authorized users.
Keywords: Biodegradation, Biosurfactant, FT-IR spectroscopy, Alkane hydroxylase, Emulsification activity, GC–MS
Introduction
Biosurfactants are surface-active molecules synthesized by bacteria, yeasts and fungi (Kumar et al. 2015; Peele et al. 2016). Biosurfactants are amphipathic molecules by both hydrophobic and hydrophilic moieties that preferentially locate at the interface between fluid phases that comprise diverse levels of polarity and hydrogen bonding, such as air/water or oil/water interfaces (Mulligan 2005; Khopade et al. 2012; Roy et al. 2015; Peele et al. 2016; Sebatini et al. 2016). Surfactants have been useful in various industries for diverse applications, such as environmental, agriculture, chemistry, cosmetics food production, medicinal, pharmaceutics and microbial-enhanced oil recovery (Freitas de Oliveira et al. 2013; Dhasayan et al. 2015). Table S1 (Supplementary material) shows the various industrial applications of biosurfactant and their source of isolations. Biosurfactants have distinctive features, including low toxicity, biodegradability, and large flexibility in operations (Abalos et al. 2004; Pathak and Keharia, 2014; Kumar et al. 2015; Mani et al. 2016). However, their production is still a challenging task. To overcome this issue, a range of inexpensive raw materials, coupled with oil wastes, plant-derived oils, lactic whey, distillery wastes and starchy substances have been tested for biosurfactant synthesis (Mukherjee et al. 2006). The reduced costs due to biosurfactant production using agricultural wastes (Gudina et al. 2011) will boost production. However, the most important issues associated with the use of cheap wastes are to discover wastes that provide an equilibrium of nutrients that allows growth along with high production as well as isolation of efficient strains, improved fermentation processes and replacement of substrates.
A broad range of microorganisms can produce biosurfactants using different substrates such as oils, alkanes, sugars, and agro-industrial wastes. Good examples are potato processing wastes and molasses (Mulligan 2005). For example, lipopeptides are synthesized by several Bacillus species. Glycolipids are synthesized by Candida and Pseudomonas species, whereas Thiobacillus thiooxidans synthesizes phospholipids. Lipid-polysaccharide compounds are produced by Acinetobacter species. Abalos et al. (2004) reported that capabilities of the rhamnolipid role by Pseudomonas aeruginosa in crude oil degradation. Microorganisms frequently produce biosurfactants during proliferation on water immiscible substrates, to make easy utilization of the substrates by the cells (Van Dyke et al. 1991; Youssef et al. 2004; Ibrahim, 2016; Parthipan et al. 2017a).
Presently, the key factor that decreases the extensive use of biosurfactants is the process economics and various approaches selected for decreasing the manufacturing expenses and building fermentation economical by means of chemical synthesis (Makkar and Cameotra 2002). This production can be achieved by production medium optimization, improvement of the efficient recovery methods and the strain development can open the way to their cheap production, throughout the enlargement of efficient processes (Mukherjee et al. 2006; Kumar et al. 2016). Thus, upcoming biosurfactant research must further favour the manufacturing cost, predominantly through the exploit of inexpensive culture media. An important factor that influences on biosurfactant production is the carbon and nitrogen sources. Additionally optimization of other environmental factors and growth conditions such as pH, agitation, temperature, and oxygen accessibility are also considerable interest (Desai and Banat 1997).
Environmental pollutions due to the release of hydrocarbons, solvents and heavy metals is a serious challenge nowadays, since these components are extremely dangerous to living organisms and indirectly contribute to the economic losses in developing countries (Sathishkumar et al. 2008; Chikere et al. 2011; Ismail et al. 2013; Ekperusi and Aigbodion 2015). Various studies were reported on degradation of xenobiotic compounds such as crude oil by indigenous microorganisms by many researchers (Hassanshahian et al. 2012; Rajasekar 2017). Although various physiochemical methods exist in literature to remediate the oil contaminations, bioremediation is considered to be one of the best choices, since they are more efficient, eco-friendly and cost effective than other methods (Ismail et al. 2013).
Biodegradative enzymes also play a key role in biodegradation of hydrocarbons. An important mechanism in alkane removal is the oxygenation of terminal methyl group. While alkane-degrading microbes own multiple genes for alkane hydroxylases, as they are highly proficient in degrading a wide range of alkanes (Van Beilen et al. 2002; Parthipan et al. 2017b). Many enzymes are involved in the hydrocarbon degradation. Good examples are methane monooxygenase, alkane monooxygenase, alcohol dehydrogenase and laccase (Parthipan et al. 2017b). A wide range of bacterial strains such as Pseudomonas sp. BP10, Stenotrophomonas nitritireducens (Jauhari et al. 2014), P. aeruginosa PSA5, Rhodococcus sp. NJ2, and Ochrobactrum intermedium (Mishra and Singh 2012) have been studied for their ability to produce these degradative enzymes during the biodegradation of hydrocarbons.
Therefore, the present study involves seven bacterial strains that are screened for biosurfactant production. Further, biosurfactant production conditions were optimized for selected biosurfactant-producing strains by providing the different carbon and nitrogen sources. The effects of pH, temperature, concentration of the carbon and nitrogen on biosurfactant production also evaluated. The role of biosurfactants towards crude oil degradation assays and the role of other degradative enzymes were also studied.
Materials and methods
Chemicals
All the chemicals used in this study, i.e., n-hexadecane, triton X-100, starch, sucrose, glycerol, maltose, mannitol, ammonium phosphate, ammonium sulphate, ammonium chloride, peptone, potassium nitrate, yeast extract, urea, hydrochloric acid, magnesium sulphate, ferrous sulphate, manganese sulphate, zinc sulphate, copper sulphate, calcium chloride, monopotassium phosphate, disodium phosphate, dipotassium phosphate, ammonium nitrate, ferric chloride, dichloromethane, Luria–Bertani (LB) medium, and sodium hydroxide of analytical grade were purchased from Himedia (Mumbai, India). Crude oil and diesel were purchased from an oil company and coconut oil was purchased from a local store.
Microorganisms and culture conditions
In this study Gram-negative bacterial strains P. stutzeri MN1, A. baumannii MN3, P. stutzeri NA3, P. aeruginosa TBH2, Chelatococcus caeni TMN2, Pseudomonas sp. TNA2 and Achromobacter xylosoxidans TTB1 were screened to evaluate their production of biosurfactants. These strains were isolated from the injection/production water collected from Indian crude oil reservoir company, Karaikal, India. Strains were identified by 16S rDNA sequence and deposited in National Center for Biotechnology Information Genbank (KU708859–KU708865) as reported in Sathishkumar et al. (2016) and Kuppusamy et al. (2017). These strains were retrieved from glycerol stock and sub-cultured in LB agar plates (g/L 10.0 tryptone, 5.0 yeast extract, 10.0 sodium chloride with 15.0 agar) and incubated at 37 °C for 24 h. Further inoculums were preferred by single colony inoculation method using LB broth (pH 7.0) and incubated in an orbital shaker (150 rpm) for 24 h at 37 °C used for further studies.
Screening for biosurfactant production
Biosurfactant production was aerobically carried out in 500 mL Erlenmeyer flask containing 200 mL of sterile minimal salt medium (MSM) (g/L 0.2 MgSo4, 0.02 CaCl2, 1.0 KH2PO4, 1.0 K2HPO4, 1.0 NH4NO3, and 0.5 FeCl3 Himedia, Mumbai, India), supplemented with 1% (v/v) glucose. Medium pH was set to 7.0 and sterilized at 121 °C for 15 min. The flasks were individually inoculated with pre-grown bacterial culture (1.6 × 104 CFU m/L), and incubated at 37 °C in an orbital shaker (150 rpm). After 5 days of incubation, the content of the flasks was centrifuged at 4 °C for 20 min at 4000×g and the supernatant was utilized for screening purposes. Biosurfactant production was tested with a series of preliminary screening assays such as drop collapse test by Jain et al. (1991), the oil displacement method (Hassanshahian 2014) and the evaluation of emulsification activity (Chen et al. 2015). All the assays were performed in triplicate and sterile distilled water was used as control.
Optimization of biosurfactant production
Based on the initial screening of the isolates, two bacterial strains, i.e., P. stutzeri NA3 and A. baumannii MN3 were selected for optimization studies. Five different parameters were selected for optimization studies such as pH, temperature, carbon, nitrogen sources, and the concentration of carbon/nitrogen sources. Both strains were sub-cultured using LB medium as described earlier.
Effect of pH
For optimization of the pH, six ranges were selected, i.e., 5.0, 6.0, 7.0, 8.0, 9.0, and 10.0. MSM medium was prepared with 1% of the glucose as sole carbon source and different pH ranges was adjusted with the help of digital pH meter using 6N HCl and 2N NaOH solutions. After the pH adjustment, the medium was sterilized at 121 °C for 15 min. Both strains NA3 and MN3 (1.6 × 104 CFU m/L) were inoculated into sterilized medium and kept at 37 °C for 5 days in an orbital shaker (150 rpm).
Effect of temperature
Five temperatures were selected for optimization, i.e., 20, 30, 40, 50, and 60 °C, since these ranges of temperature were optimum for many bacteria. MSM medium was prepared with 1% of the glucose as sole carbon source and the pH was adjusted to 8.0 and 7.0 for strain NA3 and MN3, respectively. The medium was sterilized at 121 °C for 15 min. Both strains NA3 and MN3 (1.6 × 104 CFU m/L) were inoculated and kept at different temperatures as above-mentioned for 5 days in an orbital shaker (150 rpm).
Effect of carbon
Eight carbon sources such as crude oil, coconut oil, diesel oil, sucrose, starch, glycerol, mannitol, and maltose were selected based on the their simple to complex nature for the optimization purpose. MSM medium was prepared with 1% of each carbon source and pH of the medium was adjusted to 8.0 and 7.0 for strain NA3 and MN3, respectively; further sterilized at 121 °C for 15 min. Both the bacterial strains NA3 and MN3 (1.6 × 104 CFU m/L) were inoculated and incubated at 30 and 40 °C, respectively, for 5 days in an orbital shaker (150 rpm).
Effect of nitrogen
Nitrogen is a crucial requirement for microbial growth as well as for effective biosurfactant production. Seven different nitrogen sources were selected for the optimization purpose, i.e., ammonium phosphate, ammonium sulphate, ammonium chloride, peptone, potassium nitrate, yeast extract, and urea. MSM medium was prepared without ammonium nitrate (nitrogen source) and separately replaced with each nitrogen source (1 g/L), 1% of sucrose was added as carbon source and pH of the medium was adjusted 8.0 and 7.0 for strain NA3 and MN3, respectively; further sterilized at 121 °C for 15 min. Both bacterial strains NA3 and MN3 (1.6 × 104 CFU m/L) were inoculated and incubated at 30 and 40 °C for strain NA3 and MN3, respectively, for 5 days in an orbital shaker (150 rpm).
Effect of the carbon and nitrogen concentration
Carbon and nitrogen substrates optimized in this study were further used to establish their joint concentration required for optimal biosurfactant production. Both optimized carbon and nitrogen sources were added in the MSM medium with different concentration, i.e., 1, 2, 3, 4 and 5%. pH was adjusted to 8.0 and 7.0 for strains NA3 and MN3, respectively, and sterilized at 121 °C for 15 min. Both strains NA3 and MN3 (1.6 × 104 CFU m/L) were inoculated and incubated at 30 and 40 °C, respectively, in an orbital shaker (150 rpm) for 5 days.
Analysis of optimized conditions
At end of the each optimization assay, bacterial cells were removed from surfactant-containing medium by centrifugation and the supernatant was used for the emulsification activity. Bacterial biomass was acquired as described by Santos et al. (2014). The optimum growth conditions of the each strain were confirmed by emulsification activity and bacterial biomass for each parameter. Cell-free supernatant collected from each assay optimizing the effect of the carbon and nitrogen concentration was used for quantification of biosurfactant production. The crude biosurfactant was obtained as described by Xia et al. (2011). Briefly, the supernatant was acidified to pH 2.0 using HCl and left for precipitation, the precipitated biosurfactant was pooled by centrifugation. The obtained crude biosurfactant was suspended in double-deionized water and extracted with dichloromethane. The solvent layer was collected, and the extraction steps were repeated for three times. Then the product was concentrated using a rotary evaporator and weighed to confirm the quantitative production. The surfactant collected in this method was considered as partially purified and used for characterization purposes.
Biosurfactant characterization
The biosurfactant was characterized using gas chromatography mass spectrometry (GC–MS) and Fourier-transform infrared spectroscopy (FT-IR) (Perkin–Elmer, Nicolet Nexus—470). Further purification was carried as follows: 10 mg of biosurfactants were mixed with a 5% HCl–methanol solution and 1 mL of H2O was added at the end of the reaction. Then the sample was collected with n-hexane and 1 μL of sample was injected into a gas chromatographer. GC–MS model Perkin Elmer, Clarus 680, Elite-5MS (30 m × 0.25 mm ID × 0.25 μm) was used. 1 μL of the purified sample was injected by split mode at 10:1 ratio. Helium was used as carrier gas, with flow rate of 1 mL min−1 and the working temperature of the GC injector was set to 250 °C. The gradient temperature was in the range from 60 to 300 °C at a speed of 10 °C min−1, through an isothermal phase of 6 min at the end of the analysis. The mass spectra were obtained with a m/z range of 50–600 in an ultra-high resolution mode with a acquisition speed of 6 spectra/s. The mass spectrum was matched with the NIST database. The biosurfactants functional groups were qualitatively characterized by FT-IR. The samples were pulverized by the addition of potassium bromide at 1:100 ratio and the pellet was fixed in the sample container, and analysed in the mid IR region of 400–4000 cm−1.
Biodegradation of crude oil and enzyme assays
The degradation of crude oil was carried out as described by Rahman et al. (2002). Pre-cultured individual bacterial strains and mixed consortia (each 2.1 × 104 CFU m/L) were transferred into 250 mL of Erlenmeyer flask, each containing 100 mL of optimized growth medium supplemented with 1% (v/v) sterile crude oil as carbon source with respective nitrogen sources. An uninoculated flask was also used to monitor the abiotic loss of the crude oil substrate during degradation. The flasks were incubated at optimum temperature for 7 days at 150 rpm. All experiments were performed in triplicate. At everyday intervals alkane hydroxylase and alcohol dehydrogenase activity during the biodegradation study were confirmed as described in Jauhari et al. (2014) and Parthipan et al. (2017b). Similarly, laccase enzyme was quantified as described by Kuppusamy et al. (2017).
Biodegradation of crude oil hydrocarbons was examined using GC–MS. After 7 days of incubation, the residual crude oil in the culture flask was extracted two times with an equal volume of n-hexane (Adebusoye et al. 2007) and the solvent phase was dried in a vacuum oven at 60 °C. The resultant crude oil (10 μL) was dissolved in 990 μL of n-hexane and analysed using GC–MS as previously described. Hydrocarbon degradation was expressed as the percentage of crude oil degraded versus that present in the abiotic control sample. The biodegradation efficiency (BE) percentage, based on the degradation of hydrocarbons, was calculated using the formula described by Michaud et al. (2004) and Rajasekar et al. (2007) where, BE (%) = 100—(A s × 100/A ac). Here, A s is the total area of peaks in every sample, and A ac is the total area of peaks in the proper abiotic control.
Statistical analysis
All data recorded in experiments were exposed to the analysis of variance (ANOVA), the Tukey test was used for statistical significance analyses. P < 0.05 was used for the significance of differences between means.
Results and discussion
Screening for biosurfactant production
As shown in Table 1, a total of seven bacterial strains belonging to four different genera were screened for biosurfactant production. Both primary (oil spreading and drop collapse assays) and secondary (emulsification activity) screening methods were used for the effective screening of the isolates (Korayem et al. 2015). In oil spreading assay a clear zone was measured in presence of strains NA3, MN3 and TNA2 with a range of 1.5 to 2.0 cm among the other strains such as MN1, TBH2, TMN2 and TTB1. These primary and secondary screening assays confirmed the presence of biosurfactants in the cell-free culture broth of NA3, MN3 and TNA2. The area of oil displacement in the oil spreading assay was directly proportional to the concentration of the biosurfactant in the solution. A quick flat drop was noted in drop collapse test for the strains NA3 and MN3 within 30 s followed by more than 1 min for strain MN1 and more than 2 min for the remaining strains including TBH2, TMN2, TNA2 and TTB1. The emulsification index (E24) was further used for confirming the efficient biosurfactant producers, strains NA3 and MN3 which gave the highest E24 values as 38.5 and 36.4%, respectively, than compared to other strains. This E24 value of the strains is recognized as the production of biosurfactants. In agreement with our data, several Pseudomonas and Acinetobacter species have been previously reported as efficient biosurfactant producers (Chen et al. 2012; Bao et al. 2014; Deepika et al. 2016).
Table 1.
Screening for biosurfactant production: oil spreading assays, drop collapse assay and emulsification activity of the isolates
| No. | Strain | Oil spreading | Drop collapse | Emulsification index |
|---|---|---|---|---|
| 1 | NA3 | ++ | +++ | 38.5 |
| 2 | MN1 | + | ++ | 6.94 |
| 3 | MN3 | ++ | +++ | 36.4 |
| 4 | TBH2 | + | + | 8.5 |
| 5 | TMN2 | + | + | 7.6 |
| 6 | TNA2 | ++ | + | 5.2 |
| 7 | TTB1 | + | + | 9.2 |
NA3, P. stutzeri; MN1, P. stutzeri; MN3, A. baumannii; TBH2, P. aeruginosa; TMN2, C. Caeni; TNA2, Pseudomonas sp.; TTB1, A. xylosoxidans
Oil spreading assay: ‘+’—oil spreading with a clear zone of 0.5–1.0 cm—‘++’—oil spreading with a clear zone of 1.5–2.0 cm—‘+++’—oil spreading with a clear zone of 2.0–3.0 cm
Drop collapse assay: ‘+++’—drop collapse within 1 min—‘++’—drop collapse after 1 min and ‘+’—drop collapse after 2 min of biosurfactant addition
Optimization of the substrate and growth conditions
Based on the initial screening results two strains namely P. stutzeri NA3 and A. baumannii MN3 were further selected for the optimization studies. Different carbon sources, nitrogen sources, pH, temperature and concentration of the carbon and nitrogen sources were optimized in this study (Korayem et al. 2015). The synthesis of biosurfactant was articulated in expressions of emulsification index (E24%) and bacterial biomass. The biosurfactant containing culture broth was centrifuged and supernatant was used for the measurement of emulsification activity.
Effect of pH on biosurfactant production
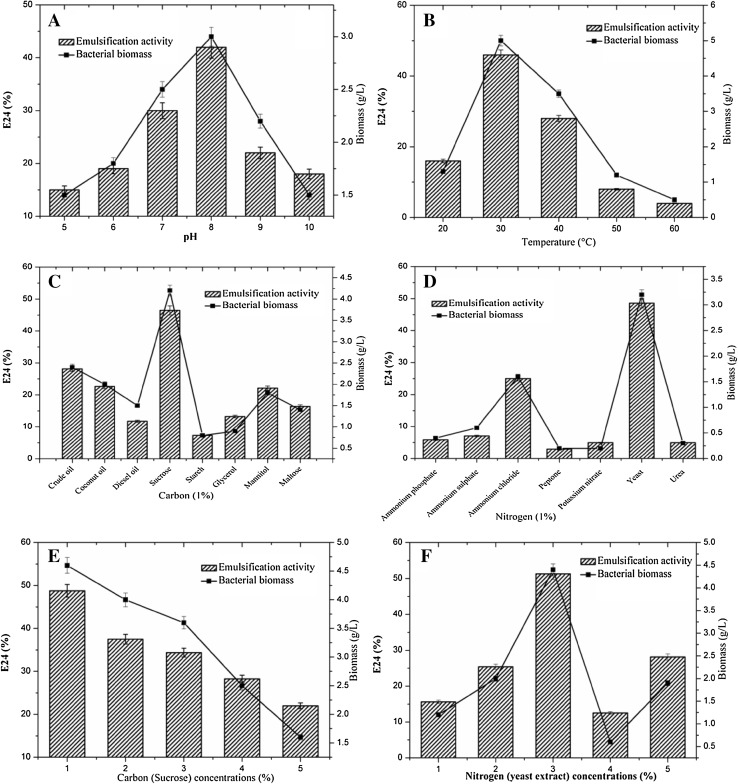
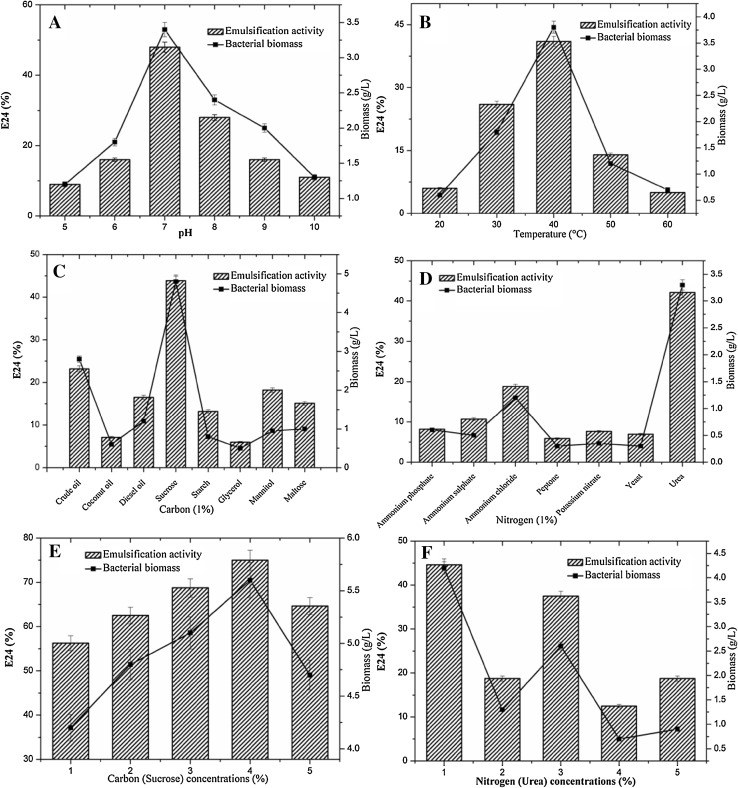
Many physiochemical factors such as pH, temperature, growth conditions and agitation were strongly influenced by the microbial growth and their metabolisms (Khopade et al. 2012). Among them pH of the production medium is a key factor for the bacterial growth. Each and every bacterium has an optimum pH level for their proficient metabolism. A minute modification in the pH level of the production medium may lead to the complete reduction of the activity. At pH 5.0, the biosurfactant synthesis was rigorously reduced and the bacterial proliferation was considerably impeded. This low pH developed unfavourable conditions for both the strains as described in Khopade et al. (2012). When the starting pH was set to 8.0 for NA3, the E24 value was increased to 42%, when increased more than pH 8.0, a decline in the biosurfactant production level was noted. Similar results were recorded for the MN3 where the optimum pH was 7.0 which matched with the previous studies too (Lotfabada et al. 2009; Khopade et al. 2012). The optimum pH for bacterial development and biosurfactant synthesis were determined to be 8.0 and 7.0 for strains NA3 and MN3 as shown in Figs. 1a and 2a. As shown by ANOVA results (Table 2), P values are lower than 0.05. The pH of the production medium plays an important role in the synthesis of sophorolipid using T. bombicola. Rhamnolipid synthesis using Pseudomonas spp. showed highest production at a pH range of 6–6.5 and declined harshly beyond pH 7.0 was also reported (Powalla et al. 1989).
Fig. 1.
Effect of different physiochemical parameters biosurfactant production by bacterial strains P. stutzeri NA3 and A. baumannii MN3. a pH, b temperature, c carbon sources, d nitrogen sources, e carbon concentration, f nitrogen concentration. Vertical bars represent the standard error of the mean based on the three independent tests
Fig. 2.
Effect of different physiochemical parameters biosurfactant production by bacterial strains A. baumannii MN3. a pH, b temperature, c carbon sources, d nitrogen sources, e carbon concentration, f nitrogen concentration. Vertical bars represent the standard error of the mean based on the three independent tests
Table 2.
Optimization of pH, temperature, carbon and nitrogen sources for biosurfactant production by strains Pseudomonas stutzeri NA3 and Acinetobacter baumannii MN3: analysis of variance (ANOVA)
| Treatment | Pseudomonas stutzeri NA3 | Acinetobacter baumannii MN3 | ||||
|---|---|---|---|---|---|---|
| df | F | P | df | F | P | |
| pH | ||||||
| 5 | 5 | 13.50 | 0.021 | 5 | 11.786 | 0.026 |
| 6 | 5 | 14.76 | 0.018 | 5 | 9.600 | 0.036 |
| 7 | 5 | 18.69 | 0.012 | 5 | 29.817 | 0.005 |
| 8 | 5 | 9.046 | 0.040 | 5 | 38.020 | 0.004 |
| 9 | 5 | 9.600 | 0.036 | 5 | 9.926 | 0.034 |
| 10 | 5 | 8.824 | 0.041 | 5 | 8.436 | 0.044 |
| Temperature | ||||||
| 20 °C | 5 | 28.680 | 0.006 | 5 | 17.246 | 0.014 |
| 30 °C | 5 | 22.072 | 0.009 | 5 | 11.616 | 0.027 |
| 40 °C | 5 | 25.835 | 0.007 | 5 | 11.906 | 0.026 |
| 50 °C | 5 | 8.824 | 0.041 | 5 | 16.769 | 0.015 |
| 60 °C | 5 | 21.600 | 0.010 | 5 | 8.517 | 0.043 |
| Carbon sources | ||||||
| Crude oil | 5 | 55.846 | 0.002 | 5 | 10.367 | 0.032 |
| Coconut oil | 5 | 11.173 | 0.029 | 5 | 28.628 | 0.006 |
| Diesel oil | 5 | 18.744 | 0.012 | 5 | 15.970 | 0.016 |
| Sucrose | 5 | 8.267 | 0.045 | 5 | 14.107 | 0.020 |
| Starch | 5 | 21.407 | 0.010 | 5 | 11.361 | 0.028 |
| Glycerol | 5 | 10.584 | 0.031 | 5 | 43.333 | 0.003 |
| Mannitol | 5 | 26.427 | 0.007 | 5 | 12.448 | 0.024 |
| Maltose | 5 | 7.983 | 0.048 | 5 | 9.047 | 0.040 |
| Nitrogen sources | ||||||
| Ammonium phosphate | 5 | 51.923 | 0.002 | 5 | 20.450 | 0.011 |
| Ammonium sulphate | 5 | 9.600 | 0.036 | 5 | 5.880 | 0.072 |
| Ammonium chloride | 5 | 18.150 | 0.013 | 5 | 9.750 | 0.035 |
| Peptone | 5 | 33.231 | 0.004 | 5 | 9.720 | 0.036 |
| Potassium nitrate | 5 | 49.148 | 0.002 | 5 | 23.520 | 0.008 |
| Yeast | 5 | 34.645 | 0.004 | 5 | 18.692 | 0.012 |
| Urea | 5 | 22.982 | 0.009 | 5 | 9.213 | 0.039 |
df degrees of freedom, F statistics, P value
Effect of temperature on biosurfactant production
Another physiochemical factor that majorly influences the production of biosurfactant is temperature of the growth medium. As shown in Figs. 1b and 2b, both strains showed the different optimal temperature of 30 and 40 °C, low temperature 20 °C was not found to be ideal temperatures for the many bacterial strains. Higher temperature of 50–60 °C also stopped many of the bacterial metabolisms. Bacterial strain NA3 showed highest E24 values (46%) at 30 °C (Khopade et al. 2012). As the incubation temperature was increased, it led to a sharp reduction in the biosurfactant production to a very low E24 value of 4% at 60 °C. Another strain MN3 showed the highest E24 values of 41% at 40 °C (Sathishkumar et al. 2008). Similar reduction in the biosurfactant production was noted with only 5% of E24 at 60 °C. Table 2 shows ANOVA results of experimental data and all the values were significant (P < 0.05). These results suggests that, both the strains NA3 and MN3 were mesophilic bacterium, which is key factor for these strains to show effective production level at moderate temperature of 30–40 °C.
Effect of carbon on biosurfactant production
The higher production of biosurfactant depends on the composition of the culture medium. Under controlled conditions, the modification of the carbon substrate in the production medium could influence the biosurfactant synthesis. As represented in Figs. 1c and 2c, eight carbon sources are screened for biosurfactant production. Among all, sucrose was found to be a favourable carbon source for both the bacterial strains (E24: 46.4% for NA3 and 43.8% for MN3) (Khopade et al. 2012). Table 2 shows ANOVA results of experimental data and all the values were found to be significant (P < 0.05). Similarly, sucrose was reported as best carbon sources for other bacteria species (Korayem et al. 2015). Besides sucrose and crude oil, coconut oil was also recognized as best production source for strain NA3, and crude oil and mannitol were the best for strain MN3. Among the carbon sources used starch and glycerol showed low E24 values for NA3 and MN3, respectively.
Effect of nitrogen on biosurfactant production
Nitrogen-rich substrates play an important role in the biosurfactant synthesis (Wu et al. 2008). The selections of nitrogen source have an effect on the biosurfactant synthesis as shown in Figs. 1d and 2d, Table 2 shows ANOVA results of experimental data and all the values were significant (P < 0.05). In sequence to acquire higher production of biosurfactant, it is obligatory to comprise controlled conditions in terms of macronutrients. Among the tested seven nitrogen sources, yeast extract gave the highest E24 values (48.5%) for the strain NA3 (Kiran et al. 2009; Khopade et al. 2012) and urea was found to be the most excellent substrate (E24 was 42.1%) for the strain MN3 (Meyer 2011; Elazzazy et al. 2015). Using ammonium salts in the form of NH4Cl led to considerable enhancement in the proliferation, but not in biosurfactant synthesis and also caused remarkable decline in pH ranges (Prieto et al. 2008; Khopade et al. 2012).
Effect of carbon and nitrogen concentration on biosurfactant production
The concentration of the substrate is an essential factor that determines the biosurfactant production rate. As shown in Figs. 1e, f and 2e, f. 1–5% of the carbon and nitrogen sources for NA3, 1% of the sucrose (E24: 48.7%) and 3% of the yeast extract (E24: 51.2%) were found to be the optional concentration for biosurfactant production. In case of MN3, 1% of the sucrose (E24: 75%) and 4% of the urea (E24: 44%) were noted as the optimum concentration of carbon and nitrogen, respectively. Table 3 shows ANOVA results of experimental data and all the values are significant (P < 0.05). Due to set up of optimized conditions including the pH, temperature, carbon and nitrogen sources, E24 values gradually increased to the maximum level compared to individual optimization conditions. Optimized concentration study as said above was used for quantitative analysis of the biosurfactant. Strain NA3 produced 3.81 g/L (Xia et al. 2011) and strain MN3 produced 4.68 g/L of biosurfactant, which are recorded as highest compared to the previous reports (Hassanshahian and Giti 2008; Xia et al. 2011).
Table 3.
Optimization of carbon and nitrogen concentration for biosurfactant production by strains Pseudomonas stutzeri NA3 and Acinetobacter baumannii MN3: analysis of variance (ANOVA)
| C/N (%) | Pseudomonas stutzeri NA3 | Acinetobacter baumannii MN3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| df | F | P | df | F | P | df | F | P | df | F | P | |
| Sucrose | Yeast extract | Sucrose | Urea | |||||||||
| 1 | 5 | 13.83 | 0.020 | 5 | 22.45 | 0.009 | 5 | 7.68 | 0.050 | 5 | 29.40 | 0.006 |
| 2 | 5 | 25.49 | 0.007 | 5 | 30.33 | 0.005 | 5 | 39.0 | 0.003 | 5 | 13.50 | 0.021 |
| 3 | 5 | 14.38 | 0.019 | 5 | 19.32 | 0.012 | 5 | 17.2 | 0.014 | 5 | 11.30 | 0.028 |
| 4 | 5 | 11.43 | 0.028 | 5 | 10.14 | 0.033 | 5 | 10.8 | 0.030 | 5 | 20.28 | 0.011 |
| 5 | 5 | 21.06 | 0.010 | 5 | 16.70 | 0.015 | 5 | 9.6 | 0.036 | 5 | 16.53 | 0.015 |
df degrees of freedom
Biosurfactant characterization
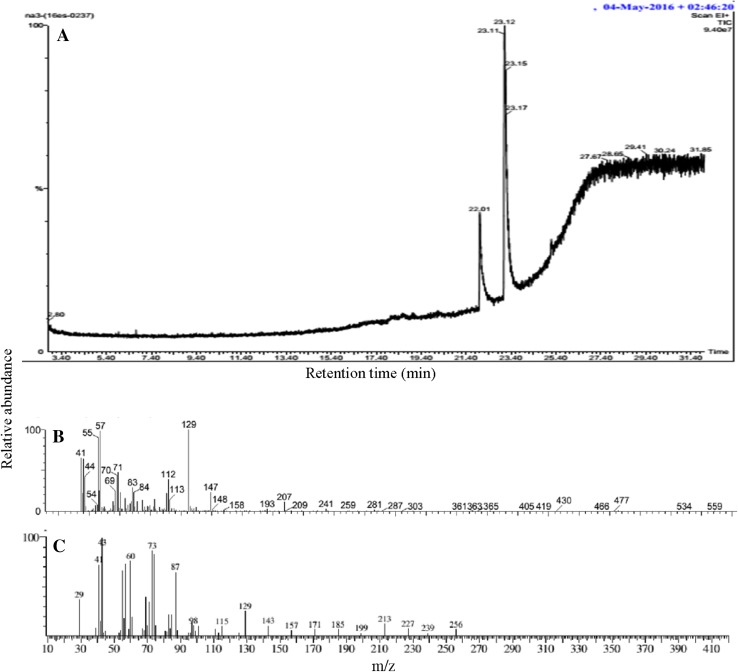
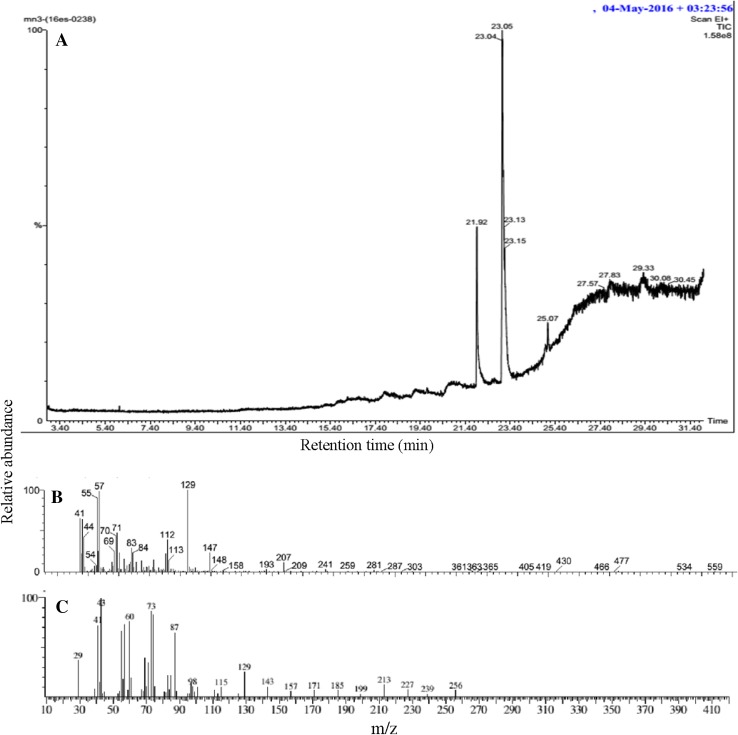
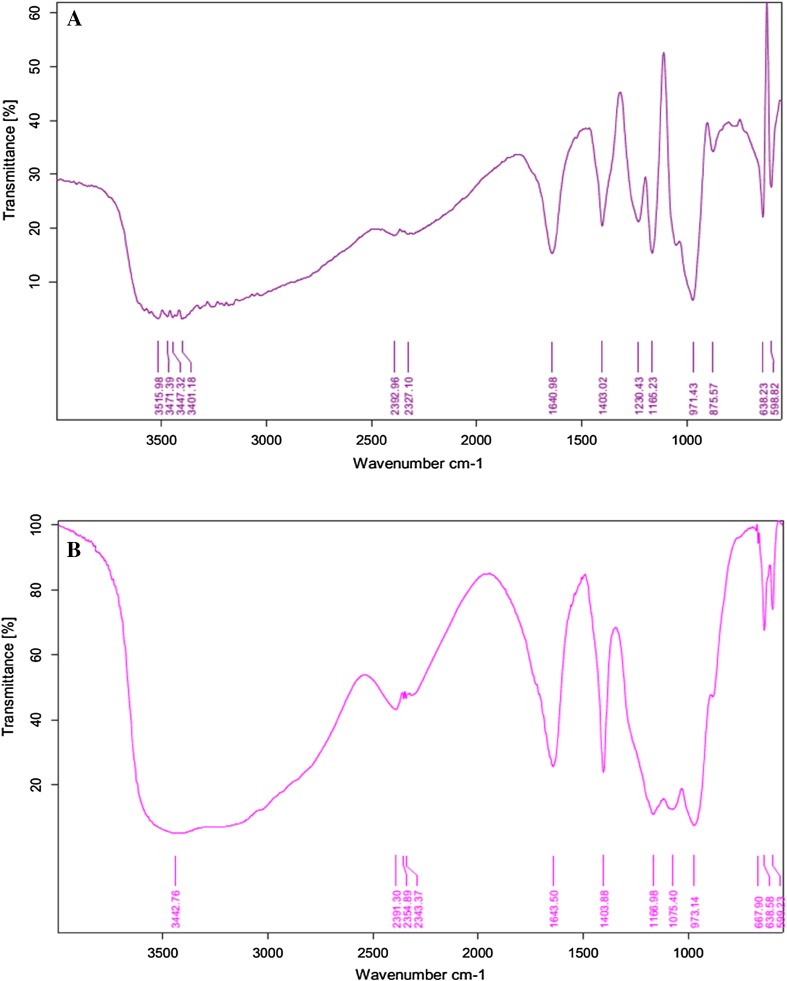
Gas chromatography analysis revealed that the biosurfactant extracted from both bacterial strains were fatty acids. Both strains P. stutzeri NA3 (Fig. 3) and A. baumannii MN3 (Fig. 4) contained fatty acids such as hexanedioic acid, bis (2-ethylhexyl) ester (C22H42O4) (Hien et al. 2013), and palmitic acid (C16H32O2) (Davila et al. 1992). FT-IR analysis of the biosurfactant produced by P. stutzeri NA3 (Fig. 5a) confirmed that it was a lipopeptide. FT-IR spectra revealed that peak at 598 cm−1 corresponding to C–I (Carbon–Iodine) bond. The peak at 638 cm−1 validated the presence of C–Br. Absorption bands at 875 and 971 cm−1 corresponds to the stretching of RCH=CH2. Intense stretching peaks at 1165 and 1640 cm−1 indicated the presence of R–NO2 groups. A peak at 1230 cm−1 was assigned to C–O stretch of esters. The transmittance at 1403 cm−1 was probably linked with the presence of aliphatic chain with C–H group. Similarly, A. baumannii MN3 also showed many analogous peaks such as C–I, C–Br, RCH=CH2, R–NO2, and C–H (Fig. 5b). In addition, a new peak was noted at 2391 cm−1 reveals the stretching of N–H group. The availability of all these functional groups firmly substantiated that biosurfactants were mainly lipopeptide in nature (Sarafin et al. 2014).
Fig. 3.
GC–MS analysis of biosurfactant from P. stutzeri NA3: GC spectrum of the biosurfactant (a), Hexanedioic acid, bis (2-ethylhexyl) ester mass spectrum (b) and Palmitic acid mass spectrum (c)
Fig. 4.
GC–MS analysis of biosurfactant from A. baumannii MN3: GC spectrum of biosurfactant (a), Hexanedioic acid, bis (2-ethylhexyl) ester mass spectrum (b) and Palmitic acid mass spectrum (c)
Fig. 5.
FT-IR spectrum of biosurfactant: P. stutzeri NA3 (a) and A. baumannii MN3 (b)
Crude oil degradation analysis
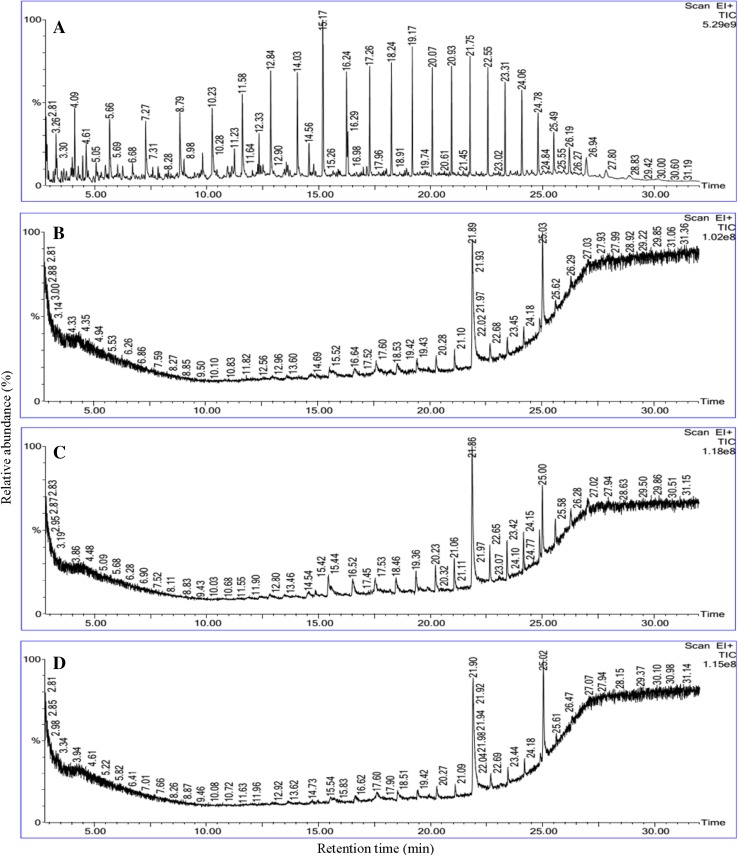
The utilization of crude oil by biosurfactant-producing bacteria was continuously monitored at the time of the biodegradation process. It was visibly noticed that inoculation of both strains in BH medium with crude oil as sole carbon source turned the medium into more turbid within 2nd day of incubation. The turbidity of the production medium increased with incubation time. At the end of the incubation period the residual crude oil was recovered for GC–MS analysis to understand the level of degradation. The gas chromatogram of crude oil degradation is shown in Fig. 6. The structural matches of the GC retention data of the crude oil, as well as the mass spectra interpretation are presented in Table 4. The biodegradation of crude oil by P. stutzeri NA3, A. baumannii MN3 and mixed consortia showed biodegradation efficiency (BE) of about 84, 78 and 86%, respectively.
Fig. 6.
Gas-chromatography characterization of crude oil degradation: abiotic control system (a), P. stutzeri NA3 (b), A. baumannii MN3 and (c), Mixed consortia (d)
Table 4.
Biodegradation efficiency of crude oil the in presence of biosurfactant-producing bacterial strains and laccase
| S. no. | RT | Compounds | RA | NA3 | BE (%) | MN3 | BE (%) | Mix | BE (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2.81 | Octane | 30 | 0 | 100 | 0 | 100 | 0 | 100 |
| 2 | 4.09 | Octane | 46 | 0 | 100 | 0 | 100 | 0 | 100 |
| 3 | 5.66 | Nonane | 40 | 0 | 100 | 0 | 100 | 0 | 100 |
| 4 | 7.27 | Decane | 34 | 0 | 100 | 0 | 100 | 0 | 100 |
| 5 | 8.79 | Undecane | 40 | 0 | 100 | 0 | 100 | 0 | 100 |
| 6 | 10.23 | Dodecane | 42 | 0 | 100 | 0 | 100 | 0 | 100 |
| 7 | 11.58 | Tridecane | 50 | 0 | 100 | 0 | 100 | 0 | 100 |
| 8 | 12.84 | Tetradecane | 66 | 0 | 100 | 0 | 100 | 0 | 100 |
| 9 | 14.03 | Pentadecane | 64 | 0 | 100 | 0 | 100 | 0 | 100 |
| 10 | 15.17 | Hexadecane | 100 | 4 | 96 | 12 | 88 | 2 | 98 |
| 11 | 16.24 | Heptadecane | 66 | 4 | 94 | 4 | 94 | 4 | 94 |
| 12 | 17.26 | Octadecane | 68 | 4 | 94 | 6 | 91 | 4 | 94 |
| 13 | 18.24 | Nonadecane | 70 | 4 | 94 | 8 | 89 | 4 | 94 |
| 14 | 19.17 | Nonadecane | 80 | 6 | 93 | 10 | 88 | 4 | 95 |
| 15 | 20.07 | Eicosane | 68 | 8 | 88 | 14 | 79 | 6 | 91 |
| 16 | 20.93 | Heneicosane | 68 | 12 | 82 | 20 | 71 | 8 | 88 |
| 17 | 21.75 | Heptadecane,2,6,10,15-tetramethyl | 72 | 72 | 0 | 70 | 3 | 70 | 3 |
| 18 | 22.55 | Docosane | 68 | 8 | 88 | 22 | 68 | 8 | 88 |
| 19 | 23.31 | 4-methyldocosane | 60 | 10 | 83 | 24 | 60 | 8 | 87 |
| 20 | 24.06 | Tetracosane | 52 | 10 | 81 | 24 | 54 | 10 | 81 |
| 21 | 24.78 | Pentacosane | 38 | 10 | 74 | 8 | 79 | 10 | 74 |
| 22 | 25.49 | Hexacosane | 56 | 52 | 7 | 44 | 21 | 50 | 11 |
| 23 | 26.19 | Heptacosane,1-chloro- | 16 | 4 | 75 | 8 | 50 | 4 | 75 |
| 24 | 26.94 | Octacosane | 10 | 4 | 60 | 6 | 40 | 2 | 80 |
| Total biodegradation efficiency (%) | 84 | 78 | 86 | ||||||
RT, retention time; RA, relative abundance (%); NA3, P. stutzeri; MN3, baumannii parvus, Mix, mixed consortia
More accurately, hydrocarbons from C8 to C28 were present in the crude oil samples. Among these hydrocarbons low molecular weight hydrocarbons, ranged between C8 and C15, such as octane nonane, decane, undecane, dodecane, tridecane, tetradecane, and pentadecane were completely removed in all the degradation systems. Remaining hydrocarbons such as hexadecane, heptadecane, octadecane, nonadecane, eicosane, heneicosane, docosane, 4-methyldocosane tetracosane, pentacosane, heptacosane 1-chloro, octacosane were degraded by 70–95%. Very low degradation efficiency was noticed for heptadecane 2,6,10,15-tetramethyl (BE was only 11%) and hexacosane (BE: below 11%). Thus, both strains have high potential to degrade aliphatic components present in the crude oil.
Degradative enzymes in biodegradation of crude oil
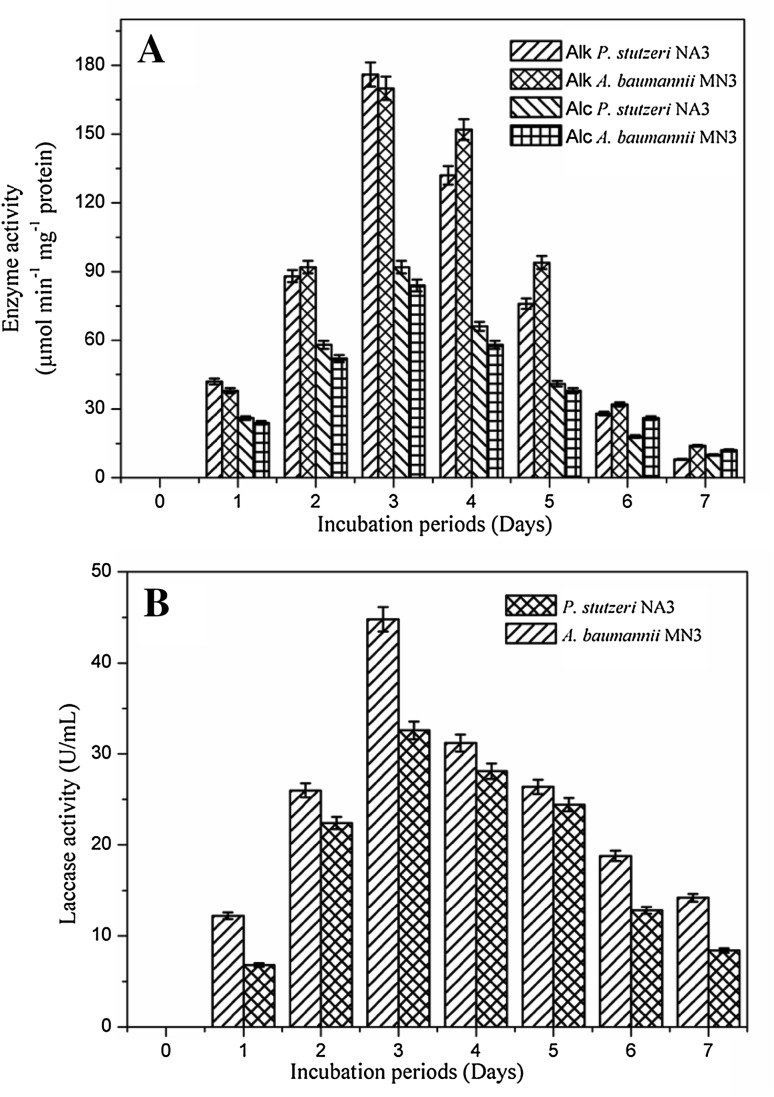
The degradative enzymes are quantified during the crude oil degradation and presented in Fig. 7a, b. Alkane hydroxylase enzyme activity was increased with incubation period; the maximum activity was recorded as 176 µmol min−1 mg−1 protein for strain NA3 and 170 µmol min−1 mg−1 protein for the strain MN3 at end of 3rd day of incubation. Activity of the alcohol dehydrogenase was not as much as alkane hydroxylase during the biodegradation (Mishra and Singh, 2012). Figure 7a represents the activity of the alcohol dehydrogenase, as 92 µmol min−1 mg−1 protein and 84 µmol min−1 mg−1 protein was recorded for the strain NA3 and strain MN3 at 3rd day. Figure 7b explains the laccase activity was also maximum after 3 days of incubation during the biodegradation process and the values are noted as 32.6 and 44.8 U/mL for the strain NA3 and MN3, respectively. As the incubation increased, the enzyme production activity was slowly declined along with the decrease in the bacterial biomass. Similarly, Pirog et al. (2010) and Parthipan et al. (2017b) also reported on the higher activity of the alkane hydroxylase enzyme than alcohol dehydrogenase in biodegradation of hexadecane by R. Erythropolis EK-1 (Pirog et al. 2010) and in crude oil degradation (Parthipan et al. 2017b). Enzyme activity found in current study was much higher/similar to the previous studies (Mishra and Singh, 2012; Parthipan et al. 2017b). Alkane hydroxylase initiates the removal of alkanes by establishing the oxygen atoms at diverse sites of alkane terminus (Ji et al. 2013).
Fig. 7.
Quantification of the degradative enzyme activity during biodegradation of crude oil by bacterial strains P. stutzeri NA3 and A. baumannii MN3: alkane hydroxylase and alcohol dehydrogenase activity (a), laccase activity (b)
Both biosurfactant-producing strains led to increased efficiency of biodegradation. Several studies show that alkanes ranged between C8 and C20 were easily utilizable as energy sources by many bacteria (Das and Mukherjee 2007; Hassanshahian and Giti 2008). Ibrahim et al. (2013) identified several bacterial genus including Achromobacter sp., Bacillus sp., Serratia sp., Sphingomonas sp. and Micrococcus sp. as crude oil degrader by producing biosurfactant, which are lipopeptide in nature. The cationic moieties of the biosurfactant attract the negatively charged bacterial membrane in contact with crude oil during degradation (Ferradji et al. 2014). Crude oil is a complex mixture of insoluble compounds, alongside n-alkanes of with different chain-lengths, which are hydrophobic and cautiously disperse in water. Thus, the synthesis of surface-active substances by bacterial strains from the degradation of short chain low molecular weight hydrocarbons leads to near the beginning solubilisation of crude oil and the turbidity of the growth medium (Ismail et al. 2013). The development of microbial cells was then encouraged by the ‘degraded’ hydrocarbons, followed by the production of supplementary emulsifying agents (Chandankere et al. 2014). As a consequence of this, higher amounts of crude oil was diffused into the culture medium, leading to a sudden increase in the culture turbidity. In this scenario, the biosurfactants synthesised by bacteria are found to be more proficient than chemical surfactants in increasing the solubility and well-organized biodegradation of petroleum hydrocarbons with highly eco-friendly in nature. In this study, the production of biosurfactants by both bacterial strains led simultaneously to the consumption of accessible hydrophobic substrates by escalating the surface area of substrates and solubility.
Conclusion
Based on the primary and secondary screening methods strains P. stutzeri NA3 and A. baumannii MN3 were identified as efficient biosurfactant producers. Physicochemical factors of the production medium were optimized using emulsification activity of the biosurfactant produced at the end of the each experiment. P. stutzeri NA3 showed highest degradation efficiency (84%) among the tested bacteria. Degradative enzymes such as alkane hydroxylase, alcohol dehydrogenase and laccase were found to play important role in the biodegradation of crude oil. This degradative enzyme and the effective biosurfactant-producing bacteria represent a promising option many applications aimed at the biodegradation of hydrocarbon and bioremediation of polluted toxic environments.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
A. Rajasekar is thankful to the Department of Biotechnology ((DBT) Government of India) for the award of the Ramalingaswami re-entry Fellowship (BT/RLF/Re-entry/17/2012), Department of Science and Technology for the young scientist award (SB/YS/LS-40/2013), University Grants Commission (MRP-MAJOR-MICRO-2013-31825) and Science and Engineering Research Board, Department of Science and Technology, Government of India (EEQ/2016/000449). P. Parthipan acknowledge the DBT, Government of India for financial support through project scheme (DBT-RLF).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s13205-017-0902-7) contains supplementary material, which is available to authorized users.
References
- Abalos A, Vinas M, Sabate J, Manresa MA, Solanas AM. Enhanced biodegradation of Casablanca crude oil by a microbial consortium in presence of a rhamnolipid produced by Pseudomonas aeruginosa AT10. Biodegradation. 2004;15:249–260. doi: 10.1023/B:BIOD.0000042915.28757.fb. [DOI] [PubMed] [Google Scholar]
- Adebusoye SA, Ilori MO, Amund OO, Teniola OD, Olatope SO. Microbial degradation of petroleum in a polluted tropical stream. World J Microbiol Biotechnol. 2007;23:1149–1159. doi: 10.1007/s11274-007-9345-3. [DOI] [Google Scholar]
- Bao M, Pi Y, Wang L, Sun P, Li Y, Cao L. Lipopeptide biosurfactant production bacteria Acinetobacter sp. D3-2 and its biodegradation of crude oil. Environ Sci Processes Impacts. 2014;16:897–903. doi: 10.1039/C3EM00600J. [DOI] [PubMed] [Google Scholar]
- Chandankere R, Yao J, Cai M, Masakorala K, Jain AK, Choig MMF. properties and characterization of biosurfactant in crude oil biodegradation by bacterium Bacillus methylotrophicus USTBa. Fuel. 2014;122:140–148. doi: 10.1016/j.fuel.2014.01.023. [DOI] [Google Scholar]
- Chen J, Huang PT, Zhang KY, Ding FR. Isolation of biosurfactant producers, optimization and properties of biosurfactant produced by Acinetobacter sp. from petroleum-contaminated soil. J Appl Microbiol. 2012;112:660–671. doi: 10.1111/j.1365-2672.2012.05242.x. [DOI] [PubMed] [Google Scholar]
- Chen C, Lin T, Shieh Y. Emulsification and antioxidation of biosurfactant extracts from chinese medicinal herbs fermentation in vitro. J Biosci Bioeng. 2015;120:387–395. doi: 10.1016/j.jbiosc.2015.02.010. [DOI] [PubMed] [Google Scholar]
- Chikere CB, Okpokwasili GC, Chikere BO. Monitoring of microbial hydrocarbon remediation in the soil. 3 Biotech. 2011;1:117–138. doi: 10.1007/s13205-011-0014-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das K, Mukherjee AK. Crude petroleum-oil biodegradation efficiency of Bacillus subtilis and Pseudomonas aeruginosa strains isolated from a petroleum-oil contaminated soil from north-east India. Bioresour Technol. 2007;98:1339–1345. doi: 10.1016/j.biortech.2006.05.032. [DOI] [PubMed] [Google Scholar]
- Davila AM, Marchal R, Vandecasteele JP. Kinetics and balance of a fermentation free from product inhibition: sophorose lipid production by Candida bombicola. Appl Microbiol Biotechnol. 1992;38:6–11. doi: 10.1007/BF00169410. [DOI] [Google Scholar]
- Deepika KV, Kalam S, Sridhar PR, Podile AR, Bramhachari PV. Optimization of rhamnolipid biosurfactant production by mangrove sediment bacterium Pseudomonas aeruginosa KVD-HR42 using response surface methodology. Biocatal Agric Biotechnol. 2016;5:38–47. [Google Scholar]
- Desai J, Banat I. Microbial production of surfactants and their commercial potential. Microbiol Mol Biol Rev. 1997;61:47–64. doi: 10.1128/mmbr.61.1.47-64.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhasayan A, Selvin J, Kiran S. Biosurfactant production from marine bacteria associated with sponge Callyspongia diffusa. 3 Biotech. 2015;5:443–454. doi: 10.1007/s13205-014-0242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekperusi OA, Aigbodion FI. Bioremediation of petroleum hydrocarbons from crude oil contaminated soil with the earthworm: Hyperiodrilus africanus. 3 Biotech. 2015;5:957–965. doi: 10.1007/s13205-015-0298-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elazzazy AM, Abdelmoneim TS, Almaghrabi OA. Isolation and characterization of biosurfactant production under extreme environmental conditions by alkali-halo-thermophilic bacteria from Saudi Arabia. Saudi J Biol Sci. 2015;22:466–475. doi: 10.1016/j.sjbs.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferradji FZ, Mnif S, Badis A, Rebbani S, Fodil D, Eddouaouda K, Sayadi S. Naphthalene and crude oil degradation by biosurfactant producing Streptomyces spp. isolated from Mitidja plain soil (North of Algeria) Int Biodeterior Biodegrad. 2014;86:300–308. doi: 10.1016/j.ibiod.2013.10.003. [DOI] [Google Scholar]
- Franzetti A, Gandolfi I, Bestetti G, Smyth TJ, Banat IM. Production and applications of trehalose lipid biosurfactants. Eur J Lipid Sci Tech. 2010;112:617–627. doi: 10.1002/ejlt.200900162. [DOI] [Google Scholar]
- Freitas de Oliveira DW, Franc IWL, Felix AKN, Martins JJL, Giro MEA, Melob VMM, Goncalves LRB. Kinetic study of biosurfactant production by Bacillus subtilis LAMI005 grown in clarified cashew apple juice. Colloids Surf B Biointerfaces. 2013;101:34–43. doi: 10.1016/j.colsurfb.2012.06.011. [DOI] [PubMed] [Google Scholar]
- Gudina EJ, Teixeira JA, Rodrigues LR. Biosurfactant-producing Lactobacilli: screening, production profiles, and effect of medium composition. Appl Environ Soil Sci. 2011;201254:9. [Google Scholar]
- Hassanshahian M. Isolation and characterization of biosurfactant producing bacteria from Persian Gulf (Bushehr Provenance) Mar Pollut Bull. 2014;86:361–366. doi: 10.1016/j.marpolbul.2014.06.043. [DOI] [PubMed] [Google Scholar]
- Hassanshahian M, Giti E. Investigation of alkane biodegradation using the microtiter plate method and correlation between biofilm formation biosurfactant production and crude oil biodegradation. Int Biodeterior Biodegrad. 2008;62:170–178. doi: 10.1016/j.ibiod.2008.01.004. [DOI] [Google Scholar]
- Hassanshahian M, Emtiazi G, Cappello S. Isolation and characterization of crude-oil-degrading bacteria from the Persian Gulf and the Caspian Sea. Mar Pollut Bull. 2012;64:7–12. doi: 10.1016/j.marpolbul.2011.11.006. [DOI] [PubMed] [Google Scholar]
- Hien LT, Yen NT, Nga WT. Biosurfactant-producing Rhodococcus ruber TD2 isolated from oil polluted water in Vung Tau coastal zone. Tap Chi Sinh Hoc. 2013;35:454–460. [Google Scholar]
- Ibrahim HMM. Biodegradation of used engine oil by novel strains of Ochrobactrum anthropi HM-1 and Citrobacter freundii HM-2 isolated from oil-contaminated soil. 3 Biotech. 2016;6:226. doi: 10.1007/s13205-016-0540-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim ML, Ijah UJJ, Manga SB, Bilbis LS, Umar S. Production and partial characterization of biosurfactant produced by crude oil degrading bacteria. Int Biodeterior Biodegrad. 2013;81:28–34. doi: 10.1016/j.ibiod.2012.11.012. [DOI] [Google Scholar]
- Ismail W, Al-Rowaihi IS, Al-Humam AA, Hamza RY, El Nayal AM, Bououdina M. Characterization of a lipopeptide biosurfactant produced by a crude-oil-emulsifying Bacillus sp. I-15. Int Biodeterior Biodegrad. 2013;84:168–178. doi: 10.1016/j.ibiod.2012.04.017. [DOI] [Google Scholar]
- Jain DK, Collins-Thompson DL, Lee H, Trevors JT. A drop-collapsing test for screening surfactant producing microorganisms. J Microbiol Methods. 1991;13:271–279. doi: 10.1016/0167-7012(91)90064-W. [DOI] [Google Scholar]
- Jauhari N, Mishra S, Kumari B, Singh SN. Bacteria-mediated aerobic degradation of hexacosane in vitro conditions. Bioresour Technol. 2014;170:62–68. doi: 10.1016/j.biortech.2014.07.091. [DOI] [PubMed] [Google Scholar]
- Jennema GE, McInerney MJ, Knapp RM, Clark JB, Feero JM, Revus DE, Menzie DE. A halotolerant, biosurfactants producing Bacillus species potentially useful for enhanced oil recovery. Dev Ind Microbiol. 1983;24:485–492. [Google Scholar]
- Ji Y, Mao G, Wang Y, Bartlam M. Structural insights into diversity and n-alkane biodegradation mechanisms of alkane hydroxylases. Front Microbiol. 2013;4:1–13. doi: 10.3389/fmicb.2013.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khopade A, Ren B, Liu XY, Mahadik K, Zhang L, Kokare C. Production and characterization of biosurfactant from marine Streptomyces species B3. J Colloid Interface Sci. 2012;367:311–318. doi: 10.1016/j.jcis.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Kiran GS, Hema TA, Gandhimathi R, Selvin J, Anto Thomas T, Ravji TR, Natarajaseenivasan K. Optimization and production of a biosurfactant from the sponge-associated marine fungus Aspergillus ustus MSF3. Colloids Surf B. 2009;73:250–256. doi: 10.1016/j.colsurfb.2009.05.025. [DOI] [PubMed] [Google Scholar]
- Kiran GS, Thomas TA, Selvin J, Sabarathnam B, Lipton AP. Optimization and characterization of a new lipopeptide biosurfactant produced by marine Brevibacterium aureum MSA13 in solid state culture. Bioresour Technol. 2010;10:2389–2396. doi: 10.1016/j.biortech.2009.11.023. [DOI] [PubMed] [Google Scholar]
- Korayem AS, Abdelhafez AA, Zaki MM, Saleh EA. Optimization of biosurfactant production by Streptomyces isolated from Egyptian arid soil using plackett–burman design. Ann Agric Sci. 2015;60:209–217. [Google Scholar]
- Kumar AP, Janardhan A, Radha S, Viswanath B, Narasimha G. Statistical approach to optimize production of biosurfactant by Pseudomonas aeruginosa 2297. 3 Biotech. 2015;5:71–79. doi: 10.1007/s13205-014-0203-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar AP, Janardhan A, Viswanath B, Monika K, Jung JY, Narasimha G. Evaluation of orange peel for biosurfactant production by Bacillus licheniformis and their ability to degrade naphthalene and crude oil. 3 Biotech. 2016;6:43. doi: 10.1007/s13205-015-0362-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuppusamy S, Sethurajan M, Kadarkarai M, Aruliah R. Biodecolourization of textile dyes by novel, indigenous Pseudomonas stutzeri L1 and Acinetobacter baumannii L2. J Environ Chem Eng. 2017;5:716–724. doi: 10.1016/j.jece.2016.12.021. [DOI] [Google Scholar]
- Lotfabada TB, Shourianc M, Roostaazada R, Najafabadi AR, Adelzadeha MR, Noghabic KA. An efficient biosurfactant-producing bacterium Pseudomonas aeruginosa MR01, isolated from oil excavation areas in south of Iran. Colloids Surf B. 2009;69:183–193. doi: 10.1016/j.colsurfb.2008.11.018. [DOI] [PubMed] [Google Scholar]
- Makkar RS, Cameotra SS. An update on the use of unconventional substrates for biosurfactant production and their new applications. Appl Microbiol Biotechnol. 2002;58:428–434. doi: 10.1007/s00253-001-0924-1. [DOI] [PubMed] [Google Scholar]
- Mani P, Dineshkumar G, Jayaseelan T, Deepalakshmi K, Ganesh Kumar C, Senthil Balan S. Antimicrobial activities of a promising glycolipid biosurfactant from a novel marine Staphylococcus saprophyticus SBPS 15. 3 Biotech. 2016;6:163. doi: 10.1007/s13205-016-0478-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer HP. Sustainability and biotechnology. Org Process Res Dev. 2011;15:180–188. doi: 10.1021/op100206p. [DOI] [Google Scholar]
- Michaud L, Lo Giudice A, Saitta M, De Domenico M, Vivia B. The biodegradation efficiency on diesel oil by two psychrotrophic antarctic marine bacteria during a two-month-long experiment. Marine Res Bull. 2004;49:405–409. doi: 10.1016/j.marpolbul.2004.02.026. [DOI] [PubMed] [Google Scholar]
- Mishra S, Singh SN. Microbial degradation of n-hexadecane in mineral salt medium as mediated by degradative enzymes. Bioresour Technol. 2012;111:148–158. doi: 10.1016/j.biortech.2012.02.049. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Das P, Sen R. Towards commercial production of microbial surfactants. Trends Biotechnol. 2006;24:509–515. doi: 10.1016/j.tibtech.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Mulligan CN. Environmental applications for biosurfactants. Environ Pollut. 2005;133:183–198. doi: 10.1016/j.envpol.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Parthipan P, Elumalai P, Karthikeyan OP, Ting YP, Rajasekar A. A review on biodegradation of hydrocarbon and their influence on corrosion of carbon steel with special reference to petroleum industry. J Environ Biotechnol Res. 2017;6(1):12–33. [Google Scholar]
- Parthipan P, Preetham E, Machuca LL, Rahman PKSM, Murugan K, Rajasekar A. Biosurfactant and degradative enzymes mediated crude oil degradation by bacterium Bacillus subtilis A1. Front Microbiol. 2017;8:193. doi: 10.3389/fmicb.2017.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak KV, Keharia H. Application of extracellular lipopeptide biosurfactant produced by endophytic Bacillus subtilis K1 isolated from aerial roots of banyan (Ficus benghalensis) in microbially enhanced oil recovery (MEOR) 3 Biotech. 2014;4:41–48. doi: 10.1007/s13205-013-0119-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peele KA, Ravi Teja ChV, Kodali VP. Emulsifying activity of a biosurfactant produced by a marine bacterium. 3 Biotech. 2016;6:177. doi: 10.1007/s13205-016-0494-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirog TP, Shevchuk TA, Klimenko LA. Intensification of surfactant synthesis in Rhodococcus erythropolis EK-1 cultivated on hexadecane. Appl Biochem Microbiol. 2010;46:599–606. doi: 10.1134/S0003683810060074. [DOI] [PubMed] [Google Scholar]
- Powalla M, Lang S, Wray V. Penta- and disaccharide lipid formation by Nocardia corynebacteroides grown on n-alkanes. Appl Microbiol Biotechnol. 1989;31:473–479. doi: 10.1007/BF00270779. [DOI] [Google Scholar]
- Prieto LM, Michelon M, Burkert JFM, Kalil SJ, Burkert CAV. The production of rhamnolipid by a Pseudomonas aeruginosa strain isolated from a southern coastal zone in Brazil. Chemosphere. 2008;71:1781–1785. doi: 10.1016/j.chemosphere.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Rahman KSM, Thahira-Rahman J, Lakshmanaperumalsamy P, Banat IM. Towards efficient crude oil degradation by a mixed bacterial consortia. Bioresour Technol. 2002;85:257–261. doi: 10.1016/S0960-8524(02)00119-0. [DOI] [PubMed] [Google Scholar]
- Rajasekar A, et al. Biodegradation of petroleum hydrocarbon and its influence on corrosion with special reference to petroleum industry. In: Heimann K, et al., editors. Biodegradation and bioconversion of hydrocarbons. Heidelberg: Springer; 2017. pp. 307–336. [Google Scholar]
- Rajasekar A, Ponmariappan S, Maruthamuthu S, Palaniswamy N. Bacterial degradation and corrosion of naphtha in transporting pipeline. Curr Microbiol. 2007;55:374–381. doi: 10.1007/s00284-007-9001-z. [DOI] [PubMed] [Google Scholar]
- Roy S, Chandni S, Das I, Karthik L, Kumar G, Bhaskara Rao KV. Aquatic model for engine oil degradation by rhamnolipid producing Nocardiopsis VITSISB. 3 Biotech. 2015;5:153–164. doi: 10.1007/s13205-014-0199-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos DKF, Brandao YB, Rufino RD, Luna JM, Salgueiro AA, Santos VA, Sarubbo LA. Optimization of cultural conditions for biosurfactant production from Candida lipolytica. Biocatal Agric Biotechnol. 2014;3:48–57. [Google Scholar]
- Sarafin Y, Donio MBS, Velmurugan S, Michaelbabu M, Citarasu T. Kocuria marina BS-15 a biosurfactant producing halophilic bacteria isolated from solar salt works in India. Saudi J Biol Sci. 2014;21:511–519. doi: 10.1016/j.sjbs.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathishkumar M, Binupriya AR, Baik SH, Yun SE. Biodegradation of crude oil by individual bacterial strains and a mixed bacterial consortium isolated from hydrocarbon contaminated areas. Clean Soil Air Water. 2008;36:92–96. doi: 10.1002/clen.200700042. [DOI] [Google Scholar]
- Sathishkumar K, Murugan K, Benelli G, Higuchi A, Rajasekar A. Bioreduction of hexavalent chromium by Pseudomonas stutzeri L1 and Acinetobacter baumannii L2. Ann Microbiol. 2016 [Google Scholar]
- Sebatini AM, Jain M, Radha P, Kiruthika S, Tamilarasan K. Immobilized lipase catalyzing glucose stearate synthesis and their surfactant properties analysis. 3 Biotech. 2016;6:184. doi: 10.1007/s13205-016-0501-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sifour M, Al-Jilawi MH, Aziz GM. Emulsification properties of biosurfactant produced from Pseudomonas aeruginosa RB 28. Pak J Biol Sci. 2007;10:1331–1335. doi: 10.3923/pjbs.2007.1331.1335. [DOI] [PubMed] [Google Scholar]
- Van Beilen JB, Smits TH, Whyte LG, Schorcht S, Rothlisberger M, Plaggemeier T, Engesser KH, Witholt B. Alkane hydroxylase homologues in gram-positive strains. Environ Microbiol. 2002;4:676–682. doi: 10.1046/j.1462-2920.2002.00355.x. [DOI] [PubMed] [Google Scholar]
- Van Dyke MI, Gulley SL, Lee H, Trevors JT. Applications of microbial biosurfactants. Biotechnol Adv. 1991;9:241–252. doi: 10.1016/0734-9750(91)90006-H. [DOI] [PubMed] [Google Scholar]
- Whang LM, Liu PWG, Ma CC, Cheng SS. Application of biosurfactant, rhamnolipid, and surfactin, for enhanced biodegradation of diesel-contaminated water and soil. J Hazard Mater. 2008;151:155–163. doi: 10.1016/j.jhazmat.2007.05.063. [DOI] [PubMed] [Google Scholar]
- Wu JY, Yeh KL, Lu WB, Lin CL, Chang JS. Rhamnolipid production with indigenous Pseudomonas aeruginosa EM1 isolated from oil-contaminated site. Bioresour Technol. 2008;99:1157–1164. doi: 10.1016/j.biortech.2007.02.026. [DOI] [PubMed] [Google Scholar]
- Xia WJ, Dong HP, Yu L, Yu DF. Comparative study of biosurfactant produced by microorganisms isolated from formation water of petroleum reservoir. Colloids Surf A Physicochem Eng Asp. 2011;392:124–130. doi: 10.1016/j.colsurfa.2011.09.044. [DOI] [Google Scholar]
- Youssef NH, Duncan KE, Nagle DP, Savage KN, Knapp RM, McInerney MJ. Comparison of methods to detect biosurfactant production by diverse microorganisms. J Microbiol Methods. 2004;56:339–347. doi: 10.1016/j.mimet.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Zosim Z, Gutnick DL, Rosenberg E. Properties of hydrocarbon-in-water emulsions stabilized by Acinetobacter RAG-1 emulsan. Biotechnol Bioeng. 1982;24:281–292. doi: 10.1002/bit.260240203. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.