Protein disulfide isomerase (PDI) was discovered over 60 years ago as an enzyme that forms disulfide bonds in newly synthesized proteins in the endoplasmic reticulum [1]. Twenty-five years ago PDI was shown to be released from activated platelets [2], opening up a new field of research on extracellular functions of this enzyme. PDI was subsequently localized to the platelet surface [3] and shown to mediate platelet aggregation [4,5]. PDI binds to and regulates activation of the αIIbβ3 integrin, the fibrinogen receptor that supports platelet aggregation and thrombosis [6–8]. Using inhibitory antibodies and targeted knockout mice, PDI has been shown to have a role in platelet accumulation and fibrin generation in vivo [7–10]. Potential substrates for PDI in coagulation include tissue factor [10,11] and factor XI [12,13]. PDI also influences platelet-dependent thrombin generation by regulating binding of coagulation factors to the platelet surface [14].
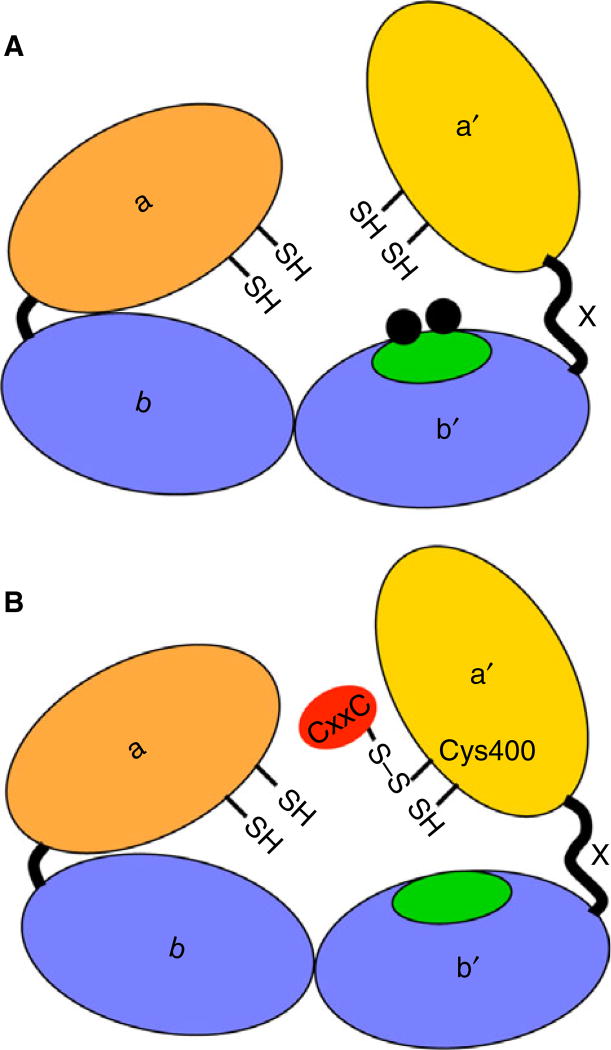
PDI contains four thioredoxin-like domains arranged in order of a-b-b′-a′, with a 19 amino acid linker between the b′ and a′ domains termed×(Fig. 1) [15]. The a and a′ domains contain the Cys-Gly-His-Cys (CGHC) active sites that catalyze the reversible oxidation and isomerization of disulfide bonds. The b and b′ domains are non-catalytic domains, with the b′ domain providing the principle substrate binding domain. Targeting PDI is a current focus for the development of antithrombotic agents and several classes of small-molecular-weight molecules that selectively inhibit PDI have been recently described. Quercetin-3-rutinoside, a commonly ingested compound in fruits, vegetables, teas and other food, was selected from a 5000-compound library by the ability to inhibit PDI [16]. This membrane-impermeable molecule inhibits PDI activity by reversibly binding to the b′x domain of PDI [17] and inhibits platelet accumulation and fibrin formation in a laser-induced cremaster arteriole injury model [16]. A phase II/III study is currently underway evaluating the ability of isoquercetin to inhibit thrombosis in patients with cancer [18]. More recently, another reversible class of PDI inhibitors called bepristats were developed and shown to inhibit platelet aggregation, and platelet accumulation and fibrin formation at the site of vessel injury [19]. The target of bepristats is also the hydrophobic pocket of the b′ domain of PDI.
Fig. 1.
Schematic of protein disulfide isomerase (PDI) inhibition strategies. (A) PDI molecule with a, b, b′,×and a′ domains. The hydrophobic pocket in the b0 domain is highlighted in green with small-molecule inhibitors like quercetin-3-rutinoside and bepristat targeting the hydrophobic pocket (black balls). (B) CxxC covalently links to reduced Cys400 to inhibit the activity of the a′ active site and platelet aggregation.
In addition to targeting the substrate binding b′ domain of PDI, the active sites of PDI are potential targets for antithrombotic activity. Although there is 36.8% sequence similarity between the a and a′ domains [15], the active sites are not functionally equivalent. Using reduced RNase as the substrate, the a domain or N-terminal active site has higher catalytic activity and functions primarily as a disulfide isomerase, whereas the a′, C-terminal, active site contributes more to the Km of PDI and functions as a disulfide oxidase [20,21]. Similarly, the active sites have different abilities to rearrange specific disulfide bonds in bovine pancreatic trypsin inhibitor [22], and the C-terminal active site is a better oxidase in the Ero1-mediated oxidative folding pathway [23]. Although these in vitro studies indicate distinct functions for each active site of PDI, until recently nothing was known about the relative importance of each active site in physiologic reactions, or in thrombosis.
A critical role for the C-terminal active site of PDI in platelet activation and thrombosis was recently reported [8]. Mice lacking PDI in vessel wall cells and platelets, and transgenic mice harboring PDI that lacks a functional C-terminal CGHC motif, had defective thrombosis in several vascular injury models. Decreased platelet accumulation and fibrin generation in a laser-induced cremaster arteriole injury were rescued by infusion of recombinant PDI containing only a functional C-terminal CGHC motif. The C-terminal active site mediated platelet aggregation and αIIbβ3 activation, as well as ATP secretion and P-selection expression through a non-αIIbβ3 substrate. The whole body knockout of PDI was lethal in embryogenesis but the PDI transgene with only a functional N-terminal active site rescued the lethal phenotype. These studies indicate the C-terminal CGHC active motif is pivotal for platelet function and thrombosis, whereas the N-terminal CGHC motif is required for murine survival. These findings suggested that inhibiting the C-terminal active site of PDI might prove useful for antithrombotic therapy while sparing the function of the N-terminal active site.
In this issue, Sousa et al. [24] demonstrate that a 12 amino acid peptide (VEFYAPWCGHCK) covalently binds to the C-terminal CGHC active motif of PDI and inhibits platelet aggregation. This PDI-targeted peptide, called CxxC, contains the C-terminal CGHC active site sequence of PDI and was previously shown by this group to inhibit PDI in the insulin reductase assay and to strongly inhibit PDI-mediated superoxide generation by leukocyte Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase [25]. CxxC dose-dependently inhibited ADP and thrombin-induced aggregation, as well as αIIbβ3 activation. A scrambled control peptide or a peptide with the Cys residues mutated to Ala did not inhibit. CxxC did not inhibit P-selectin expression, in contrast with a previous report that found a role for PDI in this process [8]; the cause of these differences is not clear. An impressive finding came from mass spectrometry analysis where the investigators demonstrated the CxxC peptide covalently linked to the reduced form of Cys400 in the C-terminal CGHC motif of PDI through a mixed disulfide bond (Fig. 1). Although the exact mechanism by which CxxC reacts with Cys400 is not clear, the authors ‘speculate that Cys397 attacks CxxC, or vise-versa, to form a mixed disulfide that is stabilized at Cys400’. The apparent stability of the covalent bond between CxxC and Cys400 raises the possibility of designing an inhibitor of PDI that reacts similarly. Because the authors didn’t retrieve the fragment containing Cys53 and Cys56 from the a domain N-terminal active site of PDI in their mass spectrometry analysis, it is not clear whether the CxxC peptide also reacts with the N-terminal CGHC motif of PDI. However, the C-terminal active site of PDI has already been demonstrated to support thrombosis [8] and therefore the strategy to target the C-terminal active site and, in particular, Cys400 provides a model for development of site-specific antithrombotic agents.
The active sites of PDI can exist in both dithiol and disulfide forms [15] but little is known about the redox state of PDI that catalyzes reactions in platelets. The CxxC peptide reacted with reduced but not oxidized PDI. This suggests CxxC targets the reduced form of the C-erminal active site on the platelet surface, and that the reduced form of PDI is important in platelet function. Because the dithiol form of PDI catalyzes isomerization and cleavage of disulfide bonds [1,15], these reactions are likely to have a role in PDI regulation of platelet function.
CxxC also decreased 3-N-maleimidyl-propionyl biotin (MPB) labeling of thiols in PDI on the surface of resting platelets without decreasing the labeling of other surface proteins, suggesting some specificity of CxxC for PDI. The inhibition of thiol labeling in PDI by CxxC suggests that PDI has free thiols in the C-terminal active site. The impermeant reducing agent, TCEP, increased MPB labeling of PDI on the surface of resting platelets by several fold. Therefore, the majority of platelet surface PDI on resting platelets is in the oxidized form, as previously reported [26]. Activation of platelets is known to increase thiols in platelet surface PDI [26,27], possibly through a transmembrane electron transport system [26,28].
A previously reported inhibitor of PDI, PACMA-31, also interacts with Cys397/Cys400 in the C-terminal active site of PDI [29]. However, this reagent was recently found to inhibit ERp57, ERp5 and thioredoxin, and to inhibit both active sites of PDI [19]. In the Sousa study, the CxxC peptide didn’t enhance inhibition of platelet aggregation by the anti-PDI antibodies BD34 and RL90, suggesting that CxxC mainly targets PDI. However, whether the CxxC peptide covalently binds to the CGHC motif in other PDI family members remains to be elucidated. If CxxC turns out to be specific for PDI it could provide a useful research tool.
Other members of the PDI family are also potential targets for antithrombotic therapy. ERp5 and ERp57, the closest homologue of PDI in platelets, are important for platelet function and thrombosis [30–35]. As with PDI, the C-terminal active site of ERp57 supports platelet aggregation [33], and platelet accumulation and fibrin generation in vivo [34]. It is not known whether inhibiting one PDI or several PDIs would provide optimal antithrombotic therapy, as the efficacy in inhibiting several PDIs will have to be balanced with potential toxicities. Inhibiting only extracellular PDIs should provide optimal antithrombotic benefit while minimizing toxicity [16]. Because the N-terminal active site is important in murine viability [8], targeting only the C-terminal active site may be another way to provide antithrombotic efficacy while also minimizing toxicity. Further studies of the detailed mechanisms by which PDIs support thrombosis should help elucidate optimal antithrombotic strategies.
Footnotes
Addendum
L. Wang and D. W. Essex wrote the manuscript.
Disclosure of Conflict of Interests
The authors have no conflict of interest.
References
- 1.Essex DW. Redox control of platelet function. Antioxid Redox Signal. 2009;11:1191–225. doi: 10.1089/ars.2008.2322. [DOI] [PubMed] [Google Scholar]
- 2.Chen K, Lin Y, Detwiler TC. Protein disulfide isomerase activity is released by activated platelets. Blood. 1992;79:2226–8. [PubMed] [Google Scholar]
- 3.Essex DW, Chen K, Swiatkowska M. Localization of protein disulfide isomerase to the external surface of the platelet plasma membrane. Blood. 1995;86:2168–73. [PubMed] [Google Scholar]
- 4.Essex DW, Li M. Protein disulphide isomerase mediates platelet aggregation and secretion. Br J Haematol. 1999;104:448–54. doi: 10.1046/j.1365-2141.1999.01197.x. [DOI] [PubMed] [Google Scholar]
- 5.Lahav J, Jurk K, Hess O, Barnes MJ, Farndale RW, Luboshitz J, Kehrel BE. Sustained integrin ligation involves extracellular free sulfhydryls and enzymatically catalyzed disulfide exchange. Blood. 2002;100:2472–8. doi: 10.1182/blood-2001-12-0339. [DOI] [PubMed] [Google Scholar]
- 6.Cho J, Kennedy DR, Lin L, Huang M, Merrill-Skoloff G, Furie BC, Furie B. Protein disulfide isomerase capture during thrombus formation in vivo depends on the presence of beta3 integrins. Blood. 2012;120:647–55. doi: 10.1182/blood-2011-08-372532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim K, Hahm E, Li J, Holbrook LM, Sasikumar P, Stanley RG, Ushio-Fukai M, Gibbins JM, Cho J. Platelet protein disulfide isomerase is required for thrombus formation but not for hemostasis in mice. Blood. 2013;122:1052–61. doi: 10.1182/blood-2013-03-492504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou J, Wu Y, Wang L, Rauova L, Hayes VM, Poncz M, Essex DW. The C-terminal CGHC motif of protein disulfide isomerase supports thrombosis. J Clin Invest. 2015;125:4391–406. doi: 10.1172/JCI80319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho J, Furie BC, Coughlin SR, Furie B. A critical role for extracellular protein disulfide isomerase during thrombus formation in mice. J Clin Invest. 2008;118:1123–31. doi: 10.1172/JCI34134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reinhardt C, von Bruhl ML, Manukyan D, Grahl L, Lorenz M, Altmann B, Dlugai S, Hess S, Konrad I, Orschiedt L, Mackman N, Ruddock L, Massberg S, Engelmann B. Protein disulfide isomerase acts as an injury response signal that enhances fibrin generation via tissue factor activation. J Clin Invest. 2008;118:1110–22. doi: 10.1172/JCI32376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langer F, Spath B, Fischer C, Stolz M, Ayuk FA, Kroger N, Bokemeyer C, Ruf W. Rapid activation of monocyte tissue factor by antithymocyte globulin is dependent on complement and protein disulfide isomerase. Blood. 2013;121:2324–35. doi: 10.1182/blood-2012-10-460493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giannakopoulos B, Gao L, Qi M, Wong JW, Yu DM, Vlachoyiannopoulos PG, Moutsopoulos HM, Atsumi T, Koike T, Hogg P, Qi JC, Krilis SA. Factor XI is a substrate for oxidoreductases: enhanced activation of reduced FXI and its role in antiphospholipid syndrome thrombosis. J Autoimmun. 2012;39:121–9. doi: 10.1016/j.jaut.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Zucker M, Seligsohn U, Yeheskel A, Mor-Cohen R. An allosteric disulfide bond is involved in enhanced activation of factor XI by protein disulfide isomerase. J Thromb Haemost. 2016;14:2202–11. doi: 10.1111/jth.13488. [DOI] [PubMed] [Google Scholar]
- 14.Jurk K, Lahav J, VAN Aken H, Brodde MF, Nofer JR, Kehrel BE. Extracellular protein disulfide isomerase regulates feedback activation of platelet thrombin generation via modulation of coagulation factor binding. J Thromb Haemost. 2011;9:2278–90. doi: 10.1111/j.1538-7836.2011.04509.x. [DOI] [PubMed] [Google Scholar]
- 15.Hatahet F, Ruddock LW. Protein disulfide isomerase: a critical evaluation of its function in disulfide bond formation. Antioxid Redox Signal. 2009;11:2807–50. doi: 10.1089/ars.2009.2466. [DOI] [PubMed] [Google Scholar]
- 16.Jasuja R, Passam FH, Kennedy DR, Kim SH, van Hessem L, Lin L, Bowley SR, Joshi SS, Dilks JR, Furie B, Furie BC, Flaumenhaft R. Protein disulfide isomerase inhibitors constitute a new class of antithrombotic agents. J Clin Invest. 2012;122:2104–13. doi: 10.1172/JCI61228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin L, Gopal S, Sharda A, Passam F, Bowley SR, Stopa J, Xue G, Yuan C, Furie BC, Flaumenhaft R, Huang M, Furie B. Quercetin-3-rutinoside inhibits protein disulfide isomerase by binding to its b’x domain. J Biol Chem. 2015;290:23543–52. doi: 10.1074/jbc.M115.666180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flaumenhaft R, Furie B. Vascular thiol isomerases. Blood. 2016;128:893–901. doi: 10.1182/blood-2016-04-636456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bekendam RH, Bendapudi PK, Lin L, Nag PP, Pu J, Kennedy DR, Feldenzer A, Chiu J, Cook KM, Furie B, Huang M, Hogg PJ, Flaumenhaft R. A substrate-driven allosteric switch that enhances PDI catalytic activity. Nat Commun. 2016;7:12579. doi: 10.1038/ncomms12579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lyles MM, Gilbert HF. Mutations in the thioredoxin sites of protein disulfide isomerase reveal functional nonequivalence of the N- and C-terminal domains. J Biol Chem. 1994;269:30946–52. [PubMed] [Google Scholar]
- 21.Kulp MS, Frickel EM, Ellgaard L, Weissman JS. Domain architecture of protein-disulfide isomerase facilitates its dual role as an oxidase and an isomerase in Ero1p-mediated disulfide formation. J Biol Chem. 2006;281:876–84. doi: 10.1074/jbc.M511764200. [DOI] [PubMed] [Google Scholar]
- 22.Darby NJ, Penka E, Vincentelli R. The multi-domain structure of protein disulfide isomerase is essential for high catalytic efficiency. J Mol Biol. 1998;276:239–47. doi: 10.1006/jmbi.1997.1504. [DOI] [PubMed] [Google Scholar]
- 23.Wang L, Li SJ, Sidhu A, Zhu L, Liang Y, Freedman RB, Wang CC. Reconstitution of human Ero1-Lalpha/protein-disulfide isomerase oxidative folding pathway in vitro. Position-dependent differences in role between the a and a’ domains of protein-disulfide isomerase. J Biol Chem. 2009;284:199–206. doi: 10.1074/jbc.M806645200. [DOI] [PubMed] [Google Scholar]
- 24.Sousa HR, Gaspar RS, Lena EML, Sda Silva SA, Fonelles JLdL, Araujo TLS, Mastrogiovanni M, Fries DM, Azevedo-Santos APS, Laurindo FRM, Torostchansky A, Paes AM. Novel antiplatelet role for a protein disulfide isomerase-targeted peptide: evidence of covalent binding to C-terminal CGHC redox motif. J Thromb Haemost. 2017 doi: 10.1111/jth.13633. [DOI] [PubMed] [Google Scholar]
- 25.de A Paes AM, Verissimo-Filho S, Guimaraes LL, Silva AC, Takiuti JT, Santos CX, Janiszewski M, Laurindo FR, Lopes LR. Protein disulfide isomerase redox-dependent association with p47(phox): evidence for an organizer role in leukocyte NADPH oxidase activation. J Leukoc Biol. 2011;90:799–810. doi: 10.1189/jlb.0610324. [DOI] [PubMed] [Google Scholar]
- 26.Burgess JK, Hotchkiss KA, Suter C, Dudman NP, Szollosi J, Chesterman CN, Chong BH, Hogg PJ. Physical proximity and functional association of glycoprotein 1balpha and protein-disulfide isomerase on the platelet plasma membrane. J Biol Chem. 2000;275:9758–66. doi: 10.1074/jbc.275.13.9758. [DOI] [PubMed] [Google Scholar]
- 27.Manickam N, Sun X, Li M, Gazitt Y, Essex DW. Protein disulphide isomerase in platelet function. Br J Haematol. 2008;140:223–9. doi: 10.1111/j.1365-2141.2007.06898.x. [DOI] [PubMed] [Google Scholar]
- 28.Essex DW, Li M, Feinman RD, Miller A. Platelet surface glutathione reductase-like activity. Blood. 2004;104:1383–5. doi: 10.1182/blood-2004-03-1097. [DOI] [PubMed] [Google Scholar]
- 29.Xu S, Butkevich AN, Yamada R, Zhou Y, Debnath B, Duncan R, Zandi E, Petasis NA, Neamati N. Discovery of an orally active small-molecule irreversible inhibitor of protein disulfide isomerase for ovarian cancer treatment. Proc Natl Acad Sci USA. 2012;109:16348–53. doi: 10.1073/pnas.1205226109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jordan PA, Stevens JM, Hubbard GP, Barrett NE, Sage T, Authi KS, Gibbins JM. A role for the thiol isomerase protein ERP5 in platelet function. Blood. 2005;105:1500–7. doi: 10.1182/blood-2004-02-0608. [DOI] [PubMed] [Google Scholar]
- 31.Holbrook LM, Sasikumar P, Stanley RG, Simmonds AD, Bicknell AB, Gibbins JM. The platelet-surface thiol isomerase enzyme ERp57 modulates platelet function. J Thromb Haemost. 2012;10:278–88. doi: 10.1111/j.1538-7836.2011.04593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu Y, Ahmad SS, Zhou J, Wang L, Cully MP, Essex DW. The disulfide isomerase ERp57 mediates platelet aggregation, hemostasis, and thrombosis. Blood. 2012;119:1737–46. doi: 10.1182/blood-2011-06-360685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang L, Wu Y, Zhou J, Ahmad SS, Mutus B, Garbi N, Hammerling G, Liu J, Essex DW. Platelet-derived ERp57 mediates platelet incorporation into a growing thrombus by regulation of the alphaIIbbeta3 integrin. Blood. 2013;122:3642–50. doi: 10.1182/blood-2013-06-506691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou J, Wu Y, Wang L, Rauova L, Hayes VM, Poncz M, Essex DW. The disulfide isomerase ERp57 is required for fibrin deposition in vivo. J Thromb Haemost. 2014;12:1890–7. doi: 10.1111/jth.12709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Passam FH, Lin L, Gopal S, Stopa JD, Bellido-Martin L, Huang M, Furie BC, Furie B. Both platelet- and endothelial cell-derived ERp5 support thrombus formation in a laser-induced mouse model of thrombosis. Blood. 2015;125:2276–85. doi: 10.1182/blood-2013-12-547208. [DOI] [PMC free article] [PubMed] [Google Scholar]