Abstract
Aim
Neutrophils are the first cells to arrive at sites of injury. Nevertheless, many inflammatory diseases are characterized by an uncontrolled infiltration and action of these cells. Cell migration depends on volume changes that are governed by ion channel activity, but potassium channels in neutrophil have not been clearly identified. We aim to test whether KCa3.1 participates in neutrophil migration and other relevant functions of the cell.
Methods
Cytometer and confocal measurements to determine changes in cell volume were used. Cells isolated from human, mouse and horse were tested for KCa3.1-dependent chemotaxis. Chemokinetics, calcium handling and release of reactive oxygen species were measured to determine the role of KCa3.1 in those processes. A mouse model was used to test for neutrophil recruitment after acute lung injury in vivo.
Results
We show for the first time that KCa3.1 is expressed in mammalian neutrophils. When the channel is inhibited by a pharmacological blocker or by genetic silencing, it profoundly affects cell volume regulation, and chemotactic and chemokinetic properties of the cells. We also demonstrated that pharmacological inhibition of KCa3.1 did not affect calcium entry or reactive oxygen species production in neutrophils. Using a mouse model of acute lung injury, we observed that Kca3.1−/− mice are significantly less effective at recruiting neutrophils into the site of inflammation.
Conclusions
These results demonstrate that KCa3.1 channels are key actors in the migration capacity of neutrophils, and its inhibition did not affect other relevant cellular functions.
Keywords: chemotaxis, KCa3.1, neutrophil
There is consensus that appropriate neutrophil migration to sites of infection or inflammation is fundamental for the correct functioning of the innate immune system. Patients affected by impaired recruitment of these leucocytes suffer from repetitive and often lethal infections. Examples are the defective neutrophil migration observed during sepsis (Reddy & Standiford 2010), or the genetic mutations that cause leucocyte adhesion deficiencies, where the absence of certain adhesion molecules dramatically reduces neutrophil chemotactic response (Schmidt et al. 2013). On the other hand, inflammatory diseases characterized by an over-amplification of neutrophilic recruitment are often more damaging than the action of the invading pathogenic microbes (Nathan 2006, Amulic et al. 2012). For example, cystic fibrosis patients develop persistent lung infections accompanied by a massive neutrophilic infiltration. This uncontrolled inflammatory response ultimately damages the lung parenchyma, which is responsible for a dramatic increase in the rate of decline in lung function (Pillarisetti et al. 2011). Similar neutrophil-induced damage has been reported in the lungs of patients affected by chronic obstructive pulmonary disease or COPD (Stockley 2002). Neutrophils are the most abundant leucocyte in the joints of individuals affected by rheumatoid arthritis, where they are thought to be major players in cartilage destruction and release of pro-inflammatory mediators (Nemeth & Mocsai 2012). Thus, in the context of an inflammatory response, the possibility to control and reduce the migration of neutrophils to injured tissues emerges as an attractive way to decrease the damage produced during acute and chronic inflammatory diseases.
Neutrophils reach tissues in response to chemoattractant molecules through a multi-step mechanism (Williams et al. 2011). Cell migration is largely dependent on the polarization of several major proteins in the plasma membrane including ion channels, and their importance in this mechanism has been recently highlighted in a comprehensive review (Schwab et al. 2012). The current model of cell migration is based on temporally and spatially separated phases of local cell swelling and shrinkage, and an essential requirement of this model is the polarization of potassium and chloride channels, whose activities are triggered by an increase in the intracellular free calcium concentration, initiating the retraction of the rear part of the migrating cell by a massive loss of KCl (Schwab 2001).
Pharmacological inhibition of IClswell, the chloride current mediated by the recently identified LRRC8A protein (reviewed in Pedersen et al. 2015) that is involved in regulatory volume decrease, can partially affect migration of human neutrophils (Volk et al. 2008). Evidence for a role of calcium-activated chloride channels has been obtained in human cells, but the identity of this channel is currently unknown (Krause & Welsh 1990). Electrophysiological recordings have demonstrated the presence of calcium-activated and voltage-dependent potassium currents in human neutrophils (von Tscharner et al. 1986, Krause & Welsh 1990), and pharmacological evidence has suggested the presence of ATP-sensitive potassium channels in rat neutrophils (Da Silva-Santos et al. 2002). However, evidence for potassium channels involved in neutrophil migration has not yet been described.
The KCa3.1 channel is a member of the extensively studied family of calcium-activated potassium channels. KCa3.1 is known to be involved in the migration process of several cell types including members of the immune system such as macrophages (Toyama et al. 2008), mast cells (Shumilina et al. 2008), monocytes (Schilling & Eder 2009) and dendritic cells (Shao et al. 2011). There are, however, no reports of KCa3.1 expression or functional role on neutrophils. In this work, we demonstrate for the first time that KCa3.1 is expressed in mammalian neutrophils and that its activity is an essential component of the migration engine in these cells. We tested whether KCa3.1 has a role in neutrophil chemoattractant-induced migration (chemotaxis) and chemoattractant-induced kinesis (chemokinesis). Our in vitro experiments show that blockade of KCa3.1 reduces both neutrophil chemotaxis and chemokinesis by altering the capacity of the cell to properly regulate cell volume, but channel inhibition does not affect intracellular calcium homeostasis or the respiratory burst. Our pharmacological observations in human neutrophils were confirmed using cells from the Kca3.1−/− mouse, and this animal model also allowed us to test the functional consequences of the absence of KCa3.1 channels in an in vivo challenge of acute lung injury.
Methods
Reagents
All chemicals were from Sigma-Aldrich unless otherwise stated (St Louis, MO, USA). TRAM-34 (1-[(2-chlorophenyl)diphenylmethyl]-1H-pyrazole) was diluted in DMSO in 100 and 10 μM stock solutions. A total of 100 nM TRAM-34 was used in all experiments. The final concentration of DMSO on external solutions used was 0.1% except in chemotactic and chemokinetic experiments where due to small experimental volumes, the final concentration of DMSO was 0.1 up to 0.27%. Control experiments were included to discard the interference of DMSO in the determinations. We observed no changes induced by DMSO with measurements. These results are in accordance with the previous observations where DMSO 1% has no effect on KCa3.1 channels (Sankaranarayanan et al. 2009).
Animals
Mice were housed at CECs animal facility. The Kca3.1 null animal generation and their genotyping have been described (Begenisich et al. 2004). Male and female mice (C57Bl6/J) aged 2–6 months were used. All experimental procedures were approved by Centro de Estudios Científicos (CECs) Institutional Animal Care and Use Committee. Nine Chilean Criollo mestizo horses (12–20 years) were used. They were housed in pasture facilities in the Veterinary Hospital of Universidad Austral de Chile, regularly de-wormed and clinically evaluated twice daily during the study. All experimental procedures in horses were approved by the Medical Ethical Committee of Universidad Austral de Chile.
Human donors
Blood was collected by venepuncture from fourteen healthy volunteers, ten correspond to males and four were females. Age of donors ranged from 25 to 45 years. Guidelines stipulated by the Medical Ethical Committee of Universidad Austral de Chile and the Declaration of Helsinki principles were followed. Approval was obtained from the Medical Ethical Committee of Universidad Austral de Chile. All donors were informed about the nature of the studies and expressed written consent to participate. Samples were treated anonymously.
Haematological analysis
Mice blood samples were obtained by retro-orbital puncture and analysed in a Sysmex KX-21N™ Automated Haematological Analyzer (Selangor, Malaysia).
Isolation of mouse neutrophils
Animals were killed by cervical dislocation. Tibias and femurs were removed, epiphyses were cut off, and bones were flushed with PBS–citrate (10 mM sodium phosphate, 2.7 mM KCl and 137 mM NaCl, pH 7.4; 0,4% w/v trisodium citrate) using a 27-G needle. Bone marrow cells were filtered through a 70-μm cell strainer (BD Bioscience, Franklin Lakes, PA, USA) and later centrifuged without brake at 600 g for 10 min. Cells were resuspended in 45% Percoll (GE Healthcare, Little Chalfont, UK), placed onto the top of a Percoll gradient (81%, 62%, 55%, 50% and 45%) and centrifuged without brake for 30 min at 1600 g; the cell band formed between 81% and 62% layers was removed and washed in PBS–citrate, placed onto a 3 mL Histopaque-1119 and centrifuged at 1600 g for 30 min. Cells were collected and washed twice with PBS–citrate, resuspended in modified Hanks’ balanced salt solution (HBSS) containing: 136 mM NaCl, 5.4 mM KCl, 1 mM MgCl2, 10 mM CaCl2, 1 mM glucose and 5.5 mM HEPES at pH 7.4.
Isolation of human neutrophils
Cells were isolated from whole blood obtained from healthy volunteers at the Universidad Austral de Chile. Briefly, blood collected in sterile tubes containing 3.8% w/v trisodium citrate was mixed with 35 mL 6% w/v dextran (average MW, 500 000) and 105 mL PBS–citrate. After 20 min at room temperature, the upper leucocyte-enriched plasma was centrifuged, and the pellet resuspended in 50% Percoll solution placed onto a Percoll gradient (82.5% and 65%) to isolate neutrophils.
Isolation of horse neutrophils
Blood was obtained from jugular puncture and put into sterile tubes containing 1 mL of 3.8% w/v trisodium citrate. To separate neutrophils, the blood was directly put into a Percoll gradient.
RT-PCR
mRNA was isolated with the Oligotex Direct mRNA kit (Promega, Fitchburg, WI, USA). Amplification of cDNAs was performed using GoTaqR Green Master Mix (Promega). PCRs were performed using 0.1 μM of specific primers. Primer pairs and expected size of the products were as follows: human KCNN4 ACTGGGCACCTTTCAGACAC and ACGTGCTTCGCCTTGTT, 194 bp; mouse KCNN4 CGGGGCACCTCACAGACACACT and CGCCGCTGACTCCTTCATCTCT, 248 bp; human TMEM16A GATTGATTCCGGTTCCAAAA and AGGGTGCTGTTTCCTGCTTA, 202 bp; human TMEM16B CTGTG-GGCTACCATGTTCCT and ACTCCGTCGTGTTTGTTTCC, 202 bp; mouse TMEM16A TTCGTCAA-TCACACGCTCTC and CTCACGCATAAACAGCTCCA, 324 bp; mouse TMEM 16B CGGATATCCCCACTGA-CATC and CTTAAGC CA-GTTCCCAGCAG, 330 bp; mouse beta-actin ATG CCAACACAGTGCTGTCT and AAGCACTTGCGG TGCACGAT, 244 bp; and human GAPDH GCAGGGGGGAGCCAAAAGGG and TGCC-AGCCCCAGCGTCAAAG, 556 bp. Human and mouse Kca3.1 PCR products were sequenced (Macrogen, Seoul, South Korea) and analysed by restriction enzymes Alw44I and DraIII (Promega).
Cytometer measurements of neutrophils
Forward scatter measurement of neutrophils was determined in the Accuri C6 (BD Bioscience), using ACCURI C6 software. A total of 2 × 106 neutrophils were incubated with 5 μM ionomycin plus TRAM-34 or the same volume of dimethyl sulfoxide (DMSO) in buffer containing 25 mM Hepes, 125 mM NaCl, 5 mM KCl, 1 mM CaCl2, 0.5 mM MgCl2, 1mM NaH2PO4, 0.1% bovine serum albumin and 0.1% glucose (pH 7.4).
Confocal microscopy
Neutrophils were loaded with calcein-AM 5 μM for 5 min (Molecular probes – Invitrogen, Waltham, MA, USA) and incubated with isotonic buffer (300 mOsm kg −1) containing (mM): 70 NaCl, 5 KCl, 0.5 MgCl2, 2 CaCl2, 10 HEPES and 140 D-manitol, pH 7.4, for 30 min at 37 °C. Excitation light was 488 nm, and emitted light was measured at wavelengths longer than 515 nm. The experiment was performed recording the calcein fluorescence of a selected area inside the cells as previously described (Niemeyer et al. 2001). Neutrophils were perfused with isotonic buffer for 5 min and then switched to hypotonic buffer (200 mOsm kg−1) containing (mM): 70 NaCl, 5 KCl, 0.5 MgCl2, 2 CaCl2, 10 HEPES and 55 D-manitol, pH 7.4, for at least 25 min. At the end of the experiments, all plates were switched to isotonic buffer to test cell integrity. Osmolality of solutions was measured in an Osmometer 3D3 (Advanced Instruments, Norwoods, MA, USA). Fluorescence was imaged using an FV1000 confocal microscope equipped with a 60X (N.A. 1.10) water immersion objective (Olympus, Tokyo, Japan). The images were obtained every 10 s using the FV1000-ASW 3.0 software.
Transwell assays
Experiments were performed in 8-μm pores (inserts) placed in 24-well plates (BD Bioscience). The lower chamber contained 700 μL of RPMI (Life Technologies, Waltham, MA, USA) complete medium with or without 1 nM N-formyl-Met-Leu-Phe (fMLP), 10 nM IL-8 (human recombinant) or 1 ng mL−1 of DNPHSA according to the origin of the neutrophils and the type of experiment. A total of 2 × 105 neutrophils were resuspended in the upper chamber (inserts) in 300 μL of complete RPMI. The plate was incubated at 37 °C for 1 h, and then, inserts were fixed with cold methanol and stained with May–Grünwald– Giemsa. Cells were counted in a bright field microscope with ×1000 magnification.
Isolation and culture of mouse bone marrow-derived mast cells (BMMCs)
Bone marrow cells were flushed from femurs, and cultured and differentiated to mast cells for 4 weeks in DMEM (Life Technologies) supplemented with 10% foetal bovine serum, 1% penicillin/streptomycin, 1% fungizone and 10 ng mL−1 IL-3 (PeproTech). Non-adherent cells in cultures were transferred into new flasks containing fresh culture medium every week. After 4 weeks, cell purity was evaluated by May–Grünwald–Giemsa and toluidine blue staining.
BMMCs sensitization
To be pre-sensitized, BMMCs were incubated in DMEM supplemented with 10% foetal bovine serum, 1% penicillin/streptomycin, 1% fungizone and 300 ng mL−1 of anti-DNP IgE (clone SPE-7) at 37 °C in a humid atmosphere (5% CO2) overnight. Cells were later stimulated with 10 ng mL−1 DNP-HSA antigen.
Chemokinesis assays
In vivo kinetic measurements of neutrophils were evaluated using microscopy real-time visualization under constant flow of HBSS 1 mM Ca2+ (bath solution). A total of 1 × 106 cells mL−1 in HBSS 1 mM Ca2+ were seeded in clean coverslips without coating molecules for 20 min at 37 °C. Cells were placed into the thermal stage chamber (Brook Industries, Lake Villa, IL, USA). Non-adherent cells were eliminated by the application of constant flow (1.5 mL min −1) of bath solution using a peristaltic pump (model 7615-72 from Ismatec SA, Cole-parmer Instrument Company, IL, USA). After 10 min of basal recordings, cells were exposed to 15 mL of bath solution containing 100 nM TRAM-34 or DMSO. Finally, the same cells were exposed to other 15 mL of bath solution with 10 nm fMLP or fMLP + TRAM-34. Stacks were collected with every 10 s using an AxioCam MRc5 (Carl Zeiss, Oberkochen, Germany). Total length of the cell path and average velocity were determined for 10–11 cells in the optical field using the Manual Tracking plugin of ImageJ.
Intracellular calcium measurements
Neutrophils were incubated with 5 mM Indo-1 AM (Life Technologies) in HBSS at a concentration of ~ 2 × 107 cells mL−1 for 30 min at 37 °C. Cells were washed with PBS, resuspended in buffer containing (mM): 25 Hepes, 125 NaCl, 5 KCl, 1 CaCl2, 0.5 MgCl2, 1 NaH2PO4, plus 0.1% bovine serum albumin and 0.1% glucose (pH 7.4) and then stimulated with 10 nM of fMLP or 100 nM IL-8 in the presence or absence of TRAM-34. Data were acquired with a Perkin-Elmer Luminescence Spectrometer LS 50B (Waltham, MA, USA), using an excitation wavelength of 330 nm, and emissions of 405 nm and 480 nm were collected. Data are expressed using the ratiometric form ratio between the absorbance at 405 nm and the isosbestic point (480 nm). The extracellular free Ca2+ concentration was determined using the Ca-EGTA Calculator v1.3 using constants from Theo Schoenmakers’ Chelator (http://maxchelator.stanford.edu/CaEGTA-TS.htm).
Reactive oxygen species production
Neutrophil’s Reactive oxygen species (ROS) generation was evaluated using the luminol-dependent chemiluminescence method (Easmon et al. 1980). Neutrophils were platted with a cell density of 3 × 105 cells/well in a 96-well flat-bottom plate in 200 μL. To induce ROS generation, we opsonized yeast with heat-inactivated serum (opsonized zymosan) at a concentration of 0.1 mg mL−1 in the presence or absence of TRAM-34. Luminol was added at a final concentration of 6.1 mM. Data were collected using a Perkin-Elmer Multilabel Reader Victor ×2 2030, at 37 °C. Kinetic of ROS production was obtained by calculating the slope from: m = Δy/Δx, where m is the slope and is defined as the difference in Y (Δy) divided by the difference in the X axis (Δx). Peak responses were obtained directly from the plots. Values are expressed as relative luminescence units (RLU).
LPS nasal inoculation, broncoalveolar lavage cell counts and lung histology
Mice were anesthetized with avertin (240 mg per kg) (i.p.) and received intranasal instillation of 100 μL LPS (2 μg mL−1, Salmonella enteritidis) or an equivalent volume of saline (0.9% NaCl). Eight or twenty hours after instillation, mice were euthanatized by Nembutal overdose (i.p.) and the trachea was exposed and cannulated with a Teflon I.V. catheter 24G. Broncoalveolar lavage (BAL) was collected with saline and cells were centrifuged once at 200 g for 7 min. The pelleted BAL cells were resuspended in PBS, and total number of leucocytes was determined using a Neubauer chamber. A total of 105 cells were cytocentrifuged (700 g for 14 min) and stained with May– Grünwald–Giemsa and differentiated according to the classical cell morphology and staining. Lungs were carefully and completely excised from the traquea and fixed in Bouin’s solution. Histological samples of 4 μm were stained with haematoxylin/eosin (H&E) and periodic acid-Schiff (PAS) for histopathological examination. Cell count was performed in 6–10 different fields per sample under 1000 × magnification.
Statistical analysis
Unless otherwise stated, all values correspond to means ± SEM. All data were analysed using SIGMAPLOT 12.3 software. The tests used are described in figure legends.
Results
Mammalian neutrophils express calcium-activated potassium but not chloride channels
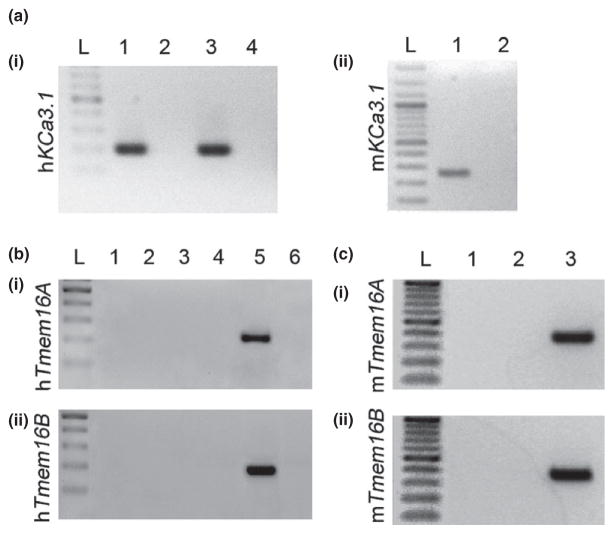
The RT-PCR analysis demonstrated that Kca3.1 mRNA is expressed in human neutrophils freshly isolated from peripheral blood and in mouse neutrophils purified from the bone marrow (Fig. 1a). We used as control the human HL-60 cell line, previously demonstrated to express functional KCa3.1 channels (Varnai et al. 1993). We found no expression of transcripts for the calcium-activated chloride channels TMEM16A and TMEM16B in human (Fig. 1b) and mouse (Fig. 1c) neutrophils.
Figure 1.
Kca3.1, Tmem16A and Tmem16B mRNA expression in mammalian neutrophils. (a) shows RTPCR products for Kca3.1 in (i) human neutrophils (lane 1) and HL-60 cells (lane 3) and (ii) mouse neutrophils (lane 1). Lanes 2 and 4 in (i) and 2 in (ii) correspond to RT-PCR negative controls. Tmem16A (i) and Tmem16B (ii) transcripts for human (b) lanes 1 and 3 in (i) and (ii), and mouse (c) lane 1 in (i) and (ii), were not detectable. Lanes 2 and 4 in (b) and lane 2 in (c) correspond to RT-PCR negative controls. Controls from MCF-7 cells are on lane 5 in (b) and the corresponding RT-PCR negative controls are on lane 6. Lane 3 in (c) are mouse colon. L indicates 100-bp ladder in all figures. Representative figures of at least 3 RT-PCRs.
KCa3.1 is required for volume regulation in neutrophils
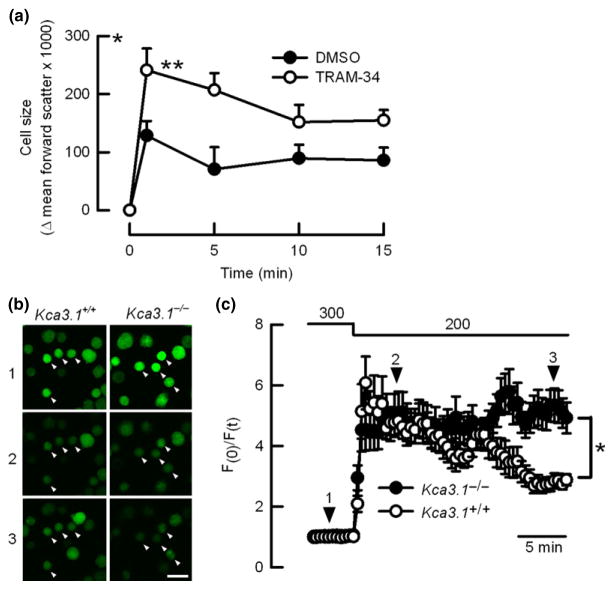
To analyse the role of KCa3.1 in volume regulation, we recorded volume changes in human neutrophils incubated with ionomycin which induces cell swelling by increasing sodium entry exchanged by calcium and/or protons (Gwag et al. 1999, Murao et al. 2005), and instantaneous opening of calcium-activated potassium channels (Krause et al. 1993). As summarized in Fig. 2(a), cells responded to ionomycin with a rapid swelling, but those pre-incubated with the KCa3.1 inhibitor TRAM-34 underwent a significantly larger swelling than control cells, evidencing impaired ability to reduce ionomycin-induced swelling when KCa3.1 is blocked. Incubation with TRAM-34 did not affect the volume of cells after ionomycin addition. In the following experiments, we recorded volume changes in calcein-loaded neutrophils from mice. We observed that both Kca3.1+/+ and Kca3.1 −/− cells incubated in isotonic buffer display similar fluorescence values and equally swell after hypotonic shock (200 mOsm kg−1) but only Kca3.1−/− neutrophils showed impaired regulated volume decrease (RVD), confirming functional expression of KCa3.1 channels in this cell type (Fig. 2c). After the hypotonic challenge, bath solution was switched again to isotonic (300 mOsm kg−1), and cells returned to fluorescence basal values (not shown).
Figure 2.
KCa3.1 regulates cell volume in neutrophils. (a) summarizes cell volume changes in human neutrophils incubated with ionomycin (5 μM) in the presence or absence of TRAM-34. n = 6 and *P < 0.05, **P < 0.01 two-way ANOVA. (b) Images are representative of calcein-loaded neutrophils (white arrowheads) observed at three different time points during the experiments presented in (c) (scale bar indicates 10 μm). (c) corresponds to traces for time course of relative fluorescence for calcein-loaded mouse neutrophils subjected to hypotonic shock (from 300 to 200 mOsm kg−1). Numbers and black arrowheads indicate the time at which pictures showed in (c) were taken. A total of 27 and 19 cells obtained from 6 Kca3.1+/+ and 4 Kca3.1−/− mice, respectively, were analysed from 3 individual experiments for each genotype. *<0.05 unpaired t-test.
Genetic deletion of KCa3.1 does not induce changes in haematological parameters in mice
Peripheral blood samples obtained from Kca3.1+/+ and Kca3.1−/− mice were analysed. No changes in the quantity and distribution of leucocytes were detected (Table 1). The observed morphology and staining pattern of Kca3.1−/− cells was identical to that of Kca3.1+/+ cells, displaying multi-lobed nucleus and typical cytoplasmatic granules (not shown).
Table 1.
Red blood cells and white blood cells distribution is not affected by Kca3.1 silencing
| Kca3.1+/+ (n = 5) | Kca3.1−/− (n = 4) | |
|---|---|---|
| Erythrocytes (106 Cells μL−1) | 8.93 ± 0.3 | 8.13 ± 0.6 |
| Leucocytes (103 Cells μL−1) | 3.42 ± 1.0 | 4.5 ± 0.7 |
| Eosinophils (103 Cells μL−1) | 0.05 ± 0.06 | 0.1 ± 0.1 |
| Lymphocytes (103 Cells μL−1) | 2.67 ± 0.8 | 3.20 ± 0.3 |
| Monocytes (103 Cells μL−1) | 0.1 ± 0.04 | 0.05 ± 0.04 |
| Neutrophils (103 Cells μL−1) | 0.7 ± 0.2 | 1.16 ± 0.5 |
Analysis of peripheral blood samples shows no significant differences in erythrocyte and leucocyte numbers between genotypes. Basophils were not detected. Non-significant differences for unpaired t-test were found for all groups.
The chemotactic response of mammalian neutrophils is impaired by pharmacological or genetic inhibition of KCa3.1
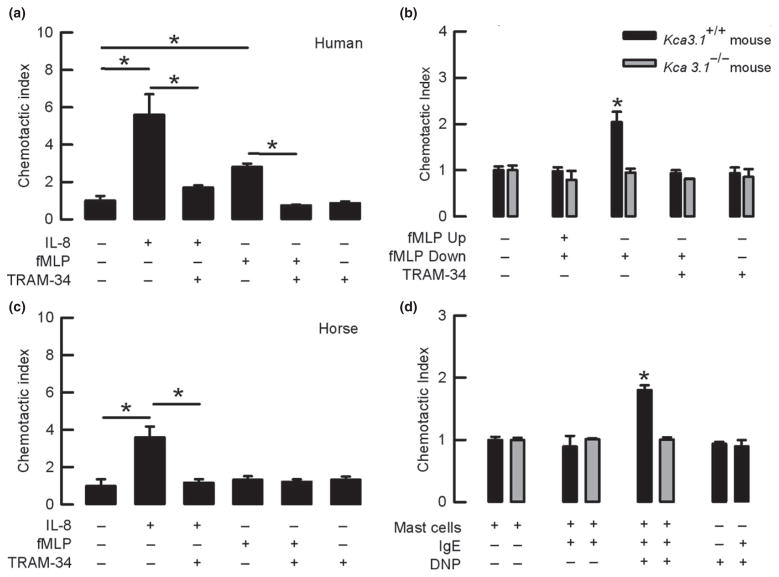
We tested whether the KCa3.1 channel plays a role in the chemotactic response of mammalian neutrophils using several chemotactic stimuli in the Transwell system. Figure 3(a) shows that human neutrophils migrate in response to both IL-8 and fMLP chemoattractant molecules but when cells were incubated with the KCa3.1 inhibitor TRAM-34, the response to IL-8 and fMLP was significantly reduced. Kca3.1+/+ mouse neutrophils increased their migration when fMLP was added in the lower chamber and not when the chemotactic gradient was broken by the addition of fMLP in both, lower and upper chambers. The Incubation of Kca3.1+/+ cells with TRAM-34 completely abolished the observed chemotactic effect (Fig. 3b). In contrast, neutrophils isolated from the Kca3.1−/− mice were unable to respond to fMLP and TRAM-34 incubation (Fig. 3b). Consistently, in horse neutrophils that are known not to migrate upon fMLP challenge (Zinkl & Brown 1982), the migratory response to IL-8 was diminished when they were exposed to TRAM-34 (Fig. 3c). Importantly, TRAM-34 did not affect human, mouse or horse cell migration in the absence of chemotactic stimulus.
Figure 3.
KCa3.1 silencing impairs chemotactic responses in mammalian neutrophils. (a) IL-8- and fMLP-induced chemotaxis is impaired by TRAM-34 in human neutrophils. *P < 0.05 (t-test) (b) Kca3.1+/+ mouse neutrophil chemotactic response to fMLP is impaired by TRAM-34. Neutrophils from Kca3.1−/− mouse do not respond to fMLP. *P < 0.05 compared to all other conditions for each group (one-way ANOVA test). (c) The chemotactic response of horse neutrophils to IL-8 is blocked by TRAM-34. *P < 0.05 (t-test). (d) Chemotactic response to mast cell degranulation is observed in Kca3.1+/+ but not in Kca3.1 −/− mouse neutrophils. *P < 0.05 compared to non-degranulated mast cells (one-way ANOVA test). n > 3 in all sets of experiments.
Mast cells are known to release chemotactic agents that attract neutrophils in vivo (Malaviya et al. 1996) and were used to recruit neutrophils in the Transwell experiments. As shown in Fig. 3(d), neutrophils isolated from Kca3.1+/+ mice migrated in response to degranulation induced by stimulation of mast cells with the antibody+ antigen complex (IgE+DNP), while neutrophils isolated from Kca3.1−/− mice did not. Control experiments using DNP or IgE+DNP in the absence of mast cells did not stimulate neutrophil migration in Kca3.1+/+ neutrophils, confirming that chemotactic agents released by mast cells were responsible for migration.
Chemokinetic parameters are altered upon KCa3.1 channel inactivation
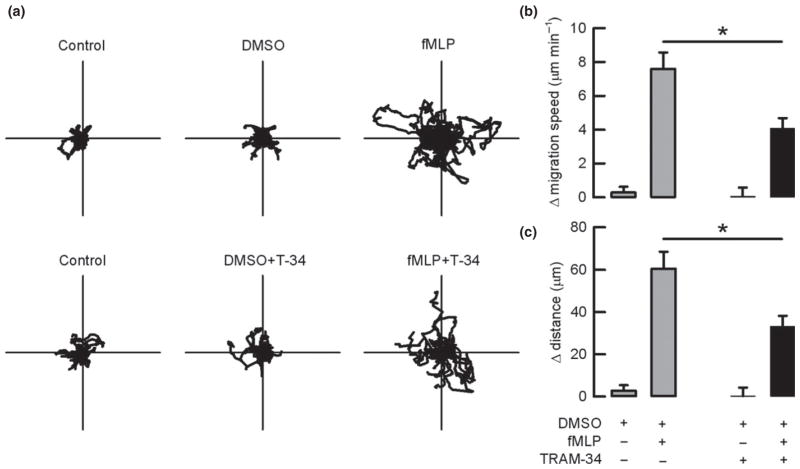
To further explore the role of KCa3.1 channels in the neutrophil migratory process, we studied chemokinesis using video microscopy. Figure 4(a) shows the trajectory of cells under the different conditions tested. Figure 4(b) and 4(c) summarizes the calculated differences in mean migration speed and the covered distance of human neutrophils respectively. As can be observed, both parameters increased when fMLP was added to the perfused solution. Human neutrophils increased migration speed in 7.6 ± 1 μm min −1 and travelled distance in 61 ± 8 μm in response to fMLP. The incubation of these cells with TRAM-34 significantly reduced the effect of fMLP on speed and distance to 4.1 ± 0.6 μm min−1 and to 33 ± 5 μm respectively. TRAM-34 tested in the absence of fMLP was also without effect.
Figure 4.
Inhibition of KCa3.1 impairs chemokinetic parameters in human neutrophils. Neutrophils were seeded in 2 plates for each experiment. (a) corresponds to individual traces for 40 cells taken from 4 independent experiments for each different condition. Polar plots have 70 μm radius. Differences in average mean migration (b) and differences in the travelled distance (c) for cells incubated with DMSO or 10 nM fMLP in the presence or absence of TRAM-34 are presented. *P < 0.005 paired t-test, n = 62 cells from 6 different experiments.
KCa3.1 inhibition does not affect the intracellular calcium homeostasis or the respiratory burst
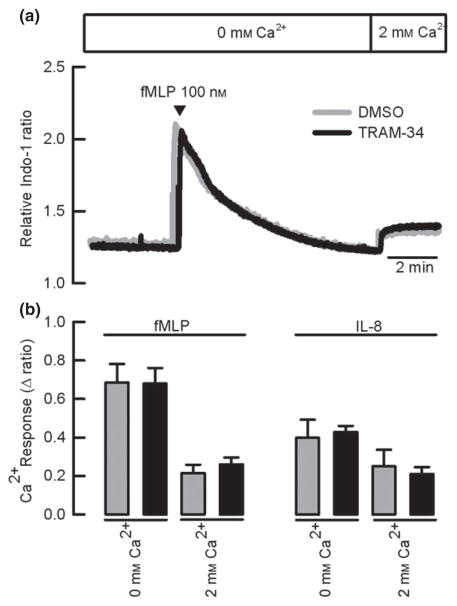
To dissociate the increase in intracellular calcium due to release from intracellular stores and that originated from the influx of calcium from the extracellular space, we used a protocol where neutrophils were stimulated with fMLP or IL-8 in the absence of external calcium and then, after the basal calcium state level has been reached, we switched extracellular free calcium from zero to 2 mM to measure influx. Addition of fMLP evoked a transient increase in calcium under extracellular free calcium conditions, with a sustained secondary increase seen on readmitting calcium to the bathing medium (Fig. 5a), and the same behaviour was observed when IL-8 was used (not shown). Data for both fMLP and IL-8 are summarized in Fig. 4(b). We observed that neither the intracellular calcium release (peak response) nor calcium influx is altered by KCa3.1 inhibition with TRAM-34.
Figure 5.
KCa3.1 inhibition does not affect calcium homeostasis in human neutrophils. (a) Representative traces for changes on intracellular calcium level when cells were stimulated with fMLP in the presence of DMSO or TRAM-34. (b) Summary of the differences for intracellular Ca2+ determined under 0 mM or 2 mM free Ca2+ on bath solution after fMLP or IL-8 stimulation. IL-8 experiments were performed as in (a). n = 4. Paired t-test analysis showed no significant effect of TRAM-34.
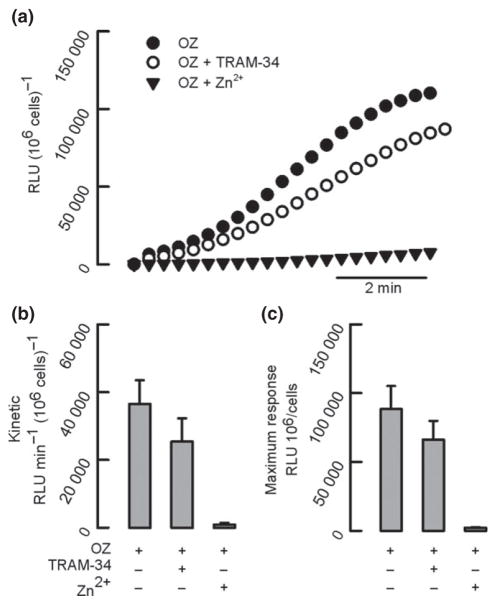
Hyperpolarization is a key step in a neutrophil’s ability to produce and release reactive oxygen species (ROS) during the respiratory burst, and potassium fluxes have been observed during this process (Reeves et al. 2002, Rada et al. 2004). We tested whether KCa3.1 channels were involved in the respiratory burst by stimulating human neutrophils with opsonized zymosan. The kinetics of ROS release were slightly reduced when cells were pre-incubated with TRAM-34 from 36 500 ± 7000 RLU min−1 (106 cells)−1 to 25 500 ± 6500 RLU min−1 (106 cells)−1 (Fig. 6b). Similarly, the maximum response shows that cells reached a value of 90 000 ± 17 000 RLU (106 cells) −1 in the absence and 66 000 ± 14 000 RLU (106 cells) −1 in the presence of TRAM-34 respectively (Fig. 6c). There were no significant differences between the groups for the rates and maximum values for ROS generation in the presence or absence of TRAM-34. As a control, in all experiments we used ZnCl2 (1 mM), a blocker of the proton channel that profoundly impairs the respiratory burst.
Figure 6.
KCa3.1 inhibition does not affect respiratory burst in human neutrophils. (a) Representative traces from an individual experiment for the respiratory burst of neutrophils stimulated with opsonized zymosan particles (OZ) in the presence of TRAM-34 or ZnCl2 (Zn2+). Kinetic (b) and maximum response (c) of ROS release are summarized. n = 6. Paired t-test analysis showed no significant effect of TRAM-34.
KCa3.1 is required for neutrophil migration in vivo
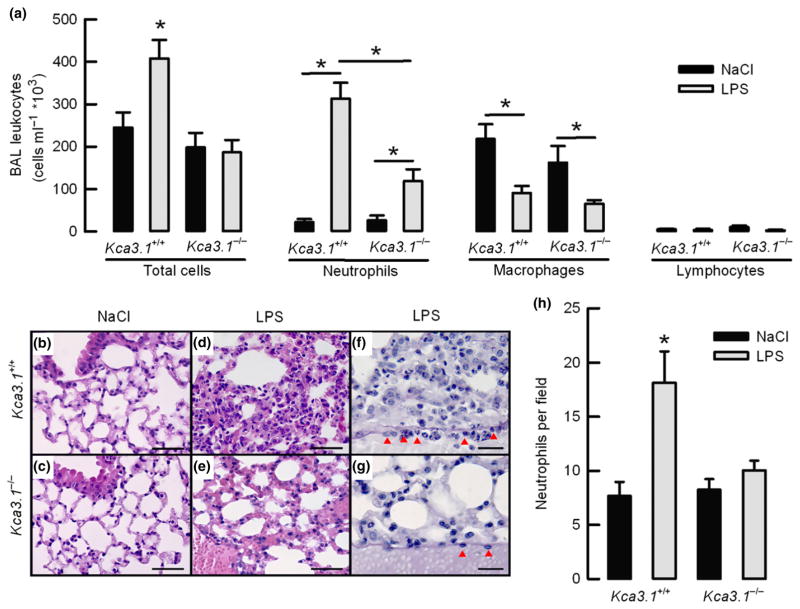
To ascertain whether KCa3.1 regulates infiltration of neutrophils in vivo, Kca3.1+/+ and Kca3.1−/− littermate mice were subjected to an acute lung injury challenge. Mice were nasally inoculated with LPS or saline (NaCl 0.9% as a control), and for each treatment, animals were separated into two different groups. In the first group, BAL samples for cell count were taken. Tissue samples for histological examinations were obtained from the second group. Fig. 7(a) summarizes the number of total leucocytes, neutrophils, macrophages and lymphocytes contained in the BAL fluid of animals tested. Examination of BAL fluid cell content confirmed that KCa3.1 function is necessary for the recruitment of neutrophils in the lungs after LPS challenge. The Kca3.1+/+ mice challenged with LPS accumulated a significantly higher number of neutrophils when compared with animals inoculated with saline, and similar results were obtained from BAL fluid samples obtained from the Kca3.1 −/−. Importantly, the number of neutrophils found in the Kca3.1 −/− mice challenged with LPS was significantly smaller than those observed in the Kca3.1+/+ mice. Both mice showed a significant reduction in the number of BAL fluid macrophages after LPS, but the number of lymphocytes was unaltered.
Figure 7.
The Kca3.1 −/− mouse exhibits a decrease in infiltrating neutrophils upon LPS delivery in the airways. (a) Number of leucocytes recovered in BAL fluid for animals instillated with nasal NaCL or LPS for 8 h. *P < 0.05 unpaired t-test. n = 4–5 for each group. (b–e) Lung histology for animals instillated for 8 h with NaCl or LPS and stained with H&E (scale bar 50 μm). (f and g) lung samples stained with PAS. Numerous neutrophils can be observed in alveoli and attached to a venule endothelium (red arrows; scale bar 20 μm). (c) Summary of neutrophil count in lung alveoli. *P < 0.05 one-way ANOVA test. n = 4 for each condition tested.
Histological examination showed that there were no perceptible differences in the lung morphology of Kca3.1+/+ and Kca3.1−/− mice inoculated with saline (Fig. 7b and 7c). LPS challenge caused lung congestion visualized as capillary hyperaemia in the lumen of capillaries of the interalveolar septum of both genotypes (Fig. 7d,e). Nevertheless a large number of neutrophils were observed in the lung parenchyma of Kca3.1+/+ mice when compared with Kca3.1−/− animals (Fig 7d,e,h). Closer examination revealed that neutrophils occupied the septa and lumen of alveoli and were usually adhered to the endothelial wall of venules in the lung parenchyma of Kca3.1+/+ mice, an event that was not observed in the blood vessels in Kca3.1−/− mice where only a few neutrophils were detected (Fig 7f,h).
Discussion
Our results show that KCa3.1 potassium channels are expressed in mammalian neutrophils and play an important role in their chemotactic response. It is important to note that silencing of KCa3.1 channels does not affect other relevant immune functions of neutrophils, such as the production of ROS, whose activity is considered to be indispensable to host defence responses. One significant observation is that the migration of neutrophils in response to different chemotactic signals is completely blocked by pharmacological inhibition with TRAM-34 or genetic deletion of Kca3.1. These results suggest that KCa3.1 opening is not coupled to the stimulation of a particular receptor and a particular signalling pathway, rather acting on downstream events related with the migration capacity of the cells. For example, neutrophils isolated from the TRPC6 channel null mice, showed impaired capacity to migrate towards a CXCR2 stimulating molecule, but displayed similar speed values than wild-type cells (Lindemann et al. 2013), an observation that could be explained by the remaining activity of KCa3.1 in the TRPC6−/− neutrophils. In fact, TRAM-34 inhibition of KCa3.1 significantly reduces both migration speed and covered distance of neutrophils after fMLP stimulation, confirming the pivotal role of KCa3.1 in the chemotactic capacity of neutrophils. Finally, our results are unlikely explained by a wide-ranging decrease in the number of receptors to chemoattractants in the membrane of Kca3.1−/− neutrophils. Analogous to our results, the response to fMLP in neutrophils isolated from a Clc3−/− mice is diminished, but the expression of fMLP receptors remained unaltered (Volk et al. 2008).
The mechanism by which KCa3.1 channels regulate migration in neutrophils acts much the same as described for several other cell types. As proposed by Schwab, the migration mechanism is a precisely coordinated series of cyclical changes in the volume of the leading edge and rear portion of the migrating cell. KCa3.1 channels open in the rear part of the cell by an increase in intracellular calcium, and potassium efflux contributes to retraction of the trailing end (Schwab 2001). Our observations are consistent with this phenomenon: (i) human neutrophils incubated with TRAM-34 showed a bigger increase in volume after ionomycin incubation when compared to controls, and (ii) confocal measurement of calcein-loaded neutrophils clearly demonstrated that genetic deletion of KCa3.1 channels from mice impairs the regulatory volume decrease in the cells after hypotonic shock. These results support the conclusion that volume dysregulation is responsible for the decreased chemotactic response observed in our experiments.
The current cell migration model predicts that the KCa3.1 activity is accompanied by calcium-activated chloride channel opening, and indeed, such currents have been recorded in neutrophils (Krause & Welsh 1990). We searched for TMEM16A and TMEM16B transcripts of the recently discovered family of calcium-activated chloride channels, but we found no expression of chloride channel in either human or mouse neutrophils. Another candidate for chloride efflux is the ClC-3 Cl−/H+ antiporter, previously linked to neutrophil motility and cell volume regulation (Volk et al. 2008). ClC-3 activity is dependent of intracellular calcium and has recently been reported to be coupled to KCa3.1 to allow ion efflux and chemotaxis in human glioma cells (Cuddapah et al. 2013). However, there is no convincing evidence for ClC-3 plasma membrane localization, and previous recordings of calcium-activated chloride channels in human neutrophils displayed outward rectification (Krause & Welsh 1990), which is absent in ClC-3 (Kasinathan et al. 2007).
The opening of KCa3.1 channels hyperpolarizes the membrane, allowing sustained calcium entry, necessary to induce, for example, the expression and release of cytokines in T lymphocytes (Di et al. 2010) and granules from mast cells (Shumilina et al. 2008). Previous evidence shows that both fMLP and IL-8 induce a two-component increase in intracellular calcium in neutrophils, displaying a rapid and transient increase mostly due to release from intracellular calcium stores, and a sustained plateau corresponding to extracellular calcium entry (Andersson et al. 1986, Wozniak et al. 1993). Our experiments demonstrated that fMLP- and IL-8-dependent depletion of the intracellular calcium stores was unaffected by the treatment of human neutrophils with TRAM-34. Intriguingly, the results obtained here showed that fMLP- and IL-8-induced calcium entry is independent of KCa3.1 activity in neutrophils, contrary to what has been reported in other cell types like T cells (Di et al. 2010), mast cells (Shumilina et al. 2008) or macrophages (Gao et al. 2010), when stimulated with different molecules. Several studies have determined that the activity of Hv1 proton channels delivers the charge compensation for the phagosome and its opening produces membrane hyperpolarization maintaining calcium entry, also essential to sustain the function of the NADPH oxidase (reviewed in DeCoursey & Hosler 2014). Hv1 channel activity might explain the unaltered calcium dynamics observed by us while KCa3.1 channels are blocked by TRAM-34 incubation. Inhibition of calcium intracellular increase has been proposed as a pharmacological target to control neutrophil-dependent inflammatory diseases in human and animals (Tintinger et al. 2009, Burgos et al. 2011). Nevertheless, besides chemotaxis, blocking calcium entry would affect several other fundamental and critical biocidal functions of neutrophils such as phagocytosis, superoxide production and granule release (Tintinger et al. 2005, Brechard & Tschirhart 2008). However, because calcium homeostasis appears to be intact in cells where KCa3.1 has been inhibited with TRAM-34, it would be expected that the function of calcium-dependent signalling and mechanisms in neutrophils lacking KCa3.1 activity are normal and can effectively respond when required. Nevertheless, we cannot rule out that calcium increase triggered by other molecules, different than IL-8 and fMLP, can be affected by KCa3.1 inhibition.
Earlier reports show that fMLP stimulates potassium-driven hyperpolarization in neutrophils (Lazzari et al. 1990) and increased potassium fluxes have been measured during the respiratory burst (Reeves et al. 2002, Rada et al. 2004). Within this context, potassium exit was postulated to provide charge compensation for superoxide anionic efflux to the phagosome during the respiratory burst, preventing depolarization of the cells. However, the molecular identification of the channel responsible for potassium exit in neutrophils has been controversial. Big conductance calcium-activated potassium channels (KCa1.1) activity was proposed to be absolutely necessary for the respiratory burst in neutrophils. But Kca1.1−/− mice and pharmacological inhibitors demonstrated that NADPH oxidase activity and killing of pathogenic microorganisms are independent of KCa1.1-induced hyperpolarization in neutrophils (Femling et al. 2006, Essin et al. 2007). Moreover, both studies found no electrophysiological evidence of KCa1.1 activity in these cells. Finally, The KCa2.3 calcium-activated potassium channel has been recently identified participating in NETosis without affecting ROS release in human neutrophils (Douda et al. 2015). As previously discussed, charge compensation due to NADPH activity involves proton exit, and our studies show that the production of ROS during the respiratory burst of human neutrophils is barely affected (about 15%) by the inhibition of KCa3.1. This result might be explained by potassium exit, which is relevant when oxidase is working at < 20% of its capacity, and at a higher workload, is proton efflux the responsible for charge compensation due to electron transfer (Lazzari et al. 1990).
Is KCa3.1 inhibition relevant to inflammatory human diseases? Observations made in the available Kca3.1−/− mouse models showed that the genetic silencing of the channel leads to mild phenotypes affecting the volume regulation of T cells and erythrocytes (Begenisich et al. 2004), and a slight elevation of systemic blood pressure (Si et al. 2006). The absence of KCa3.1 also inhibits intestinal calcium-dependent chloride secretion and produces a non-obstructive dehydration of faeces (Flores et al. 2007), and these animals were also found to have mild and progressive splenomegaly (Grgic et al. 2009a). But surprisingly, and most importantly, even though the KCa3.1 channel functionally expresses in several cells of the immune system, no severe immunologic phenotype has been reported. Our own examination of haematological parameters suggests that the rate of proliferation and maturation of neutrophil precursors, as well as their release from the bone marrow to the circulation and clearance, are not disturbed in the Kca3.1−/−, as we did not observe circulating immature myeloid cells. Similar observations were previously reported in another mouse strain (Grgic et al. 2009a). Such findings discard that the Kca3.1 −/− mouse presented with neutropenia and that this haematological condition could be responsible for the diminished number of neutrophils observed after the LPS challenge in the lungs of the Kca3.1−/− animals.
KCa3.1 inhibition has proven to be beneficial in a series of models and diseases that involve activation of cells of the immune system and inflammation such as experimental autoimmune encephalomyelitis (Reich et al. 2005), atherogenesis in humans and mice (Toyama et al. 2008), anaphylactic shock (Shumilina et al. 2008), renal fibrosis (Grgic et al. 2009b) and inflammatory bowel disease (Di et al. 2010). The whole animal experiments described in this work are consistent with KCa3.1 being a good therapeutic target for inflammatory diseases. We found that genetic silencing of KCa3.1 significantly reduced the number of neutrophils in the BAL and lung tissue of animals subjected to acute lung injury by LPS instillation and such observations support a crucial role for KCa3.1 channels in the neutrophil migration machinery. But some caution must be exercised when interpreting the results of whole animal studies. Silencing of Kca3.1 gene is also affecting the function of other cells of the immune system and endothelium (Grgic et al. 2005), which can also influence the migration of neutrophils (Shumilina et al. 2008, Di et al. 2010).
Reports of KCa3.1 mutations in human diseases are scarce. This could point towards two options. Mutations in the Kca3.1 gene do not occur in humans, or because Kca3.1 mutations do not produce severe phenotypes (like what has been observed in mice), they remain undetected. Nevertheless, single nucleotide polymorphisms of the promoter region in the human gene can enhance Kca3.1 expression and worsen the inflammatory disease of patients affected by Crohn (Simms et al. 2010). These observations, in conjunction with the results obtained in animals, strongly suggest that Kca3.1 activity might favour undesired inflammatory responses.
In summary, our results demonstrate that KCa3.1 channels are important for neutrophil migration in mammals. The inhibition of KCa3.1 channels had only modest effects on other relevant functions of neutrophils, an important consideration for future therapeutic applications. Our results obtained with the Kca3.1−/− mouse and the pharmacological blocker TRAM-34 support the possibility of KCa3.1 inhibition as a mechanism to prevent inflammatory cell infiltration. Overall, inhibition of KCa3.1 channels seems to be an attractive and safe target to contain the unwanted side effects of neutrophils overactivity.
Acknowledgments
This research was supported by Fondecyt grants 11100408 (C.A.F.), 1110464 (C.D.F.) and 11090292 (P.E.). The Centro de Estudios Científicos (CECs) is funded by the Centers of Excellence Base Financing Program of Conicyt.
Footnotes
Conflicts of interest
None.
The authors would like to thank the staff of CECs animal facility for their untiring work; Luis Galietta and Francisco Sepúlveda for their discussions, suggestions and critical reading of the manuscript; and Rodrigo Lerchundi for his helpful assistance in confocal measurements.
References
- Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: from mechanisms to disease. Annu Rev Immunol. 2012;30:459–489. doi: 10.1146/annurev-immunol-020711-074942. [DOI] [PubMed] [Google Scholar]
- Andersson T, Dahlgren C, Pozzan T, Stendahl O, Lew PD. Characterization of fMet-Leu-Phe receptor-mediated Ca2 + influx across the plasma membrane of human neutrophils. Mol Pharmacol. 1986;30:437–443. [PubMed] [Google Scholar]
- Begenisich T, Nakamoto T, Ovitt CE, Nehrke K, Brugnara C, Alper SL, Melvin JE. Physiological roles of the intermediate conductance, Ca2 +-activated potassium channel Kcnn4. J Biol Chem. 2004;279:47681–47687. doi: 10.1074/jbc.M409627200. [DOI] [PubMed] [Google Scholar]
- Brechard S, Tschirhart EJ. Regulation of superoxide production in neutrophils: role of calcium influx. J Leukoc Biol. 2008;84:1223–1237. doi: 10.1189/jlb.0807553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos RA, Conejeros I, Hidalgo MA, Werling D, Hermosilla C. Calcium influx, a new potential therapeutic target in the control of neutrophil-dependent inflammatory diseases in bovines. Vet Immunol Immunopathol. 2011;143:1–10. doi: 10.1016/j.vetimm.2011.05.037. [DOI] [PubMed] [Google Scholar]
- Cuddapah VA, Turner KL, Seifert S, Sontheimer H. Bradykinin-induced chemotaxis of human gliomas requires the activation of KCa3.1 and ClC-3. J Neurosci. 2013;33:1427–1440. doi: 10.1523/JNEUROSCI.3980-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva-Santos JE, Santos-Silva MC, de Cunha FQ, Assreuy J. The role of ATP-sensitive potassium channels in neutrophil migration and plasma exudation. J Pharmacol Exp Ther. 2002;300:946–951. doi: 10.1124/jpet.300.3.946. [DOI] [PubMed] [Google Scholar]
- DeCoursey TE, Hosler J. Philosophy of voltagegated proton channels. J R Soc Interface. 2014;11:20130799. doi: 10.1098/rsif.2013.0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di L, Srivastava S, Zhdanova O, Ding Y, Li Z, Wulff H, Lafaille M, Skolnik EY. Inhibition of the K+ channel KCa3.1 ameliorates T cell-mediated colitis. Proc Natl Acad Sci U S A. 2010;107:1541–1546. doi: 10.1073/pnas.0910133107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douda DN, Khan MA, Grasemann H, Palaniyar N. SK3 channel and mitochondrial ROS mediate NADPH oxidase-independent NETosis induced by calcium influx. Proc Natl Acad Sci U S A. 2015;112:2817–2822. doi: 10.1073/pnas.1414055112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easmon CS, Cole PJ, Williams AJ, Hastings M. The measurement of opsonic and phagocytic function by Luminol-dependent chemiluminescence. Immunology. 1980;41:67–74. [PMC free article] [PubMed] [Google Scholar]
- Essin K, Salanova B, Kettritz R, Sausbier M, Luft FC, Kraus D, Bohn E, Autenrieth IB, Peschel A, Ruth P, Gollasch M. Large-conductance calcium-activated potassium channel activity is absent in human and mouse neutrophils and is not required for innate immunity. Am J Physiol Cell Physiol. 2007;293:C45–C54. doi: 10.1152/ajpcell.00450.2006. [DOI] [PubMed] [Google Scholar]
- Femling JK, Cherny VV, Morgan D, Rada B, Davis AP, Czirjak G, Enyedi P, England SK, Moreland JG, Ligeti E, Nauseef WM, DeCoursey TE. The antibacterial activity of human neutrophils and eosinophils requires proton channels but not BK channels. J Gen Physiol. 2006;127:659–672. doi: 10.1085/jgp.200609504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores CA, Melvin JE, Figueroa CD, Sepulveda FV. Abolition of Ca2 +-mediated intestinal anion secretion and increased stool dehydration in mice lacking the intermediate conductance Ca2 +-dependent K+ channel Kcnn4. J Physiol. 2007;583:705–717. doi: 10.1113/jphysiol.2007.134387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao YD, Hanley PJ, Rinné S, Zuzarte M, Daut J. Calcium-activated K(+) channel (K(Ca)3.1) activity during Ca(2 + ) store depletion and store-operated Ca(2 + ) entry in human macrophages. Cell Calcium. 2010;48:19–27. doi: 10.1016/j.ceca.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Grgic I, Eichler I, Heinau P, Si H, Brakemeier S, Hoyer J, Kohler R. Selective blockade of the intermediate-conductance Ca2+-activated K+ channel suppresses proliferation of microvascular and macrovascular endothelial cells and angiogenesis in vivo. Arterioscler Thromb Vasc Biol. 2005;25:704–709. doi: 10.1161/01.ATV.0000156399.12787.5c. [DOI] [PubMed] [Google Scholar]
- Grgic I, Kaistha BP, Paschen S, Kaistha A, Busch C, Si H, Kohler K, Elsasser HP, Hoyer J, Kohler R. Disruption of the Gardos channel (KCa3.1) in mice causes subtle erythrocyte macrocytosis and progressive splenomegaly. Pflugers Arch. 2009a;458:291–302. doi: 10.1007/s00424-008-0619-x. [DOI] [PubMed] [Google Scholar]
- Grgic I, Kiss E, Kaistha BP, Busch C, Kloss M, Sautter J, Muller A, Kaistha A, Schmidt C, Raman G, Wulff H, Strutz F, Grone HJ, Kohler R, Hoyer J. Renal fibrosis is attenuated by targeted disruption of KCa3.1 potassium channels. Proc Natl Acad Sci U S A. 2009b;106:14518–14523. doi: 10.1073/pnas.0903458106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwag BJ, Canzoniero LM, Sensi SL, Demaro JA, Koh JY, Goldberg MP, Jacquin M, Choi DW. Calcium ionophores can induce either apoptosis or necrosis in cultured cortical neurons. Neuroscience. 1999;90:1339–1348. doi: 10.1016/s0306-4522(98)00508-9. [DOI] [PubMed] [Google Scholar]
- Kasinathan RS, Foller M, Lang C, Koka S, Lang F, Huber SM. Oxidation induces ClC-3-dependent anion channels in human leukaemia cells. FEBS Lett. 2007;581:5407–5412. doi: 10.1016/j.febslet.2007.10.042. [DOI] [PubMed] [Google Scholar]
- Krause KH, Welsh MJ. Voltage-dependent and Ca2(+)-activated ion channels in human neutrophils. J Clin Invest. 1990;85:491–498. doi: 10.1172/JCI114464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause KH, Demaurex N, Jaconi M, Lew DP. Ion channels and receptor-mediated Ca2 + influx in neutrophil granulocytes. Blood Cells. 1993;19:165–173. [PubMed] [Google Scholar]
- Lazzari KG, Proto P, Simons ER. Neutrophil hyperpolarization in response to a chemotactic peptide. J Biol Chem. 1990;265:10959–10967. [PubMed] [Google Scholar]
- Lindemann O, Umlauf D, Frank S, Schimmelpfennig S, Bertrand J, Pap T, Hanley PJ, Fabian A, Dietrich A, Schwab A. TRPC6 regulates CXCR2-mediated chemotaxis of murine neutrophils. J Immunol. 2013;190:5496–5505. doi: 10.4049/jimmunol.1201502. [DOI] [PubMed] [Google Scholar]
- Malaviya R, Ikeda T, Ross E, Abraham SN. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-alpha. Nature. 1996;381:77–80. doi: 10.1038/381077a0. [DOI] [PubMed] [Google Scholar]
- Murao H, Shimizu A, Hosoi K, Iwagaki A, Min KY, Kishima G, Hanafusa T, Kubota T, Kato M, Yoshida H, Nakahari T. Cell shrinkage evoked by Ca2+-free solution in rat alveolar type II cells: Ca2+-regulation of Na+–H+ exchange. Exp Physiol. 2005;90:203–213. doi: 10.1113/expphysiol.2004.028837. [DOI] [PubMed] [Google Scholar]
- Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- Nemeth T, Mocsai A. The role of neutrophils in autoimmune diseases. Immunol Lett. 2012;143:9–19. doi: 10.1016/j.imlet.2012.01.013. [DOI] [PubMed] [Google Scholar]
- Niemeyer MI, Cid LP, Barros LF, Sepulveda FV. Modulation of the two-pore domain acid-sensitive K+ channel TASK-2 (KCNK5) by changes in cell volume. J Biol Chem. 2001;276:43166–43174. doi: 10.1074/jbc.M107192200. [DOI] [PubMed] [Google Scholar]
- Pedersen SF, Klausen TK, Nilius B. The identification of a volume-regulated anion channel: an amazing Odyssey. Acta Physiol. 2015;213:868–881. doi: 10.1111/apha.12450. [DOI] [PubMed] [Google Scholar]
- Pillarisetti N, Williamson E, Linnane B, Skoric B, Robertson CF, Robinson P, Massie J, Hall GL, Sly P, Stick S, Ranganathan S Australian Respiratory Early Surveillance Team for Cystic F. Infection, inflammation, and lung function decline in infants with cystic fibrosis. Am J Respir Crit Care Med. 2011;184:75–81. doi: 10.1164/rccm.201011-1892OC. [DOI] [PubMed] [Google Scholar]
- Rada BK, Geiszt M, Kaldi K, Timar C, Ligeti E. Dual role of phagocytic NADPH oxidase in bacterial killing. Blood. 2004;104:2947–2953. doi: 10.1182/blood-2004-03-1005. [DOI] [PubMed] [Google Scholar]
- Reddy RC, Standiford TJ. Effects of sepsis on neutrophil chemotaxis. Curr Opin Hematol. 2010;17:18–24. doi: 10.1097/MOH.0b013e32833338f3. [DOI] [PubMed] [Google Scholar]
- Reeves EP, Lu H, Jacobs HL, Messina CG, Bolsover S, Gabella G, Potma EO, Warley A, Roes J, Segal AW. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature. 2002;416:291–297. doi: 10.1038/416291a. [DOI] [PubMed] [Google Scholar]
- Reich EP, Cui L, Yang L, Pugliese-Sivo C, Golovko A, Petro M, Vassileva G, Chu I, Nomeir AA, Zhang LK, Liang X, Kozlowski JA, Narula SK, Zavodny PJ, Chou CC. Blocking ion channel KCNN4 alleviates the symptoms of experimental autoimmune encephalomyelitis in mice. Eur J Immunol. 2005;35:1027–1036. doi: 10.1002/eji.200425954. [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan A, Raman G, Busch C, Schultz T, Zimin PI, Hoyer J, Kohler R, Wulff H. Naphtho[1,2-d]thiazol-2-ylamine (SKA-31), a new activator of KCa2 and KCa3.1 potassium channels, potentiates the endothelium-derived hyperpolarizing factor response and lowers blood pressure. Mol Pharmacol. 2009;75:281–295. doi: 10.1124/mol.108.051425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling T, Eder C. Lysophosphatidylcholine- and MCP-1-induced chemotaxis of monocytes requires potassium channel activity. Pflugers Arch. 2009;459:71–77. doi: 10.1007/s00424-009-0710-y. [DOI] [PubMed] [Google Scholar]
- Schmidt S, Moser M, Sperandio M. The molecular basis of leukocyte recruitment and its deficiencies. Mol Immunol. 2013;55:49–58. doi: 10.1016/j.molimm.2012.11.006. [DOI] [PubMed] [Google Scholar]
- Schwab A. Function and spatial distribution of ion channels and transporters in cell migration. Am J Physiol Renal Physiol. 2001;280:F739–F747. doi: 10.1152/ajprenal.2001.280.5.F739. [DOI] [PubMed] [Google Scholar]
- Schwab A, Fabian A, Hanley PJ, Stock C. Role of ion channels and transporters in cell migration. Physiol Rev. 2012;92:1865–1913. doi: 10.1152/physrev.00018.2011. [DOI] [PubMed] [Google Scholar]
- Shao Z, Makinde TO, Agrawal DK. Calciumactivated potassium channel KCa3.1 in lung dendritic cell migration. Am J Respir Cell Mol Biol. 2011;45:962–968. doi: 10.1165/rcmb.2010-0514OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumilina E, Lam RS, Wolbing F, Matzner N, Zemtsova IM, Sobiesiak M, Mahmud H, Sausbier U, Biedermann T, Ruth P, Sausbier M, Lang F. Blunted IgE-mediated activation of mast cells in mice lacking the Ca2 +-activated K+ channel KCa3.1. J Immunol. 2008;180:8040–8047. doi: 10.4049/jimmunol.180.12.8040. [DOI] [PubMed] [Google Scholar]
- Si H, Heyken WT, Wolfle SE, Tysiac M, Schubert R, Grgic I, Vilianovich L, Giebing G, Maier T, Gross V, Bader M, de Wit C, Hoyer J, Kohler R. Impaired endothelium-derived hyperpolarizing factor-mediated dilations and increased blood pressure in mice deficient of the intermediate-conductance Ca2 +-activated K+ channel. Circ Res. 2006;99:537–544. doi: 10.1161/01.RES.0000238377.08219.0c. [DOI] [PubMed] [Google Scholar]
- Simms LA, Doecke JD, Roberts RL, Fowler EV, Zhao ZZ, McGuckin MA, Huang N, Hayward NK, Webb PM, Whiteman DC, et al. KCNN4 gene variant is associated with ileal Crohn’s Disease in the Australian and New Zealand population. Am J Gastroenterol. 2010;105:2209–2217. doi: 10.1038/ajg.2010.161. [DOI] [PubMed] [Google Scholar]
- Stockley RA. Neutrophils and the pathogenesis of COPD. Chest. 2002;121:151S–155S. doi: 10.1378/chest.121.5_suppl.151s. [DOI] [PubMed] [Google Scholar]
- Tintinger G, Steel HC, Anderson R. Taming the neutrophil: calcium clearance and influx mechanisms as novel targets for pharmacological control. Clin Exp Immunol. 2005;141:191–200. doi: 10.1111/j.1365-2249.2005.02800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tintinger GR, Steel HC, Theron AJ, Anderson R. Pharmacological control of neutrophil-mediated inflammation: strategies targeting calcium handling by activated polymorphonuclear leukocytes. Drug Des Devel Ther. 2009;2:95–104. [PMC free article] [PubMed] [Google Scholar]
- Toyama K, Wulff H, Chandy KG, Azam P, Raman G, Saito T, Fujiwara Y, Mattson DL, Das S, Melvin JE, Pratt PF, Hatoum OA, Gutterman DD, Harder DR, Miura H. The intermediate-conductance calcium-activated potassium channel KCa3.1 contributes to atherogenesis in mice and humans. J Clin Invest. 2008;118:3025–3037. doi: 10.1172/JCI30836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Tscharner V, Prod’hom B, Baggiolini M, Reuter H. Ion channels in human neutrophils activated by a rise in free cytosolic calcium concentration. Nature. 1986;324:369–372. doi: 10.1038/324369a0. [DOI] [PubMed] [Google Scholar]
- Varnai P, Demaurex N, Jaconi M, Schlegel W, Lew DP, Krause KH. Highly co-operative Ca2 + activation of intermediate-conductance K+ channels in granulocytes from a human cell line. J Physiol. 1993;472:373–390. doi: 10.1113/jphysiol.1993.sp019952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk AP, Heise CK, Hougen JL, Artman CM, Volk KA, Wessels D, Soll DR, Nauseef WM, Lamb FS, Moreland JG. ClC-3 and IClswell are required for normal neutrophil chemotaxis and shape change. J Biol Chem. 2008;283:34315–34326. doi: 10.1074/jbc.M803141200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MR, Azcutia V, Newton G, Alcaide P, Luscinskas FW. Emerging mechanisms of neutrophil recruitment across endothelium. Trends Immunol. 2011;32:461–469. doi: 10.1016/j.it.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak A, Betts WH, Murphy GA, Rokicinski M. Interleukin-8 primes human neutrophils for enhanced superoxide anion production. Immunology. 1993;79:608–615. [PMC free article] [PubMed] [Google Scholar]
- Zinkl JG, Brown PD. Chemotaxis of horse polymorphonuclear leukocytes to N-formyl-L-methionyl-L-leucyl-L-phenylalanine. Am J Vet Res. 1982;43:613–616. [PubMed] [Google Scholar]