Abstract
Fe-S cofactors are composed of iron and inorganic sulfur in various stoichiometries. A complex assembly pathway conducts their initial synthesis and subsequent binding to recipient proteins. In this minireview, we discuss how discovery of the role of the mammalian cytosolic aconitase, known as iron regulatory protein 1 (IRP1), led to the characterization of the function of its Fe-S cluster in sensing and regulating cellular iron homeostasis. Moreover, we present an overview of recent studies that have provided insights into the mechanism of Fe-S cluster transfer to recipient Fe-S proteins.
Keywords: energy metabolism, iron-response element (IRE), iron-sulfur protein, metalloenzyme, mitochondrial respiratory chain complex, HSC20, HSPA9, ISCU, iron-sulfur cluster biogenesis
Discovery of a regulatory role for an iron-sulfur cluster in iron regulatory protein 1
Interest in understanding how mammalian cells regulated iron uptake and distribution in the 1980s led to the discovery of a post-transcriptional regulatory mechanism, which was found to be crucial for cellular and systemic iron homeostasis in vertebrates. It was known that the expression of a major iron storage protein, ferritin (Ft),2 was primarily regulated at the translational rather than transcriptional level (1, 2). However, it was not possible to dissect how ferritin levels were controlled under different conditions of iron availability until the genes encoding H and L ferritin were cloned in 1984 (2). At that time, gel-shift assays were commonly used to demonstrate direct binding of specific transcription factors to DNA sequences. A similar approach revealed that one or more cytosolic proteins were bound to the 5′-untranslated (5′-UTR) region of ferritin transcripts in mammalian cells (3–5). Moreover, it appeared that the binding factors reflected the iron status of the cell, as ferritin translation and gel-shift binding activity were reduced in cells that were iron-deficient, while conversely increasing in cells that were iron-loaded. The region of the ferritin transcripts responsible for mediating translational regulation was identified and subsequently named the IRE, for iron-responsive element. The IRE was defined through mutagenesis and sequence homology as a short stem-loop structure located near the 5′-end of the ferritin transcript. Intensive efforts to identify cytosolic factors that bound to the IRE resulted in cloning of two major cytosolic regulatory proteins, iron regulatory proteins 1 and 2 (IRP1 and IRP2) (5). It was immediately apparent, upon inspection of its primary amino acid sequence, that IRP1 was remarkably similar to mitochondrial aconitase, which was a well-characterized Fe-S protein. A cubane [Fe4-S4] cluster in the IRP1 active-site cleft was known to be critical for the reversible aconitase-mediated conversion of citrate to isocitrate (6), which is essential for cholesterol and fatty acid metabolism, as citrate is the substrate of ATP-citrate lyase, which generates acetyl-coenzyme A utilized for cholesterol and lipid biosynthesis. Additionally, citrate has important regulatory roles in glycolysis and fatty acid synthesis and oxidation (7). Isocitrate is also metabolized by the cytosolic NADP-dependent isocitrate dehydrogenase to generate α-ketoglutarate and NADPH; the latter is an essential cofactor for several enzymatic reactions involved in glutathione metabolism and lipid and cholesterol biosynthesis (7). Interestingly, IRP1 was found to readily convert from an Fe-S enzyme, which exhibited cytosolic aconitase activity, to an apo-form, which was able to bind with high affinity to the IREs present in several messenger RNAs (mRNAs) encoding proteins involved in iron homeostasis (8, 9). IRP1 binding to the 5′-UTR of the Ft transcript was found to sterically interfere with translation of the Ft mRNA and therefore with ferritin synthesis (10).
Multiple studies characterized critical structural properties and sequence features of functional IREs, which were used to identify novel IRE-containing transcripts (11). A three-dimensional structure of a classical IRE is depicted in Fig. 1. It consists of a lower base-paired stem, an unpaired cytosine that bulges out from the stem, and a five base-paired upper stem upon which a 6-membered loop rests. The loop contains base-pairing between positions 1 and 5 and a highly conserved G residue. Using informatics approaches followed by biochemical assays, functional IREs were identified in the 5′-UTR of at least seven other transcripts, including the erythroid form of aminolevulinic acid synthase (ALAS2), which is the rate-limiting enzyme for heme synthesis (11), mitochondrial aconitase (12), HIF2α (13), and the iron exporter ferroportin (14), in addition to the ferritin H and L transcripts (3). Five IREs are present at the 3′-UTR of the transcript of transferrin receptor 1, where binding of IRPs protects the mRNA from degradation and thereby increases levels of transferrin receptor 1 in iron-depleted cells (15). Another important iron transcript encodes the divalent metal transporter-1 (DMT1), which is found on the apical membrane of duodenal enterocytes and in endosomes, where it is responsible for the export of iron from recycling vesicles to the cytosol. DMT1 mRNA contains an IRE at its 3′-UTR, which enables cells to increase expression of the iron transporter under conditions of iron deficiency (16). An IRE was also identified in the 5′-UTR of the Alzheimer's amyloid precursor protein transcript, pointing to a possible iron-dependent regulation of intracellular amyloid precursor protein levels (17). The transcript of CDC14A, a cell cycle regulatory protein, also contains a potentially functional IRE in its 3′-UTR (18). Other advanced techniques may reveal additional functional IREs in mammalian transcripts (19).
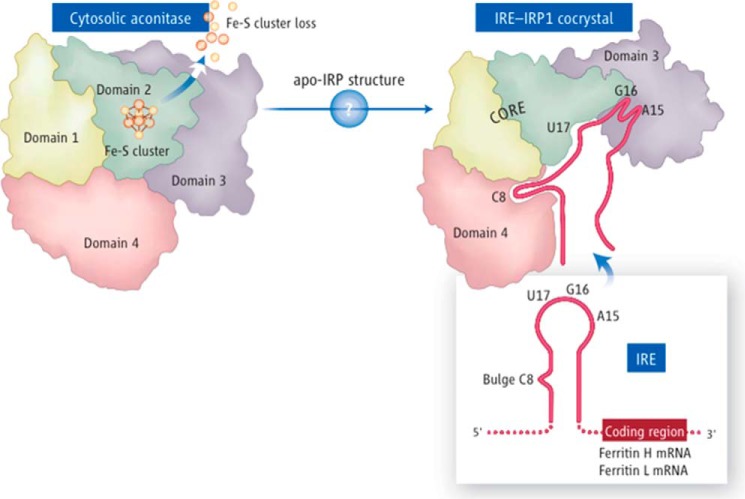
Figure 1.
IRP1 alternates between function as a cytosolic aconitase, which contains a [Fe4-S4] cluster in the active-site cleft, to an apoprotein form that lacks the cluster and binds to IRE stem-loop structures present in several iron transcripts. Upon binding, IRP1 represses translation of multiple transcripts that contain IREs near the 5′-end of the transcript and stabilizes mRNAs that contain IREs at the 3′-UTR from endonucleolytic degradation. Apo-IRP1 undergoes a large conformational change that creates a complex IRE-binding pocket, in which the bulge C binds to domain 4, and three residues of the loop make finger-like binding projections into newly accessible regions of domain 3. The length of the upper stem of the IRE optimizes the distance between its two main IRP contact points, resulting in high affinity binding.
The mechanism of iron sensing was subsequently supported by solution of the crystal structures of cytosolic aconitase with its intact cubane cluster (20) and of the IRE-IRP1 complex, which formed in iron-depleted conditions upon loss of the Fe-S cluster from IRP1 (21). Important features of the high-affinity IRE-IRP complex included a large conformational change of domains 3 and 4 of the protein, with the IRE projecting its bulge C and A15, G16, and U17 residues of the loop into the newly accessible binding pockets of the IRP1 apoprotein. Thus, the presence or absence of the iron-sulfur cluster of IRP1 was crucial in determining whether the protein would function as a cytosolic aconitase or as a post-transcriptional regulatory protein that increased levels of available cellular iron by decreasing synthesis of the iron sequestration protein, ferritin, and enhancing translation of the iron uptake proteins, transferrin receptor 1 and DMT1.
When IRP1 was cloned, a second IRE-binding protein, IRP2, was also identified (5, 22, 23). IRP2 was found to be rapidly degraded by the ubiquitin proteasomal system in iron-replete cells (24, 25), to lack aconitase activity, and to contain an extra 73 amino acid domain enriched in cysteine and proline residues and located 139 amino acids downstream of the translation initiation codon. The role of the 73 amino acid domain remains uncharacterized. IRP2 is not known to ligate an Fe-S cluster, even though the cluster-coordinating cysteines are conserved between IRP1 and IRP2. IRP2 functions as an IRE-binding protein, which appears to have nearly complete overlapping and redundant functions to IRP1. It probably arose from a gene duplication event, which likely enabled the two IRPs to assume slightly different roles in overall physiology (26).
Both IRP1 and IRP2 are ubiquitously expressed in all cell types, although at different ratios (27, 28). Cells that lack both IRPs fail to survive the blastocyst stage, whereas animals that lack either IRP1 or IRP2 live to be adults (29). However, IRP1 or IRP2 knock-out mice present with dramatically different phenotypes that likely reflect the differential dependence of specific cell types on each IRP to achieve full function of the IRE-IRP regulatory system. IRP2 knock-out animals develop adult onset neurodegenerative disease (30–32), with prominent loss of motor neurons and abnormal balance and gait (33), along with anemia (32, 34) and erythropoietic protoporphyria (32). In contrast, animals that lack IRP1 develop polycythemia, cardiac fibrosis, and pulmonary hypertension attributable to high HIF2α-mediated induction of erythropoietin expression, caused by failure to repress translation of the HIF2α transcript through IRP1 binding to the 5′-UTR IRE of HIF2α mRNA in erythropoietin-synthesizing cells of the kidney (35, 36).
How iron-sulfur clusters are synthesized and trafficked in mammalian cells
Upon discovering the role of an Fe-S cluster in regulation of intracellular iron metabolism, researchers began to focus on the question of how mammalian cells synthesize Fe-S clusters. It was known that Fe-S clusters could assemble from iron and inorganic sulfur under anaerobic conditions in organic solvents (37), but comparable conditions are not present in mammalian cells. The key to elucidating the mechanism of Fe-S biogenesis in living cells was provided by studies in the bacterium Azotobacter vinelandii, which was found to have three operons devoted to Fe-S cluster biogenesis, one of which, the specialized nif operon, encoded proteins involved solely in Fe-S cluster assembly for nitrogenase (38). The subsequent identification of the Escherichia coli isc− and suf− gene cluster components revealed that the core machinery for Fe-S cluster biogenesis is evolutionarily highly conserved to engineer a complex series of biogenesis reactions, although the operons are activated under different conditions. The isc operon is constitutively expressed, whereas the suf gene cluster is highly activated under oxidative stress conditions (39). Orthologs of the bacterial isc genes have been identified in yeast, plants, and animals (40), whereas the suf system is present mainly in cyanobacteria and plants (40, 41). Intense studies in bacteria and yeast have provided most of the insights into the mechanism of Fe-S cluster assembly, which starts with sulfur mobilization from the substrate cysteine by a cysteine desulfurase (42). A main Fe-S cluster scaffold protein, physically bound to the cysteine desulfurase, provides the cysteine ligands to coordinate the newly synthesized cluster; concurrently, a not yet identified donor protein provides iron, whereas ferredoxin supplies electrons required to achieve the final electronic configuration of the nascent Fe-S cluster. Finally, a cochaperone/chaperone system assists in transferring the cluster to recipient Fe-S apoproteins (Fig. 2).
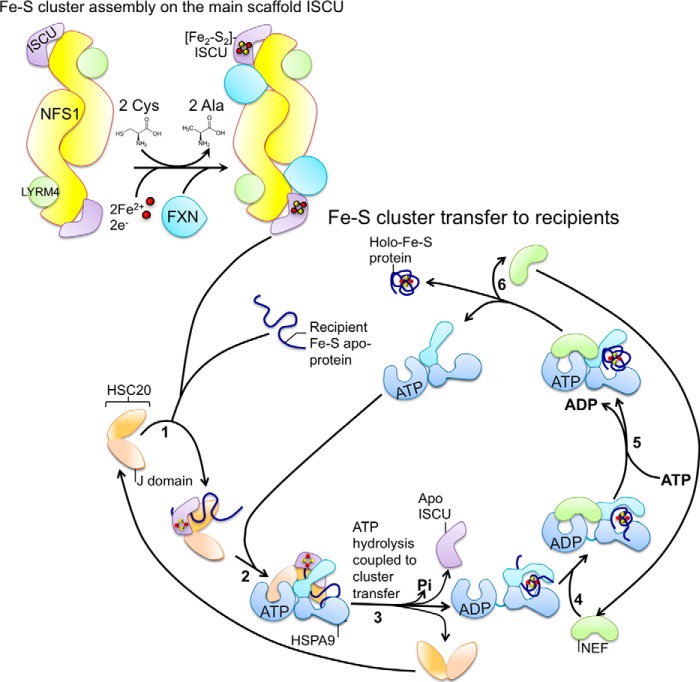
Figure 2.
Model for how highly conserved basic Fe-S biogenesis machinery generates and transfers Fe-S clusters. Fe-S cluster assembly on the main scaffold protein ISCU: nascent Fe-S clusters are initially assembled on the main scaffold protein ISCU. A cysteine desulfurase NFS1 forms a dimer to which monomers of the primary scaffold ISCU are proposed to bind at either end, based on the solved structure of the bacterial machinery. LYRM4 is a structural component of the core complex in eukaryotes, and it is required for the activity of NFS1, which, aided by its cofactor pyridoxal phosphate, provides sulfur, removed from cysteine, for the nascent cluster. Frataxin is part of the core complex, potentially binding in a pocket-like region between NFS1 and ISCU. The cluster assembles upon ISCU when iron is provided together with the reducing equivalents needed to generate the final electronic configuration of the cluster. Fe-S cluster transfer to recipients: cluster transfer from ISCU to recipient apoproteins is assisted by a dedicated chaperone/co-chaperone (HSPA9/HSC20) system that facilitates cluster release from the primary scaffold ISCU and transfer to recipient apoproteins or to intermediate carriers, which then target specific recipients. The co-chaperone HSC20 starts the functional cycle of the cognate system by associating with the scaffold protein ISCU, which is loaded with an Fe-S cluster, and with a recipient Fe-S apoprotein (step 1). Step 2, ISCU binds to the HSP70 chaperone (HSPA9 in mammalian cells) in a two-step process, which involves the transient interaction of the J-domain of HSC20 with the NBD of the ATP-bound state of HSPA9, and the interaction of ISCU with the SBD. ATP-bound HSPA9 is in the open conformation, which exhibits the substrate-binding cavity to allow the interaction with ISCU. Step 3, simultaneous association of ISCU and the interaction of the NBD of HSPA9 with the J-domain of HSC20 lowers the activation energy for the hydrolysis of ATP. Hydrolysis of ATP and the coupled conformational change in the SBD of HSPA9 is proposed to facilitate cluster release from ISCU and transfer to the recipient protein. Step 4, a nucleotide exchange factor (NEF), which exhibits high affinity for the ADP-bound state of the HSP70 chaperone, binds to the HSP70-client complex and exchanges ADP with ATP in the NBD (step 5). The client, which has folded into the native conformation driven by the energy provided by hydrolysis of ATP and has acquired its Fe-S cluster, is finally released (step 6).
As the genes encoding components of the Fe-S biogenesis pathway were identified in the 1990s, it was also possible to search for their orthologs in the partially sequenced human genome. The human ortholog of the sulfur-mobilizing enzyme, now known as NFS1, was found to have a conserved function in mammalian cells (43) and to form a complex with the human ortholog of the scaffold protein NifU, named ISCU, in both the mitochondrial matrix and in cytosolic/nuclear fractions (44). In addition, the eukaryotic cysteine desulfurase, Nfs1 in yeast, was discovered to depend on an obligate binding partner, Isd11, for function (45, 46), and a single ISD11 ortholog, also known as LYRM4, was identified in the human genome (47). Based on sequence homology with bacterial and yeast ferredoxins, two genes, FDX1 and FDX1L, were identified in human, but only one, FDX1L, was initially thought to be dedicated to Fe-S biogenesis (48). A subsequent study contrasted with this conclusion and found that the two human ferredoxins have redundant functions and are both involved in Fe-S cluster biogenesis (49). A single ferredoxin reductase, FDXR (49), and a single homolog of the cochaperone HSCB, now known as HSC20, were identified in the human genome (50), whereas the search for the ortholog of the bacterial chaperone HscA, essential for Fe-S biogenesis, was complicated by the presence of several closely related heat shock 70 proteins (HSP70s) in the human genome. Recently, the mitochondrial HSP70 protein, HSPA9, was found to be the cognate chaperone of HSC20 involved in Fe-S biogenesis (50). Thus, mammalian cells contain the framework needed for basic biogenesis of Fe-S cofactors.
Importantly, the core ISC components have been identified in the mitochondria, cytosol, and nucleus of mammalian cells (43, 47, 50–53), suggesting that Fe-S cluster biogenesis may have evolved in multiple subcellular compartments of eukaryotes. Particularly, a single NFS1 gene identified in the human genome generates two distinct isoforms through alternative utilization of in-frame AUGs (43). The mitochondrial isoform is generated by initiation at the first AUG of the NFS1 transcript and contains a mitochondrial targeting sequence at the N terminus, which undergoes cleavage to yield a mature mitochondrial protein of 47 kDa in size. An alternative isoform is generated by initiation of translation at the second in-frame AUG, lacks the first 60 residues of the mitochondrial precursor form, and resides in both the cytosol and nucleus. Cytosolic NFS1 (c-NFS1) was proved to be an active cysteine desulfurase, which was able to abstract sulfur from cysteine, and form a complex with the cytosolic isoform of the main scaffold protein ISCU1 (54). c-NFS1 is also efficiently involved in sulfur mobilization for molybdenum cofactor biosynthesis (55), and cytosolic/nuclear isoforms of NFS1 were found to be required for cell viability and for the post-transcriptional modification of tRNAs (56, 57). Previous studies have also shown that alternative splicing of the ISCU transcript gives rise to mitochondrial and cytosolic isoforms of the main scaffold protein (ISCU2 and ISCU1, respectively) in mammalian cells (44), and subsequent functional analyses revealed the importance of ISCU1 for maintenance/repair of the [Fe4-S4] cluster of cytosolic aconitase (52). The cochaperone HSC20 is the sole human DnaJ type III protein dedicated to Fe-S cluster transfer to recipients (50). Mutations in HSC20 and in its orthologs cause defects in Fe-S protein activities, mitochondrial iron accumulation, and impaired mitochondrial respiration in human cell lines (50) and in multiple experimental systems, including yeast (58) and fly (59). Knock-out of HSC20 in mammalian cells prevented assembly of the mitochondrial respiratory chain, due to the crucial role of the cochaperone in the biogenesis of the Fe-S cluster containing complexes (53, 60, 61). HSC20 is an essential Fe-S biogenesis factor and it has also been found in the cytosol of mammalian cells (50, 53). These findings differed from studies of the yeast model system, Saccharomyces cerevisiae, in which it was asserted that de novo Fe-S cluster biogenesis occurred exclusively in the mitochondrial matrix, whereas the cytoplasmic Fe-S assembly machinery depended on the export of a sulfur-containing compound (X-S), perhaps through the ABC transporter Atm1, for incorporation into cytosolic and nuclear Fe-S proteins (62). Studies in Arabidopsis thaliana led to the identification of a physiological substrate of the ABC transporter ATM3, which is the functional ortholog of yeast Atm1 and mammalian ABCB7. ATM3 was found to selectively transport oxidized glutathione and glutathione polysulfide, likely from the mitochondrial matrix into the cytosol (63).
After several decades of studies, a general model emerged in which biogenesis of Fe-S clusters depended on formation of a multimeric complex between the cysteine desulfurase NFS1, its binding partner ISD11, the scaffold protein ISCU1, and ferredoxins (see Fig. 2). In addition, frataxin, the human ortholog of yeast Yfh1 and bacterial CyaY, was identified as an essential component of the Fe-S biogenesis pathway through studies of the human neurodegenerative disease Friedreich ataxia (64). Several experimental evidences led to the proposal that frataxin functions as an allosteric effector of the cysteine desulfurase, by aiding conformational changes that enable NFS1 to transfer inorganic sulfur to the nascent cluster on the main scaffold ISCU (65). Subsequent to cluster assembly on ISCU, a chaperone/cochaperone system, consisting of HSPA9/HSC20 in mammalian cells, physically interacts with ISCU and facilitates cluster release from the scaffold for incorporation into recipient proteins (see Fig. 2) (53, 66). Although several structures of the bacterial biogenesis complexes have been published, the structure of the mammalian assembly complex may differ significantly (99). Two of the most challenging matters in obtaining crystals of the biogenesis complexes relate to the transient nature of the interaction of several components of the pathway, such as frataxin and the ferredoxins (67), and the complication that arises from the presence of numerous accessory proteins such as the acyl carrier protein (68), along with monothiol glutaredoxins and members of the putative “A-type” scaffold family, which are thought to function as intermediate carriers (69–71). Importantly, the exact roles of the proposed scaffold proteins NFU1 and BolA3 remain to be elucidated (51, 72–74).
How are Fe-S clusters delivered to correct recipient proteins?
Although much has been learned about the biogenesis of mammalian Fe-S proteins, one of the main unanswered questions in the field regarded how nascent Fe-S clusters were specifically targeted to the correct recipients, and not to proteins, such as those containing cysteine-rich zinc finger motifs, which could potentially ligate an Fe-S cluster but specifically bind to zinc. In addition, some Fe-S clusters are deeply buried within proteins, suggesting that they must be inserted during folding, and in some cases, they may contribute to the tertiary structure of the protein, by drawing distal cysteine residues into proximity (75, 76).
The cochaperone HSC20 was considered a possible crucial intermediary between the core biogenesis machinery and recipient Fe-S proteins for its ability to bridge synthesis of nascent clusters to the transfer to selected targets. To evaluate whether HSC20 had binding partners other than HSPA9 and ISCU, a yeast two-hybrid screening was performed between HSC20 and potential binding partners of a human library. Excitingly, the known iron-sulfur protein SDHB was identified in multiple clones of the library as an HSC20 interacting partner (53). Extensive deletional and mutational analyses of the sequences of SDHB that were able to bind HSC20 led to the identification of a motif in SDHB that represented the main molecular determinant of the interaction with HSC20. The motif consisted of a tripeptide referred to as the LYR motif, because leucine (L) often occupies the first position, tyrosine (Y) occupies the second position, and arginine (R) occupies the third position. Two such motifs were present in SDHB, one located near the N terminus, and the second closer to the C terminus (see Fig. 3). Multiple sequence alignments of the SDHB primary sequence from bacteria to human revealed that the LYR motifs were highly conserved. The basic features of the motif were the presence of leucine, isoleucine, or valine in the first position, tyrosine or phenylalanine in the second position, and lysine or arginine in the third position.
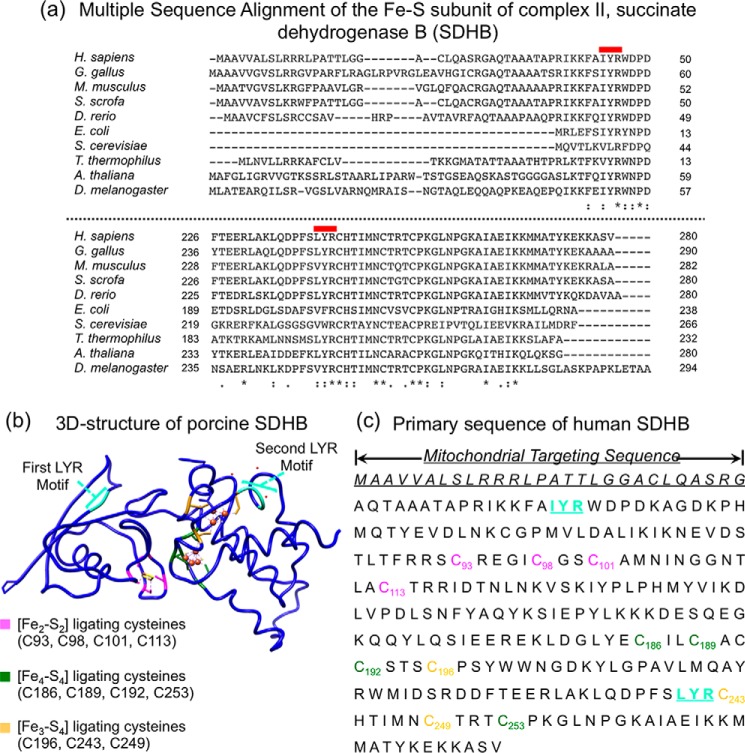
Figure 3.
SDHB contains two highly conserved LYR motifs that are essential for Fe-S cluster incorporation. a, multiple sequence alignment of the Fe-S subunit of complex II, SDHB. The two LYR motifs that engage the Fe-S transfer complex are highly conserved in human, yeast, plants, and bacteria. b, ribbon representation of the three-dimensional structure of porcine SDHB (Protein Data Bank code 3SFD, 96% identical to human) and primary sequence of human SDHB (c). The two LYR motifs are shown in cyan. The cysteine residues that coordinate the [Fe2-S2] cluster are in magenta, and the ligands of [Fe4-S4] and of [Fe3-S4] clusters are shown in green and yellow, respectively.
SDHB, the Fe-S subunit of mitochondrial complex II, contains three Fe-S clusters of different nuclearities, a [Fe2-S2] cluster, a [Fe4-S4] cluster, and a [Fe3-S4] cluster. Upon inspection of the structure of SDHB, it appeared that the clusters were deeply buried in the mature protein and that they could not be inserted after folding, but rather they underwent ligation to the cysteine residues during folding of the primary peptide into the final functional conformation of SDHB.
Biogenesis of complex II was known to depend on an accessory factor, SDHAF1, also known as LYRM8, for proper maturation. However, the molecular role of this assembly factor had remained unknown. Biochemical and functional studies revealed that the LYR motif of SDHAF1 was essential for Fe-S cluster incorporation into SDHB (60), by directly engaging an Fe-S transfer complex formed of HSC20/HSPA9/holo-ISCU. Importantly, SDHB mutations lead to paraganglioma and renal cancer, and many of these pathogenic mutations were found to alter either the LYR motifs or the known cysteinyl ligands of the Fe-S clusters (77).
The Fe-S cluster of the Rieske protein, UQCRFS1, is essential for acquisition of mitochondrial complex III (CIII) activity, although the mechanism for Fe-S cluster transfer had not previously been elucidated. LYRM7, a known accessory protein involved in biogenesis of mitochondrial complex III, was identified as an HSC20 interacting partner (53). Biochemical approaches were undertaken to dissect the last steps of CIII assembly and led to the molecular characterization of a novel complex III assembly intermediate, composed of LYRM7 bound to UQCRFS1 and to a component of the Fe-S cluster biogenesis machinery, consisting of HSC20, its cognate chaperone HSPA9, and the holo-scaffold ISCU. Direct binding of HSC20 to the LYR motif of LYRM7 in a pre-assembled UQCRFS1-LYRM7 intermediate in the mitochondrial matrix was found to facilitate transfer of an Fe-S cluster from holo-ISCU to UQCRFS1 (61). It would be interesting to investigate whether the S. cerevisiae orthologs of LYRM7 and UQCRFS1 (Mzm1 and Rip1, respectively), which were also found to form a complex (78), interact with the Fe-S transfer complex, which in yeast consists of the cochaperone Jac1, the specialized chaperone Ssq1, and the scaffold protein Isu. Although the Fe-S biogenesis machinery is highly conserved from bacteria to human, there are several differences between the chaperone/cochaperone system of most organisms compared with S. cerevisiae. Most eukaryotes utilize a single multifunctional mitochondrial chaperone or Hsp70 protein, whereas S. cerevisiae and a subset of fungi also express a highly specialized Hsp70 involved in Fe-S cluster biogenesis, named Ssq1 (79). In addition, the cochaperone Jac1 dedicated to Fe-S cluster biogenesis in yeast strains that encode Ssq1 has a truncated N terminus (79) compared with human HSC20, in which an extended N-terminal domain contains two CXXC modules (Cys-41/Cys-44 and Cys-58/Cys-61) that coordinate a zinc ion in vitro (80). The physiological relevance of the zinc finger domain of HSC20 remains to be elucidated, although it may facilitate dimerization of the co-chaperone.
The biogenesis of the mitochondrial respiratory chain is a modular process in which intricate membrane-bound complexes are ultimately formed, aided by multiple accessory factors that coordinate the synthesis and sequential interactions of individual subunits encoded by both the nuclear and mitochondrial genomes. Numerous assembly intermediates have been previously identified (see Fig. 4) (81–84), and recent studies suggest that HSC20 is intimately involved in complex I formation through its role in Fe-S delivery to nascent subunits, where proteomics and biochemical interactions revealed that all five Fe-S cluster subunits of mitochondrial complex I were identified as HSC20 interacting partners (61).
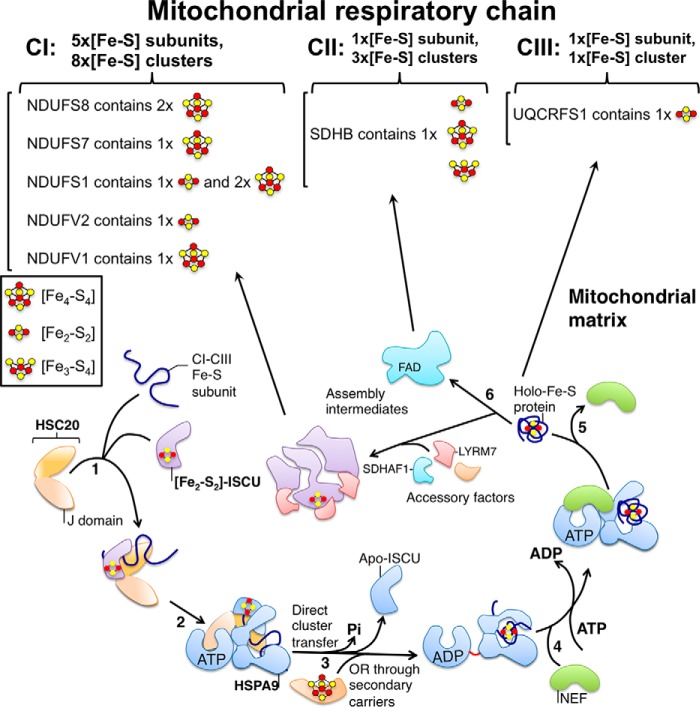
Figure 4.
Single adaptable cochaperone scaffold complex delivers nascent Fe-S clusters to mammalian respiratory chain complexes I–III. HSC20 binds the main scaffold protein ISCU and a recipient Fe-S cluster-ligating subunit of complexes I–III of the respiratory chain (step 1). The transient interaction of the J-domain of HSC20 with the nucleotide-binding domain of HSPA9 activates the ATPase activity of the chaperone (step 2). The hydrolysis of ATP is coupled to a conformational change in HSPA9, which is proposed to facilitate cluster release from ISCU and transfer to the recipient protein (step 3). Secondary carriers such as ISCA1/2, NFU1, may also mediate or facilitate transfer of Fe-S clusters from ISCU to Fe-S clients (step 3). NEF, which has high affinity for the ADP-bound state of HSPA9, exchanges ADP with ATP (step 4). The Fe-S protein, which has folded into the native conformation driven by the energy provided by hydrolysis of ATP, and has acquired its Fe-S cluster, is released from the transfer complex (steps 5 and 6). As the biogenesis of the mitochondrial OXPHOS system is a modular and complex process, the newly synthesized Fe-S subunits of complexes I–III likely interact with accessory factors, which coordinate the assembly of a large number of proteins into assembly intermediates, prior to their integration into functional respiratory complexes.
The question of how Fe-S clusters are incorporated into complex I was previously addressed in studies of yeast Yarrowia lipolitica, in which the evolutionarily conserved Fe-S protein Ind1 was proposed to facilitate insertion of Fe-S cofactors into subunits of complex I (85). The human ortholog, NUBPL, was assigned a similar role (86). However, in-depth characterization of the Ind1 ortholog in Arabidopsis thaliana, INDH, demonstrated that INDH was a mitochondrial translation factor required for the expression of multiple complex I subunits, and compromise of INDH function did not specifically inhibit Fe-S delivery (87). Consistent with a role of Ind1 in mitochondrial translation is the fact that respiratory complexes III and IV were also mildly affected in NUBPL-depleted cells (88).
Fe-S clusters are transferred from the scaffold protein ISCU to recipient proteins as outlined in Fig. 4. As has already been discussed, the core Fe-S components of the biogenesis machinery are also present in the mammalian cytosol. It has become increasingly important to understand biogenesis of Fe-S clusters for cytosolic and nuclear Fe-S proteins because of their critical roles in DNA metabolism (89–92). The exact role of the Fe-S clusters in DNA metabolism proteins has been long debated. Interestingly, recent studies suggest that the redox state of Fe-S repair enzymes is crucial for their ability to find and repair DNA lesions (93). Prior to the most recent exciting investigations, it was postulated that the main role of the Fe-S clusters in DNA metabolism enzymes was to provide structural stability (94). Because most Fe-S proteins readily undergo single electron oxidation/reductions, activity of Fe-S proteins may be tethered to the general reducing power of the cell, and oxidation of Fe-S proteins may diminish their repair and growth functions when cells are depleted of reducing power.
Notably, Fe-S clusters are crucial for many RNA metabolism proteins such as ABCE1, which is a crucial factor in RNA translation (95, 96), opening the possibility that other RNA metabolism-associated proteins may be unrecognized iron-sulfur proteins.
Thus, it is possible that hundreds of Fe-S proteins are present in mammalian cells, waiting to be identified (97). If Fe-S proteins prove to be fairly abundant, it would affect the calculations about cysteines, disulfide bonds, and redox status, as many cysteines would be engaged in ligating Fe-S cofactors (98). Bioinformatics approaches can foster identification of candidate Fe-S proteins, in combination with sophisticated biochemical techniques that enable researchers to study these labile prosthetic groups under protected conditions (75).
This work was supported by the Eunice Kennedy Shriver NICHD Intramural Research Program. This is the third article in the Thematic Minireview series “Metals in Biology 2017: Iron transport, storage, and the ramifications.”The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- Ft
- ferritin
- SDHB
- succinate dehydrogenase B
- IRE
- iron-response element
- IRP
- iron regulatory protein
- NBD
- nucleotide-binding domain
- SBD
- substrate-binding domain
- NEF
- nucleotide exchange factor.
References
- 1. Zahringer J., Baliga B. S., and Munro H. N. (1979) Relative abundance of specific messenger-RNA species in the free mRNP fraction of rat liver. FEBS Lett. 108, 317–320 [DOI] [PubMed] [Google Scholar]
- 2. Leibold E. A., Aziz N., Brown A. J., and Munro H. N. (1984) Conservation in rat liver of light and heavy subunit sequences of mammalian ferritin. Presence of unique octopeptide in the light subunit. J. Biol. Chem. 259, 4327–4334 [PubMed] [Google Scholar]
- 3. Hentze M. W., Caughman S. W., Rouault T. A., Barriocanal J. G., Dancis A., Harford J. B., and Klausner R. D. (1987) Identification of the iron-responsive element for the translational regulation of human ferritin mRNA. Science 238, 1570–1573 [DOI] [PubMed] [Google Scholar]
- 4. Leibold E. A., and Munro H. N. (1988) Cytoplasmic protein binds in vitro to a highly conserved sequence in the 5′ untranslated region of ferritin heavy- and light-subunit mRNAs. Proc. Natl. Acad. Sci. U.S.A. 85, 2171–2175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rouault T. A., Tang C. K., Kaptain S., Burgess W. H., Haile D. J., Samaniego F., McBride O. W., Harford J. B., and Klausner R. D. (1990) Cloning of the cDNA encoding an RNA regulatory protein–the human iron-responsive element-binding protein. Proc. Natl. Acad. Sci. U.S.A. 87, 7958–7962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kennedy M. C., Emptage M. H., Dreyer J. L., and Beinert H. (1983) The role of iron in the activation-inactivation of aconitase. J. Biol. Chem. 258, 11098–11105 [PubMed] [Google Scholar]
- 7. Tong W. H., and Rouault T. A. (2007) Metabolic regulation of citrate and iron by aconitases: role of iron-sulfur cluster biogenesis. Biometals 20, 549–564 [DOI] [PubMed] [Google Scholar]
- 8. Kaptain S., Downey W. E., Tang C., Philpott C., Haile D., Orloff D. G., Harford J. B., Rouault T. A., and Klausner R. D. (1991) A regulated RNA binding protein also possesses aconitase activity. Proc. Natl. Acad. Sci. U.S.A. 88, 10109–10113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kennedy M. C., Mende-Mueller L., Blondin G. A., and Beinert H. (1992) Purification and characterization of cytosolic aconitase from beef liver and its relationship to the iron-responsive element binding protein. Proc. Natl. Acad. Sci. U.S.A. 89, 11730–11734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Haile D. J., Rouault T. A., Harford J. B., Kennedy M. C., Blondin G. A., Beinert H., and Klausner R. D. (1992) Cellular regulation of the iron-responsive element binding protein: disassembly of the cubane iron-sulfur cluster results in high-affinity RNA binding. Proc. Natl. Acad. Sci. U.S.A. 89, 11735–11739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dandekar T., Stripecke R., Gray N. K., Goossen B., Constable A., Johansson H. E., and Hentze M. W. (1991) Identification of a novel iron-responsive element in murine and human erythroid δ-aminolevulinic acid synthase mRNA. EMBO J. 10, 1903–1909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim H. Y., LaVaute T., Iwai K., Klausner R. D., and Rouault T. A. (1996) Identification of a conserved and functional iron-responsive element in the 5′-untranslated region of mammalian mitochondrial aconitase. J. Biol. Chem. 271, 24226–24230 [DOI] [PubMed] [Google Scholar]
- 13. Sanchez M., Galy B., Muckenthaler M. U., and Hentze M. W. (2007) Iron-regulatory proteins limit hypoxia-inducible factor-2α expression in iron deficiency. Nat. Struct. Mol. Biol. 14, 420–426 [DOI] [PubMed] [Google Scholar]
- 14. Abboud S., and Haile D. J. (2000) A novel mammalian iron-regulated protein involved in intracellular iron metabolism. J. Biol. Chem. 275, 19906–19912 [DOI] [PubMed] [Google Scholar]
- 15. Binder R., Horowitz J. A., Basilion J. P., Koeller D. M., Klausner R. D., and Harford J. B. (1994) Evidence that the pathway of transferrin receptor mRNA degradation involves an endonucleolytic cleavage within the 3′ UTR and does not involve poly(A) tail shortening. EMBO J. 13, 1969–1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gunshin H., Allerson C. R., Polycarpou-Schwarz M., Rofts A., Rogers J. T., Kishi F., Hentze M. W., Rouault T. A., Andrews N. C., and Hediger M. A. (2001) Iron-dependent regulation of the divalent metal ion transporter. FEBS Lett. 509, 309–316 [DOI] [PubMed] [Google Scholar]
- 17. Rogers J. T., Randall J. D., Cahill C. M., Eder P. S., Huang X., Gunshin H., Leiter L., McPhee J., Sarang S. S., Utsuki T., Greig N. H., Lahiri D. K., Tanzi R. E., Bush A. I., Giordano T., and Gullans S. R. (2002) An iron-responsive element type II in the 5′-untranslated region of the Alzheimer's amyloid precursor protein transcript. J. Biol. Chem. 277, 45518–45528 [DOI] [PubMed] [Google Scholar]
- 18. Sanchez M., Galy B., Dandekar T., Bengert P., Vainshtein Y., Stolte J., Muckenthaler M. U., and Hentze M. W. (2006) Iron regulation and the cell cycle: identification of an iron-responsive element in the 3′-untranslated region of human cell division cycle 14A mRNA by a refined microarray-based screening strategy. J. Biol. Chem. 281, 22865–22874 [DOI] [PubMed] [Google Scholar]
- 19. Castello A., Horos R., Strein C., Fischer B., Eichelbaum K., Steinmetz L. M., Krijgsveld J., and Hentze M. W. (2013) System-wide identification of RNA-binding proteins by interactome capture. Nat. Protoc. 8, 491–500 [DOI] [PubMed] [Google Scholar]
- 20. Dupuy J., Volbeda A., Carpentier P., Darnault C., Moulis J. M., and Fontecilla-Camps J. C. (2006) Crystal structure of human iron regulatory protein 1 as cytosolic aconitase. Structure 14, 129–139 [DOI] [PubMed] [Google Scholar]
- 21. Walden W. E., Selezneva A. I., Dupuy J., Volbeda A., Fontecilla-Camps J. C., Theil E. C., and Volz K. (2006) Structure of dual function iron regulatory protein 1 complexed with ferritin IRE-RNA. Science 314, 1903–1908 [DOI] [PubMed] [Google Scholar]
- 22. Samaniego F., Chin J., Iwai K., Rouault T. A., and Klausner R. D. (1994) Molecular characterization of a second iron-responsive element binding protein, iron regulatory protein 2. Structure, function, and post-translational regulation. J. Biol. Chem. 269, 30904–30910 [PubMed] [Google Scholar]
- 23. Guo B., Yu Y., and Leibold E. A. (1994) Iron regulates cytoplasmic levels of a novel iron-responsive element-binding protein without aconitase activity. J. Biol. Chem. 269, 24252–24260 [PubMed] [Google Scholar]
- 24. Vashisht A. A., Zumbrennen K. B., Huang X., Powers D. N., Durazo A., Sun D., Bhaskaran N., Persson A., Uhlen M., Sangfelt O., Spruck C., Leibold E. A., and Wohlschlegel J. A. (2009) Control of iron homeostasis by an iron-regulated ubiquitin ligase. Science 326, 718–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Salahudeen A. A., Thompson J. W., Ruiz J. C., Ma H. W., Kinch L. N., Li Q., Grishin N. V., and Bruick R. K. (2009) An E3 ligase possessing an iron-responsive hemerythrin domain is a regulator of iron homeostasis. Science 326, 722–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rouault T. A. (2006) The role of iron regulatory proteins in mammalian iron homeostasis and disease. Nat. Chem. Biol. 2, 406–414 [DOI] [PubMed] [Google Scholar]
- 27. Meyron-Holtz E. G., Ghosh M. C., Iwai K., LaVaute T., Brazzolotto X., Berger U. V., Land W., Ollivierre-Wilson H., Grinberg A., Love P., and Rouault T. A. (2004) Genetic ablations of iron regulatory proteins 1 and 2 reveal why iron regulatory protein 2 dominates iron homeostasis. EMBO J. 23, 386–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Meyron-Holtz E. G., Ghosh M. C., and Rouault T. A. (2004) Mammalian tissue oxygen levels modulate iron-regulatory protein activities in vivo. Science 306, 2087–2090 [DOI] [PubMed] [Google Scholar]
- 29. Smith S. R., Ghosh M. C., Ollivierre-Wilson H., Hang Tong W., and Rouault T. A. (2006) Complete loss of iron regulatory proteins 1 and 2 prevents viability of murine zygotes beyond the blastocyst stage of embryonic development. Blood Cells Mol. Dis. 36, 283–287 [DOI] [PubMed] [Google Scholar]
- 30. Zumbrennen-Bullough K. B., Becker L., Garrett L., Hölter S. M., Calzada-Wack J., Mossbrugger I., Quintanilla-Fend L., Racz I., Rathkolb B., Klopstock T., Wurst W., Zimmer A., Wolf E., Fuchs H., Gailus-Durner V., et al. (2014) Abnormal brain iron metabolism in Irp2 deficient mice is associated with mild neurological and behavioral impairments. PLoS ONE 9, e98072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. LaVaute T., Smith S., Cooperman S., Iwai K., Land W., Meyron-Holtz E., Drake S. K., Miller G., Abu-Asab M., Tsokos M., Switzer R. 3rd., Grinberg A., Love P., Tresser N., and Rouault T. A. (2001) Targeted deletion of the gene encoding iron regulatory protein-2 causes misregulation of iron metabolism and neurodegenerative disease in mice. Nat. Genet. 27, 209–214 [DOI] [PubMed] [Google Scholar]
- 32. Cooperman S. S., Meyron-Holtz E. G., Olivierre-Wilson H., Ghosh M. C., McConnell J. P., and Rouault T. A. (2005) Microcytic anemia, erythropoietic protoporphyria, and neurodegeneration in mice with targeted deletion of iron-regulatory protein 2. Blood 106, 1084–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jeong S. Y., Crooks D. R., Wilson-Ollivierre H., Ghosh M. C., Sougrat R., Lee J., Cooperman S., Mitchell J. B., Beaumont C., and Rouault T. A. (2011) Iron insufficiency compromises motor neurons and their mitochondrial function in Irp2-null mice. PLoS ONE 6, e25404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Galy B., Ferring D., Minana B., Bell O., Janser H. G., Muckenthaler M., Schümann K., and Hentze M. W. (2005) Altered body iron distribution and microcytosis in mice deficient in iron regulatory protein 2 (IRP2). Blood 106, 2580–2589 [DOI] [PubMed] [Google Scholar]
- 35. Ghosh M. C., Zhang D. L., Jeong S. Y., Kovtunovych G., Ollivierre-Wilson H., Noguchi A., Tu T., Senecal T., Robinson G., Crooks D. R., Tong W. H., Ramaswamy K., Singh A., Graham B. B., Tuder R. M., et al. (2013) Deletion of iron regulatory protein 1 causes polycythemia and pulmonary hypertension in mice through translational derepression of HIF2alpha. Cell Metab. 17, 271–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Anderson S. A., Nizzi C. P., Chang Y. I., Deck K. M., Schmidt P. J., Galy B., Damnernsawad A., Broman A. T., Kendziorski C., Hentze M. W., Fleming M. D., Zhang J., and Eisenstein R. S. (2013) The IRP1-HIF-2α axis coordinates iron and oxygen sensing with erythropoiesis and iron absorption. Cell Metab. 17, 282–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Venkateswara Rao P., and Holm R. H. (2004) Synthetic analogues of the active sites of iron-sulfur proteins. Chem. Rev. 104, 527–559 [DOI] [PubMed] [Google Scholar]
- 38. Jacobson M. R., Brigle K. E., Bennett L. T., Setterquist R. A., Wilson M. S., Cash V. L., Beynon J., Newton W. E., and Dean D. R. (1989) Physical and genetic map of the major nif gene cluster from Azotobacter vinelandii. J. Bacteriol. 171, 1017–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Selbach B. P., Pradhan P. K., and Dos Santos P. C. (2013) Protected sulfur transfer reactions by the Escherichia coli Suf system. Biochemistry 52, 4089–4096 [DOI] [PubMed] [Google Scholar]
- 40. Barras F., Loiseau L., and Py B. (2005) How Escherichia coli and Saccharomyces cerevisiae build Fe/S proteins. Adv. Microb. Physiol. 50, 41–101 [DOI] [PubMed] [Google Scholar]
- 41. Xu X. M., Adams S., Chua N. H., and Møller S. G. (2005) AtNAP1 represents an atypical SufB protein in Arabidopsis plastids. J. Biol. Chem. 280, 6648–6654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zheng L., White R. H., Cash V. L., Jack R. F., and Dean D. R. (1993) Cysteine desulfurase activity indicates a role for NIFS in metallocluster biosynthesis. Proc. Natl. Acad. Sci. U.S.A. 90, 2754–2758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Land T., and Rouault T. A. (1998) Targeting of a human iron-sulfur cluster assembly enzyme, nifs, to different subcellular compartments is regulated through alternative AUG utilization. Mol. Cell 2, 807–815 [DOI] [PubMed] [Google Scholar]
- 44. Tong W. H., and Rouault T. (2000) Distinct iron-sulfur cluster assembly complexes exist in the cytosol and mitochondria of human cells. EMBO J. 19, 5692–5700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Adam A. C., Bornhövd C., Prokisch H., Neupert W., and Hell K. (2006) The Nfs1 interacting protein Isd11 has an essential role in Fe/S cluster biogenesis in mitochondria. EMBO J. 25, 174–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wiedemann N., Urzica E., Guiard B., Müller H., Lohaus C., Meyer H. E., Ryan M. T., Meisinger C., Mühlenhoff U., Lill R., and Pfanner N. (2006) Essential role of Isd11 in mitochondrial iron-sulfur cluster synthesis on Isu scaffold proteins. EMBO J. 25, 184–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shi Y., Ghosh M. C., Tong W. H., and Rouault T. A. (2009) Human ISD11 is essential for both iron-sulfur cluster assembly and maintenance of normal cellular iron homeostasis. Hum. Mol. Genet. 18, 3014–3025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sheftel A. D., Stehling O., Pierik A. J., Elsässer H. P., Mühlenhoff U., Webert H., Hobler A., Hannemann F., Bernhardt R., and Lill R. (2010) Humans possess two mitochondrial ferredoxins, Fdx1 and Fdx2, with distinct roles in steroidogenesis, heme, and Fe/S cluster biosynthesis. Proc. Natl. Acad. Sci. U.S.A. 107, 11775–11780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shi Y., Ghosh M., Kovtunovych G., Crooks D. R., and Rouault T. A. (2012) Both human ferredoxins 1 and 2 and ferredoxin reductase are important for iron-sulfur cluster biogenesis. Biochim. Biophys. Acta 1823, 484–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Uhrigshardt H., Singh A., Kovtunovych G., Ghosh M., and Rouault T. A. (2010) Characterization of the human HSC20, an unusual DnaJ type III protein, involved in iron-sulfur cluster biogenesis. Hum. Mol. Genet. 19, 3816–3834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tong W. H., Jameson G. N., Huynh B. H., and Rouault T. A. (2003) Subcellular compartmentalization of human Nfu, an iron-sulfur cluster scaffold protein, and its ability to assemble a [4Fe-4S] cluster. Proc. Natl. Acad. Sci. U.S.A. 100, 9762–9767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tong W. H., and Rouault T. A. (2006) Functions of mitochondrial ISCU and cytosolic ISCU in mammalian iron-sulfur cluster biogenesis and iron homeostasis. Cell Metab. 3, 199–210 [DOI] [PubMed] [Google Scholar]
- 53. Maio N., Singh A., Uhrigshardt H., Saxena N., Tong W. H., and Rouault T. A. (2014) Cochaperone binding to LYR motifs confers specificity of iron-sulfur cluster delivery. Cell Metab. 19, 445–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Li K., Tong W. H., Hughes R. M., and Rouault T. A. (2006) Roles of the mammalian cytosolic cysteine desulfurase, ISCS, and scaffold protein, ISCU, in iron-sulfur cluster assembly. J. Biol. Chem. 281, 12344–12351 [DOI] [PubMed] [Google Scholar]
- 55. Marelja Z., Mullick Chowdhury M., Dosche C., Hille C., Baumann O., Löhmannsröben H. G., and Leimkühler S. (2013) The l-cysteine desulfurase NFS1 is localized in the cytosol where it provides the sulfur for molybdenum cofactor biosynthesis in humans. PLoS ONE 8, e60869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nakai Y., Umeda N., Suzuki T., Nakai M., Hayashi H., Watanabe K., and Kagamiyama H. (2004) Yeast Nfs1p is involved in thio-modification of both mitochondrial and cytoplasmic tRNAs. J. Biol. Chem. 279, 12363–12368 [DOI] [PubMed] [Google Scholar]
- 57. Mühlenhoff U., Balk J., Richhardt N., Kaiser J. T., Sipos K., Kispal G., and Lill R. (2004) Functional characterization of the eukaryotic cysteine desulfurase Nfs1p from Saccharomyces cerevisiae. J. Biol. Chem. 279, 36906–36915 [DOI] [PubMed] [Google Scholar]
- 58. Kim R., Saxena S., Gordon D. M., Pain D., and Dancis A. (2001) J-domain protein, Jac1p, of yeast mitochondria required for iron homeostasis and activity of Fe-S cluster proteins. J. Biol. Chem. 276, 17524–17532 [DOI] [PubMed] [Google Scholar]
- 59. Uhrigshardt H., Rouault T. A., and Missirlis F. (2013) Insertion mutants in Drosophila melanogaster Hsc20 halt larval growth and lead to reduced iron-sulfur cluster enzyme activities and impaired iron homeostasis. J. Biol. Inorg. Chem. 18, 441–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Maio N., Ghezzi D., Verrigni D., Rizza T., Bertini E., Martinelli D., Zeviani M., Singh A., Carrozzo R., and Rouault T. A. (2016) Disease-causing SDHAF1 mutations impair transfer of Fe-S clusters to SDHB. Cell Metab. 23, 292–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Maio N., Kim K. S., Singh A., and Rouault T. A. (2017) A single adaptable cochaperone-scaffold complex delivers nascent iron-sulfur clusters to mammalian respiratory chain complexes I–III. Cell Metab. 25, 945–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lill R., Srinivasan V., and Mühlenhoff U. (2014) The role of mitochondria in cytosolic-nuclear iron-sulfur protein biogenesis and in cellular iron regulation. Curr. Opin. Microbiol. 22, 111–119 [DOI] [PubMed] [Google Scholar]
- 63. Schaedler T. A., Thornton J. D., Kruse I., Schwarzländer M., Meyer A. J., van Veen H. W., and Balk J. (2014) A conserved mitochondrial ATP-binding cassette transporter exports glutathione polysulfide for cytosolic metal cofactor assembly. J. Biol. Chem. 289, 23264–23274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Schulz J. B., and Pandolfo M. (2013) 150 years of Friedreich ataxia: from its discovery to therapy. J. Neurochem. 126, 1–3 [DOI] [PubMed] [Google Scholar]
- 65. Bridwell-Rabb J., Fox N. G., Tsai C. L., Winn A. M., and Barondeau D. P. (2014) Human frataxin activates Fe-S cluster biosynthesis by facilitating sulfur transfer chemistry. Biochemistry 53, 4904–4913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Vickery L. E., and Cupp-Vickery J. R. (2007) Molecular chaperones HscA/Ssq1 and HscB/Jac1 and their roles in iron-sulfur protein maturation. Crit. Rev. Biochem. Mol. Biol. 42, 95–111 [DOI] [PubMed] [Google Scholar]
- 67. Kim J. H., Frederick R. O., Reinen N. M., Troupis A. T., and Markley J. L. (2013) [2Fe-2S]-ferredoxin binds directly to cysteine desulfurase and supplies an electron for iron-sulfur cluster assembly but is displaced by the scaffold protein or bacterial frataxin. J. Am. Chem. Soc. 135, 8117–8120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Van Vranken J. G., Jeong M. Y., Wei P., Chen Y. C., Gygi S. P., Winge D. R., and Rutter J. (2016) The mitochondrial acyl carrier protein (ACP) coordinates mitochondrial fatty acid synthesis with iron-sulfur cluster biogenesis. Elife 5, e17828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wingert R. A., Galloway J. L., Barut B., Foott H., Fraenkel P., Axe J. L., Weber G. J., Dooley K., Davidson A. J., Schmidt B., Paw B. H., Shaw G. C., Kingsley P., Palis J., Schubert H., et al. (2005) Deficiency of glutaredoxin 5 reveals Fe-S clusters are required for vertebrate haem synthesis. Nature 436, 1035–1039 [DOI] [PubMed] [Google Scholar]
- 70. Ye H., Jeong S. Y., Ghosh M. C., Kovtunovych G., Silvestri L., Ortillo D., Uchida N., Tisdale J., Camaschella C., and Rouault T. A. (2010) Glutaredoxin 5 deficiency causes sideroblastic anemia by specifically impairing heme biosynthesis and depleting cytosolic iron in human erythroblasts. J. Clin. Invest. 120, 1749–1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mapolelo D. T., Zhang B., Randeniya S., Albetel A. N., Li H., Couturier J., Outten C. E., Rouhier N., and Johnson M. K. (2013) Monothiol glutaredoxins and A-type proteins: partners in Fe-S cluster trafficking. Dalton Trans. 42, 3107–3115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Cameron J. M., Janer A., Levandovskiy V., Mackay N., Rouault T. A., Tong W. H., Ogilvie I., Shoubridge E. A., and Robinson B. H. (2011) Mutations in iron-sulfur cluster scaffold genes NFU1 and BOLA3 cause a fatal deficiency of multiple respiratory chain and 2-oxoacid dehydrogenase enzymes. Am. J. Hum. Genet. 89, 486–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Navarro-Sastre A., Tort F., Stehling O., Uzarska M. A., Arranz J. A., Del Toro M., Labayru M. T., Landa J., Font A., Garcia-Villoria J., Merinero B., Ugarte M., Gutierrez-Solana L. G., Campistol J., Garcia-Cazorla A., et al. (2011) A fatal mitochondrial disease is associated with defective NFU1 function in the maturation of a subset of mitochondrial Fe-S proteins. Am. J. Hum. Genet. 89, 656–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Melber A., Na U., Vashisht A., Weiler B. D., Lill R., Wohlschlegel J. A., and Winge D. R. (2016) Role of Nfu1 and Bol3 in iron-sulfur cluster transfer to mitochondrial clients. Elife 5, e15991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Maio N., and Rouault T. A. (2016) Mammalian Fe-S proteins: definition of a consensus motif recognized by the co-chaperone HSC20. Metallomics 8, 1032–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Rouault T. A. (2015) Mammalian iron-sulphur proteins: novel insights into biogenesis and function. Nat. Rev. Mol. Cell Biol. 16, 45–55 [DOI] [PubMed] [Google Scholar]
- 77. Saxena N., Maio N., Crooks D. R., Ricketts C. J., Yang Y., Wei M. H., Fan T. W., Lane A. N., Sourbier C., Singh A., Killian J. K., Meltzer P. S., Vocke C. D., Rouault T. A., and Linehan W. M. (2016) SDHB-deficient cancers: the role of mutations that impair iron-sulfur cluster delivery. J. Natl. Cancer Inst. 108, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Atkinson A., Smith P., Fox J. L., Cui T. Z., Khalimonchuk O., and Winge D. R. (2011) The LYR protein Mzm1 functions in the insertion of the Rieske Fe/S protein in yeast mitochondria. Mol. Cell. Biol. 31, 3988–3996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Pukszta S., Schilke B., Dutkiewicz R., Kominek J., Moczulska K., Stepien B., Reitenga K. G., Bujnicki J. M., Williams B., Craig E. A., and Marszalek J. (2010) Co-evolution-driven switch of J-protein specificity towards an Hsp70 partner. EMBO Rep. 11, 360–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Bitto E., Bingman C. A., Bittova L., Kondrashov D. A., Bannen R. M., Fox B. G., Markley J. L., and Phillips G. N. Jr. (2008) Structure of human J-type co-chaperone HscB reveals a tetracysteine metal-binding domain. J. Biol. Chem. 283, 30184–30192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Smith P. M., Fox J. L., and Winge D. R. (2012) Biogenesis of the cytochrome bc1 complex and role of assembly factors. Biochim. Biophys. Acta 1817, 276–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Stroud D. A., Surgenor E. E., Formosa L. E., Reljic B., Frazier A. E., Dibley M. G., Osellame L. D., Stait T., Beilharz T. H., Thorburn D. R., Salim A., and Ryan M. T. (2016) Accessory subunits are integral for assembly and function of human mitochondrial complex I. Nature 538, 123–126 [DOI] [PubMed] [Google Scholar]
- 83. Van Vranken J. G., Na U., Winge D. R., and Rutter J. (2015) Protein-mediated assembly of succinate dehydrogenase and its cofactors. Crit. Rev. Biochem. Mol. Biol. 50, 168–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Guerrero-Castillo S., Baertling F., Kownatzki D., Wessels H. J., Arnold S., Brandt U., and Nijtmans L. (2017) The assembly pathway of mitochondrial respiratory chain complex I. Cell Metab. 25, 128–139 [DOI] [PubMed] [Google Scholar]
- 85. Bych K., Kerscher S., Netz D. J., Pierik A. J., Zwicker K., Huynen M. A., Lill R., Brandt U., and Balk J. (2008) The iron-sulphur protein Ind1 is required for effective complex I assembly. EMBO J. 27, 1736–1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Sheftel A. D., Stehling O., Pierik A. J., Netz D. J., Kerscher S., Elsässer H. P., Wittig I., Balk J., Brandt U., and Lill R. (2009) Human ind1, an iron-sulfur cluster assembly factor for respiratory complex I. Mol. Cell. Biol. 29, 6059–6073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wydro M. M., Sharma P., Foster J. M., Bych K., Meyer E. H., and Balk J. (2013) The evolutionarily conserved iron-sulfur protein INDH is required for complex I assembly and mitochondrial translation in Arabidopsis [corrected]. Plant Cell 25, 4014–4027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Calvo S. E., Tucker E. J., Compton A. G., Kirby D. M., Crawford G., Burtt N. P., Rivas M., Guiducci C., Bruno D. L., Goldberger O. A., Redman M. C., Wiltshire E., Wilson C. J., Altshuler D., Gabriel S. B., et al. (2010) High-throughput, pooled sequencing identifies mutations in NUBPL and FOXRED1 in human complex I deficiency. Nat. Genet. 42, 851–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Gari K., León Ortiz A. M., Borel V., Flynn H., Skehel J. M., and Boulton S. J. (2012) MMS19 links cytoplasmic iron-sulfur cluster assembly to DNA metabolism. Science 337, 243–245 [DOI] [PubMed] [Google Scholar]
- 90. Stehling O., Vashisht A. A., Mascarenhas J., Jonsson Z. O., Sharma T., Netz D. J., Pierik A. J., Wohlschlegel J. A., and Lill R. (2012) MMS19 assembles iron-sulfur proteins required for DNA metabolism and genomic integrity. Science 337, 195–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Stehling O., Mascarenhas J., Vashisht A. A., Sheftel A. D., Niggemeyer B., Rösser R., Pierik A. J., Wohlschlegel J. A., and Lill R. (2013) Human CIA2A-FAM96A and CIA2B-FAM96B integrate iron homeostasis and maturation of different subsets of cytosolic-nuclear iron-sulfur proteins. Cell Metab. 18, 187–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Odermatt D. C., and Gari K. (2017) The CIA targeting complex is highly regulated and provides two distinct binding sites for client iron-sulfur proteins. Cell Rep. 18, 1434–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. O'Brien E., Holt M. E., Thompson M. K., Salay L. E., Ehlinger A. C., Chazin W. J., and Barton J. K. (2017) The [4Fe4S] cluster of human DNA primase functions as a redox switch using DNA charge transport. Science 355, eaag1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kuo C. F., McRee D. E., Fisher C. L., O'Handley S. F., Cunningham R. P., and Tainer J. A. (1992) Atomic structure of the DNA repair [4Fe-4S] enzyme endonuclease III. Science 258, 434–440 [DOI] [PubMed] [Google Scholar]
- 95. Barthelme D., Scheele U., Dinkelaker S., Janoschka A., Macmillan F., Albers S. V., Driessen A. J., Stagni M. S., Bill E., Meyer-Klaucke W., Schünemann V., and Tampé R. (2007) Structural organization of essential iron-sulfur clusters in the evolutionarily highly conserved ATP-binding cassette protein ABCE1. J. Biol. Chem. 282, 14598–14607 [DOI] [PubMed] [Google Scholar]
- 96. Heuer A., Gerovac M., Schmidt C., Trowitzsch S., Preis A., Kötter P., Berninghausen O., Becker T., Beckmann R., and Tampé R. (2017) Structure of the 40S-ABCE1 post-splitting complex in ribosome recycling and translation initiation. Nat. Struct. Mol. Biol. 24, 453–460 [DOI] [PubMed] [Google Scholar]
- 97. Rouault T. A. (2015) Iron-sulfur proteins hiding in plain sight. Nat. Chem. Biol. 11, 442–445 [DOI] [PubMed] [Google Scholar]
- 98. Jones D. P., and Sies H. (2015) The redox code. Antioxid. Redox Signal. 23, 734–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Cory S. A., Van Vranken J. G., Brignole E. J., Patra S., Winge D. R., Drennan C. L., Rutter J., and Barondeau D. P. (2017) Structure of human Fe-S assembly subcomplex reveals unexpected cysteine desulfurase architecture and acyl-ACP-ISD11 interactions. Proc. Natl. Acad. Sci. U.S.A. 114, E5325–E5334 [DOI] [PMC free article] [PubMed] [Google Scholar]