Abstract
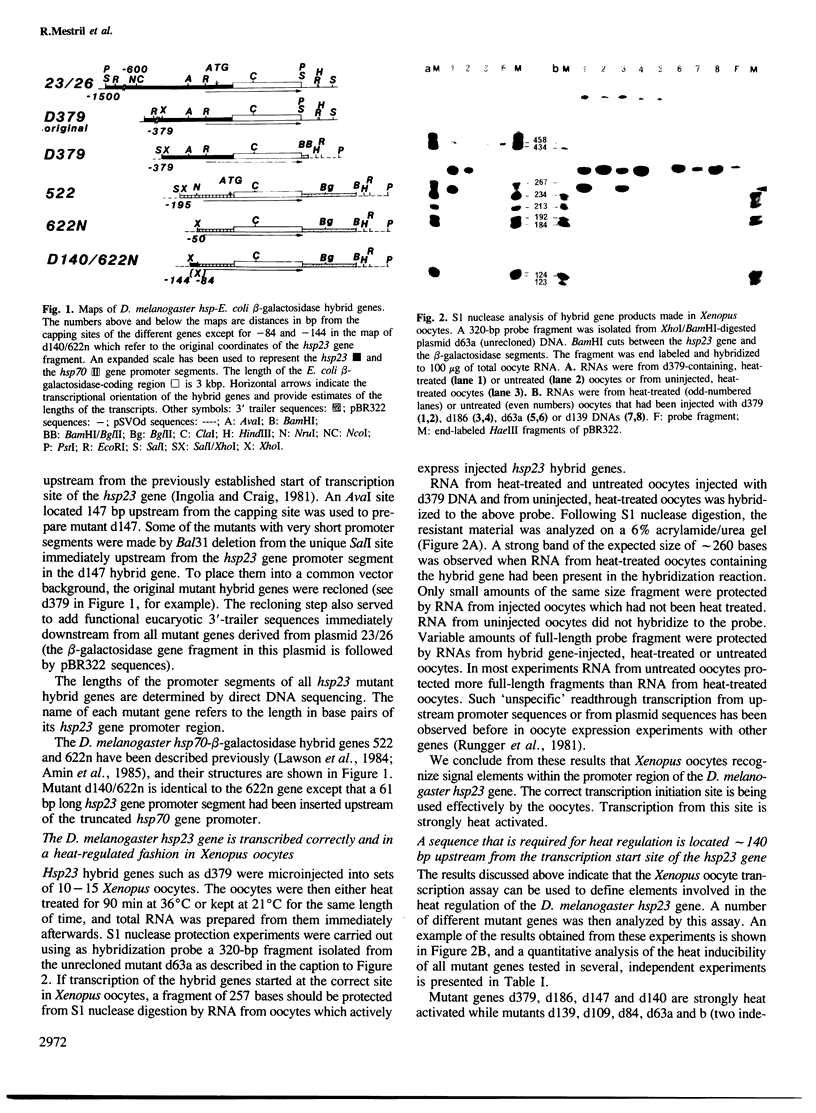
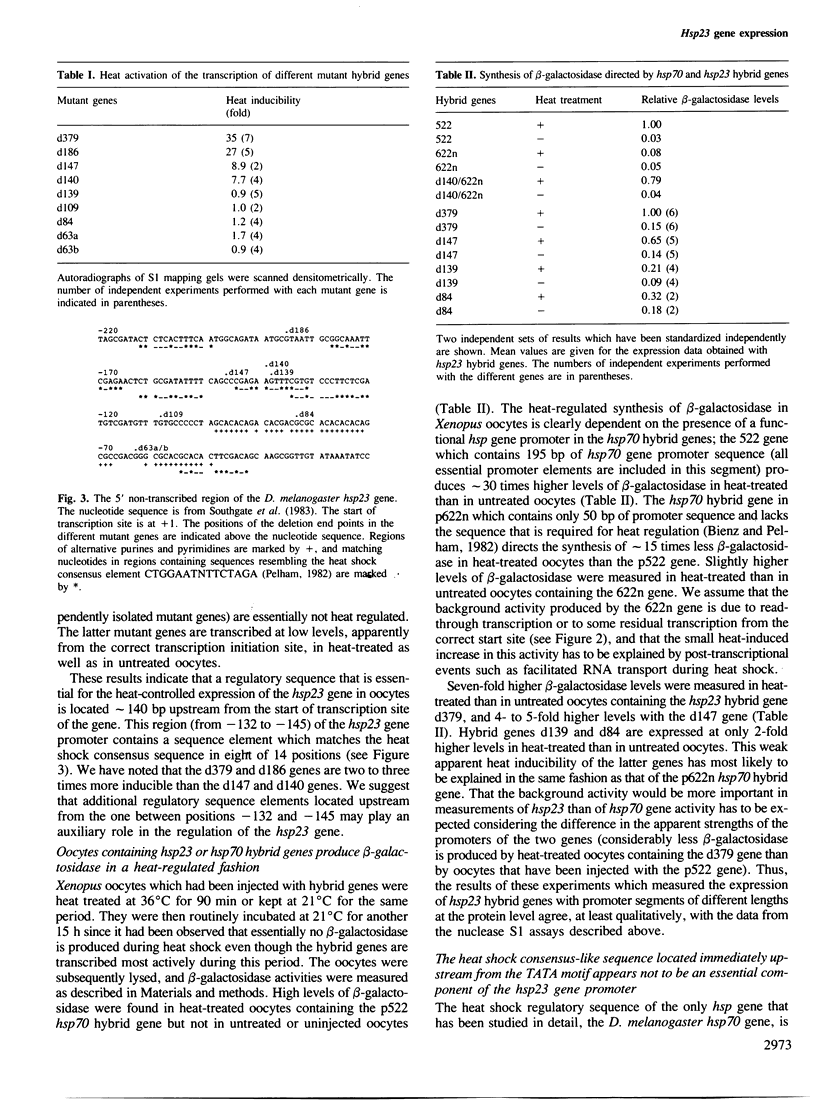
The expression of Drosophila melanogaster hsp23-Escherichia coli beta-galactosidase hybrid genes containing different segments of the 5' non-transcribed sequence of the hsp23 gene has been examined at the RNA and protein levels in Xenopus oocytes. Transcription of the hybrid genes is initiated correctly. Mutant genes with hsp23 gene promoter segments of at least 140 bp in length are strongly heat-activated while genes with shorter promoter segments are expressed constitutively and at low levels. This maps an element required for the heat-controlled expression of the D. melanogaster hsp23 gene to a region, approximately 140 bp upstream from the start of the transcription site, which contains a sequence (CGAGAAGTT-TCGTGT) that is closely related to the one responsible for the heat regulation of the hsp70 gene. These findings demonstrate the importance of this regulatory sequence for a second hsp gene and support the notion that hsp genes are heat-regulated by a common mechanism. The functional element in the hsp23 gene promoter is located greater than 80 bp further upstream from the TATA box than the relevant element in the hsp70 gene promoter. Even though other related sequences are present further upstream and downstream from the functional element, they play at most an auxiliary role in the regulation of hsp23 gene expression.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amin J., Mestril R., Lawson R., Klapper H., Voellmy R. The heat shock consensus sequence is not sufficient for hsp70 gene expression in Drosophila melanogaster. Mol Cell Biol. 1985 Jan;5(1):197–203. doi: 10.1128/mcb.5.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner M., Bonner J. J. The induction of gene activity in drosophilia by heat shock. Cell. 1979 Jun;17(2):241–254. doi: 10.1016/0092-8674(79)90150-8. [DOI] [PubMed] [Google Scholar]
- Ayme A., Southgate R., Tissières A. Nucleotide sequences responsible for the thermal inducibility of the Drosophila small heat-shock protein genes in monkey COS cells. J Mol Biol. 1985 Apr 20;182(4):469–475. doi: 10.1016/0022-2836(85)90233-5. [DOI] [PubMed] [Google Scholar]
- Bienz M., Pelham H. R. Expression of a Drosophila heat-shock protein in Xenopus oocytes: conserved and divergent regulatory signals. EMBO J. 1982;1(12):1583–1588. doi: 10.1002/j.1460-2075.1982.tb01359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienz M. Xenopus hsp 70 genes are constitutively expressed in injected oocytes. EMBO J. 1984 Nov;3(11):2477–2483. doi: 10.1002/j.1460-2075.1984.tb02159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzin C. H., Bournias-Vardiabasis N. Teratogens induce a subset of small heat shock proteins in Drosophila primary embryonic cell cultures. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4075–4079. doi: 10.1073/pnas.81.13.4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappello J., Zuker C., Lodish H. F. Repetitive Dictyostelium heat-shock promotor functions in Saccharomyces cerevisiae. Mol Cell Biol. 1984 Apr;4(4):591–598. doi: 10.1128/mcb.4.4.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Martinez-Arias A., Shapira S. K., Chou J. Beta-galactosidase gene fusions for analyzing gene expression in escherichia coli and yeast. Methods Enzymol. 1983;100:293–308. doi: 10.1016/0076-6879(83)00063-4. [DOI] [PubMed] [Google Scholar]
- Corces V., Pellicer A., Axel R., Meselson M. Integration, transcription, and control of a Drosophila heat shock gene in mouse cells. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7038–7042. doi: 10.1073/pnas.78.11.7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingolia T. D., Craig E. A. Primary sequence of the 5' flanking regions of the Drosophila heat shock genes in chromosome subdivision 67B. Nucleic Acids Res. 1981 Apr 10;9(7):1627–1642. doi: 10.1093/nar/9.7.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireland R. C., Berger E., Sirotkin K., Yund M. A., Osterbur D., Fristrom J. Ecdysterone induces the transcription of four heat-shock genes in Drosophila S3 cells and imaginal discs. Dev Biol. 1982 Oct;93(2):498–507. doi: 10.1016/0012-1606(82)90137-3. [DOI] [PubMed] [Google Scholar]
- Klein R. D., Selsing E., Wells R. D. A rapid microscale technique for isolation of recombinant plasmid DNA suitable for restriction enzyme analysis. Plasmid. 1980 Jan;3(1):88–91. doi: 10.1016/s0147-619x(80)90037-2. [DOI] [PubMed] [Google Scholar]
- Lawson R., Mestril R., Schiller P., Voellmy R. Expression of heat shock-beta-galactosidase hybrid genes in cultured Drosophila cells. Mol Gen Genet. 1984;198(2):116–124. doi: 10.1007/BF00328710. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight S. L. Functional relationships between transcriptional control signals of the thymidine kinase gene of herpes simplex virus. Cell. 1982 Dec;31(2 Pt 1):355–365. doi: 10.1016/0092-8674(82)90129-5. [DOI] [PubMed] [Google Scholar]
- Mellon P., Parker V., Gluzman Y., Maniatis T. Identification of DNA sequences required for transcription of the human alpha 1-globin gene in a new SV40 host-vector system. Cell. 1981 Dec;27(2 Pt 1):279–288. doi: 10.1016/0092-8674(81)90411-6. [DOI] [PubMed] [Google Scholar]
- Mirault M. E., Southgate R., Delwart E. Regulation of heat-shock genes: a DNA sequence upstream of Drosophila hsp70 genes is essential for their induction in monkey cells. EMBO J. 1982;1(10):1279–1285. doi: 10.1002/j.1460-2075.1982.tb00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran L., Mirault M. E., Tissières A., Lis J., Schedl P., Artavanis-Tsakonas S., Gehring W. J. Physical map of two D. melanogaster DNA segments containing sequences coding for the 70,000 dalton heat shock protein. Cell. 1979 May;17(1):1–8. doi: 10.1016/0092-8674(79)90289-7. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. A regulatory upstream promoter element in the Drosophila hsp 70 heat-shock gene. Cell. 1982 Sep;30(2):517–528. doi: 10.1016/0092-8674(82)90249-5. [DOI] [PubMed] [Google Scholar]
- Probst E., Kressmann A., Birnstiel M. L. Expression of sea urchin histone genes in the oocyte of Xenopus laevis. J Mol Biol. 1979 Dec 15;135(3):709–732. doi: 10.1016/0022-2836(79)90173-6. [DOI] [PubMed] [Google Scholar]
- Rungger D., Türler H. DNAs of simian virus 40 and polyoma direct the synthesis of viral tumor antigens and capsid proteins in Xenopus oocytes. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6073–6077. doi: 10.1073/pnas.75.12.6073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirotkin K., Davidson N. Developmentally regulated transcription from Drosophila melanogaster chromosomal site 67B. Dev Biol. 1982 Jan;89(1):196–210. doi: 10.1016/0012-1606(82)90307-4. [DOI] [PubMed] [Google Scholar]
- Southgate R., Ayme A., Voellmy R. Nucleotide sequence analysis of the Drosophila small heat shock gene cluster at locus 67B. J Mol Biol. 1983 Mar 25;165(1):35–57. doi: 10.1016/s0022-2836(83)80241-1. [DOI] [PubMed] [Google Scholar]
- Voellmy R., Rungger D. Transcription of a Drosophila heat shock gene is heat-induced in Xenopus oocytes. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1776–1780. doi: 10.1073/pnas.79.6.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver R. F., Weissmann C. Mapping of RNA by a modification of the Berk-Sharp procedure: the 5' termini of 15 S beta-globin mRNA precursor and mature 10 s beta-globin mRNA have identical map coordinates. Nucleic Acids Res. 1979 Nov 10;7(5):1175–1193. doi: 10.1093/nar/7.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]




