Abstract
DNA remodeling during endoreplication appears to be a strong developmental characteristic in orchids. In this study, we analyzed DNA content and nuclei in 41 species of orchids to further map the genome evolution in this plant family. We demonstrate that the DNA remodeling observed in 36 out of 41 orchids studied corresponds to strict partial endoreplication. Such process is developmentally regulated in each wild species studied. Cytometry data analyses allowed us to propose a model where nuclear states 2C, 4E, 8E, etc. form a series comprising a fixed proportion, the euploid genome 2C, plus 2–32 additional copies of a complementary part of the genome. The fixed proportion ranged from 89% of the genome in Vanilla mexicana down to 19% in V. pompona, the lowest value for all 148 orchids reported. Insterspecific hybridization did not suppress this phenomenon. Interestingly, this process was not observed in mass-produced epiphytes. Nucleolar volumes grow with the number of endocopies present, coherent with high transcription activity in endoreplicated nuclei. Our analyses suggest species-specific chromatin rearrangement. Towards understanding endoreplication, V. planifolia constitutes a tractable system for isolating the genomic sequences that confer an advantage via endoreplication from those that apparently suffice at diploid level.
Keywords: endoreplication, genome imbalance, cytogenetics, Vanilla, cytometry, genome size
Introduction
In general, nuclear DNA is considered as stably transmitted through replication or endoreplication (Arias and Walter 2007). Mechanisms protecting DNA from over or underreplication via epigenetic marks are now well documented within individuals and between generations. Contrasting with this strong generic mechanism to ensure whole DNA transmission, cases exist of genomic DNA remodeling during development in eukaryotic cells (Zufall et al. 2005).
A first concept of “chromatin diminution” was initially exposed by Boveri (1887), describing massive fragmentation of chromosomes during the early divisions of nematode embryonic development. Further examples of DNA rearrangements are now better known, mostly from the animal kingdom and insects. The elimination of a chromosome occurs during sex determination in Sciarid flies (Sanchez and Perondini 1999; Goday and Esteban 2001), whereas DNA is reorganized into a satellite chromosome during copepod development (Degtyarev et al. 2004; Drouin 2006).
In another case, part of nuclear DNA is eliminated in specific cell lineages that correspond to developmental programs, as typified in the vertebrate lamprey where extensive DNA reorganization occurs between germinal and somatic cell lineages (Smith et al. 2009). In this case, up to 20% of DNA is excised.
Precise excision may also be performed in order to assemble coding sequences in a specialized nucleus entirely devoted to gene expression, whereas complete genomic DNA is maintained in a separate nucleus devoted to sexual reproduction. This has been observed in Tetrahymena thermophila where a RNA-guided DNA deletion occurred (Yao and Chao 2005) or in Paramecium tetraurelia through homology-dependent Internal Eliminated Sequences (Duret et al. 2008). Beyond simple chromatin diminution or DNA elimination, diverse mechanisms of genome imbalance seem to occur during development. Underreplication may be one of such mechanism to regulate cell function, especially during endoreplication processes. Endoreplication allows endonuclear chromosome duplication without cytokinesis (D’Amato 1964). Generally, it appears to involve full genome replication (Barow and Jovtchev 2007). However, in some endoreplicating cell types as the trophoblast giant cells (TGC) of the rodent placenta, it was shown that large segments of parallel chromatin constitute up to 1000-fold amplification during developmental process (Hannibal et al. 2014; Cross 2014; Neiman et al. 2017). In this situation, endoreplication seems to be a mechanism to regulate cell function, although the molecular mechanisms of such process are unknown.
Plant cells may provide the experimental model for such studies, as endoreplication is widely present in the plant kingdom. Canonical in Arabidopsis thaliana, endoreplication is observed throughout development (Brown et al. 1991; Galbraith et al. 1991). It may be associated with cell elongation (e.g., in hypocotyls, Kondorosi et al. 2000), in organ development (e.g., in fleshy fruits, Bourdon et al. 2012), in hosting symbionts (e.g., the Medicago/Sinorhizobium symbiosis where both partners endoreplicate [Kondorosi et al. 2013]) and in pathogen interactions (e.g., nematode infection of plants [de Almeida Engler and Gheysen 2013]). Transcriptional activity increased strongly in the endocycled cells (Bourdon et al. 2012).
Kausch and Horner (1984), studying the differentiation of foliar ideoblasts in the orchid Vanilla planifolia, questioned the ability of plant cells to perform differential DNA replication. They described cell lineages rising from the meristem with more and more chromocentres, dispersion of preprophase heterochromatin, with neither mitosis nor polytene chromosomes. From Feulgen microdensitometry they concluded: “DNA content values above the 8C level do not fit the geometrical order which is found if the total genome is replicated during each endo-cycle, a result indicating differential DNA replication” for c.50% of the genome. Such simple endoreplication of tiny portions of genomic DNA has also been observed in a histone methylating mutant (Jacob et al. 2010), suggesting a true case for DNA remodeling mechanism in plant cells.
The previous observations of Kaush and Horner on orchids have been extended and appear to be specific of this plant group. Indeed, as substantiated herein, a developmentally regulated partial endoreplication appears in all orchids of subfamily Vanilloideae analyzed so far (Bory et al. 2008; Lepers-Andrzejewski et al. 2010; Trávníček et al. 2015). In Vanilla spp., all somatic nuclei appear to contain two copies of the holoploid genome, plus additional copies of 19–83% of the genome in a binary series. Importantly, Hriˇbová et al. (2016) have recently reported the first attempt to decipher molecular mechanisms involved in such process, outlining the difficulty of such studies.
In order to better analyze, this DNA remodeling process and to provide a precise map for further research of the molecular mechanisms involved, genome content of 87 species of orchids have been analyzed for partial endoreplication. A combination of flow cytometry and nuclei imaging approaches has described nuclear architecture and karyology features in order to further decipher the potential correlation between size of nuclei and partial endoreplication process.
Our results collated with previously published reports show that, across many sections of orchids, the endoreplicating portion is not progressive (as suggested by the abbreviation PPE in previous reports), but a fixed fraction characteristic of a given species, a process we now term strict partial endoreplication (SPE). This leads to formation of highly asymmetric nuclei where part of genetic information is only two copies (diploid) whereas the rest is amplified up to 64-fold. It is not suppressed by hybridization between species. Surprisingly, it has not been found in the most popular commercial orchids massively produced by in vitro meristem culture, such as Phalaenopsis spp.
The results permit the establishment of a phenomenological model and are discussed along with experimental strategies to elucidate the mechanisms behind this novel process of DNA remodeling and its biological significance.
Materials and Methods
Plant Material
The panel of orchids studied included species of Vanilla focusing on tropical and subtropical species (see table 3), other orchids chosen to cover other subfamilies or sections, and finally taxa which simply appeared during biodiversity studies around the Mediterranean Sea or in the Balkans (Siljak-Yakovlev et al. 2010, Bou Dagher-Kharrat et al. 2013, Pustahija et al. 2013). Some of the V. ×tahitensis and hybrids plants were issued from in vitro culture and have been genotypically verified.
Table 3.
Endoreplication Mode of 75 Taxa (85 Accessions) from Orchidaceae, Compiled with Reports Concerning 49 Additional Taxa, Covering 65 Genera Overall
| Species | Subfamily | Localitya | Mode Conventional or Partial Endoreplication or Noneb | Highest Degree Typically Observedc | Replicate Fraction P (%) | 1C-Value Mean (±sd) (pg) | 2n* | Ploidy (x) | Individuals Studied for P; 2C | Reference Standardd | Publication or Collaboratore |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Acineta superba (Kunth) Rchb. | Epidendroideae | conv | 5.01 (0.06) | CC | T2015 | ||||||
| Aerangis ellisii (B.S. Williams) Schltr. | Epidendroideae | conv | 1.48 (0.04) | G | T2015 | ||||||
| Aerangis luteoalba var. rhodosticta (Kraenzl.) J. L. Stewart | Epidendroideae | conv | 1.45 (0.01) | 42 | G | T2015 | |||||
| Anacamptis palustris (Jacq.) R.M.Bateman,Pridgeon & M.W.Chasesyn. Orchis palustris Jacq. | Orchidoideae | France Fos | PE | 32E | 37(0) | 4.27(0.0) | 2 | A | Fridlender A. | ||
| Anacamptis palustris × purpurea hybridssyn. Orchis palustris × purpurea hybrids | Orchidoideae | France Fos | PE | 32E | 37(0.1) | 4.27(0.0) | 3 | A | Fridlender A. | ||
| Anacheilium fragrans (Sw.) Acuña | Epidendroideae | MNHN-JB-17442 | conv | 8C | 100 | 0.84 | 2; 1 | A | |||
| Angraecum eburneum Bory subsp. superbum (Thouars) H.Perrier | Epidendroideae | MNHN-JB-348 | PE | 8E | 87 | 5.11 | 2; 1 | ||||
| Angraecum eichlerianum Kraenzl. | Epidendroideae | MNHN-JB-354 | PE | 16E | 87 | 3.61 | 38 | 2; 1 | |||
| Angraecum praestans Schltr. | Epidendroideae | conv | 2.95 (0.03) | G | T2015 | ||||||
| Ansellia africana Lindl. | Epidendroideae | MNHN-JB-504 | conv | 32C | 103 | 1.62 (0.14) | 42 | 2; 2 | B | ||
| Brassia arcuigera Rchb. f. | Epidendroideae | MNHN-572 | PE | 8E | 94 | 2.75 | 2; 1 | B | |||
| Bulbophyllum careyanum Sprengel | Epidendroideae | MNHN-JB-16979 | conv | 32C | 99 | 1.73 | 38 | 2; 1 | A | ||
| Bulbophyllum cocoinum Bateman ex Lindley | Epidendroideae | MNHN-JB-17091 | PE | 16E | 93 | 2.53 | 38 | 2; 1 | B | ||
| Bulbophyllum echinolabium J.J. Sm. | Epidendroideae | conv | 2.04 (0.03) | G | T2015 | ||||||
| Bulbophyllum lepidum (Blume) J. J. Sm. | Epidendroideae | MNHN-JB-17021 | conv | 8C | 101 | 2.06 | 38 | 2; 1 | A | ||
| Bulbophyllum medusae (Lindl.) Rchb. f. | Epidendroideae | conv | 1.29 (0.02) | 38 | CC | T2015 | |||||
| Bulbophyllum rufinum Rchb. f. | Epidendroideae | MNHN-JB | conv | 32C | 98 | 1.66 | 2; 1 | F | |||
| Cattleya mossiae C.Parker ex Hook. | Epidendroideae | MNHN-JB-17180 | conv | 16C | 97 | 2.77 | 1; 1 | F | |||
| Chamaeangis odoratissima (Rchb. f.) Schltr. | Epidendroideae | MNHN-JB-791 | conv | 16C | 97 | 1.81 | 50 | 1; 1 | B | ||
| Chelonistele sulphurea (Blume) Pfitzer | Epidendroideae | conv | 2.67 (0.02) | CC | T2015 | ||||||
| Chysis bractescens Lindl. | Epidendroideae | MNHN | conv | 16C | 101 | 1.80 (0.03) | 3; 2 | B | |||
| Cleisostoma racemiferum (Lindl.) Garay | Epidendroideae | conv | 1.80 (0.04) | 38 | G | T2015 | |||||
| Cleisostoma subulatum Blume | Epidendroideae | conv | 2.24 (0.04) | 38 | G | T2015 | |||||
| Coelogyne assamica Linden & Rchb. f. | Epidendroideae | conv | 2.97 (0.04) | G | T2015 | ||||||
| Coelogyne cristata Lindl. | Epidendroideae | MNHN-JB-51482 | none | 4C | 97 | 3.40 | 40 | 2; 1 | F | ||
| Coelogyne fimbriata Lindl. | Epidendroideae | conv | 3.53 (0.05) | 40 | G | T2015 | |||||
| Cynorkis guttata Hermans & P.J. Cribb | Orchidoideae | PE | 21.1 | 6.85 (0.03) | CC | T2015 | |||||
| Cypripedium calceolus L. | Cypripedioideae | Orsay France | PE | 16E | 84 | 38.7 (0.34) | 20 | 3 | E | ||
| Dendrobium glomeratum Rolfe | Epidendroideae | conv | 2.23 (0.03) | G | T2015 | ||||||
| Dendrobium wattii (Hook.) Rchb. f. | Epidendroideae | conv | 1.46 (0.05) | CC | T2015 | ||||||
| Dendrochilum tenellum (Nees & Meyen) Ames | Epidendroideae | MNHN-JB-32330 | none | 4C | 98 | 1.14 | 1; 1 | ||||
| Dendrochilum latifolium Lindl. | Epidendroideae | MNHN-JB-2788 | none | 4C | 98 | 1.14 | 1; 1 | B | |||
| Dracula astute (Rchb.) Luer | Epidendroideae | PE | 36.1 | 1.93 (0.03) | H | T2015 | |||||
| Dracula chestertonii (Rchb.) Luer | Epidendroideae | PE | 34.6 | 2.14 (0.03) | H | T2015 | |||||
| Encyclia tampensis (Lindl.) Small | Epidendroideae | MNHN-JB-2857 | conv | 16C | 100 | 1.30 | 2; 1 | A | |||
| Epidendrum falcatum Lindl. | Epidendroideae | MNHN-JB-43085 | none | 4C | 100 | 3.89 | 2; 1 | B | |||
| Epidendrum nocturnum Jacquin | Epidendroideae | MNHN-JB-17464 | conv | 16C | 100 | 1.52 | 1; 1 | A | |||
| Epidendrum rigidum Jack. | Epidendroideae | conv | 1.21 (0.01) | CC | T2015 | ||||||
| Epipactis consimilis D. Don. | Epidendroideae | Liban | none | trace 4C | – | 13.7 | 40 | 1 | B2013 | ||
| Eria lasiopetala (Willd.) Ormero d.f. | Epidendroideae | MNHN-JB-17104 | PE | 32E | 87 | 3.09 | 3; 1 | A | |||
| Eria micholitzii Kraenzl. | Epidendroideae | MNHN-JB-30988 | PE | 16E | 90 | 1.03 | 2; 1 | B | |||
| Eria vieillardii Rchb. f. | Epidendroideae | MNHN-JB-17617 | conv | 8C | 102 | 2.38 | 2; 1 | ||||
| Gastrochilus calceolaris (Buch.-Ham ex J.E.Sm.) D.Don | Epidendroideae | conv | 2.74 (0.03) | 38 | CC | T2015 | |||||
| Gomphichis macbridei C.Schweinf. | Orchidoideae | PE | 49.4 | 7.03 (0.07) | H | T2015 | |||||
| Gymnadenia conopsea (L.) R.Br. | Orchidoideae | Czech Rep & Slovakia | PE | 60 | 3.81 | 40 | 4 | E | T2011 | ||
| Gymnadenia densiflora (Wahlenb.) A.Dietr. | Orchidoideae | Czech Rep & Slovakia | PE | 75 | 3.40 | 40 | 4 | CC | T2011 | ||
| Himantoglossum hircinum (L.) Spreng. | Orchidoideae | Orsay France | PE | 5.8 | E | ||||||
| Liparis crenulata (Blume) Lindl. | Epidendroideae | PE | 76.4 | 1.88 (0.02) | G | T2015 | |||||
| Liparis sparsiflora Aver. | Epidendroideae | conv | 2.43 (0.03) | CC | T2015 | ||||||
| Lockhartia biserra (Rich.) Hoehne | Epidendroideae | MNHN-JB-32474 | conv | 64C | 105 | 1.14 (0.00) | 4; 3 | B | |||
| Ludisia discolour (Ker Gawl.) A. Rich | Orchidoideae | PE | 61.1 | 1.1 (0.03) | H | T2015 | |||||
| Lycaste skinneri Lindl. var. rosea | Epidendroideae | MNHN-JB | conv | 8C | 95 | 1.73 | 2; 1 | A | |||
| Masdevallia ignea Rchb. f. | Epidendroideae | PE | 54.1 | 1.44 (0.04) | G | T2015 | |||||
| Masdevallia veitchiana Rchb. f. | Epidendroideae | PE | 46.1 | 1.69 (0.01) | G | T2015 | |||||
| Maxillaria tenuifolia Lindl. | Epidendroideae | MNHN-JB-60348 | conv | 8C | 98 | 2.90 (0.13) | 3; 2 | ||||
| Maxillaria violacea-punctata Rchb.f. | Epidendroideae | MNHN | conv | 8C | 97 | 3.78 | 1; 1 | F | |||
| Miltoniopsis roezlii (Rchb. f.) God.-Leb. | Epidendroideae | conv | 1.75 (0.02) | G | T2015 | ||||||
| Myrmecophila tibicinis (Batem.) Rolfe | Epidendroideae | MNHN-JB | conv | 16C | 97 | 1,72 | 1; 1 | F | |||
| Neobenthamia gracilis Rolfe | Epidendroideae | conv | 0.58 (0.02) | CC | T2015 | ||||||
| Neotinea tridentata (Scop.) R.M.Bateman | Epidendroideae | Lebanon Nahr Ibrahim 70m | PE | 39 | 9.26 | 42 | 6 | E | B2013 | ||
| Neuwiedia zollingeri var. javanica (J.J.Sm.) de Vogel | Apostasioideae | conv | 3.24 (0.05) | CC | T2015 | ||||||
| Oeceoclades maculata (Lindl.) Lindl | Epidendroideae | MNHN-30881 | PE | 64E | 81 | 1.09 (0.01) | 3; 2 | F | |||
| Oeceoclades gracillima (Schltr.) Garay & P.Taylor. | Epidendroideae | MNHN-33968 | PE | 64E | 84 | 1.35 | 2; 1 | A | |||
| Oeceoclades petiolata (Schltr.) Garay & P.Taylor | Epidendroideae | MNHN-32168 | PE | 8E | 86 | 1.07 (0.01) | 3; 2 | F | |||
| Oncidium cf. reflexum Lindl. | Epidendroideae | conv | 2.88 (0.04) | CC | T2015 | ||||||
| Oncidium flexuosum Lodd. | Epidendroideae | MNHN | none | 4C | 99 | 0.94 | 56 | 1; 1 | B | ||
| Oncidium sotoanum R.Jiménez & Hágsater | Epidendroideae | conv | 2.73 (0.05) | CC | T2015 | ||||||
| Ophrys fusca Link | Orchidoideae | Lebanon Botmeh 1100m | PE | 16E | 82 | 10.49 | 36,72 | E | B2013 | ||
| Orchis anatolica Boiss. | Orchidoideae | Lebanon Baakline 940m | PE | 16E | 39 | 10.16 | E | B2013 | |||
| Orchis maculata subsp. macrostachys (Tineo) Soó | Orchidoideae | Lebanon | PE | 32E | 82 | 2.84 (0.24) | 4 | A | B2013 | ||
| Orchis purpurea Huds. | Orchidoideae | France Saint Maximin | PE | 32E | 54 | 5.80 | 42 | 7 | A | Fridlender A. | |
| Pabstiella tripterantha (Rchb. f.) Luer | Epidendroideae | conv | 0.74 (0.01) | G | T2015 | ||||||
| Panisea uniflora (Lindl.) Lindl. | Epidendroideae | conv | 2.83 (0.02) | 40 | G | T2015 | |||||
| Paphiopedilum callosum (Rchb.f.) Stein | Cypripedioideae | PE | 81.1 | 28.62 (0.37) | 32 | CC | T2015 | ||||
| Paphiopedilum lawrenceanum (Rchb.f.) Pfitzer | Cypripedioideae | MNHN | PE | 4E | 70 | 28.28 | 36 | 2; 1 | |||
| Paphiopedilum malipoense S.C.Chen & Z.H.Tsi | Cypripedioideae | MNHN-JB-28557 | PE | 8E | 59 | 28.05 | 26 | 1; 1 | F | ||
| Paphiopedilum purpuratum (Lindl.) Stein | Cypripedioideae | PE | 70.3 | 34.56 (0.99) | 40 | H | T2015 | ||||
| Phalaenopsis sp. Blume, hybrid | Epidendroideae | MNHN & commerce | conv | 64C | 99 | 4.23 | 4 | D, E | |||
| Pholidota articulata Lindl. | Epidendroideae | MNHN-JB-33306 Cambodge | none | 4C | 99 | 2.23 (0.04) | 7; 5 | F | |||
| Phragmipedium besseae var. dalessandroi (Dodson & O.Gruss) A.Moon & P.J.Cribb | Cypripedioideae | conv | 7.95 (0.09) | 24 | H | T2015 | |||||
| Platanthera bifolia (L.) Rchb. | Orchidoideae | BH Kladanj | PE | 16E | 79 (3.1) | 6.87 (0.05) | 42* | 2* | 4; 5 | C | S2010, P2013 |
| Pleurothallis schweinfurthii Garay | Epidendroideae | conv | 0.65 (0.01) | CC | T2015 | ||||||
| Pleurothallis vittata Lindl. | Epidendroideae | MNHN-JB-52119 | PE | 16E | 90 | 2.75 | 2; 1 | A | |||
| Ponthieva parvilabris (Lindl.) Rchb.f. | Orchidoideae | PE | 46.0 | 4.27 (0.08) | G | T2015 | |||||
| Porroglossum peruvianum H.R.Sweet | Epidendroideae | PE | 32.5 | 2.16 (0.14) | G | T2015 | |||||
| Psychopsis papilio (Lindl.) H.G.Jones | Epidendroideae | PE | 48.0 | 6.82 (0.19) | CC | T2015 | |||||
| Rhipidoglossum rutilum (Rchb. f.) Schltr. | Epidendroideae | conv | 1.92 (0.04) | G | T2015 | ||||||
| Scaphosepalum bicolor Luer & R. Escobar | Epidendroideae | conv | 0.43 (0.01) | G | T2015 | ||||||
| Scaphyglottis albida (Rchb.f.) Schltr | Epidendroideae | MNHN-JB-18567 | conv | 101 | 0.68 | 1; 1 | A | ||||
| Stanhopea embreei Dodson | Epidendroideae | conv | 2.16 (0.02) | H | T2015 | ||||||
| Stelis argentata Lindl. | Epidendroideae | MNHN-JB-18622 | conv | 8C | 101 | 1.91 | 30 | 2; 1 | F | ||
| Stelis pachyglossa (Lindl.) Pridgeon & M.W.Chase | Epidendroideae | conv | 0.74 (0.01) | CC | T2015 | ||||||
| Stenoglottis longifolia Hooker f. | Orchidoideae | PE | 37.5 | 2.82 | G | T2015 | |||||
| Trichotosia velutina Kraenzl. | Epidendroideae | MNHN-JB-62002 Cambodge | PE | 16E | 82 | 2.81 | 2; 1 | A | |||
| Trisetella andreettae Luer | Epidendroideae | PE | 44.5 | 0.89 (0.01) | CC | T2015 | |||||
| Trudelia cristata (Lindl.) Senghas | Epidendroideae | MNHN-JB-13208 Nepal | conv | 16C | 98 | 1.77 | 1; 1 | F | |||
| Vanilla africana Lindl. | Vanilloideae | CR0103, CR0107 Africa | PE | 2.91 (0.04) | 5 | D | B2010 | ||||
| Vanilla albida Blume | Vanilloideae | CR0058, CR0793 Thailand | PE | 57 | 2.76 (0.04) | B2010 | |||||
| Vanilla aphylla Blume | Vanilloideae | PE | 37 | 2.75 (0.08) | CC | T2015 | |||||
| Vanilla bahiana Hoehne | Vanilloideae | CR0098 Brazil | PE | 2.07 (0.05) | 5 | D | B2010 | ||||
| Vanilla (cf.) cribbiana Soto Arenas | Vanilloideae | CR0119 | PE | 2.52 (0.06) | 6 | D | |||||
| Vanilla chamissonis Klotzsch | Vanilloideae | CR0666 Sao Paulo | PE | 42 | 3.97 (0.07) | 6 | D | B2010 | |||
| Vanilla crenulata Rolfe | Vanilloideae | CR0091 Africa | PE | 41 | 3.23 (0.01) | 5 | D | B2010 | |||
| Vanilla (cf.) grandiflora Lindl. | Vanilloideae | CR0693 Guyana | PE | 41 | 3.23 (0.0) | 5 | D | ||||
| Vanilla humblotii Rchb. f. | Vanilloideae | CR0108, CR0871 Comoros | PE | 3.86 (0.07) | 5 | D | |||||
| Vanilla imperialis Kraenzl. | Vanilloideae | CR0796 CR0797 Africa | PE | 45 | 3.02 (0.05) | 4 | D | ||||
| Vanilla insignis Ames | Vanilloideae | CR0087 | PE | 1.92 (0.03) | 8 | D | |||||
| Vanilla leprieuri R. Portères | Vanilloideae | CR0109 French Guyana | PE | 58 | 2.50 (0.01) | 4 | D | ||||
| Vanilla lindmaniana Kraenzl. | Vanilloideae | CR0682 self CR1643 | PE | 2.52 (0.10) | 9 | D | |||||
| Vanilla madagascariensis Rolfe | Vanilloideae | CR0142, CR0812, CR0821, CR1647, Madagascar | PE | 3.12 (0.22) | 7 | D | |||||
| Vanilla mexicana Mill. (syn. V. inodora Shiede) | Vanilloideae | 8 localities Guadeloupe | PE | 32E | 83.1 (3.6) | 2.38 (0.051) | 26–32* | 2 | 18 | D | Barre N. & Silvestre D. |
| Vanilla odorata C. Presl | Vanilloideae | CR0686 America | PE | 43 | 2.48 (0.06) | B2010 | |||||
| Vanilla palmarum (Salzm. ex Lindl.) Lindl. | Vanilloideae | CR0083 Bahia | PE | 46 | 2.21 (0.19) | 3 | D | ||||
| Vanilla phalaenopsis Reichb. f. ex Van Houtte | Vanilloideae | CR0146 Africa | PE | 3.66 (0.07) | 4 | D | |||||
| Vanilla pierrei Gagnep. | Vanilloideae | Cambodia Dep Sihanoukville Kbal Chhay 10°37′27.12″N/103°31′28.87″E, 125m #985 | PE | 64E | 37 | 2.54 (0.03) | 5 | B | Telepova M. | ||
| Vanilla planifolia Jacks. ex Andrews | Vanilloideae | EVT Tahiti | PE | 256E in leaves | 28.4 (0.3) | 2.30 | 25,26, 28, 30, 32, 26–32* | 2 | 32 | A | |
| Vanilla planifolia Jacks. ex Andrews | Vanilloideae | PE | 26.2 | 2.31 (0.05) | 2 | H | T2015 | ||||
| Vanilla planifolia Jacks. ex Andrews | Vanilloideae | CR0631, CR0649 Reunion Is. | PE | 2.26 (0.08) | 2 | 10 | D | ||||
| Vanilla planifolia Jacks. ex Andrews | Vanilloideae | CR0068 Reunion Is. | PE | 2.31 (0.03) | 2 | 1 | D | ||||
| Vanilla planifolia Jacks. ex Andrews | Vanilloideae | S21-26, S29 Reunion Is. | PE | 2.22 (0.08) | 2 | 33 | D | ||||
| Vanilla planifolia Jacks. ex Andrews | Vanilloideae | CR0630, CR0645 Reunion Is. | PE | 3.29 (0.07) | 3 | 12 | D | ||||
| Vanilla planifolia Jacks. ex Andrews | Vanilloideae | CR0641, CR802 Reunion Is. | PE | 4.43 (0.06) | 4 | 2 | D | ||||
| Vanilla polylepis Summerh. | Vanilloideae | CR0705 Africa | PE | 44 | 3.10 (0.04) | 3 | D | ||||
| Vanilla pompona Schiede complex | Vanilloideae | CRnnnn | PE | 3.50 (0.42) | 2 | 180 | A, D | ||||
| Vanilla pompona Schiede | Vanilloideae | EVT Tahiti CR0018 | PE | 64E | 19.1 | 3.51 (0.16) | 3228–32* | 2 | 5 | A | |
| Vanilla roscheri Rch b.f. | Vanilloideae | CR1444 South Africa | PE | 3.25 (0.0) | 2 | D | GIgant R. | ||||
| Vanilla ×tahitensis J.W. Moore | Vanilloideae | CR0017 Reunion Is (origin French Polynesia) | PE | 2.18 (0.05) | 2 | 10 | D | ||||
| Vanilla ×tahitensis J.W.Moore | Vanilloideae | Tahiti | PE | 64E | 26.8 | 2.113 (0.058) | 22–31* | 2 | 5 | A | |
| Vanilla ×tahitensis cv tahiti H5-27 × V. pompona CR0018 hybrids | Vanilloideae | Tahiti (EVT # HY0502-009) | PE | 64E | 21.1 (1.1) | 2.796 (0.082) | 22–31* | 2 | 65; 65 | A | |
| Vanilla ×tahitensis cv tahiti H5-27 × V. pompona CR0018 hybrids | Vanilloideae | Tahiti (ETV HY502-137) | PE | 64E | 21 | 5.30 (0.046) | 47–59* | 4 | 2; 2 | A | |
| Vanilla trigonocarpa Hoehne | Vanilloideae | CR0069 Brazil (Alagoinhas) | PE | 4.11 (0.04) | B2010 | ||||||
| Vanilla wightiana Lindl. | Vanilloideae | CR0707 South Western Ghats | PE | 2.53 (0.0) | |||||||
| Warczewiczella timbiensis P.Ortiz | Epidendroideae | conv | 3.31 (0.03) | CC | T2015 | ||||||
| Zygopetalum maculatum (Kunth) Garay | Epidendroideae | MNHN | PE | 8E | 90 | 5.45 | 2; 1 | F |
2n∗ chromosome numbers were determined by the authors; all others (no asterisk) are taken from the literature.
Locality or origin: BH: Bosnia and Herzegovina; CR: BRC Vatel Cirad PVBMT Université de la Réunion; MNHN—JB: Museum National d’Histoire Naturelle, Jardin Botanique, Paris.
PE = Partiel Endoreplication; conv = Classical whole-genome endoreplication; none = no endoreplication above 4C.
Nuclei with C-value or with endoreplication state E.
Calibration standard species (Gif-Orsay): A: Solanum lycopersicum Mill. cv. “Roma”; B: Petunia hybrida (Hook) Vilm. cv. “PxPC6”; C: Pisum sativum L. cv. “Long Express”, D: Artemisia arborescens L. (origin: Crete, 2C = 11.43 pg, Garcia et al. 2006); E: Triticum aestivum L. cv. “Chinese Spring”; F: Salvia brachyodon Vandas (2n = 14; 2C = 0.95 pg), from Siljak-Yakovlev et al. (2010). Other calibration standards in Trávníček et al. (2015) CC: Pisum sativum L. cv. “Ctirad” (2C = 8.76 pg); G: Solanum pseudocapsicum (2.59 pg); H: Vicia faba “Inovec” (26.90 pg).
Sources of data or collaboration. For 2C only: B2010, Bory et al. (2010); B2013, Bou Dagher-Kharrat et al. (2013); P2013, Pustahija et al. (2013); S2010, Siljak-Yakovlev et al. (2010); For 2C and P: T2011, Trávníček et al. (2011); T2015, Trávníček et al. (2015).
Flow Cytometry
Cytometry analyses were performed using the usual parameters described in Brown et al. (2010). Internal references used for cytometry were Solanum lycopersicum L. “Montfavet 63-5” (2C = 1.99 pg, Lepers-Andrzejewski et al. 2011), Petunia hybrida Vilm. “PxPc6”, Pisum sativum L. “Long Express”, Triticum aestivum L. “Triple Dirk” (2C = 2.85 pg, 8.37 pg and 30.90 pg, respectively, Marie and Brown 1993), Artemisia arborescens L. (origin: Crete, 2C = 11.43 pg, Garcia et al. 2006) and Salvia brachyodon Vandas (2n = 14; 2C = 0.95 pg, from Siljak-Yakovlev et al. 2010). The conversion is 1 pg DNA = 978 Mbp (Doležel et al. 2003).
Terminology Used and Measurement of the Fixed Part of the Genome Which Does Not Endoreplicate
C-value is the DNA content of a holoploid genome with chromosome number (n) (corresponding to the haploid complement). Nuclei with the holoploid genome of a diploid plant contain 2C DNA (Greilhuber et al. 2005).
The symbol E represents an Endoreplication state, and designates nuclear populations that have undergone endoreplication cycles (2E, 4E, 6E, etc.).
R (for DNA Relative fluorescence intensity) is the DNA index. It corresponds to relative fluorescence intensity of endoreplicated nuclei compared with the 2C peak.
The component F represents the Fixed part of the haploid genome which does not endoreplicate. The component P represents the part potentially Participating in endoreplication. P and F are proportions (and not amounts) of the genome (without units). In the classical endoreplication of Arabidopsis thaliana, F is null and P is 1 (F + P =1). Note that this terminology differs from the one used by Travnicek (Trávníček et al. 2015) where P is a DNA amount (not a proportion) of the replicated part of the 2C genome.
In our work, P and F are proportions of the genome (without units) whereas p and f are amounts (typically pg). Note that, as proportions, F and P have the same value whether referring to the haploid or to the diploid genome. By contrast, the absolute quantity p (pg) in the haploid is doubled to 2p (pg) in the diploid nucleus (the italic lower-case indicating absolute units). In quantitative terms, the haploid nucleus is (1f + 1p) pg, and diploid nucleus is (2f + 2p) pg. So 4E nuclei have four copies of the part of the genome which replicate, and two copies of the rest of the genome which does not replicate, in total 2f + 4p (pg). The 8E nuclei have 2f + 8p (pg), etc.
The P value (fig. 2 and table 1) is most simply assessed from R, the relative fluorescence intensity (I, arbitrary units) of peak#2 (the first endocycle population) to peak#1 (2C nuclei), also termed the DNA Index:
| (1) |
Fig. 2.—
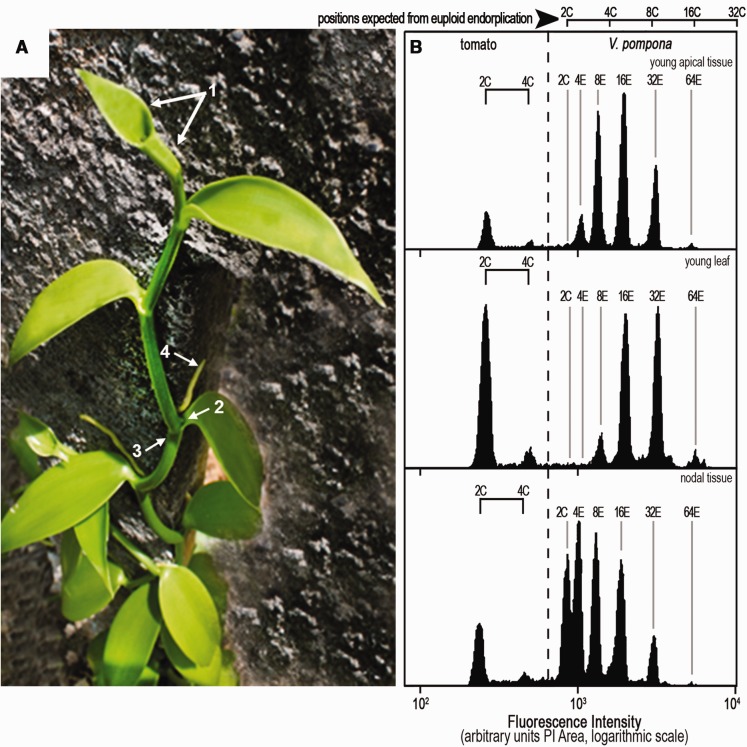
DNA histograms of Vanilla tissues. (A) Vanilla planifolia. In vanilla plants: population of 2C nuclei was expected when using the emerging apex or a very young leaf (enclosing the apex, arrow 1), the heart of a lateral bud (arrow 2), or notably the node (arrow 3); very young aerial root (arrow 4). (B) DNA histograms from Vanilla pompona somatic tissues (tomato as internal standard). A solid horizontal bar () represents the increment equivalent to a doubling, for example, 2C–4C for tomato. Note that euploid nuclei are sometimes difficult to assess in young apical tissue and young leaf, complicating the estimations of genome-size and of the endoreplication process itself. Contrastingly, in nodal tissue, the 2C nuclei, essential for assessing genome-size, constituted exceptionally a fifth of this sample of orchid nuclei. Populations of larger nuclei were also evident: 4E, 8E, 16E, 32E, and a trace of 64E.
Table 1.
Nuclear Classes in Cytometry Samples from Vanilla planifolia Tissues
| Tissue | Nuclear Populations | |||||
|---|---|---|---|---|---|---|
| (a) | 2C | 4E | 8E | 16E | 32E | 64E |
| Young leaf | + | + | + | + | ||
| Mature leaf | + | + | + | + | (+) | |
| Leaf epidermal peel | + | + | ||||
| Stem | (+) | + | + | + | + | |
| Chopped seeds | + | + | + | + | + | + |
| Interpeak Ratio * Mean (sd) | 1.28 (0.01) | 1.44 (0.02) | 1.62 (0.03) | 1.75 (0.02) | 1.84 (0.07) | |
| Number of Measures | 3 | 11 | 18 | 9 | 3 | |
| (b) | ||||||
| Aerial Roots (mm) | Nuclear Populations (%) | |||||
| 2C+4C | 4E+8E | 16E | >16E | |||
| 0–1 | trace | 64 | 22 | 14 | ||
| 1–2 | 30 | 60 | 10 | |||
| 2–4 | 2 | 30 | 69 | |||
| 4–12 | 0 | 25 | 75 | |||
| Dissected Axillary Bud | 95 | 5 | ||||
Interpeak Ratio is the fluorescence intensity (I, arbitrary units) of peak n to peak (n − 1).
When any 2C population was minor and poorly defined, the deduction of P became less precise. Factor P was thus calculated from the mean intensities of 4E, 8E, and 16E nuclear peaks relative to 2C, taking the geometric average (Px= [(R2 − 1) + (R3 − R2) + (R4 − R3)]/7) of the three estimates of P.
Genome Analyses
Genome size, DNA histograms, and base composition were obtained as detailed in Lepers-Andrzejewski et al. (2011) using initially an EPICS Elite cytometer, then a MoFlo ASTRIOS 6-way sorter (Beckman-Coulter). A CyFlow SL cytometer (Partec) with 532 nm laser was used occasionally for data comparison between different machines. Nuclei were identified by a gate on 488 nm Side-Scatter and propidium iodide (PI)-Area (log scales) and the cytogram of PI-Area versus PI-Height signals served to select singlets, eliminating doublets and detecting any degradation. PI was used at 70–100 µg/ml.
Base Composition
Base composition was assessed following Godelle et al. (1993). This protocol relies upon the differential staining observed with the AT-dependent dye bisbenzimide Hoechst 33342, the GC-dependent dye chromomycin and the intercalant propidium iodide.
Nuclei Sorting
Nuclei were sorted directly onto two aligned three-well microscope glass slides (Superfrost®, CML France, http://www.cml.fr) prepared with 20 µl of a cushion comprising 500 mM sucrose, 50% nuclear isolation buffer and 2% formaldehyde (Bourdon et al. 2011). Six populations were sampled simultaneously in this way. Nuclei were stained with DAPI used in a 5–10mg/ml range.
Nuclei Imaging and 3D Reconstruction
For karyology study, cytogenetic techniques were as detailed in Lepers-Andrzejewski et al. (2011). For nuclei imaging, slide wells of sorted DAPI-stained nuclei were completed, if necessary, with additional buffer containing DAPI (4′,6-diamidino-2-phenylindole), then sealed with coverslips. The quality of nuclei sorting was checked on a Reichert DIC/epifluorescence microscope with a Retiga2000 camera (QImaging). For 3D reconstruction, nuclei were observed with a spinning disk microscope (Roper Scientific, Evry, France), inverted TE Eclipse with 100x NA1.40 oil objective (Nikon), and 0.1 µm Z-steps. Image analysis and processing, except for deconvolution, was performed with ImageJ software (http://rsb.info.nih.gov/ij); Wayne Rasband, National Institutes of Health, Bethesda, MD).To obtain an accurate measure of nuclear and nucleolar volumes in 3D, it was necessary to correct the z-axis distortion caused by the refractive indexes mismatch between the oil objective and the mount medium (a mix of nuclei isolation buffer and sucrose cushion, see above). Z-stacks of 10 µm diameter fluorescent beads were employed to compute a linear correction factor, adapting a strategy described by Ferko et al. (2006). Such process allowed precise measurements of volumes from z-stacks of the beads, which were within 2.6% of volumes theoretically computed, under the assumption of perfect sphericity, from the surface of a maximal projection of the stack (n = 16 measures). The same correction (Δz microscope/Δz focus = 0.48) was then applied to the z-stacks of DAPI-stained nuclei sorted by cytometry. After Huygens deconvolution (Scientific Volume Imaging, Hilversum, The Netherlands). Parameters: cmle algorithm, 40 iterations, quality threshold 0.1, signal to noise ratio 10, background 20. Images depth: 8 bit), the z-corrected stacks were binarized and the ImageJ “3D Object Counter” plugin (Fabrice Cordelières) was employed to compute a “chromatin volume.” These volumes correlated tightly with the DNA quantities evaluated by cytometry. In parallel, segmentation, at a lower threshold, of non-deconvolved stacks, in which some DAPI signal is still present in the nucleolar regions, allowed the reconstruction of a “whole nuclei” volume. Nucleolar volumes were then obtained by subtracting the “chromatin volume” from the “whole nuclei”, and, when necessary, filtering out manually peripheral spurious particles corresponding to dispersed low density chromatin regions.
Three-dimensional surface rendering of deconvoluted z-stacks of ploidy-sorted nuclei was performed with the UCSF Chimera software47 version 1.8 (Resource for Biocomputing, Visualization and Informatics at the University of California, San Francisco, supported by NIGMS P41-GM103311).
Results
Endoreplication is Only Partial in Four Vanilla spp
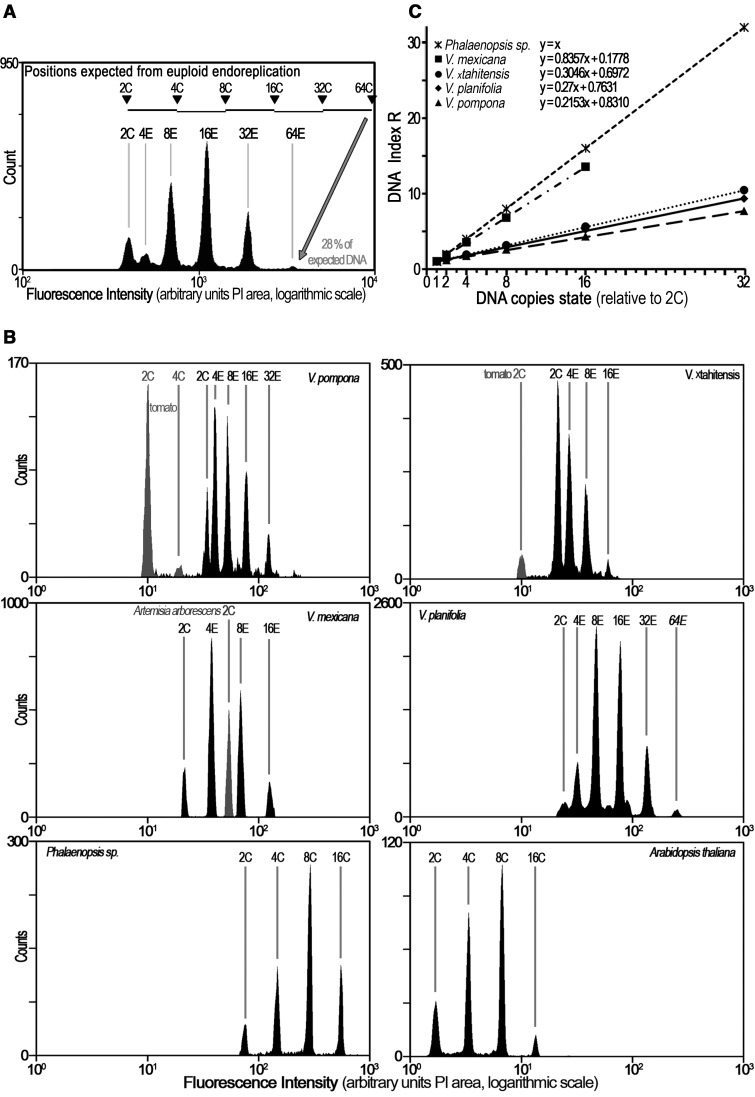
In order to handle with precision the large range of endoreplicated nuclei present in each sample, a histogram of nuclear DNA levels was obtained using a logarithmic amplifier. DNA histogram from nuclei extracted from young leafy apex of Vanilla planifolia is shown in figure 1. Fluorescence intensity of DNA staining revealed a series of peaks (fig. 1A). However, the peak position (or interpeak values) did not mirror the profiles expected from a classical euploid endoreplication process (i.e., 4C, 8C, 16C, etc.). It rather suggested a partial endoreplication of the genome. The peak positions were assigned as 4E, 8E, 16E, 32E, 64E according the endoreplication state (E). At the 64E position, a comparison of the C-value and E-value outlined that only 28% of the expected 64C-DNA were present in Vanilla planifolia nuclei.
Fig. 1.—
Partial endoreplication in four Vanilla spp. contrasting with euploid endoreplication. (A) DNA histogram of young leafy apex from Vanilla planifolia. Note the 2C nuclear population and endoreplicated nuclear populations 4E, 8E, 16E, 32E, and 64E from young leafy apex of an in vitro Vanilla planifolia plant. (B) DNA histograms from V. pompona, V. ×tahitensis; V. mexicana; V. planifolia, and Phalaenopsis sp. (tomato or Artemisia arborescens as internal standard.) The peaks correspond to 2C, 4E, 8E, 16E, 32E, and 64E. For Phalaenopsis sp. these are simply 2C–64C. (C) Plotting DNA index R as a function of DNA copies state allows determination of the endoreplicated proportion P. Regression lines for each species are shown, with their respective functions and correlation coefficients R2 (in all cases >0.999). The estimated function of each graph is y = P x + F, where P is the endoreplicated proportion, and F the fixed proportion of DNA. Each point is the mean of at least 30 measurements. Note that P value varies between Vanilla species, being the lowest in Vanilla pompona.
Similar DNA histograms of endoreplicated nuclei were observed in three other species of Vanilla, that is, Vanilla pompona, Vanilla ×tahitensis, and Vanilla mexicana (fig. 1B). These Vanilla histogram profiles contrasted with histograms obtained with foliar nuclei from Arabidopsis thaliana (2C = 0.330 pg) or from the orchid Phalaenopsis sp. (2C = 8.46 pg) which showed normal euploid endoreplication, resulting in a binary exponential of endocycles 2C, 4C, 8C, etc. nuclei (fig. 1B, bottom line) characteristic of the so-called “whole-genome endoreplication” (Trávníček et al. 2015). These results strongly suggest that endoreplication in those four Vanilla species was only partial.
To complete these observations, the relative fluorescence intensity of endoreplicated nuclei of the four Vanilla species was compared with the 2C peak, defining the DNA index R. (fig. 1C). Here again, the evolution of DNA index was clearly lower in the four Vanilla species studied when compared with normal euploid endoreplication described in Phalaenopsis sp, further illustrating that the endoreplication was not whole genome, but partial.
Interestingly, the profiles of partial endoreplication appeared to vary between species, suggesting that the proportion of the genome potentially Participating in endoreplication (P) was different from one plant species to another, both in relative and absolute terms. As shown in figure 1C, the P fraction of Vanilla mexicana was higher than in Vanilla pompona, being the lowest (see also table 2).
Table 2.
Mean P-Factors of Five Vanilla spp. and of 65 Diploid F1-Hybrids, with Their Quantitative 2C and 2p Equivalents
| Population | 2C (sd) (pg) | P-factor mean* (sd) | 2p equivalent from P × 2C (pg) |
|---|---|---|---|
| V. mexicana | 4.759 (0.101) | 0.831 (0.036) | 3.955 |
| V. pierrei | 5.087 (0.061) | 37 | 1.88 |
| V. planifolia | 4.59 | 0.284 (0.003) | 1.304 |
| V. pompona | 7.015 (0.311) | 0.1910 | 1.340 |
| V. ×tahitensis | 4.226 (0.116) | 0.2675 | 1.130 |
| Hybrids V. ×tahitensis × V. pompona | 5.592 (0.163) | 0.2107 (0.0114) | 1.178 (0.002) |
| Theoretical midpoint between parents V. t × V. p | 5.621 | 0.2293 | 1.235 |
The next step was therefore to investigate the extent and the features of these partial endoreplication processes in various tissues of Vanilla spp.
Occurrence of a Strict Partial Endoreplication in Vanilla planifolia
In order to assess any variability in the endoreplication process within one plant, the nuclear populations from different parts of the plant were analyzed, as illustrated for Vanilla pompona or Vanilla planifolia (table 1 and fig. 2).
Partial endoreplication occurred in all the tissues studied (table 1). However the profiles of the nuclear DNA histograms changed according to the tissue sampled, the plant age and the conditions of growth, etc. (fig. 2). Their interpretation must be done with serious attention to avoid erroneous estimations of genome-size and of the endoreplication process itself, as described below.
As illustrated in figure 2, the frequency of each endocycle population in the sample differs from tissue to tissue.
In Vanilla somatic tissues, surprisingly, euploid nuclei were difficult to identify. In nuclear suspensions from leaves—whether very young, expanding or mature—the dominant class was typically 8E or 16E nuclei, and the highest level of endoreplication was generally 32E or 64E (fig. 2B). The 2C orchid nuclei were undetected in the histogram from young apical tissue (fig. 2B, top), while some 4E nuclei were present. Similarly, the first peak of orchid nuclei in this sample from a young leaf was the 8E population: 2C and 4E were not detected (fig. 2B, middle). In the root, nuclear populations were also often lacking a population of 2C nuclei. Similarly, in nuclei extracted from vigorous young aerial roots of V. planifolia, 2C and 4C nuclei were detected as traces only in the distal 2 mm segments (table 2b). Samples from older tissue above 1 mm in these roots contained only endocycle nuclei. Endoreplication processes were hardly detectable in the pod and in the seeds where a large 2C or 4C populations were present (data not shown).
Still, the 2C nuclei position is essential for assessing genome-size and therefore its absence may led to erroneous estimations of genome-size and of the endoreplication process itself.
Euploid nuclei were however well present in meristematic tissues. In this monocot family, the meristems lie in the nodes and their axillary buds (fig. 2A), and in the apices of roots and aerial roots. Taking nodal tissue, the 2C nuclei constituted exceptionally a fifth of this sample of orchid nuclei. Populations of larger nuclei were also evident: 4E, 8E, 16E, 32E, and a trace of 64E. The node of Vanilla stem therefore appears as the most reliable tissue for cytometric genome-size analysis requiring 2C nuclei (fig. 2B, bottom)
Despite of the variations recorded in the histogram profiles through the various samples, a striking observation is that the progression of partial endoreplication remained unchanged—as the Interpeak Ratios did not vary (table 1a). This observation defines the partial replication process as a strict event regulated all along the endocycle on vanilla nuclei. We will thus term the mechanism described above as strict partial endoreplication (SPE) process. That means that the P fraction of the genome participating to endoreplication is constant in the whole plant.
Cytological Features of Vanilla Nuclei Undergoing Strict Partial Endoreplication
We investigated both morphological (evolution of nuclei and nucleoli volumes during continuous endocycles) and biochemical features (base composition analyses) of endoreplicated nuclei in Vanilla planifolia in order to correlate flow cytometry data with structural or molecular information.
First of all, nuclear population of Vanilla planifolia was imaged by microscopy to investigate potential morphological features associated with endoreduplication processes (fig. 3). The nucleolar size is a well-known indicator of transcriptional activity, which is expected to be stronger in endoreplicated nuclei as previously shown for tomato pericarp nuclei (Bourdon et al. 2012). Volume of endocycle nuclei increased proportionally to their DNA value. In other words, the quantitative DNA staining (the parameter for sorting) and the nuclear volumes (from imaging) proved to be coherent with what has been observed in other endoreplicating tissues. An 8-fold increase in (partial) genome copy number from 2 to 16 has resulted in only a 3.5-fold increase in nuclear volume. Contrastingly, the nucleolar proportion (and absolute volume) accounted from 2% to 12% of nuclear volumes, increasing with endoreplication. For instance although the 4E nuclei have only 28% more DNA than the 2C euploid nuclei, they were twice the volume and their nucleoli were almost four times in volume. In this structural study, no nuclear subdomain was observed that might correspond to one full euploid copy “kept to the side/in reserve.”
Fig. 3.—
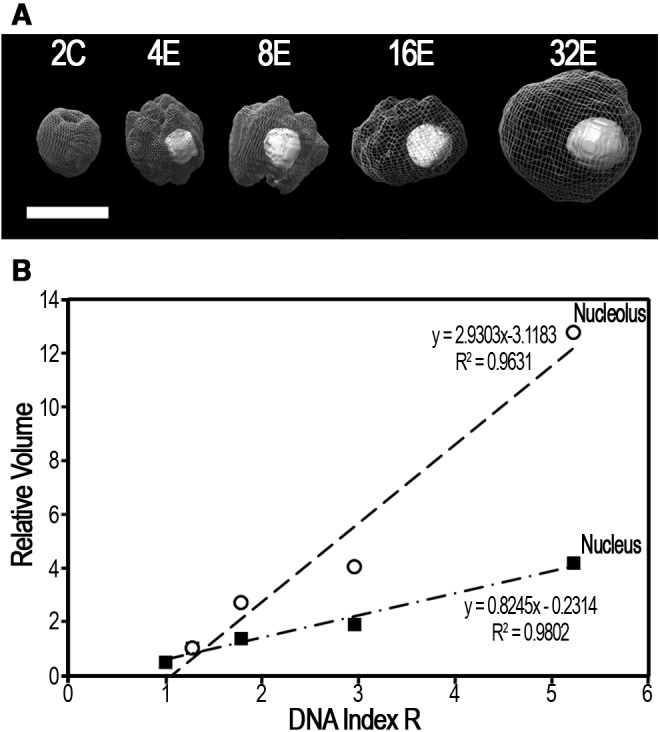
Relative volumes of nuclei and nucleoli increase with DNA Index R. (A) 3-dimensional surface rendering of deconvolved z-stacks of representative ploidy-sorted nuclei. For each sample, 10–40 nuclei were assessed. Scale bar: 5 µm. (B) Nuclear and nucleolar volumes (µm3) were, respectively, normalized to those ones of the 4E, and represented in function of DNA Index R. Nucleolus from 2C-nuclei was too small and undetectable for volume estimation.
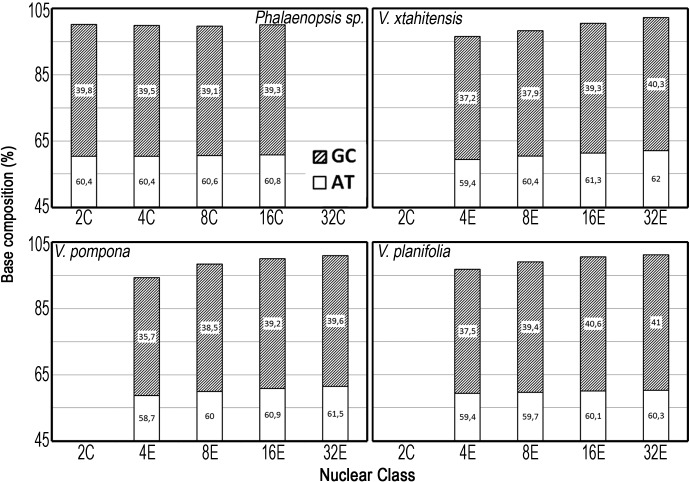
Secondly, we questioned a potential correlation between strict partial endoreplication and the evolution of DNA base composition of nuclear populations (fig. 4). For Vanilla planifolia, V. pompona and V. ×tahitensis, the genome base composition of endoreplicated nuclei was not significantly different from that of the 2C nuclei, that is, 39.6% GC (sd 0.5%). The sum of the two estimations, AT and GC, should of course approach 100% as observed for Phalaenopsis sp. (fig. 4) for which the various classes of endoreplicated nuclei displayed homogeneous properties towards the stains. Surprisingly, the endoreplicated nuclei showed a drift in this 3-way comparison: as strict partial endoreplication progressed, the stainability of the nuclei of the three Vanilla spp. evolved. Their chromatin apparently became less accessible to the intercalary dye propidium iodide, or relatively more accessible to the nonintercalating dyes.
Fig. 4.—
Base-composition analysis of nuclear populations 2C, 4E, 8E, 16E, and 32E using fluorescent stains: the accessibility of chromatin to stains changes with partial endoreplication. AT% deduced from Hoechst 33342 and GC% deduced from chromomycin A3 versus propidium iodide. The average base composition of the Vanilla spp. was GC = 39.6% (sd 0.52%) from two replicated experiments both taking ten samples for each stain (and 4–5 peaks within each histogram). Phalaenopsis sp. had GC = 39.4%. The average CV of cytometry histograms was 4.35%, V. pompona being inferior with average CV = 5.3%. Note the evolution of the stainability properties in the three species of Vanilla.
To sum up, nuclei imaging allowed to confirm that the transcription activity increased with E state, confirming that the P fraction is indeed transcribed. Analysis of base composition by cytochemistry however did not permit to conclude on evolution of base composition through endocycles, but did outline a possible variation in chromatin concentration between species.
Strict Partial Endoreplication Occurs Across Vanilla spp. with a Species Specific P Factor
The next question was to know if the strict partial endoreplication process was a generic feature in Vanilla spp. Strict partial endoreplication was consistently observed across the 25 Vanilla spp. and two hybrids examined (table 3), confirming our first reports (Bory et al. 2008; Lepers-Andrzejewski et al. 2011). Each species had a characteristic P factor, whatever the source of material, including those originating from different geographical regions. This observation reported for four species in table 2, included V. ×tahitensis which was recorded as an ancestral hybrid (Lepers-Andrzejewski et al. 2010), involving Vanilla planifolia as one of the genitors.
Pursuing the notion that this phenomenon might be an epigenetic adaptation, we examined 65 F1 hybrid plants from the cross V. ×tahitensis (♀) × V. pompona (♂) with, respectively, 2C = 4.226 pg and 7.015 pg and P = 0.2675 and 0.1910 (table 2).
All hybrids showed partial endoreplication with not a single case where the phenomenon was suppressed. Firstly, the hybrids appear from their 2C value to have the equivalent of a haploid complement from each parent: their mean genome-size (5.592 pg) sits nicely near the midpoint between the 2C values of the two parents (5.621 pg) (fig. 5). But secondly hybrid P factors skewed from the midpoint between the parents (0.2293) to the low value characteristic of V. ×tahitensis (table 2 and fig. 5). In other words, the F1-hybrids had—as expected—a balanced mixture of the parental chromatin, but somatic editing during endoreplication skewed in favor of the more compact V. ×tahitensis chromatin (see fig. 4 and related text). Such skewed distribution of the P-factor can only arise if in somatic nuclei the endocycle participating part of the larger V. pompona is underrepresented. In our case, it appears as if the more compact genome of V. ×tahitensis (its 2p component is smaller, only 84% that of V. pompona) is preferentially retained, may be to face the needs of somatic expansion (fig. 5).
Fig. 5.—
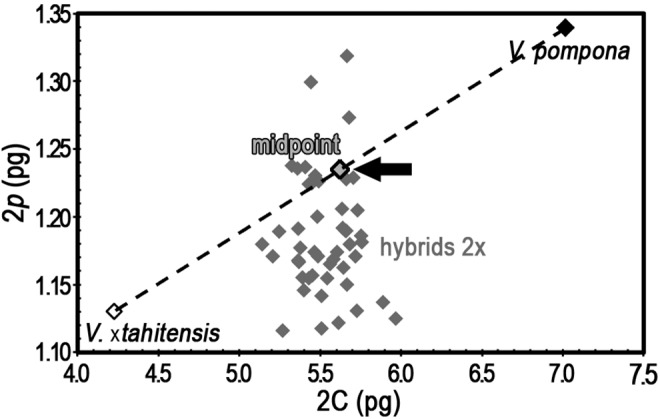
Hybridization does not repress the partial nature of endoreplication. Genome-size (2C pg) versus the quantity of DNA replicating at the first endocycle (2p pg) for 65 diploid hybrids and the parents V. ×tahitensis and V. pompona. The theoretical midpoint between the parents is indicated by an arrow. See table 2 (grey row) for data. 2p is expressed in absolute units (pg) to avoid using proportions when the relevant genome-sizes differ. The mean genome-size of the hybrids was close to the mean between the parent species (arrowhead) (5.592 vs. 5.621 pg, see table 1), reflecting a balanced mixture of the parental chromatin. Note that hybrid 2p equivalent skewed from the midpoint between the parents to the low value characteristic of V. ×tahitensis.
Strict Partial Endoreplication Occurs in Many Sections of Orchidaceae
The Orchidaceae family is the largest of the monocots, comprising c. 25,000 species, with genome size ranging c. 168-fold (Leitch et al. 2009). Table 3 is a compilation of observations relative to partial endoreplication in 136 accessions corresponding to 126 species and three hybrids across 68 genera of this family and those in the comprehensive study by Trávníček et al. (2015). Strict partial endoreplication appears to be the rule in subfamilies Orchidoideae, Vanilloideae, and Cypripedioideae. In a sampling of 134 accessions, 77 were presenting strict partial endoreplication, 50 displayed conventional endoreplication, and only seven did not show endoreplication after the 4C state. As analyzed from this table 3, the Vanilloideae and Orchidoideae were definitely the most representative subfamilies of Orchidaceae for SPE (respectively, 36 out 36 samples, 16 out 16 samples), meanwhile the Cypripedioideae exhibited five cases of SPE but one case of conventional endoreplication. Epidendroideae subfamily presented a mix of DNA replication processes (20 cases of SPE, 48 cases of conventional endoreplication, seven with none endoreplication).
Where the extent of partial endoreplication was differentially measured in parents, the hybrid progeny also displayed the process. Conversely, most samples from the Epidendroideae subfamily showed euploid endocycles, as did the one Apostasioideae in Trávníček et al. (2015). A spontaneous hybrid population, Anacamptis palustris × purpurea, found near a highway in Fos, France (pers. obs. Alain Fridlender) displayed partial endoreplication like its parents.
Discussion
As in numerous other plant families, developmentally regulated endoreplication occurs in the tissues of many orchids. Developmental pattern, molecular mechanisms and cell biological implications of endoreplication have been addressed in many reports (Barow and Meister 2003; Barow and Jovtchev 2007; Lee et al. 2009; Breuer et al. 2014). Endoreplication is one morphogenetic factor conducive to cell enlargement: in tomato pericarp, increasing endoreplication is associated with increasing transcriptional activity and a highly invaginated nuclear membrane ensuring nucleo-cytoplasmic exchange, as envisaged in the karyoplasmic ratio theory (Chevalier et al. 2013; Pirrello et al. 2014). Coordination between organelles and genomes is part of this concept (Raynaud et al. 2005; Chevalier et al. 2013). In terms of cell energetics and metabolism, endoreplication appears as an economical mechanism for somatic growth without the full cost of cytokinesis.
Orchids present a distinct case of developmentally regulated endoreplication that is a strict partial endoreplication (SPE), an original remodeling process of genomic DNA. Full genetic information is conserved in the nuclei of somatic tissues, but only part of it is selectively amplified through up to five rounds of endoreplication. In Vanilla species, this leads to formation of highly asymmetric nuclei where part of the genetic information is stable in dual copies (fraction F) whereas the rest (fraction P) is amplified up to 64 times. This is associated with a marginal nuclear volume increase compared with the strong increase of nucleolar volumes. The implication, for somatic development, is that a part of the genome may be minimized while another part is amplified in line with the putative advantages of endoreplication. Interestingly, the fact that in hybrid plants the endoreplicating part had properties closest to the parent which had the smaller (more compact) genome, might suggest privileged mechanisms to insure the needs of somatic expansion in the hybrid population.
Still, endoreplication with such massive genome imbalance is unparalleled, and raises numerous questions.
Strict Partial Endoreplication (SPE) versus Progressive Partial Replication (PPE)
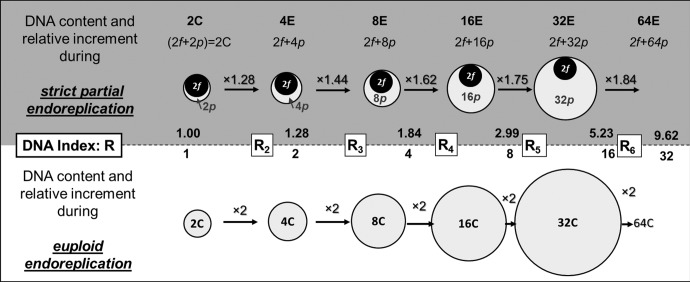
In a previous study, we had already reported that the peak-to-peak ratios of nuclear DNA levels tended towards a doubling as endocycles progressed (Bory et al. 2008). At the time we coined the term “progressively partial endoreplication” to describe such endoreplication process in orchids, a term, used recently in Trávníček et al. (2015), as “PPE”, and in Hriˇbová et al. (2016). However, our present results demonstrate that there is nothing progressive in the delimitation of the participating matrix: it is endoreplicating as a constant matrix. Accordingly, we have decided to shift to “strict partial endoreplication” (SPE) as the most pertinent expression. A phenomenological model to illustrate this original process of DNA remodeling is presented figure 6 with chi-squared evaluation of robustness. The evaluation of the P and F proportions (or the related quantities p and f) for each sample revealed species specificity. In our panel, this factor P ranged from 19% in Vanilla pompona to almost euploid endoreplication (P = 100%) in many Epidendroideae. Experimental error suggests that any values of P ≥ 95% may be interpreted as classical “whole-genome endoreplication”, to use the term of Trávníček et al. (2015).
Fig. 6.—
A general model for strict partial endoreplication. In this cartoon, the area of each object faithfully reflects its relative size. This example is based upon leafy apex of diploid Vanilla planifolia: 2n = 2x = 32 with 2C = 4.59 pg and P = 0.284 ± 0.003 (32 estimates) so 2p = 1.30 pg in the diploid nucleus whereas the major nonreplicating component is 2f = 3.29 pg. Canonical euploid endoreplication yields nuclear classes of 2C, 4C, 8C, 16C, 32C, 64C, etc. Strict partial endoreplication yields nuclear classes termed 2C, 4E, 8E, 16E, 32E, where E symbolizes a state related to the number of copies of the endoreplicated DNA. F is the fixed proportion of the haploid genome which cannot endoreplicate and P the part potentially participating in endoreplication. The term “strict” supports the observation that F and P are constant during development of a given plant. The italic lower cases (f, p) denote the respective DNA quantities of F and P fraction. R is the DNA index. The DNA index of the second peak in a partial endoreplication series, R2, gives an initial estimation of P = (R2 − 1), 1 being the P value in classical endoreplication.
Biological Significance of SPE
One main question is to understand how the genome imbalance is managed in terms of chromosome organization in the endoreduplicated nuclei. Several hypotheses may be proposed:
-
a)
Base composition in whole nuclei in the Vanilla genus, shows a GC content around 39.6%, as often found in monocots. In Musa acuminata this is typical of protein-coding sequences (D’Hont et al. 2012). This value differs from the one reported by Jersáková et al. (2013), which, using a different algorithm, reported atypically GC content low values in the subfamily Apostasioideae (34–37.4%). Although our cytometry approach does not permit to detect minor deletions or changes, it still revealed some differences in the conformation of endoreplicated chromatin, which may potentially result from epigenetic marks. In classical plant endoreplication, the chromosomes are uniformly polytenic (Bourdon et al. 2011). In the case of strict partial endoreplication, large segments of chromatin amplified up to 64-fold may be parallel structures so that part of a chromosome is polytene, other parts not. This would be closely related to the mechanism reported for trophoblast giant cells of the placenta (Hannibal et al. 2014).
-
b)
Alternatively, in these somatic tissues, are cycles of whole genome duplication followed by selective elimination of DNA? None of the cytometric data point towards this hypothesis. Wherever it occurs, apoptosis or similar chromatin degradation is immediately evident in DNA histograms obtained from cytometry. We did not observe such events in any of the analyzed samples. In Vanilla pompona such a mechanism would imply degradation of 81% of a freshly duplicated genome, whereas the diploid genome must be protected. This would be an energy-inefficient procedure. Yet, the pioneering observations of foliar ideoblasts in Vanilla planifolia had described increasing chromocentres, dispersion of preprophase heterochromatin, with neither mitosis nor polytene chromosomes (Kausch and Horner 1984). These authors assessed DNA loss at ∼50%, but they had probably also overlooked the true 2C nuclei. In fact, loss of ∼50% was probably evaluated from comparison between the 4E and 8E nuclei. In Cymbidium ceres, no evidence for polytene chromosomes was found neither by electron microscopy nor through enlarged endochromocentres despite apparent differential replication including an AT-rich satellite (Nagl and Rücker 1976). Hriˇbová et al. (2016) demonstrated, using EdU incorporation in nuclei of root meristems of the orchid Ludisia dicolor, that endoreplication involves only part of the nuclear DNA, allowing them to reject a model where whole-genome endoreplication would be followed by selective DNA elimination. By Illumina sequencing of DNA from flow sorted nuclei, either 2C or endoreplicated (2C+2p) [our nomenclature], it was shown that the proportion of high repetitive DNA sequences (LTR retroelements, tandem repeats, rDNA sequences, etc.) in this endoreplicated fraction was less than in the initial 2C genome, but no specific element seemed to be the target of this endoreplication. The observed orchid Ludisia dicolor has a small genome and is, advantageous for sequencing, but P = 59% means that the endoreplicated fraction is quite high. Comparing the replicated fraction with the initial genome would have higher contrast if the studied orchid had a low P-value, as does Vanilla pompona with P = 19% (but a large genome, 2C = 7.015 pg).
-
c)
Might the P-factor reveal massive epigenetic adaptation? Apparently not, given that it appears to be particularly stable and characteristic for a given Vanilla species despite the diverse origins and high polymorphism in the field. Then, what could be the nature of the “F” region versus the “P” fraction in the genome?
-
d)
May the P factor indicate a specific genome protection? An advantage might be to favor the integrity of the genome through relative isolation of one euploid genome during plant growth, because the active chromatin is more exposed to transformation from interactions with plants, microorganisms, insects, etc. After all, the Orchidaceae are highly promiscuous interactors with other organisms. Plant genomes generally have an aggressive epigenomic surveillance system to purge invasive sequences such as young Long Terminal repeat Retrotransposons (Michael 2014). Such retrotransposons may amplify up during endoreplication by a “copy-and-paste” mechanism. This is probably not possible in the Fixed fraction, and possibly in one full euploid copy “kept to the side, in reserve”, thereby offering some protection of the genome.
-
e)
What is the nature of the F fraction (DNA that does not undergo endoreplication)? In Vanilla spp. the F fraction finally ranged from 81% to 17% of the genome. One may speculate that it corresponds essentially to noncoding DNA, highly repeated sequences and transposable elements contributing to chromosome architecture and regulation. Yet the dual copy number of this part of the genome is sufficient to ensure proper orchid development. Although plants do not maintain a germinal cell lineage, these orchids produce true haploid gametes despite their genome imbalance in somatic tissues. Apparently, the holoploid genome is distinctively marked to permit precise euploid gametogenesis.
Repression of replication of certain regions of the genome has been reported in other systems, notably Drosophila (Nordman et al. 2012, Frawley and Orr-Weaver 2015). Underreplicated regions are generally silent (Nordman et al. 2012, Orr-Weaver 2015), meaning that it may be advantageous to repress amplification of genome regions which display very few genes, like in heterochromatic regions. In Drosophila, it has been shown that Suppressor of UnderReplicated protein (SUUR), associated to PCNA constitute a complex which binds chromatin in a cell specific manner (Nordman and Orr-Weaver 2015; Orr-Weaver 2015). In the absence of SUUR there is constitutive DNA damage within heterochromatin whereas euchromatin needs SUUR constitutive expression to generate DNA damage. SUUR-mediated repression of DNA replication is associated with this DNA damage and genome instability within under replicated regions while the fork progresses. In Drosophila polytene chromosomes, it was shown that there were 112 underreplicated regions of 60–480 kb in size (Yarosh and Spradling 2016). Whether the same mechanism holds in the strict partial endoreplication (SPE) mechanism that we and others (Bory et al. 2008; Lepers-Andrzejewski et al. 2010; Hriˇbová et al. 2016) reported has to be addressed.
Experimental Approaches and Perspectives
Our data corroborated the finding of Trávníček et al. (2015) showing that the identification of the 2C population was essential to assess genome size and strict partial endoreplication in orchids. We believe that many genome-size assessments in orchids are erroneous as the 2C nuclear population has been overlooked in preparations from foliar tissue for cytometry. Published 2C data on Orchidaceae must therefore be used with particular caution. This has already been raised (Bory et al. 2008; Lepers-Andrzejewski et al. 2010; Trávníček et al. 2011, 2015): we ourselves have made the error with Vanilla planifolia, V. pompona, etc., Table 3 updates our estimates. The use of a plant tissue clearly expressing a 2C population will facilitate the manipulation and quality of nuclei. Trávníček et al. (2015) proposed that ovaries and pollinaria were favorable tissues for reliable levels of true 2C nuclei. From our studies, we believe that the nodes from plants of Vanilla planifolia are a first choice for isolating elements linked to partial endoreplication. This is a tractable system adequate and rich in 2C, ideal for understanding endoreplication, via genome sequencing. This is especially so when in vitro plants are available.
It also becomes clear that any attempt to genetically modify orchids should aim at tissue that is particularly rich in 2C nuclei. Otherwise, the eventual recombinants may have an imbalanced genome. We recall that early protocols to modify Arabidopsis thaliana by genetic transformation used endoreplicating petioles that then yielded unwanted polyploid individuals (Sangwan et al. 1992).
A comparative genomic approach to obtain sequences indicative of the F and P fractions could use DNA obtained from nuclei sorted by cytometry, taking on one side 2C nuclei (2f +2p) and on the other 32E endoreplicated nuclei (e.g., 2f + 32p). This DNA would be used to prepare a BAC library. With a six-way sorting cytometer, one can purify nuclei from each of the nuclear classes in parallel, in order to follow the progressive nuclear imbalance.
In order to identify a putative nuclear compartment characterized by intense epigenetic marks, dual immunolabeling of nuclear suspensions, which were then analyzed by multi-color cytometry could be used. This has been performed in Arabidopsis thaliana to quantify two epigenetic marks on nuclei from different classes (Bourbousse et al. 2015) or in tomato pericarp nuclei to assess RNA polymerase states (Pirrello et al. 2014). These approaches could serve to quantify (by cytometry) and map (by imaging) epigenetics marks, nuclear structure and nuclear compartmentation along the endocycle process.
However, no doubt the most urgent need is for sequencing enriched DNA in order to have putative markers of the F (Fixed) and P (Participating in endoreplication) components which, in epigenetic terms, might be denoted F-chromatin and P-chromatin. Phalaenopsis sp. (table 3) may be used as a reference material with canonical euploid endoreplication.
Last but not the least, Vanilla is a key resource in the tropical areas. The challenge for promoting sustainable production of Vanilla is based on our ability to protect the wild species through understanding of their genome evolution and their conservation. This biodiversity should allow improvement of the cultivated Vanilla spp. Furthermore, molecular analyses of orchids should lead to a better understanding of the fundamental contributions of endoreplication in plant development—as here an edited copy of the significant part of the genome is available.
Acknowledgments
We thank colleagues identified below for their precious samples and interest in the theme. In this respect, thanks are due to Magda Bou Dagher-Kharrat (Université Saint-Joseph, Mar Roukos, Mkalles, Lebanon); Fatima Pustahija, (University of Sarajevo, Zagrebacka 20, Bosnia and Herzegovina); Michel Grisoni (Cirad, Pôle de Protection des Plantes, 97410 St Pierre, France); Alain Fridlender, (Université de Provence, Marseille); Nicolas Barre (Association Guadeloupéenne d’Orchidophilie); Daniel Silvestre (Parc National Guadeloupe); Rodolphe Gigant (Université de la Réunion); Danièle Roque (Centre de Ressources Biologiques Plantes Tropicales (CRB-PT), Guadeloupe); Marpha Telepova and Denis Larpin (Museum National d’Histoire Naturelle, Jardin Botanique, Paris, and Russian Academy of Sciences Komarov Botanical Institute, Herbarium (LE). The contributions of Olivier Catrice (Imagif) and Cécile Raynaud (IPS2) have been valued. We thank Romain Le Bars (I2BC) for assistance in quantitative imaging.
The present work has benefited from the core facilities of Imagerie‐Gif, (http://www.i2bc.paris-saclay.fr), member of IBiSA (http://www.ibisa.net). It was supported by the Programme d’Investissement d’Avenir “France‐BioImaging” (Agence Nationale de la Recherche ANR‐10‐ISBN‐04‐01), and the Labex “Saclay Plant Science” (Agence Nationale de la recherche, ANR‐11‐IDEX‐0003‐02). It was carried out with the financial support of the French Agence Nationale de la Recherche through the Endorepigen grant (ANR-09-GENM-105), Vabiome (ERANET Netbiome #11-EBIM-005-01, #11-EBIM-005-05, #11-EBIM-005-04 and #11-EBIM-005-06). S.B. and M.B. planned and performed the experiments. S.B., M.D., S.S.Y., M.B., and B.S.J. contributed to the manuscript writing. MWB and SB performed the quantitative imaging. S.L., S.S.Y., and P.B. prepared the Vanilla biological material, S.L. and S.S.Y. performed the Cytogenetics observations; S.B., N.M., M.W., and M.B. performed the Cytometric analyses.
Literature Cited
- de Almeida Engler J, Gheysen G. 2013. Nematode-induced endoreplication in plant host cells: why and how? Mol Plant Microbe Interact. 26:17–24. [DOI] [PubMed] [Google Scholar]
- d’Amato F. 1964. Endopolyploidy as a factor in plant tissue development. Caryologia 17:41–52. [Google Scholar]
- Arias EE, Walter JC. 2007. Strength in numbers: preventing rereplication via multiple mechanisms in eukaryotic cells. Genes Dev. 21:497–518. [DOI] [PubMed] [Google Scholar]
- Barow M, Jovtchev G. 2007. Endopolyploidy in plants and its analysis by flow cytometry In: Dolezel J, Greilhuber J, Suda J, editors. Flow cytometry with plant cells. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co; p. 349–372. [Google Scholar]
- Barow M, Meister A. 2003. Endopolyploidy in seed plants is differently correlated to systematics, organ, life strategy and genome size. Plant Cell Environ. 26:571–584. [Google Scholar]
- Bory S, Brown S, Duval MF, Besse P. 2010. Evolutionary processes and diversification in the genus Vanilla In: Odoux E, Grisoni M, editors. Vanilla. Boca Raton: Taylor & Francis Books; p. 15–29. [Google Scholar]
- Bory S, et al. 2008. Natural polyploidy in Vanilla planifolia (Orchidaceae). Genome 51:816–826. [DOI] [PubMed] [Google Scholar]
- Bou Dagher-Kharrat M, et al. 2013. Nuclear DNA C-values for biodiversity screening: case of the Lebanese flora. Plant Biosyst. 147:1228–1237. [Google Scholar]
- Bourbousse C, et al. 2015. Light signaling controls nuclear architecture reorganization during seedling establishment. Proc Natl Acad Sci U S A. 112(21):E2836–E2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdon M, et al. 2011. In planta quantification of endopolyploidy using Fluorescent In Situ Hybridization (FISH). Technical Advance. Plant J. 66:1089–1099. [DOI] [PubMed] [Google Scholar]
- Bourdon M, et al. 2012. Evidence for karyoplasmic homeostasy during endoreplication and a ploidy-dependent increase in gene transcription during tomato fruit growth. Development 139:3817–3826. [DOI] [PubMed] [Google Scholar]
- Boveri T. 1887. Über Differenzierung der Zellkerne während der Furchung des Eies von Ascaries megalocephala. Anat Anz. 2:297–344. [Google Scholar]
- Breuer C, Braidwood L, Sugimoto K. 2014. Endocycling in the path of plant development. Curr Opin Plant Biol. 17:78–85. [DOI] [PubMed] [Google Scholar]
- Brown SC, Devaux P, Marie D, Bergounioux C, Petit P. 1991. Cytométrie en flux: Application à l’analyse de la ploïdie chez les végétaux. Biofutur 105:1–14. [Google Scholar]
- Brown S, Catrice O, Siljak-Yakovlev S, Mergaert P, Satiat-Jeunemaître B. (2010) Le cycle et l’endoréplication chez les végétaux. Dans: Cycle cellulaire et cytométrie en flux In: Ronot X, Grunwald D, Mayol J-F, editors. Lavoisier, Paris: Tec & Doc; pp. 190–213, 274–276. [Google Scholar]
- Chevalier C, et al. 2013. Endoreduplication and fruit growth in tomato: evidence in favour of the karyoplasmic ratio theory. J Exp Bot. 65:2731–2746. [DOI] [PubMed] [Google Scholar]
- Cross JC. 2014. More of a good thing or less of a bad thing: gene copy number variation in polyploid cells of the placenta. PLoS Genet. 10(5):e1004330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degtyarev SV, et al. 2004. The molecular structure of the DNA fragments eliminated during chromatin diminution in Cyclops kolensis. Genome Res. 14:2287–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Hont A, et al. 2012. The banana (Musa acuminata) genome and the evolution of monocotyledonous plants. Nature 488:213–219. [DOI] [PubMed] [Google Scholar]
- Drouin G. 2006. Chromatin diminution in the copepod Mesocyclops edax: diminution of tandemly repeated DNA families from somatic cells. Genome 49:657–665. [DOI] [PubMed] [Google Scholar]
- Doležel J, Bartoš J, Voglmayr H, Greilhuber J. 2003. Nuclear DNA content and genome size of trout and human. Cytometry 51:127–129. [DOI] [PubMed] [Google Scholar]
- Duret L, et al. 2008. Analysis of sequence variability in the macronuclear DNA of Paramecium tetraurelia: a somatic view of the germ line. Genome Res. 18:585–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferko MC, Patterson BW, Butler PJ. 2006. High-resolution solid modeling of biological samples imaged with 3D fluorescence microscopy. Microsc Res Tech. 648– 655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frawley LE, Orr-Weaver TL. 2015. Polyploidy. Curr Biol. 25:R345–R361. [DOI] [PubMed] [Google Scholar]
- Galbraith DW, Harkins KR, Knapp S. 1991. Systemic endopolyploidy in Arabidopsis thaliana. Plant Physiol. 96:985–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia S, Garnatje T, Twibell JD, Vallès J. 2006. Genome size variation in the Artemisia arborescens complex (Asteraceae, Anthemideae) and its cultivars. Genome 49:244–253. [DOI] [PubMed] [Google Scholar]
- Greilhuber J, Doležel J, Lysak MA, Bennett MD. 2005. The origin, evolution and proposed stabilization of the terms “genome size” and “C-value” to describe nuclear DNA contents. Ann Bot. 95:255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goday C, Esteban MR. 2001. Chromosome elimination in Sciarid flies. Bioessays 23:242–250. [DOI] [PubMed] [Google Scholar]
- Godelle B, Cartier D, Marie D, Brown S, Siljak-Yakovlev S. 1993. A heterochromatin study demonstrating the non-linearity of fluorometry useful for calculating genomic base-composition. Cytometry 14A:618–626. [DOI] [PubMed] [Google Scholar]
- Hannibal RL, et al. 2014. Copy number variation is a fundamental aspect of the placental genome. PLoS Genet. 10(5):e1004290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hriˇbová E, et al. 2016. The enigma of progressively partial endoreplication: new insights provided by flow cytometry and next generation sequencing. Genome Biol Evol. 8(6):1996–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob Y, et al. 2010. Regulation of heterochromatic DNA replication by histone H3 lysine 27 methyltransferases. Nature 466:987–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jersáková J, et al. 2013. Genome size variation in Orchidaceae subfamily Apostasioideae: fi the phylogenetic gap. Bot J Linn Soc. 172:95–105. [Google Scholar]
- Kausch AP, Horner HT. 1984. Increased nuclear DNA content in raphide crystal idioblasts during development in Vanilla planifolia L. (Orchidaceae). Eur J Cell Biol. 33:7–12. [PubMed] [Google Scholar]
- Kondorosi E, Roudier F, Gendreau E. 2000. Plant cell-size control: growing by ploidy?. Curr Opin Plant Biol. 3:488–492. [DOI] [PubMed] [Google Scholar]
- Kondorosi E, Mergaert P, Kereszt A. 2013. A paradigm for endosymbiotic life: cell differentiation of Rhizobium bacteria provoked by host plant factors. Annu Rev Microbiol. 67:611–628. [DOI] [PubMed] [Google Scholar]
- Lee HO, Davidson JM, Duronio RJ. 2009. Endoreplication: polyploidy with purpose. Genes Dev. 23:2461–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitch I, et al. 2009. Genome size diversity in orchids: consequences and evolution. Ann Bot. 104:469–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepers-Andrzejewski S, Brunschwig C, Collard FX, Dron M. 2010. Morphological, chemical, sensory and genetic specificities of Tahitian vanilla In: Odoux E, Grisoni M, editors. Vanilla, Boca Raton, USA: Taylor & Francis Books; p. 205–228. [Google Scholar]
- Lepers-Andrzejewski S, Siljak-Yakovlev S, Brown SC, Wong M, Dron M. 2011. Diversity and dynamics of plant genome size: an example of polysomaty from a cytogenetic study of Tahitian Vanilla (Vanilla xtahitensis, Orchidaceae). Am J Bot. 98:986–997. [DOI] [PubMed] [Google Scholar]
- Marie D, Brown SC. 1993. A cytometric exercise in plant DNA histograms, with 2C values for seventy species. Biol Cell 78:41–51. [DOI] [PubMed] [Google Scholar]
- Michael TP. 2014. Plant genome size variation: bloating and purging DNA. Brief Funct Genomics 13:255–256. [DOI] [PubMed] [Google Scholar]
- Nagl W, Rücker W. 1976. Effects of phytohormones on thermal denaturation profiles of Cymbidium DNA: indication of differential DNA replication. Nucleic Acid Res. 3(8):2033–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neiman M, Beaton MJ, Hessen DO, Jeyasingh PD, Weider LJ. 2017. Endopolyploidy as a potential driver of animal ecology and evolution. Biol Rev Camb Philos Soc. 92:234–247. [DOI] [PubMed] [Google Scholar]
- Nordman J, Li S, Eng T, MacAlpine D, Orr-Weaver TL. 2012. Developmental control of the DNA replication and transcription programs. Genome Res. 21:175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordman JT, Orr-Weaver TL. 2015. Understanding replication fork progression, stability, and chromosome fragility by exploiting the suppressor of underreplication protein. Bioessays 37:857–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr-Weaver TL. 2015. When bigger is better: the role of polyploidy in organogenesis. Trends Genet. 31:307–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirrello J, et al. 2014. How fruit developmental biology makes use of flow cytometry approaches. Cytometry 85A(2):115–125. [DOI] [PubMed] [Google Scholar]
- Pustahija P, et al. 2013. Small genomes dominate in plants growing on serpentine soils in West Balkans, an exhaustive study of 8 habitats covering 308 taxa. Plant Soil 373:427–453. [Google Scholar]
- Raynaud C, et al. 2005. Cell and plastid division are coordinated through the pre-replication factor AtCDT1. Proc Natl Acad Sci U S A. 102:8216–8221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez L, Perondini ALP. 1999. Sex determination in sciarid flies: a model for the control of differential X-chromosome elimination. J Theor Biol. 197:247–259. [DOI] [PubMed] [Google Scholar]
- Sangwan R, Bourgeois Y, Brown SC, Vasseur G, Sangwan-Norreel B. 1992. Characterization of competent cells and early events of Agrobacterium-mediated genetic transformation in Arabidopsis thaliana. Planta 188:439–456. [DOI] [PubMed] [Google Scholar]
- Siljak-Yakovlev S, et al. 2010. Towards a genome size and chromosome number database of Balkan flora: C-values in 343 taxa with novel values for 242. Adv Sci Lett. 3:190–213. [Google Scholar]
- Smith JJ, Antonacci F, Eichler EE, Amemiya CT. 2009. Programmed loss of millions of base pairs from a vertebrate genome. Proc Natl Acad Sci U S A. 106:11212–11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trávníček P, et al. 2011. Remarkable coexistence of multiple cytotypes of the Gymnadenia conopsea aggregate (the fragrant orchid): evidence from flow cytometry. Ann Bot. 107:77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trávníček P, et al. 2015. Challenges of flow-cytometric estimation of nuclear genome size in orchids, a plant group with both whole-genome and progressively partial endoreplication. Cytometry 87A(10):958–966. [DOI] [PubMed] [Google Scholar]
- Yao MC, Chao JL. 2005. RNA-guided DNA deletion in Tetrahymena: an RNAi-based mechanism for programmed genome rearrangements. Annu Rev Genet. 39:537–559. [DOI] [PubMed] [Google Scholar]
- Yarosh W, Spradling A. 2016. Incomplete replication generates somatic DNA alterations within Drosophila polytene salivary gland cells. Genes Dev 28:1840–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zufall RA, Robinson T, Katz LA. 2005. Evolution of developmentally regulated genome rearrangements in eukaryotes. J Exp Zoolog B Mol Dev Evol. 304:448–455. [DOI] [PubMed] [Google Scholar]