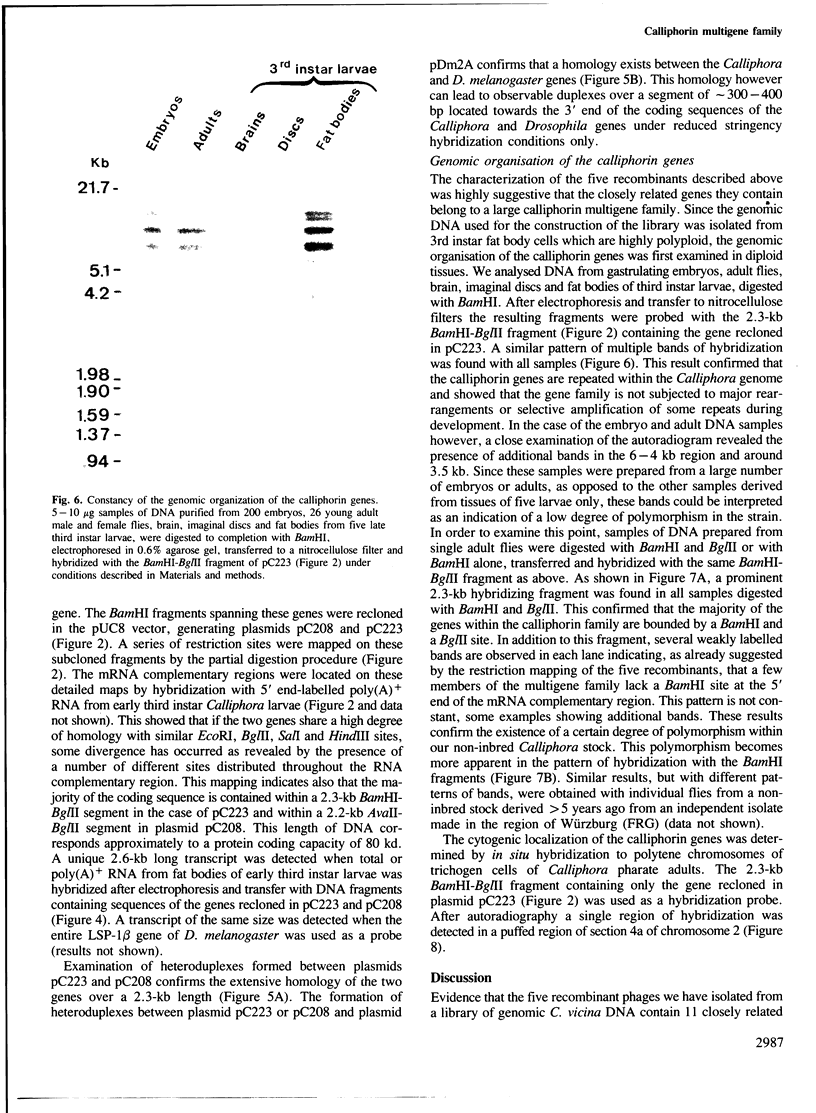
Abstract
A library of Calliphora vicina genomic DNA was constructed in the λEMBL3 vector and screened for recombinant phages containing chromosomal segments encoding calliphorin, the major larval serum protein (LSP) of Calliphora. A large series of recombinants hybridizing with in vitro labelled poly(A)+ RNA from Calliphora larval fat bodies and with specific probes derived from the LSP-1 genes of Drosophila melanogaster was isolated. Five of these phages, chosen at random, were shown by hybrid selection to retain calliphorin mRNA specifically. Eleven calliphorin mRNA-homologous regions were located on restriction maps of these phages by hybridization with 5' end-labelled poly(A)+ RNA from Calliphora larval fat bodies. Each phage contains at least two calliphorin genes arranged in direct repeat orientation and seperated by 3.5–5 kb intergenic regions. The genes display similar but not identical restriction patterns. Filter hybridization and heteroduplex analysis indicate that they share a detectable homology with the LSP-1β gene of D. melanogaster. Whole genome Southern analysis showed that these genes belong to a large family of closely related calliphorin genes which were found by in situ hybridization to polytene chromosomes of trichogen cells to be clustered in region 4a of chromosome 2 of Calliphora vicina.
Keywords: calliphorin, Calliphora vicina, gene evolution, multigene family, polymorphism
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alonso A., Flytzanis C. N., Schätzle U., Scheller K., Sekeris C. E. Purification and reverse transcription of the messenger RNA coding for the insect protein, calliphorin, isolated from larvae of the blowfly, Calliphora vicina R.-D. Eur J Biochem. 1979 Mar;94(2):601–608. doi: 10.1111/j.1432-1033.1979.tb12930.x. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Brock H. W., Roberts D. B. Location of the LSP-1 Genes in Drosophila Species by IN SITU Hybridization. Genetics. 1983 Jan;103(1):75–92. doi: 10.1093/genetics/103.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Gross-Bellard M., Oudet P., Chambon P. Isolation of high-molecular-weight DNA from mammalian cells. Eur J Biochem. 1973 Jul 2;36(1):32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kejzlarová-Lepesant J., Brock H. W., Moreau J., Dubertret M. L., Billault A., Lepesant J. A. A complete and a truncated U1 snRNA gene of Drosophila melanogaster are found as inverted repeats at region 82E of the polytene chromosomes. Nucleic Acids Res. 1984 Dec 11;12(23):8835–8846. doi: 10.1093/nar/12.23.8835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp D. J., Thomson J. A., Peacock W. J., Higgins T. J. Messenger RNA for the insect storage protein calliphorin: in vitro translation and chromosomal hybridization analyses of a 20 S poly(A)-RNA fraction. Biochem Genet. 1978 Apr;16(3-4):355–371. doi: 10.1007/BF00484091. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Lepesant J. A., Kejzlarova-Lepesant J., Garen A. Ecdysone-inducible functions of larval fat bodies in Drosophila. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5570–5574. doi: 10.1073/pnas.75.11.5570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepesant J. A., Levine M., Garen A., Lepesant-Kejzlarvoa J., Rat L., Somme-Martin G. Developmentally regulated gene expression in Drosophila larval fat bodies. J Mol Appl Genet. 1982;1(5):371–383. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Munn E. A., Feinstein A., Greville G. D. The isolation and properties of the protein calliphorin. Biochem J. 1971 Sep;124(2):367–374. doi: 10.1042/bj1240367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Ribbert D. Chromomeres and puffing in experimentally induced polytene chromosomes of Calliphora erythrocephala. Chromosoma. 1979 Oct 1;74(3):269–298. doi: 10.1007/BF01190743. [DOI] [PubMed] [Google Scholar]
- Ricciardi R. P., Miller J. S., Roberts B. E. Purification and mapping of specific mRNAs by hybridization-selection and cell-free translation. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4927–4931. doi: 10.1073/pnas.76.10.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller K., Karlson P. Effects of ecdysteroids on RNA synthesis of fat body cells in Calliphora vicina. J Insect Physiol. 1977;23(2):285–291. doi: 10.1016/0022-1910(77)90043-9. [DOI] [PubMed] [Google Scholar]
- Sekeris C. E., Scheller K. Calliphorin, a major protein of the blowfly: correlation between the amount of protein, its biosynthesis, and the titer of translatable calliphorin-mRNA during development. Dev Biol. 1977 Aug;59(1):12–23. doi: 10.1016/0012-1606(77)90236-6. [DOI] [PubMed] [Google Scholar]
- Smith D. F., McClelland A., White B. N., Addison C. F., Glover D. M. The molecular cloning of a dispersed set of developmentally regulated genes which encode the major larval serum protein of D. melanogaster. Cell. 1981 Feb;23(2):441–449. doi: 10.1016/0092-8674(81)90139-2. [DOI] [PubMed] [Google Scholar]
- Smith H. O., Birnstiel M. L. A simple method for DNA restriction site mapping. Nucleic Acids Res. 1976 Sep;3(9):2387–2398. doi: 10.1093/nar/3.9.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tahara T., Kuroiwa A., Obinata M., Natori S. Multi-gene structure of the storage protein genes of Sarcophaga peregrina. J Mol Biol. 1984 Mar 25;174(1):19–29. doi: 10.1016/0022-2836(84)90362-0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson J. A., Radok K. R., Shaw D. C., Whitten M. J., Foster G. G., Birt L. M. Genetics of lucilin, a storage protein from the sheep blowfly, Lucilia cuprina (Calliphoridae). Biochem Genet. 1976 Feb;14(1-2):145–160. doi: 10.1007/BF00484881. [DOI] [PubMed] [Google Scholar]
- Truett M. A., Jones R. S., Potter S. S. Unusual structure of the FB family of transposable elements in Drosophila. Cell. 1981 Jun;24(3):753–763. doi: 10.1016/0092-8674(81)90101-x. [DOI] [PubMed] [Google Scholar]
- Wolfe J., Akam M. E., Roberts D. B. Biochemical and immunological studies on larval serum protein 1, the major haemolymph protein of Drosophila melanogaster third-instar larvae. Eur J Biochem. 1977 Sep 15;79(1):47–53. doi: 10.1111/j.1432-1033.1977.tb11782.x. [DOI] [PubMed] [Google Scholar]
- Wyatt G. R., Pan M. L. Insect plasma proteins. Annu Rev Biochem. 1978;47:779–817. doi: 10.1146/annurev.bi.47.070178.004023. [DOI] [PubMed] [Google Scholar]