Abstract
We evaluated how regulatory support services provided by University of Illinois at Chicago's Center for Clinical and Translational Science may reduce Institutional Review Board (IRB) turnaround times. IRB applications were categorized by receipt of any regulatory support, and amount of support received. Turnaround time included total turnaround time, time for IRB review, and time for investigators to modify protocols. There were no differences in any turnaround times for supported versus non-supported applications. However, for supported applications, those receiving more intensive support had total turnaround times 16.0 days (standard error 7.62, p<0.05) faster than those receiving less intensive support. Receiving higher regulatory support may be associated with faster approval of IRB submissions.
Keywords: Institutional Review Boards, CTSA evaluation, clinical research, translational research, evaluation research
Introduction
Institutional Review Boards (IRBs), which determine whether or not federal regulations, ethical standards, and human subject protection requirements are met when conducting federally-funded scientific research, are critical and necessary components of research infrastructure. However, researchers have also expressed challenges in working with IRBs, often indicating that the time necessary to complete the IRB review process (a.k.a., IRB turnaround time) is burdensome and/or a barrier to scientific progress (Abbott & Grady, 2011). Reducing IRB turnaround time has been identified as an important objective of the National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA) program. IRB turnaround time is defined as the time from the date of initial application submission to the IRB to the date of IRB approval (National Institutes of Health, 2014). This performance measure has been examined in recent years in attempts to improve IRB efficiency without compromising human subject protections (Hall, Hanusa, Stone, Ling, &Arnold, 2015; Cleaton-Jones, 2010). This measure has also been identified by the Evaluation Key Function Committee of the National Center for Advancing Translational Sciences (NCATS) as an important metric for assessing the efficiency and outcomes of clinical research (CTSA Program Common Metrics Operational Guideline, 2016; CTSA Program Common Metrics Booklet, 2015).
Reducing IRB turnaround time would contribute to decreasing the time needed to move from research to practice to policy. Trochim, Kane, Graham, and Pincus (2011) describe a process marker model for evaluating the efficiency of translational research, which proposes that translational research is a continuous process that moves through several definable markers and ultimately leads to health impacts and outcomes. While many research activities and outcomes do not have direct influences on health impacts, specific markers along the translational continuum, including IRB submission date and IRB approval date, can nevertheless be used to measure, and reduce, the time taken by the overall translational process (Trochim et al., 2011). Empirically, research has suggested that waiting to obtain IRB approval can delay project initiation (Silberman & Kahn, 2011), and investigators have cited the growing costs associated with IRB submission (Gordon, Kessinger, Mann, & Prentice, 2003). Difficulties may be compounded for research that engages vulnerable populations and for multisite studies which can require approval from multiple IRBs (Check, Weinfurt, Dombeck, Kramer, & Flynn, 2013).
The University of Illinois at Chicago (UIC) Center for Clinical and Translational Science (CCTS) is an NIH CTSA awarded center which helps to coordinate resources and services to advance health research and improve the translation of scientific discoveries into effective health services, programs, and resources. The CCTS offers a comprehensive set of research support services and educational programs to researchers at the University, including a regulatory support service which assists investigators with IRB applications. IRB applications are submitted to and reviewed by the Office for the Protection of Research Subjects (OPRS) at UIC. OPRS is accredited by the Association for the Accreditation of Human Research Protection Programs, Inc (AAHRPP). OPRS provides general information, guidelines, and tips for IRB submission on their website, but otherwise does not provide individualized guidance for submissions unless consultations are requested by investigators. The CCTS provides a range of regulatory support services to investigators developing IRB applications. Services include answering questions only, reviewing a minor portion, major portion, or entire submission, and assisting with drafting and submitting all required documents. In this paper, we evaluate the effects of CCTS regulatory support services on the turnaround time required to obtain approval of initial IRB applications at UIC over a seven-year period.
Methods
Data Sources
The unit of analysis in this evaluation was IRB applications involving human subjects research. We examined the approval times associated with initial IRB applications that did and did not receive CCTS regulatory service assistance using data systematically collected by the UIC OPRS RiSC© Web data system. The data reported represent UIC IRB applications first submitted to OPRS between August 1, 2008 and June 31, 2014. There were a total of 4,955 initial submissions during that time period, of which 3,646 (73.5%) were submitted from one of the University's six health science colleges (Applied Health Sciences, Dentistry, Medicine, Nursing, and Pharmacy, and School of Public Health). Applications submitted by the University's non-health sciences colleges were excluded from analyses because they were less likely to involve clinical and/or translational research. In fact, only 5 of those 1,309 applications received regulatory support services. Applications that were reviewed by IRBs external to UIC, primarily the Western IRB, were also excluded from analysis as their review process could be quite different, resulting in a final analytic sample of 3,526 applications.
Measures
There were two independent variables of interest in this evaluation. One was a dichotomous indicator of receipt of any CCTS regulatory support services prior to submission of an IRB application. The second was an indicator of the amount of regulatory support contributed to each application that did receive services. When services were provided, regulatory support staff routinely assigned effort codes to applications representing the level of work involved. A 5-point ordinal level of effort scale was developed to represent the continuum of regulatory support services that were provided:
Effort Level 1: regulatory support staff answered questions and/or verbally provided advice to investigators (n = 4; 4.55% of all applications receiving support services);
Effort Level 2: regulatory support staff reviewed a minor portion of IRB application materials (e.g., only 1-2 documents; n = 18; 20.5%);
Effort Level 3: regulatory support staff reviewed a major portion of IRB application materials, and minor editing was necessary (n = 12; 13.6%);
Effort Level 4: regulatory support staff reviewed all IRB application materials, and a high level of editing and input was necessary (n = 31; 35.2%); and
Effort Level 5: regulatory support staff drafted and submitted all IRB application materials on behalf of the investigator (n = 23; 26.1%).
Given the relatively small total number of applications receiving regulatory support services, these effort levels were collapsed for analyses to denote applications receiving more intensive support (effort levels 4–5) versus applications receiving less intensive support (effort levels 1–3).
Three measures of IRB turnaround time were examined as dependent variables in the analyses. These included total turnaround time and its two components: IRB processing and review time and principal investigator (PI) response time. Total turnaround time is defined as the number of days between the date of submission of a complete IRB application to the UIC OPRS and the date the application was formally approved by one of the four campus IRBs assigned to its review. IRB processing and review time is that portion of total IRB turnaround time that is required for applications to be processed and reviewed by OPRS staff and IRB board members. It is defined as the number of days between the date of submission of a complete IRB application and the date of correspondence between OPRS and investigators regarding the outcome of their application. In addition to formal approval, review outcomes can include requests for additional information or clarifications regarding the research protocol and/or protocol modifications. PI response time is the remaining portion of total turnaround time during which investigators prepare responses to IRB requests for clarifications and/or modifications. It is defined as the number of days between the date of receipt of OPRS correspondence requesting changes and the date of submission of formal responses from investigators addressing those requests. If more than one round of changes or modifications was needed, the number of days for IRB processing and for PI response for each round were respectively summed to represent total IRB processing time and total PI response time.
Receipt of any regulatory support services, the total number of hours of regulatory support services received, and the effort level of services received served as this evaluation's main independent variables. Several covariates were also examined. These included IRB review level (full, expedited, or exempt), the initial year of submission of IRB applications (to account for temporal differences in IRB review), and whether applications represented multisite investigations. We also assessed whether applications were submitted by investigators in the UIC College of Medicine (54.6% of all applications), as applications originating from the College of Medicine may have important disciplinary differences and also raise more complex ethical issues, thus taking longer to review. Finally, we created a composite variable indicating whether the IRB required modifications to or deferral of an application until further information was provided.
Statistical Analyses
Both bivariate and multivariate analyses were employed to determine the degree to which receipt of CCTS regulatory support services was associated with length of time required to complete initial review. Due to non-normality in the distributions of turnaround times and the existence of outliers, bivariate analyses relied on Wilcox on-Mann-Whitney tests to evaluate differences in IRB turnaround between submitted applications that did versus did not receive any CCTS regulatory support services. Among applications that did receive regulatory support, differences in IRB turnaround times were also evaluated between those receiving more versus less intensive regulatory support. As secondary analyses, we also used two-sample t-tests to examine whether overall CCTS regulatory support and level of support were associated with the number of protocol modifications required in the IRB review process.
As our non-parametric tests produced similar results to parametric tests, we employed generalized linear regression models for multivariate analyses to control for potential confounders. These models also adjusted for the investigator-clustered nature of IRB applications to capture any variance in turnaround time that might be associated with differences across investigators. The 3,526 reviewed applications were nested within 1,656 principal investigators, who each submitted between 1 and 101 applications.
Results
Of the 3,526 applications submitted from one of the six UIC health science colleges, most received expedited review (N=1,895; 53.7%). A further 547 applications (15.5%) received full (i.e., convened) review, and 1,084 applications (30.7%) were classified as exempt. A total of 89 applications received CCTS regulatory support services. Among all applications in the sample, the median turnaround time between initial submission and final approval was 28 days (mean=40.8 days; SD=40.7). Median IRB processing and review time was 18 days (mean=24.0 days; SD=21.5), and median PI response time to reviews was 6 days (mean=16.8 days; SD=26.2). As expected, the time necessary to obtain final approval varied by the level of IRB review required. Full review proposals required the longest turnaround time, with a median of 70 days (mean=80.2 days; SD=48.0). For expedited reviews, median turnaround time was 33 days (mean=43.3 days; SD=36.6), followed by 10 days (mean=16.6 days; SD=22.7) for exempt reviews. Appendix Tables A and B provide additional details regarding turnaround times by type of review and year. As exempt reviews cover research activity that is considered low-risk, with relatively quick turnaround, many such reviews did not opt for CCTS support – only 6 (0.55%) out of the 1,084 exempt reviews received regulatory support. Thus, our present analyses focused primarily on full and expedited reviews.
Overall Effects of Regulatory Support Services
Figure 1 depicts differences in median turnaround times for applications that received CCTS regulatory support compared to those that did not request such support, stratified by full reviews and expedited reviews. Median total turnaround time for full reviews receiving regulatory support services was 70.5 days (mean=68.7 days; SD=30.8), compared to 70 days (mean=81.0 days; SD=48.8) for those not receiving regulatory support. For expedited reviews, median total turnaround time for those receiving regulatory support was 34 days (mean=52.6 days; SD=43.5), compared to 33 days (mean=43.0 days; SD=36.4) for non-supported applications. Wilcoxon-Mann-Whitney tests showed that both of these differences were not significant (p=0.297 for full reviews; p=0.163 for expedited reviews). Somewhat surprisingly, expedited reviews that received regulatory support services experienced significantly longer IRB processing and review times than expedited reviews that did not receive services (median=25 days and 20 days, respectively, p=0.040; Figure 1).
Figure 1.
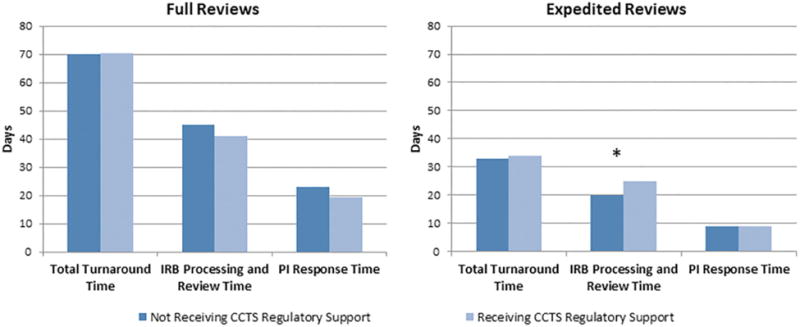
Median Turnaround Times for Full and Expedited Reviews by CCTS Regulatory Support Receipt: August 2008 to June 2014. Note. CCTS = Center for Clinical and Translational Science; IRB = institutional review board; PI = principal investigator. *p < .05.
We found that CCTS regulatory support was associated with a larger number of modifications when looking at all applications: supported applications required an average of 1.34 (SD=0.88) modifications and non-supported applications required an average of 1.02 (SD=0.96) modifications (t=3.1, df=3524, p=0.002). However, among full reviews, supported applications actually required significantly fewer modifications than non-supported applications: 1.41 (SD=0.66) versus 1.88 (SD=0.92) modifications, respectively (t=2.9, df=545, p=0.004). Among expedited reviews, there was no significant difference in the number of modifications required for applications receiving regulatory support and those not receiving support.
Results of generalized linear regression models examining the effects of receipt of any CCTS regulatory support are presented in Table 1. Separate models were estimated for total turnaround time, IRB processing and review time, and PI response time. After adjusting for covariates, there were no significant differences in any of the three turnaround time outcome measures between applications receiving regulatory support and those not receiving support. Interestingly, total turnaround time decreased by an average of 1.82 days each year between 2008 and 2014 (although not always in a linear manner, see Appendix Tables A and B). Most of this decrease was due to reductions in IRB processing and review time.
Table 1. Generalized linear regression of CCTS regulatory support receipt and covariates on turnaround time measures: August 2008 – June 2014 (N=3,526).
| Total Turnaround Time | IRB Processing and Review Time | PI Response Time | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Predictor | b | SEb | b | SEb | b | SEb |
| CCTS Regulatory Support Received (1=yes) | -1.40 | 4.37 | 1.48 | 2.31 | -2.78 | 2.97 |
| Year Application First Submitted | -1.82*** | 0.37 | -2.37*** | 0.17 | 0.58* | 0.28 |
| Investigator from College of Medicine (1=yes) | 6.46*** | 1.32 | 2.44*** | 0.66 | 4.19*** | 0.96 |
| IRB Review Level (‘Full’ as reference) | ||||||
| Exempt | -41.1*** | 2.84 | -27.4*** | 1.22 | -13.9*** | 2.07 |
| Expedited | -28.6*** | 2.75 | -19.4*** | 1.17 | -9.26*** | 1.98 |
| Multisite Application (1=yes) | -0.45 | 1.44 | -0.61 | 0.75 | 0.18 | 1.08 |
| Protocol Modifications Required (1=yes) | 33.3*** | 1.01 | 12.3*** | 0.63 | 20.9*** | 0.66 |
p < 0.05;
p < 0.01;
p < 0.001
Note: CCTS – Center for Clinical and Translational Science. IRB – Institutional Review Board. PI – principal investigator. SE – standard error.
Effects of Level of Regulatory Support
Next, the association between level of regulatory support provided and turnaround time was examined. Analyses were limited to 88 total applications for which regulatory support was provided, since one application with regulatory support was missing an effort code. Figure 2 depicts differences in total turnaround time for applications that received regulatory support, based on full versus expedited review and effort level provided (more intensive versus less intensive support). Among full reviews, those receiving more intensive support had a median total turnaround time of 46 days (mean=55.1 days; SD=26.1), while those receiving less intensive support had a turnaround of 84.5 days (mean=88.3 days; SD=26.6), a significant difference at p=0.003. Among expedited reviews, median turnaround times were 31 days (mean=43.8 days; SD=43.7) for applications receiving more intensive support and 57 days (mean=66.7 days; SD=40.9) for those receiving less intensive support; p=0.021. Full reviews that received more intensive support also had significantly shorter IRB processing and review times and PI response times than full reviews with less intensive support (median=35.5 versus 59.5 days, p=0.007 and median=9.5 versus 30 days, p=0.012, respectively). Expedited reviews that received more intensive support had significantly shorter PI response times than those with less intensive support.
Figure 2.
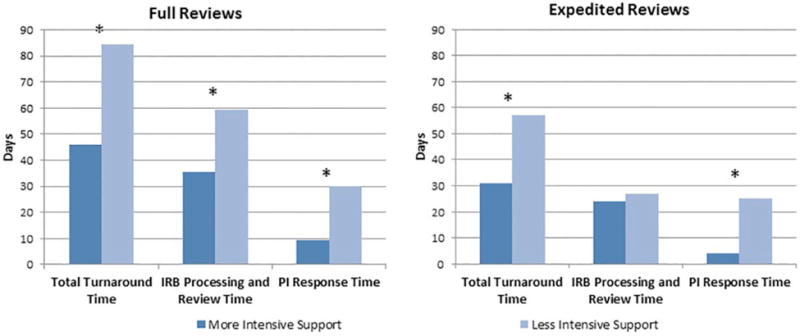
Median Turnaround Times for Full and Expedited Reviews Receiving CCTS Regulatory Support by Level of Support: August 2008 to June 2014. Note. CCTS = Center for Clinical and Translational Science; IRB = institutional review board; PI = principal investigator. *p < .05.
Overall among applications with regulatory support, those receiving more intensive support needed an average of 1.19 modifications (SD=0.89), compared with 1.56 modifications (SD=0.82) for those receiving less intensive support, a difference that was borderline significant (t=1.97, df=86, p=0.052). Among full reviews, applications receiving more intensive support also required significantly fewer modifications than those receiving less intensive support: 1.20 (SD=0.52) versus 1.71 (SD=0.73) modifications, respectively (t=2.4, df=32, p=0.022). However, there were no significant differences in number of modifications required for applications receiving more versus less intensive support among expedited reviews.
Generalized linear regression models (Table 2) restricted to the 88 applications with regulatory support also revealed a positive association between level of effort and total turnaround time. After adjusting for covariates, applications receiving more intensive regulatory support obtained final approval on average 16.0 days (p<0.05) faster than applications receiving less intensive support. However, neither time due to IRB processing and review or due to PI response were significantly affected by level of support received.
Table 2. Generalized linear regression of CCTS regulatory support level and covariates on turnaround time measures: August 2008 – June 2014 (N=88).
| Total Turnaround Time | IRB Processing and Review Time | PI Response Time | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Predictor | b | SEb | b | SEb | b | SEb | |
| Received More Intensive Regulatory Support (1=yes) | -16.0* | 7.62 | -4.25 | 4.43 | -7.97 | 5.00 | |
| Year Application First Submitted | -5.97** | 1.93 | -4.47*** | 1.04 | -1.35 | 1.40 | |
| Investigator from College of Medicine (1=yes) | 6.90 | 6.50 | 1.25 | 3.22 | 4.44 | 5.04 | |
| IRB Review Level (‘Full’ as reference) | |||||||
| Exempt | -44.9*** | 8.76 | -27.3*** | 5.52 | -15.3** | 5.64 | |
| Expedited | -9.27 | 7.05 | -8.55* | 3.80 | 0.99 | 5.50 | |
| Multi-Site Application (1=yes) | -3.93 | 6.33 | 3.28 | 3.98 | -6.39 | 3.32 | |
| Protocol Modifications Required (1=yes) | 35.9*** | 6.81 | 16.8*** | 4.21 | 18.8*** | 3.39 | |
p < 0.05;
p < 0.01;
p < 0.001
Note: CCTS – Center for Clinical and Translational Science. IRB – Institutional Review Board. PI – principal investigator. SE – standard error.
Discussion
When examining all applications that received regulatory support, we found that those that had received more intensive support experienced shorter total IRB turnaround times than applications with less intensive support. However, in our adjusted analyses, more intensive support was not associated with either investigator response times or IRB processing and review times in particular. Other research does indicate that many of the longest delays surrounding IRB approval may occur due to lengthy investigator response times (Hall et al., 2015; Tzeng, Wu, &Hsu, 2015). While we did find some indication of this in unadjusted analyses, our adjusted analyses may have been hampered by small sample sizes, or may suggest that other factors play more important roles in turnaround times. Notably, we did find that receiving more intensive support was associated with needing fewer modifications, a significant determinant of all three turnaround time measures. CCTS support could thus be linked to turnaround time indirectly, through modifications. Reducing the number of modifications each protocol needs to go through could reduce investigator burden as well as IRB workload. Especially as IRBs may be constrained to operate under finite resources and personnel even if research applications increase (Cleat on-Jones, 2012), the regulatory support services provided by the CCTS could assist in streamlining the overall process.
Although necessary, IRB submission can impose a not inconsequential burden on researchers, especially for multisite studies (Green, Lowery, Kowalski, & Wyszewianski, 2006). In addition to the initial application, submissions often require additional revision of the application, consent documents, or other forms. The support services provided by the CCTS can assist investigators in a variety of ways in navigating this process, from answering basic questions about the submission process to helping the researcher craft the most clear and concise description of the research being proposed and the potential human subjects risks involved. This assistance may also be another way to support multi-institutional or multisite research, whose more complex application process may result in additional delays to research initiation (Dyrbye et al., 2007). Further research should evaluate how, in addition to the streamlining of IRB processes and central review boards (Greene & Geiger, 2006), external support from CTSA-provided services can encourage participation in and promote efficiency of multisite research.
We did find that for expedited reviews only, receiving any type of regulatory support increased IRB processing and review time. It could be that investigators who requested CCTS assistance have the least experience in IRB submission, and thus their applications required the most amount of time to review. However, after controlling for relevant covariates, we found that receiving any type of regulatory support did not significantly impact total IRB turnaround time, IRB processing and review time, or PI response time. Another study on research ethics committees in the UK, analogous to IRBs in the United States, also reached similar conclusions. That study found that assigning ethics officers to applications before formal review to identify possible issues and to discuss such issues with applicants did not increase the proportion of applications that were approved on first review and did not reduce the time to a committee decision (Dixon-Woods et al., 2016). However, this study also reported challenges in that ethics officers were not always able to meet with applicants, and did not consistently anticipate issues in applications for which research ethics committees later required modifications.
Our null findings regarding overall CCTS regulatory support may also suggest a wide variety in terms of actual support received by investigators. Indeed, there could be a certain threshold of support that confers improvements in turnaround time. Our results among applications receiving more versus less regulatory support do suggest this possibility, but further examination is nevertheless warranted to fully understand the impacts of certain regulatory support services on IRB turnaround. We also found that total turnaround time decreased over time, regardless of review level or CCTS support. Overall increases in efficiency and effectiveness by OPRS staff and IRB reviewers over the study period could also have overshadowed any effects of the CCTS regulatory support services. Finally, our findings could have been due to the small numbers of reviews that utilized CCTS services (only 88 reviews), which could have limited our ability to detect significance.
This study has some limitations. First, only a very small percentage of the total applications received regulatory support, which could have prevented us from detecting significant associations, especially in adjusted analyses, and limits our ability to generalize our findings. In addition, the operational measures of IRB turnaround time employed in this research do not include the time investigators spend preparing the initial paperwork for their IRB applications. This initial preparation time can also be expected to vary, depending on factors such as the complexity of the research protocol, the experience of the investigators, and the level of technical support available. Future research should continue to develop methods to assess this presently unmeasured component of the IRB submission process. Finally, this evaluation was restricted to a single university, and anecdotal evidence suggests wide variability, or at least perceptions of wide variability, in the degree of burden and time requirements necessary to comply with the IRB review process across institutions (Varley et al., 2016).
This work also has several strengths. It provides an empirical evaluation of a core support service at a research intensive CTSA-funded institution that directly addresses the CTSA Program's overarching goal of accelerating clinical and translational research. The data, obtained from multiple sources, represent a complete accounting of the relevant human subjects research conducted at this institution over a seven year time period. We also successfully disaggregated total IRB turnaround time, facilitating our ability to understand the effects of receiving regulatory support services on individual components of the IRB review process.
In conclusion, we found significant differences in IRB turnaround times between applications receiving more intensive regulatory support from the UIC CCTS and applications receiving less intensive support. The CSTA Program endeavors to support all aspects of clinical and translational science, and services such as regulatory consultation will be essential in ensuring timely study initiation and effective research practice.
Best Practices.
Regulatory support services may be helpful in reducing investigator burden in IRB applications. Particularly for new investigators or multisite research, assistance from organizations such as the CTSA hubs can help accelerate research initiation.
Research Agenda.
As described above, additional research should evaluate how regulatory support services may help streamline the approval of multisite research, as application procedures for such projects can be quite complex. In addition, larger sample sizes, perhaps achieved through collaborations between multiple CTSA hubs, can help clarify the impact of regulatory support, independent of time trends and other potential confounders. Research should assess which specific services (one-on-one consultations, protocol editing services, IRB workshops, etc.) may be most useful to investigators, and whether a certain amount of support needs to be provided in order to result in benefits in turnaround time. Especially as the IRB submission process may vary tremendously between different institutions, collaboration between universities may also contribute to establishing a set of best practices for reducing IRB turnaround times. Finally, research also needs to examine pre-submission effort, and whether support services can effectively assist researchers in preparing the protocol and other initial paperwork for their IRB applications.
Educational Implications.
Data show that of IRB applications receiving regulatory support from UIC's CCTS, those that received more intensive support needed fewer modifications and experienced shorter total turnaround times than those that received less intensive support.
Acknowledgments
We thank Lynn Podraza and Sandra Rahbe, University of Illinois at Chicago (UIC) Center for Clinical and Translational Science (CCTS) Regulatory Support staff who provided the support services being evaluated here. We also thank the UIC Office for the Protection of Research Subjects for providing access to the IRB turnaround data analyzed here. This research was supported by the UIC CCTS, Award Number UL1TR000050 and Award Number UL1TR002003 from the National Center for Research Resources.
Funding: This research was supported by the University of Illinois at Chicago (UIC) Center for Clinical and Translational Science (CCTS), Award Number UL1TR000050 and Award Number UL1TR002003 from the National Center for Research Resources.
Biographies
Pankaja Desai is currently the Director of Research at Alliance of Chicago Community Health Services. She was the Assistant Director of Evaluation and Tracking at the UIC CCTS at the time of the paper and oversaw data collection. She managed projects evaluating the impact of CCTS services, and helped conceptualize the paper and write the manuscript.
Priyanka Nasa is a Research Specialist for Evaluation and Tracking at the UIC CCTS. She works on projects evaluating the impact of CCTS services on research output. She contributed to collecting and analyzing the data for the paper.
Jackie Soo is the Assistant Director of Evaluation and Tracking at the UIC CCTS. She oversees a number of projects assessing the utilization and quality of CCTS services, including regulatory support, and their effects on research output. She contributed to writing and editing the final manuscript.
Cunhui Jia is currently a Clinical Application Specialist at Abbvie. At the time of the paper she worked as a Research Assistant in the Institute for Health Research and Policy at UIC. Her responsibilities involved providing statistical support for the UIC CCTS, and she contributed to analyzing the data for the paper.
Michael L. Berbaum is the Director of the Design and Analysis core at the UIC CCTS. His research interests include assessing the statistical effects of clustering, missing data, and sample selection in health studies. He contributed to the conceptualization and analysis of the paper.
James H. Fischer is the Director of OPRS at UIC and Professor in the Department of Pharmacy Practice at the College of Pharmacy. His research interests include pharmacometrics and the clinical pharmacology of antiepileptic drugs. He contributed to the conceptualization of the paper and editing of the final manuscript.
Timothy P. Johnson directs Evaluation and Tracking for the UIC CCTS. He is also Professor of Public Administration and Director of the Survey Research Laboratory at UIC. He contributed to the conceptualization, analysis, and writing of the paper.
Appendix
Table A. Turnaround time measures for full reviews, by year.
| Year | Total Turnaround Time | IRB Processing and Review Time | PI Response Time | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Median | Mean | SD | Median | Mean | SD | Median | |
| Overall (N=547) | 80.2 | 48.0 | 70.0 | 47.5 | 21.1 | 45.0 | 32.7 | 34.8 | 23.0 |
| 2008 (N=57) | 86.0 | 47.0 | 74.0 | 56.4 | 24.0 | 53.0 | 29.6 | 29.7 | 20.0 |
| 2009 (N=103) | 94.0 | 48.2 | 86.0 | 53.5 | 18.3 | 51.0 | 40.4 | 36.4 | 28.0 |
| 2010 (N=87) | 80.3 | 36.1 | 76.0 | 54.8 | 23.4 | 55.0 | 25.6 | 20.8 | 22.0 |
| 2011 (N=96) | 79.4 | 53.3 | 67.0 | 50.5 | 21.9 | 46.0 | 28.9 | 38.0 | 20.0 |
| 2012 (N=87) | 70.6 | 44.2 | 57.0 | 38.2 | 15.8 | 35.0 | 32.4 | 33.5 | 23.0 |
| 2013 (N=83) | 74.0 | 50.5 | 59.0 | 37.4 | 14.8 | 35.0 | 36.6 | 40.8 | 23.0 |
| 2014 (N=34) | 70.6 | 55.3 | 60.5 | 36.2 | 18.7 | 33.0 | 34.3 | 40.5 | 26.5 |
Note: IRB – Institutional Review Board; PI – Principal Investigator; SD – standard deviation.
Table B. Turnaround time measures for expedited reviews, by year.
| Year | Total Turnaround Time | IRB Processing and Review Time | PI Response Time | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Median | Mean | SD | Median | Mean | SD | Median | |
| Overall (N=1895) | 43.3 | 36.6 | 33.0 | 24.5 | 18.0 | 20.0 | 18.8 | 26.2 | 9.00 |
| 2008 (N=145) | 50.9 | 32.7 | 48.0 | 32.8 | 18.0 | 31.0 | 18.1 | 22.2 | 11.0 |
| 2009 (N=343) | 47.9 | 35.3 | 38.0 | 29.0 | 18.1 | 25.0 | 18.9 | 23.4 | 9.00 |
| 2010 (N=312) | 52.7 | 37.7 | 45.0 | 32.7 | 21.3 | 28.0 | 20.0 | 24.5 | 12.0 |
| 2011 (N=325) | 42.5 | 37.3 | 31.0 | 25.1 | 18.5 | 20.0 | 17.4 | 25.1 | 9.00 |
| 2012 (N=318) | 29.9 | 28.3 | 20.0 | 16.8 | 14.4 | 13.0 | 13.1 | 20.7 | 4.50 |
| 2013 (N=317) | 39.8 | 39.0 | 30.0 | 18.0 | 11.6 | 16.0 | 21.8 | 32.0 | 11.0 |
| 2014 (N=135) | 43.0 | 41.4 | 32.0 | 17.4 | 10.8 | 16.0 | 25.6 | 35.5 | 14.0 |
Note: IRB – Institutional Review Board; PI – Principal Investigator; SD – standard deviation.
Footnotes
The Authors declare no other conflicts of interest.
References
- Abbott L, Grady C. A systematic review of the empirical literature evaluating IRBs: what we know and what we still need to learn. Journal of Empirical Research on Human Research Ethics. 2011;6:3–19. doi: 10.1525/jer.2011.6.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Check DK, Weinfurt KP, Dombeck CB, Kramer JM, Flynn KE. Use of central institutional review boards for multicenter clinical trials in the United States: a review of the literature. Clinical Trials. 2013;10:560–567. doi: 10.1177/1740774513484393. [DOI] [PubMed] [Google Scholar]
- Cleaton-Jones P. Process error rates in general research applications to the Human Research Ethics Committee (Medical) at the University of the Witwatersrand: a secondary data analysis. South African Journal of Bioethics & Law. 2010;3:20–24. [Google Scholar]
- Cleaton-Jones P. Applications and secretariat workload at the University of the Witwatersrand Human Research Ethics Committee (Medical) 2002-2011: a case study. South African Journal of Bioethics & Law. 2012;5:38–44. [Google Scholar]
- Clinical and Translational Science Awards Common Metrics Booklet. CTSA Program Annual Principal Investigator Meeting; Dec 16-17, 2015; Washington DC. 2015. Retrieved from https://ctsacentral.org/wp-content/uploads/CTSA-Common-Metrics-Booklet-12-16-2015.pdf. [Google Scholar]
- CTSA Program Common Metrics Operational Guideline: IRB Duration. Clinical and Translational Science Awards. 2016 Retrieved from http://www.tuftsctsi.org/wp-content/uploads/2016/08/IRB-Duration-Operational-Guidelines-8.3.2016-v-1.3.pdf.
- Dixon-Woods M, Foy C, Hayden C, Al-Shahi Salman R, Tebbutt S, Schroter S. Can an ethics officer role reduce delays in research ethics approval? A mixed-method evaluation of an improvement project. BMJ Open. 2016;6:e011973. doi: 10.1136/bmjopen-2016-011973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyrbye LN, Thomas MR, Mechaber AJ, Eacker A, Harper W, Massie FS, Jr, Shanafelt TD. Medical education research and IRB review: an analysis and comparison of the IRB review process at six institutions. Academic Medicine. 2007;82:654–660. doi: 10.1097/ACM.0b013e318065be1e. [DOI] [PubMed] [Google Scholar]
- Gordon BG, Kessinger A, Mann SL, Prentice ED. The impact of escalating regulatory requirements on the conduct of clinical research. Cytotherapy. 2003;5:309–313. doi: 10.1080/14653240310002225. [DOI] [PubMed] [Google Scholar]
- Green LA, Lowery JC, Kowalski CP, Wyszewianski L. Impact of institutional review board practice variation on observational health services research. Health Services Research. 2006;41:214–230. doi: 10.1111/j.1475-6773.2005.00458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene SM, Geiger AM. A review finds that multicenter studies face substantial challenges but strategies exist to achieve Institutional Review Board approval. Journal of Clinical Epidemiology. 2006;59:784–790. doi: 10.1016/j.jclinepi.2005.11.018. [DOI] [PubMed] [Google Scholar]
- Hall DE, Hanusa BH, Stone RA, Ling BS, Arnold RM. Time required for institutional review board review at one Veterans Affairs medical center. JAMA Surgery. 2015;150:103–109. doi: 10.1001/jamasurg.2014.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Health. National Center for Advancing Translational Sciences, Clinical and Translational Science AwardU 54, RFA-TR-14-009. 2014 Posted 9/12/14. Retrieved from http://grants.nih.gov/grants/guide/rfa-files/RFA-TR-14-009.html.
- Silberman G, Kahn KL. Burdens on research imposed by institutional review boards: the state of the evidence and its implications for regulatory reform. The Milbank Quarterly. 2011;89:599–627. doi: 10.1111/j.1468-0009.2011.00644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trochim W, Kane C, Graham M, Pincus HA. Evaluating translational research: a process marker model. Clinical and Translational Science. 2011;4:153–162. doi: 10.1111/j.1752-8062.2011.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzeng DS, Wu YC, Hsu JY. Latent variable modeling and its implications for institutional review board review: variables that delay the reviewing process. BMC Medical Ethics. 2015;16:57. doi: 10.1186/s12910-015-0050-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- University of Illinois at Chicago. Center for Clinical and Translational Science website. Retrieved from http://www.ccts.uic.edu/content/uics-home-clinical-translational-research.
- Varley PR, Feske U, Gao S, Stone RA, Zhang S, Monte R, Hall DE. Time required to review research protocols at 10 Veterans Affairs Institutional Review Boards. Journal of Surgical Research. 2016;204:481–489. doi: 10.1016/j.jss.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]


