Graphical abstract
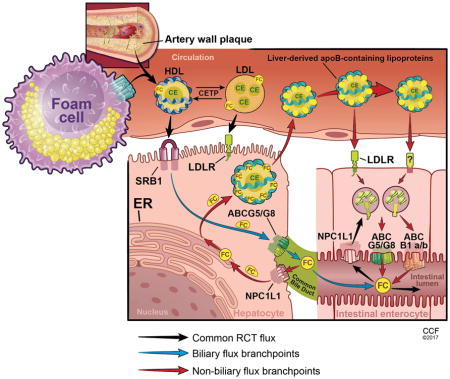
Elimination of excess cholesterol by the reverse cholesterol transport (RCT) pathway opposes atherosclerotic cardiovascular disease. RCT begins with the mobilization of excess free cholesterol (FC) from macrophage foam cells in the artery wall to high density lipoproteins (HDL). Following esterification of cholesterol in the plasma compartment by lecithin cholesterol:acyltransferase (LCAT), cholesteryl esters (CE) can be selectively delivered to the liver via the class B type 1 scavenger receptor (SR-BI). Alternatively, cholesterol ester transfer protein (CETP) may exchange CE for triglycerides on apolipoprotein B (apoB)-containing lipoproteins, which can deliver cholesterol to hepatocytes by receptor-mediated endocytosis. Cholesterol elimination from the liver requires transport across the canalicular surface by the ABCG5/G8 transporter or the bile salt export protein (BSEP, ABCB11) following cholesterol conversion to primary bile acids. However, a fraction of cholesterol is reabsorbed in the proximal small intestine via Niemann Pick C1-Like 1 (NPC1L1) and bile acids in the distal small intestine through the combined actions of the apical sodium dependent bile acid transporter (ASBT) and intestinal bile acid transporter (IBAT), thereby limiting neutral and acidic sterol loss from the body.
Under a variety of experimental conditions in which biliary cholesterol secretion is disrupted, fecal neutral sterol content remains constant or is increased indicating the existence of a non-hepatobiliary pathway for cholesterol excretion in rodents. Direct evidence for such a pathway in the small intestine was first provided by van der Velde and colleagues, who demonstrated its existence using an intestinal perfusion model.1 Subsequently, a series of studies provided both direct and indirect evidence for the rerouting of cholesterol from the biliary pathway through liver-derived apoB-containing lipoproteins for transintestinal cholesterol excretion (TICE) in mice. Investigations into the molecular mediators of TICE within the enterocyte, from uptake at the basolateral surface, intracellular transport, and secretion into the intestinal lumen are still in their infancy. However, LDLR, ABCG5/G8, and P-glycoprotein (ABCB1a/b) have each been implicated in the process.2 While the elucidation of the molecular underpinnings that support TICE await discovery, it is clear that TICE can fully compensate for a variety of disruptions in biliary cholesterol secretion in mice and that the pathway is regulated by dietary, pharmacological, and microbial factors (reviewed in 3). However, the relative contribution of TICE to cholesterol excretion under physiological conditions remains unclear and its role in humans is controversial.
Groen and colleagues (pp**) attempted to address the first of these two questions using an innovative approach akin to parabiosis. Bile was diverted from the common bile duct of paired rats and rerouted to the proximal small intestine of each partner animal such that the two shared a common enterohepatic bile acid pool. The pair was treated with ezetimibe to block the reabsorption of cholesterol secreted by the liver into bile and by the small intestine into the lumen. Rat macrophage foam cells containing radiolabeled cholesterol were then intraperitoneally injected into one of the paired animals. Since the bile of the injected rat was diverted to its partner, the appearance of radiolabeled cholesterol in the feces of the injected rat was used to determine the contribution of TICE to macrophage RCT. The hepatobiliary contribution was determined by measuring radiolabeled sterol in the feces of the biliary diversion recipient rat. Under these conditions, TICE accounted for approximately 20% of macrophage RCT.
This creative study clearly establishes TICE as a contributor to macrophage-derived cholesterol elimination in a conscious, preclinical rodent model. However, an important consideration is that the experiment was conducted under the non-physiologic condition of ezetimibe treatment. On one hand, ezetimibe was an essential tool to prevent the non-injected rat from reabsorbing radiolabeled cholesterol and sending it back to its donor via the hepatobiliary pathway. On the other hand, ezetimibe treatment fundamentally alters intestinal cholesterol metabolism resulting in increased cholesterol synthesis and reduced expression of liver X receptor (LXR) target genes such as ABCG5/G8 and ABCA1.4, 5 In addition, ezetimibe is a pharmacological stimulator of TICE since it blocks the reabsorption of cholesterol originating from both the liver and small intestine.
Another caveat to the study is the absence of a gallbladder in rats and the continual secretion of bile into the intestinal lumen. Although it has been reported that rats, mice, and humans have similar rates of TICE, it will be important to determine the relative contributions of TICE and hepatobiliary cholesterol excretion to macrophage RCT in an “parabileosis” animal model in which the delivery of gallbladder bile to the intestinal lumen is intermittent and coordinated with food intake.
While this novel approach reaffirms earlier evidence for both biliary and non-biliary contributions to macrophage RCT, true quantitation remains elusive. Irrespective of the absolute contribution, the critical question that remains is how to therapeutically stimulate RCT to provide benefit to those suffering from atherosclerotic cardiovascular disease. TICE is an attractive pathway given that accelerating hepatobiliary cholesterol secretion is expected to promote saturation of gallbladder bile and increase the risk of gallstone formation. Recent studies, again by the Groen group, demonstrate that TICE has a highly dynamic range and impressive capacity to export cholesterol into the feces in mice treated with an farnesoid X receptor (FXR) agonist (de Boer et al., Gastroenterology, in press). Similarly, recent studies indicate that TICE is active in humans and is stimulated by ezetimibe treatment.6 Thus, the rodent models not only recapitulate features of TICE in humans, but also suggest that TICE is a druggable pathway. Further investigation into the key mediators of TICE and their regulation in the intestine are critical to unlock the therapeutic potential of this anti-atherosclerotic pathway.
References
- 1.van der Velde AE, Vrins CLJ, van den Oever K, Kunne C, Oude Elferink RPJ, Kuipers F, Groen AK. Direct intestinal cholesterol secretion contributes significantly to total fecal neutral sterol excretion in mice. Gastroenterology. 2007;133:967–975. doi: 10.1053/j.gastro.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 2.Le May C, Berger JM, Lespine A, Pillot B, Prieur X, Letessier E, Hussain MM, Collet X, Cariou B, Costet P. Transintestinal cholesterol excretion is an active metabolic process modulated by pcsk9 and statin involving abcb1. Arterioscler Thromb Vasc Biol. 2013;33:1484–1493. doi: 10.1161/ATVBAHA.112.300263. [DOI] [PubMed] [Google Scholar]
- 3.Temel RE, Brown JM. A new model of reverse cholesterol transport: Enticeing strategies to stimulate intestinal cholesterol excretion. Trends in pharmacological sciences. 2015;36:440–451. doi: 10.1016/j.tips.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Engelking LJ, McFarlane MR, Li CK, Liang G. Blockade of cholesterol absorption by ezetimibe reveals a complex homeostatic network in enterocytes. J Lipid Res. 2012;53:1359–1368. doi: 10.1194/jlr.M027599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Repa JJ, Turley SD, Quan G, Dietschy JM. Delineation of molecular changes in intrahepatic cholesterol metabolism resulting from diminished cholesterol absorption. J Lipid Res. 2005;46:779–789. doi: 10.1194/jlr.M400475-JLR200. [DOI] [PubMed] [Google Scholar]
- 6.Jakulj L, van Dijk TH, de Boer JF, Kootte RS, Schonewille M, Paalvast Y, Boer T, Bloks VW, Boverhof R, Nieuwdorp M, Beuers UH, Stroes ES, Groen AK. Transintestinal cholesterol transport is active in mice and humans and controls ezetimibe-induced fecal neutral sterol excretion. Cell Metab. 2016;24:783–794. doi: 10.1016/j.cmet.2016.10.001. [DOI] [PubMed] [Google Scholar]


