Abstract
In this study, we described the generation and immunogenicity of the Zika Virus (ZIKV) envelope protein (E) domain III (DIII) as a protein subunit vaccine candidate. ZIKV EDIII (zEDIII) was rapidly produced in E. coli in inclusion bodies. ZIKV EDIII was solubilized, refolded and purified to >95% homogeneity with a one-step Ni2+ affinity chromatography process. Further analysis revealed that zEDIII was refolded properly and demonstrated specific binding to an anti-zEDIII monoclonal antibody that recognizes a zEDIII conformational epitope. Subcutaneous immunization of mice with 25 and 50 μg of zEDIII was performed over a period of 11 weeks. zEDIII evoked ZIKV-specific and neutralizing antibody response with titers that exceed the threshold that correlates with protective immunity against ZIKV. The antigen-specific IgG isotypes were predominantly IgG1 and splenocyte cultures from immunized mice secreted IFN-gamma, IL-4 and IL-6. Notably, zEDIII-elicited antibodies did not enhance the infection of dengue virus in Fc gamma receptor (FcγR)-expressing cells. This study provided a proof of principle for the further development of recombinant protein-based subunit vaccines against ZIKV.
Keywords: Zika virus, Antigen, Vaccine, Envelope protein, Domain III (DIII), Neutralizing immunity, Antibody-dependent enhancement (ADE)
Introduction
Zika virus (ZIKV) belongs to the genus Flavivirus in the family Flaviviridae, and is closely related to the four serotypes of dengue virus (DENV), West Nile virus (WNV), tick-borne encephalitis virus (TBEV), and yellow fever virus (YFV) [1]. Recent ZIKV outbreaks have been linked to the development of severe fetal abnormalities that include microcephaly and Guillain-Barre’ syndrome [2, 3]. In 2015, over 1.5 million people were infected with ZIKV in Brazil and the World Health Organization has warned that ZIKV is “spreading explosively” and four million people could be infected in American countries within next 12 months [4]. However, currently there is no approved vaccine for human use. Therefore, there is an urgent need for the development of an effective prophylactic vaccine to prevent ZIKV infection.
Like other flaviviruses, the ZIKV Envelope (E) glycoprotein mediates viral assembly, attachment to cellular receptors, and is essential for the subsequent membrane fusion involved in viral entry [1]. It is also a major target of host antibody responses [1]. Studies have revealed that ZIKV E shares a three-domain architecture with the E proteins of DENV and other related flaviviruses [5]. The domain III of flavivirus E protein (EDIII) contains the cellular receptor-binding motifs and importantly, the majority of the type-specific neutralizing epitopes that induce strong host antibody responses and/or protective immunity are mapped to this domain [6, 7]. Recently, EDIII of ZIKV (zEDIII) has been found to be targeted by several different ZIKV-specific antibodies with distinct yet potent neutralizing activities [8]. Since neutralizing antibodies have been considered to be correlate with protection for approved vaccines against YFV and TBEV, as well as having been demonstrated to play important roles in the protection against infection by many flaviviruses [9, 10], the potential of zEDIII in inducing potent neutralizing antibodies renders it a prime candidate as an effective subunit vaccine against ZIKV. Hence, we investigated the potential of zEDIII as an effective subunit vaccine against ZIKV in this study.
Material and methods
Construction of DIII expression vectors
The coding DNA sequence of ZIKV E protein of strain PRVABC59 (amino acid 1-403, Genbank Acc.No. AMC13911) was synthesized using the original Genbank sequence (Integrated DNA Technologies, IA). The EDIII coding sequence was amplified by PCR and cloned into the pET28a bacterial expression vector with restriction enzymes BamH1 and XhoI (MilliporeSigma, MA). The pET28a vector provides the start codon and an N-terminal hexa-histidine tag (His6) tag for Ni2+ affinity chromatography-based purification (Fig 1A). The resulting plasmid, pET28a-His6-zEDIII was transformed into E. coli BL-21 cells for expression as previously described [11].
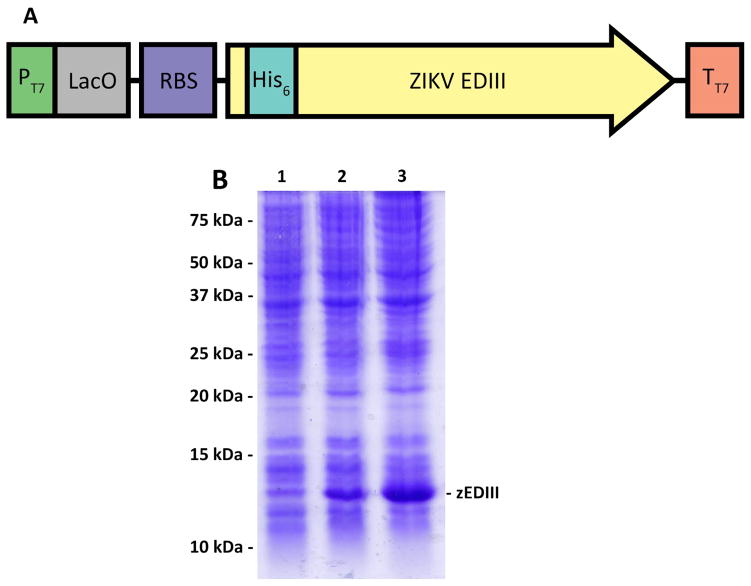
Figure 1. Expression of zEDIII in E. coli.
(A) zEDIII expression is driven by the T7 promoter (PT7) under the control of the lac repressor. A ribosomal binding site (RBS) is provided upstream of the zEDIII coding sequence to enhance its translational efficiency. The coding sequence of zEDIII is fused to an 18-bp sequence that codes for a hexa-histidine tag (His6) at its N-terminus for efficient purification and detection of the target protein. (B) Samples of the E. coli BL-21 culture were collected at various time points after induction with IPTG and total cellular proteins were analyzed by a 15% SDS-PAGE under reducing condition, followed by Coomassie blue staining. Lane 1, Total protein sample from non-induced E. coli as a negative control; Lanes 2 and 3, total protein samples from E. coli collected 4 and 12 hr post IPTG induction.
Expression, refolding and purification of ZIKV EDIII from E. coli
zEDIII was expressed in E. coli, refolded using an oxidative protocol, and purified by immobilized metal anion chromatography (IMAC) with a Ni His.Bind column as described previously [12]. Details of these methods are provided in Supplementary material.
SDS-PAGE, Western blot, and ELISAs
SDS-PAGE and western blot were used to characterize the size, identity, and purity of the recombinant zEDIII. The specific recognition of refolded zEDIII by mAbs that bind to ZIKV EDIII-specific conformational epitopes was determined by ELISA as described previously [13]. The titers of zEDIII-specific total IgG and the IgG1 and IgG2c subtypes in mouse serum were also determined by ELISAs as previously published [14]. Endpoint titers were defined as the highest reciprocal serum dilution that yielded an OD450 >2-fold over background values. Geometric mean titer (GMT) was calculated for each group at various time points, and was used to express the titers of the zEDIII-specific IgG. Details of the SDS-PAGE, western blot and ELISA methods are provided in Supplementary material.
Neutralization and antibody-dependent enhancement Assay
A plaque reduction neutralization test (PRNT) was used to measure ZIKV-specific neutralizing antibodies as previously published [15]. Neutralizing antibody titers were expressed as the reciprocal of the highest dilution of serum that neutralized ≥ 50% of ZIKV. The enhancing activities of zEDIII-elicited antibodies for DENV infection was determined by an antibody-dependent enhancement (ADE) assay as previously described [16]. Details of the PRNT and ADE method are provided in the Supplementary material.
Mouse immunization
All animal work was approved by the institutional animal care and use committee and carried out in accordance with the NIH guide for the care and use of laboratory animals. Six-week old female C57BL/6 mice were divided into 5 groups (n = 6 per group). Mice in group 1 received PBS with TiterMax Gold (TMG, MilliporeSigma, MA) as mock immunized control. Groups 2 and 3 received 25 μg and 50 μg of zEDIII with TMG per dosage, respectively. Group 4 received PBS with aluminum hydroxide gel (alum, InvivoGen, CA) as another mock control. Group 5 received 25 μg of zEDIII with alum. On Day 0, each mouse was injected subcutaneously with 100 μl of material containing saline, 25 μg or 50 μg purified zEDIII protein in PBS with TMG or alum as adjuvant (zEDIII Protein solution: TMG or alum volume ratio = 1:1). Mice were boosted on days 21, 42 and 63 using the same dosage and immune protocol as in the 1st immunization. Blood samples were collected from the retro-orbital vein on Day -7 before the immunization (pre-immune sample) and on days 14 (2 week), 35 (5 week), 56 (8 week) and 77 (11 week) after the 1st immunization. On day 84 (12 week), mice were euthanized and the spleens aseptically removed for in vitro splenocyte cultures.
Spleen cell culture and cytokine production measurement
Single-cell suspensions of the spleens from immunized mice were prepared by mechanical dissociation and splenocyte culture supernatants were collected 48 hr after stimulation to determine cytokine production as described previously [17]. Details of these methods are provided in Supplementary material.
Statistical analyses
Analysis of biochemical and immunological data was performed using GraphPad Prism software version 6.07 (GraphPad, CA). Kd of zEDIII binding to ZV54 was determined by non-linear regression analysis using a one-site binding model. Comparisons of zEDIII-specific total IgG, IgG1 and IgG2c titers, cytokine concentrations, and neutralization potency between groups was performed using t-tests. Comparison of IgG1/IgG2c ratio between samples collected at various time points was performed by one-way ANOVA. A p value of <0.05 indicated statistically significant differences.
Results
Expression and refolding of ZIKV E DIII
The coding sequence of zEDIII (from amino acid 303 to 403 of E protein) was cloned into expression vector pET28a (Fig 1A) and transformed into E. coli cells. To determine the optimum time for zEDIII accumulation, E. coli samples were taken every 4 hr after adding Isopropyl β-D-1-thiogalactopyranoside (IPTG) to the bacterial culture and zEDIII induction was analyzed by Coomassie blue staining analysis of SDS-PAGE of total E. coli protein. Expression of a protein with the predicted molecular weight of His6-zEDIII (14.5 kDa) was detected 4 hr after IPTG induction and a higher level of accumulation of this protein was achieved after 12 hr incubation (Fig 1B). Further analysis with both SDS-PAGE and western blotting indicated that zEDIII was produced in the inclusion bodies (data not shown). Sarcosine was used to solubilize inclusion bodies and zEDIII was refolded to recover its native conformation by consecutive dialysis with buffers containing reduced/oxidized glutathione.
Purification and characterization of ZIKV E DIII
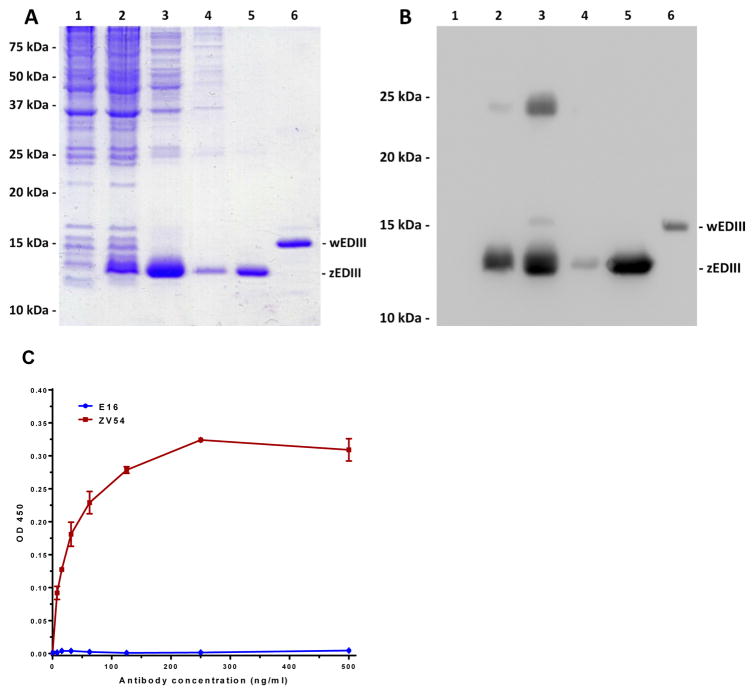
The availability of an efficient purification scheme is an essential component for zEDIII to become a viable vaccine candidate. Since zEDIII was tagged with a His6 tag on its N-terminus, we developed a one-step purification procedure based on Ni2+-based IMAC. Samples from various purification steps were analyzed by SDS-PAGE (Fig 2A) and Western blot analysis (Fig 2B). The result confirmed that zEDIII was produced in the inclusion bodies of E. coli cells (Fig 2A and B, Lane 3). Ni2+ affinity chromatography efficiently removed the remaining E. coli host proteins (Fig 2A and B, Lane 4), and purified zEDIII to greater than 95% homogeneity (Fig 2A and B, Lane 5). A cross-reactive band was detected in fractions of total protein lysate and solubilized inclusion bodies (Fig 2B, Lanes 2 – 3), suggesting a potential zEDIII-containing protein complex. However, this cross-reactive protein/protein complex was successfully removed by Ni2+ affinity chromatography and only the expected zEDIII band with the predicted molecular mass was detected in the purified zEDIII fraction (Fig 2A and B, Lane 5).
Figure 2. Purification and characterization of recombinant zEDIII.
zEDIII was purified from E. coli and analyzed on 15% SDS-PAGE gels and either visualized with Coomassie blue staining (A), or transferred to a PVDF membranes followed by Western analysis with HisDetector™ Ni-HRP (B). Lane 1: non-induced E. coli protein control; Lane 2: total protein after IPTG induction; Lane 3: Solubilized inclusion bodies; Lane 4: Ni2+ IMAC flow through; Lane 5: Ni2+ IMAC elute; Lane 6: E. coli-produced WNV EDIII as a reference sample. Purified zEDIII was further analyzed by ELISA to access its specific binding to monoclonal antibodies that recognize EDIII conformational epitopes (C). Serial dilutions of ZV54 and E16 mAbs that recognize a conformational epitope on EDIII of ZIKV and WNV, respectively, were incubated in microtiter wells coated with zEDIII and detected with an HRP-conjugated anti-mouse gamma antibody. Mean ± SD of samples from three independent experiments is presented.
To confirm the authenticity and proper folding of the purified zEDIII, we examined its binding to ZV54 mAb generated against ZIKV EDIII and E16 mAb against WNV EDIII. E16 has been shown to be WNV specific and only bind a conformational epitope on the lateral ridge of WNV EDIII [18]. Similarly, ZV54 is ZIKV specific and binds a lateral ridge conformational epitope on zEDIII that consists of 4 discontinuous structural elements of the native zEDIII [8]. Therefore, recognition of a recombinant zEDIII by ZV54 will be indicative of its proper folding. Indeed, ELISA analysis demonstrated that zEDIII bound ZV54 specifically with high affinity (Kd = 0.2nM) but did not show any binding to E16 (Fig 2C). Additionally, zEDIII did not bind to 6D8, an anti-Ebola IgG isotype control (data not shown). Together, these results demonstrated that zEDIII can be purified to high homogeneity and purified zEDIII was folded into a conformation that resembles the native viral zEDIII on the surface of ZIKV.
Immune responses evoked by immunization of zEDIII
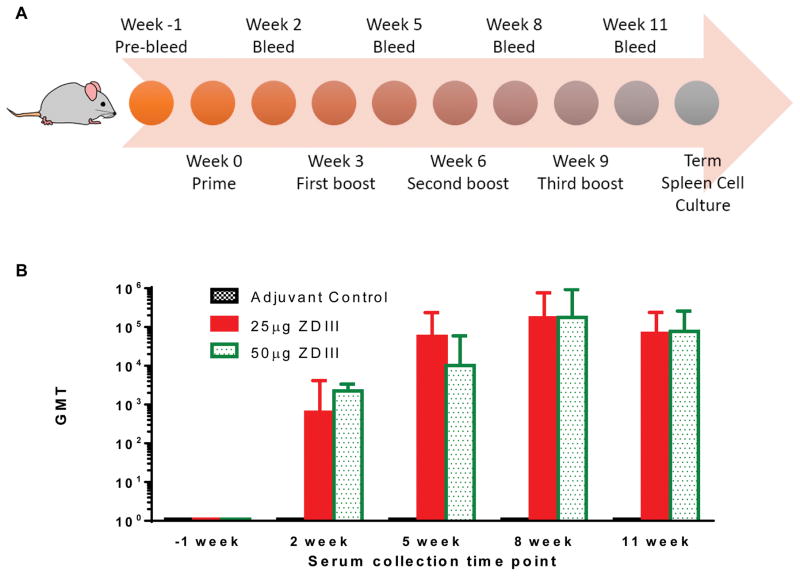
C57BL/6 mice were injected subcutaneously with four doses of zEDIII with TMG or alum as adjuvant over an 11-week time period (Fig 3A). Mice were divided into 5 groups (n = 6 per group), with groups 1 and 4 as the negative control groups injected with PBS with TMG and alum adjuvant, respectively, groups 2 and 3 with 25 μg and with 50 μg of zEDIII with TMG, and group 5 with 25 μg of zEDIII with alum, respectively. Individual serum zEDIII-specific antibody responses were measured by ELISA and GMT was calculated for each group at various time points. Samples collected from the control adjuvant group throughout the entire experiment course and pre-immune sera for all groups taken prior to the first immunization (day 0) were negative for the presence of anti-zEDIII IgG (titer < 10) (Fig 3B). All mice in groups immunized with 25 μg and 50 μg of zEDIII with TMG responded after the first administration and IgG titers increased after each of the first three antigen’s delivery and reached its peak at week 8, two weeks after the third immunization (Fig 3B). Antibody titers at week 11 (two weeks after the fourth dose) were similar to those of week 8 for groups 2 and 3 (Fig 3B). This suggests that the last immunization did not significantly further boost the zEDIII-specific antibody response. The amplitude of the zEDIII-specific IgG response did not show a dose-dependent trend between the two dosages throughout the immunization scheme (p = 0.71) (Fig 3B), possibly due to the high dosage used. The pattern of zEDIII-specific IgG response with alum as adjuvant is almost identical to that with TMG (Fig S1). In addition, the antigen-specific IgG titers from mice injected with zEDIII + alum are similar or higher than that of mice received zEDIII + TMG throughout the immunization course (p = 0.41) (Fig S1).
Figure 3. Time course of zEDIII-specific IgG responses in mice upon subcutaneous delivery of recombinant zEDIII.
C57BL/6 mice (n = 6 per group) were immunized subcutaneously with four doses of zEDIII with TMG as the adjuvant over an 11-week time period (A). Antigen was injected on weeks 0, 3, 6 and 9 with the indicated dosage. Blood samples were collected on weeks -1 (preimmune bleed), 2, 5, 8, and 11 (2 weeks after each antigen injection) and serum zEDIII-specific antibody was measured by ELISA (B). The y axis shows the geometric mean titers (GMT) and the error bars show the 95% level of confidence of the mean.
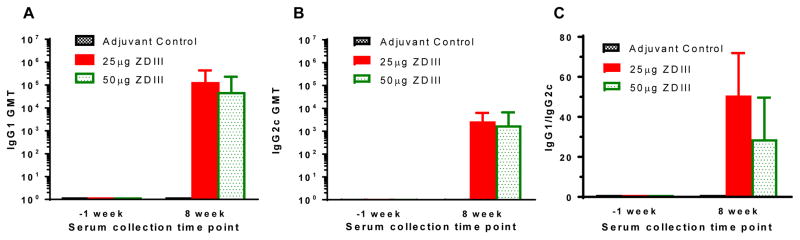
Antigen-specific IgG1 and IgG2c subtypes were evaluated by ELISA for serum samples from mice that were immunized with 25 and 50 μg of zEDIII. As shown in Figure 4, zEDIII induced robust IgG response of both IgG1 (Fig 4A) and IgG2c (Fig 4B) subtypes with higher titers of IgG1 at week 8 (Fig 5C), suggesting a Th2-type biased response stimulated by zEDIII antigen with TMG as the adjuvant. Similar results were also obtained for sera collected at weeks 5 and 11 (data not shown). Statistical analysis indicates that there is no significant difference between the two dosage groups in IgG1 (p = 0.18) or IgG2c (p =0.33) titers, or in ratios of IgG1/IgG2c (p = 0.5). In addition, the ratio of IgG1/IgG2c for both dosage groups did not vary significantly between weeks 5, 8, and 11 (p = 0.52 for the 25 μg group, p = 0.47 for the 50 μg group). The ratio of IgG1/IgG2c in mice that received zEDIII with alum as the adjuvant is also similar to that of zEDIII + TMG-injected mice (data not shown).
Figure 4. Anti-zEDIII IgG subtypes of serum samples from immunized mice.
Serum samples collected at week -1 and 8 from mice that were immunized with the indicated antigen (TMG adjuvanted) were analyzed by ELISA for zEDIII-specific IgG1 (A) and IgG2c (B) titers. Geometric means titers (GMT) and 95% level of confidence of the mean of mice in each immunization group from several independent measurements are presented. The ratio between specific IgG1 and IgG2c antibody responses was calculated for each individual mouse in treatment groups and the mean IgG1/IgG2c ratio and the standard deviation of the mean (SD) from several independent measurements are presented (C).
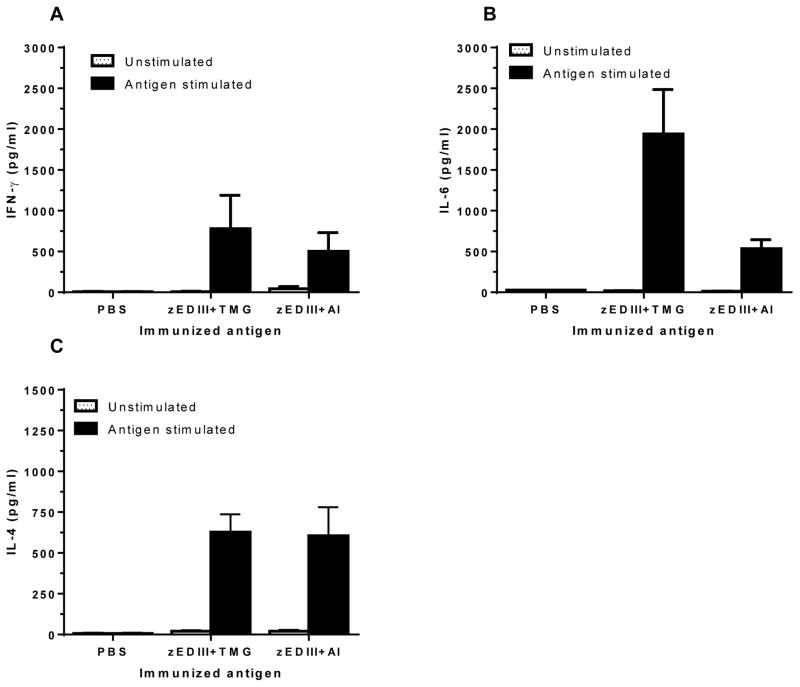
Figure 5. Cytokine production of splenocytes from Immunized mice.
Spleen cells from mice immunized with PBS + adjuvant, 25 μg zEDIII protein + TMG adjuvant, or 25 μg zEDIII + alum adjuvant (Al) were stimulated with zEDIII for 48 hr. The production of IFN-γ (A), IL-6 (B), and IL-4 (C) was quantitated by ELISA. Mean concentration (pg/ml) and SD from three independent experiments with technical triplicates for each sample are presented. Compared with the control mice received PBS + adjuvant alone, significant differences (p < 0.02) in the induction of cytokines are observed.
Th1- and Th2-type cytokine (i.e. IFN-γ, IL-4 and IL-6) production by splenocytes from immunized mice was also measured 48hr after in vitro stimulation with zEDIII or CoA (positive control). IFN-γ, IL-4 and IL-6 production was robustly stimulated by Con A, indicating the competency of splenocytes in producing cytokines upon stimulation in vitro (data not shown). No significant cytokine titers were detected from splenocytes of mice receiving PBS after in vitro stimulation with zEDIII (Fig 5). In contrast, splenocytes from zEDIII-injected mice produced significant levels of IFN-γ (Fig 5A), IL-6 (Fig 5B), and IL-4 (Fig 4C) with that of IL-6 and IL-4 being higher (TMG as the adjuvant) or similar (Alum as the adjuvant) to that of IFN-γ. These results indicate that zEDIII induced a balanced cellular immune response probably with a more Th2-type bias, corroborating the results of the humoral response above.
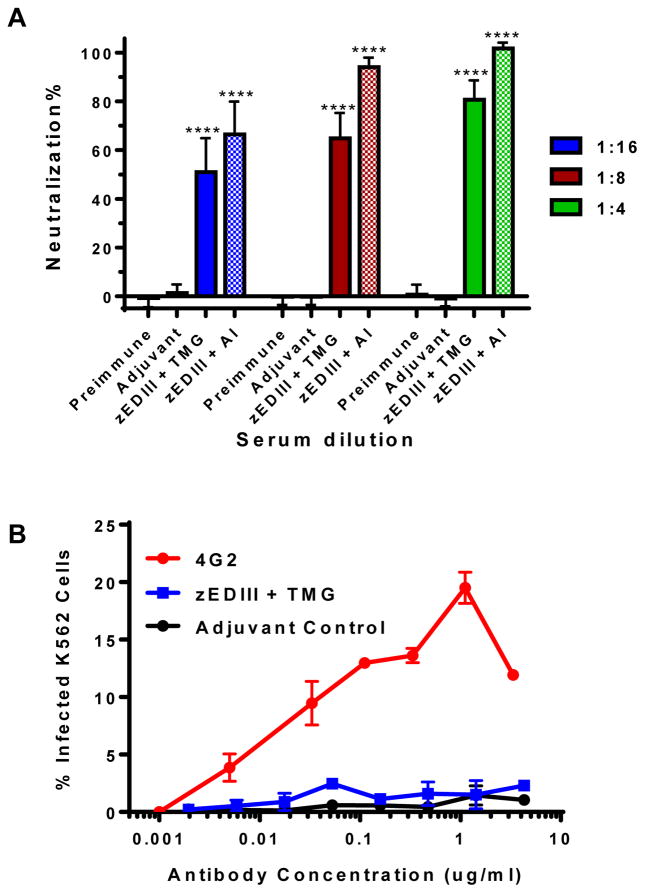
The ability of induced antibodies in response to zEDIII immunization to confer protection against ZIKV infection was examined by a PRNT assay. Incubation of ZIKV with preimmune sera or sera from mice inoculated with PBS and adjuvant did not reduce ZIKV infection (Fig 6A). In contrast, incubation of anti-zEDIII serum with ZIKV conferred potent neutralizing effects (p <0.0001 comparing anti-zEDIII serum versus adjuvant alone sera). Specifically, ZIKV infection was reduced by > 50% and >80% when incubated with sera of 1/16 and 1/4 dilutions, respectively with TMG as the adjuvant; and reduced by > 66% and >94% with sera of equivalent dilutions when alum was used as the adjuvant (Fig 6A).
Figure 6. Neutralization of ZIKV and enhancement of DENV infection by antibodies in anti-zEDIII serum. (A) Neutralization.
Pooled sera from week -1 (preimmune bleed) or week 11 of mice received PBS + Adjuvant or 25 μg of zEDIII + TMG, or week 5 of mice received 25 μg of zEDIII + alum (Al) were serially diluted and incubated with 100 PFU of ZIKV prior to infection of Vero cells. A PRNT assay was performed as described in Materials and Methods to assess ZIKV-specific neutralizing antibodies in the sera. Mean neutralization% and SD from three independent experiments with technical triplicates for each sample are presented. **** indicates p values < 0.0001 of zEDIII-immunized serum compared to that of PBS + adjuvant control, which were determined by unpaired t-test. (B) Antibody-dependent enhancement of DENV infection. Serial dilutions of IgGs from week 11 pooled sera of mice received PBS + Adjuvant (adjuvant control) or 25 μg of zEDIII + TMG were mixed with DENV-2 and added to FcγR expressing K562 cells. Anti-DENV2 E mAb 4G2 was used as an ADE positive control. Cells were then fixed, permeabilized and analyzed by flow cytometry for DENV infection of cells after 48hr incubation.
Antibody-dependent enhancement activity of IgGs from zEDIII-injected mice
One of the challenges of vaccine development for ZIKV is the risk of antibody-dependent enhancement (ADE) of heterologous flavivirus (e.g. DENV) infection. As such, we investigated if zEDIII-based antigen would induce IgGs that have a different ADE profile in vitro compared to that of 4G2, an anti-DENV-2 E domain II (EDII) mAb that cross-reactive with E of other flaviviruses [19]. 4G2 efficiently promoted ADE of DENV-2 infection in K562 cells that express the human FcγR (Fig 6B). In contrast, IgGs isolated from zEDIII-injected mice (50 μg with TMG, week 11) displayed no significant ADE activity for DENV-2 similar to IgGs from the negative control mice that received PBS and adjuvant (Fig 6B). PRNT analysis revealed that high concentrations of zEDIII-evoked IgGs used in the ADE assay have neutralizing activity (data not shown), confirming the lack of ADE is not due to insufficient amount anti-zEDIII IgGs in the assay.
Discussion
The widespread epidemics of the current ZIKV outbreak and its clinical effects on fetuses in pregnant women call for the urgent development of vaccines. Recently, three types of ZIKV vaccine candidates were evaluated in animal models [20, 21]. These studies demonstrated that a purified inactivated ZIKV and a plasmid DNA that expresses an optimized ZIKV premembrane (prM) and E protein (prM-E) provided complete protection against ZIKV challenges in both mouse and rhesus monkey models [20, 21]. In addition, a recombinant rhesus adenovirus serotype 52 vector vaccine candidate that expresses the same ZIKV prM-E protein as in the naked plasmid vaccine candidate also protected non-human primates against ZIKV challenge [21]. While these studies have laid the foundation for ZIKV vaccine development, risk factors associated with these vaccine candidates including incomplete inactivation, unfavorable host responses to viral vectors, and the potential of ADE for heterologous flavivirus (e.g. DENV) infection, call for the development of safer ZIKV vaccines, particularly for pregnant women.
Here, we demonstrate for the first time that immunization of recombinant zEDIII protein elicited a strong antigen-specific response with a ZIKV neutralization titer that has been shown to correlate with protection in mice against challenges of both Brazil and Puerto Rico strains of ZIKV [20]. zEDIII has been recently revealed as the domain of the E protein that binds cellular receptor and contains epitopes of potently neutralizing antibodies against ZIKV [8]. These zEDIII-specific antibodies have been shown to be ZIKV specific and recognize three spatially distinct epitopes in zEDIII. Furthermore, they have shown neutralizing potency against African, Asian and American strains of ZIKV and some of them protected mice against a lethal ZIKV challenge [8]. These results and the ability of EDIII of flaviviruses to independently fold into a functional domain [12] suggest that recombinant zEDIII is an appealing vaccine candidate. In this study, zEDIII was facilely produced in large quantity in E. coli. The distribution of zEDIII in the inclusion bodies offered an advantage in separating zEDIII from most of E. coli soluble proteins. In addition, the inclusion bodies were solubilized effectively and zEDIII was refolded to display its native conformation. This is supported by that the refolded zEDIII was specifically recognized by ZV54, a protective anti-ZIKV mAb that binds a large conformational epitope spanning 4 distinct regions of zEDIII [8]. This result also indicates that key ZIKV neutralization determinants at the lateral ridge region are preserved in our zEDIII protein preparations. Furthermore, zEDIII can be purified to >95% homogeneity by a simple and a scalable purification scheme.
The proof-of-principle of inducing protective immunity against ZIKV by vaccination was demonstrated in mice by immunization with inactivated ZIKV or a plasmid DNA that drives the expression of ZIKV prM-E proteins [20]. The results of this groundbreaking research revealed the mechanism of immune protection against ZIKV. Specifically, protective efficacy against both Brazil strain (Brazil ZKV2015) and Puerto Rico strain (PRVABC59) was found to correlate with E-specific antibody titers (log titers > 2.35–3.2) and neutralization antibody titers (>10, established against strain PRVABC59), and protection can be mediated by vaccine-evoked IgG alone [20]. Furthermore, the same ZIKV E-specific IgG-mediated protective mechanism was confirmed in a non-human primate model [21]. Our results indicated that zEDIII also induced potent antigen-specific humoral response, as well as ZIKV-neutralizing antibody response. Specifically, zEDIII with TMG adjuvant elicited high antigen-specific IgG titers at week 2 (log titer > 2.8) and week 5 (log titer > 3.9). The zEDIII-specific IgG titer and ZIKV neutralization titer (which was established with the same ZIKV PRVABC59 strain as in previous studies [20, 21]) at week 8 were >170,000 (log titer >5.23) and 16, respectively, exceeding the minimal threshold of E-specific and neutralizing antibody titers required for protection against both ZKV2015 and PRVABC59 strains in the mouse model. In addition, when alum, an adjuvant that has been approved for human applications was used in place of TMG, it elicited a stronger IgG response with a zEDIII-specific IgG log titer and ZIKV neutralization titer ( >4.58 and >16, respectively) that also exceed the threshold required for protection as early as at week 5. Together with the findings that (1) the EDIII of flavivirus contains the majority of epitopes that induce protective immunity [6, 7] and (2) zEDIII-specific antibodies protect mice effectively against lethal challenges of several strains of ZIKV [8], our results suggest that our vaccination regime with EDIII has at least equivalent potency in eliciting humoral response against ZIKV in mice as the reported DNA or inactivated virus-based vaccines and may also provide protective immunity in mice. Since vaccine-elicited antibody responses, especially those with neutralizing titers > 10, have been found to correlate to protection in humans against YFV and TBEV [22–24], our data suggest the possibility of developing zEDIII-based vaccines against ZIKV for humans.
It is interesting that zEDIII with TMG or alum adjuvant evoked both Th1 and Th2 types of IgGs and cytokines, suggesting the induction of specific and potent balanced humoral and cellular immune responses with a Th2 bias in immunized mice. These results are not totally unexpected, as studies with flavivirus antigens showed that TMG and alum tends to induce a Th2 type-biased responses [25]. Generally, Th1 response is more preferable for treating viral infection, however, the evidence of protective immunity provided by DNA and inactivated virus-based vaccine formulated with alum - a Th2-biased adjuvant, suggests a Th2-biased response is also just effective.
In our vaccination regime, the protective titer was reached after the delivery of zEDIII boost, suggesting this formulation may gave a lower immunogenicity than that of the inactivated ZIKV in spite of the relatively high dosage of the zEDIII antigen [20]. This is not unexpected because it has been observed that soluble protein-based subunit vaccines, especially those formulated with sub-optimal adjuvants, generally have relatively lower immunogenicity than those based on inactivated or live-attenuated viruses [26]. Further experiments are warranted to identify more immunogenic zEDIII-based antigen formats such as zEDIII-presenting virus-like particles (VLPs) and the optimal adjuvant to enhance the potency of zEDIII so that protective immunity can be provided with a minimal amount of zEDIII dosage and minimal number of antigen delivery. Further study of the protective potency in animal models especially with the optimized form of zEDIII antigen and adjuvant combination is also warranted in the future. The current construct of zEDIII carries a His tag for facile purification. Although the His tag is a short and poorly immunogenic sequence [27], its attachment to zEDIII might interfere with the immunogenicity of zEDIII or raise concerns for His tag-specific immunogenicity if the current zEDIII construct were used for human applications. Even though no His-tagged vaccine has been licensed for human use, vaccine candidates with His tag have successfully made through Phase I and Phase II clinical trials, showing no safety concerns [28]. We project that the aforementioned EDIII optimization process for enhancing its immunogenicity will lead to zEDIII-displaying formats (e.g. VLPs) that eliminate the need of the His tag for purification, as well as enhance the potency of the zEDIII antigen so that it will be safe and potent enough for potential human applications.
The use of zEDIII-based ZIKV vaccines offers several advantages over the published DNA, inactivated virus or adenovirus vector-based vaccine candidates. First, as a recombinant protein-based subunit vaccine, zEDIII will have the best safety profile compared with inactivated virus and viral vector-based subunit vaccines, due to the virtual nonexistence of possible incomplete inactivation or unfavorable host responses to viral vectors. Additionally, carefully chosen recombinant protein-based subunit vaccines including zEDIII have the advantage of specifically targeting well-defined neutralizing epitopes and avoiding epitopes with pathological effects. This point is particularly crucial for vaccines against ZIKV and other flaviviruses due to the risk of ADE. ADE occurs because cross-reactive but sub-neutralizing antibodies (including vaccine elicited antibodies) can form complexes with the infecting flavivirus that bind to Fc gamma receptor (FcγR)-bearing myeloid cells, resulting in increased viral uptake and infection [29]. ADE has been implicated for DENV. Individuals who were previously infected or vaccinated against one serotype of DENV may be more at risk to develop the more severe dengue hemorrhagic fever/dengue shock syndrome (DHF/DSS) through ADE if they are exposed to another serotype of DENV during secondary infection [30]. In addition, antibodies against DENV and ZIKV have been found to cross-react with each other and can enhance the replication of the other virus in vitro, strongly indicating ADE may occur between these two geographically co-circulating viral diseases [31–33]. Therefore, minimizing the risk of ADE of heterologous flavivirus infection in people vaccinated against ZIKV should be an important consideration for ZIKV vaccine development. Recent structural studies indicate that the majority of the exposed residues that are conserved between ZIKV and other flavivirus E proteins are located in the fusion loop and the adjacent region of domain II (zEDII) [34]. Similarly, the majority of DENV cross-reactive antibodies in human humoral response to ZIKV E protein have modest neutralizing activity and are targeted to epitopes on domain I (zEDI) or zEDII [33], which corroborate the findings in other flaviviruses [35, 36]. Also in consistent with the results from other flaviviruses, antibodies against zEDIII epitopes are ZIKV-specific and are overall highly potent in neutralizing ZIKV and protective against ZIKV challenge in mice [8, 33]. Furthermore, while it was shown that zEDI/zEDII-specific antibodies enhanced DENV infection both in vitro and in vivo, zEDIII-specific antibodies did not show ADE activity for DENV infection [33]. Indeed, our results revealed that antibodies elicited by zEDIII antigen did not exhibit ADE activity for DENV-2 infection. These results indicate that our protein-based zEDIII vaccine candidate may offer additional safety advantages over the current candidates based on inactivated virus, adenovirus vector or DNA, which all produce the full-length ZIKV E protein, and thereby, have the potential to induce zDI/zDII-targeted subneutralizing antibodies and enhance DENV infection in vaccinated subjects. This safety issue is particularly important for ZIKV vaccines as pregnant women are the focus of the target population.
Overall, the robust production of zEDIII, its effective refolding and purification, its potent immunogenicity that induces IgG titers that correlates with protective immunity, and the lack of ADE for DENV infection indicate that zEDIII is a promising vaccine candidate against ZIKV. Collectively, our study has provided the proof of principle and suggested the feasibility for the further development of recombinant protein-based subunit vaccines against ZIKV with potency and potentially enhanced safety.
Supplementary Material
Acknowledgments
We thank Dr. F. Bai for the 4G2 mAb and J. Kilbourne for her technical assistance in animal studies. We also thank A. Esqueda and J. Hurtado for the critical reading of the manuscript. This work was supported in part by a grant from National Institute of Allergy and Infectious Diseases (NIAID) # R33AI101329 to QC
Footnotes
Conflicts of Interest
The authors declare that there is no conflict of interests regarding the publication of this article.
References
- 1.Lazear HM, Diamond MS. Zika Virus: New Clinical Syndromes and Its Emergence in the Western Hemisphere. J Virol. 2016;90:4864–75. doi: 10.1128/JVI.00252-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Attar N. ZIKA virus circulates in new regions. Nat Rev Micro. 2016;14:62. [Google Scholar]
- 3.Cao-Lormeau V-M, Blake A, Mons S, Lastère S, Roche C, Vanhomwegen J, et al. Guillain-Barré Syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. The Lancet. 2016;387:1531–9. doi: 10.1016/S0140-6736(16)00562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Samarasekera U, Triunfol M. Concern over Zika virus grips the world. The Lancet. 2016;387:521–4. doi: 10.1016/S0140-6736(16)00257-9. [DOI] [PubMed] [Google Scholar]
- 5.Dai L, Song J, Lu X, Deng YQ, Musyoki AM, Cheng H, et al. Structures of the Zika Virus Envelope Protein and Its Complex with a Flavivirus Broadly Protective Antibody. Cell Host and Microbe. 2016;19:696–704. doi: 10.1016/j.chom.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 6.Oliphant T, Engle M, Nybakken G, Doane C, Johnson S, Huang L, et al. Development of a humanized monoclonal antibody with therapeutic potential against West Nile virus. Nature Medicine. 2005;11:522–30. doi: 10.1038/nm1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sukupolvi-Petty S, Austin SK, Engle M, Brien JD, Dowd KA, Williams KL, et al. Structure and Function Analysis of Therapeutic Monoclonal Antibodies against Dengue Virus Type 2. J Virol. 2010;84:9227–39. doi: 10.1128/JVI.01087-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao H, Fernandez E, Dowd Kimberly A, Speer Scott D, Platt Derek J, Gorman Matthew J, et al. Structural Basis of Zika Virus-Specific Antibody Protection. Cell. 2016;166:1016–27. doi: 10.1016/j.cell.2016.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belmusto-Worn VE, Sanchez JL, McCarthy K, Nichols R, Bautista CT, Magill AJ, et al. Randomized, double-blind, phase III. Pivotal field trial of the comparative immunogenicity, safety, and tolerability of two yellow fever 17D vaccines (ARILVAX™ and YF-VAX®) in healthy infants and children in Peru. American Journal of Tropical Medicine and Hygiene. 2005;72:189–97. [PubMed] [Google Scholar]
- 10.Heinz FX, Holzmann H, Essl A, Kundi M. Field effectiveness of vaccination against tick-borne encephalitis. Vaccine. 2007;25:7559–67. doi: 10.1016/j.vaccine.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 11.Lai H, He J, Hurtado J, Stahnke J, Fuchs A, Mehlhop E, et al. Structural and functional characterization of an anti-West Nile virus monoclonal antibody and its single-chain variant produced in glycoengineered plants. Plant Biotechnology Journal. 2014;12:1098–107. doi: 10.1111/pbi.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He J, Peng L, Lai H, Hurtado J, Stahnke J, Chen Q. A Plant-Produced Antigen Elicits Potent Immune Responses against West Nile Virus in Mice. Biomed Res Int. 2014;2014:10. doi: 10.1155/2014/952865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lai H, He J, Engle M, Diamond MS, Chen Q. Robust production of virus-like particles and monoclonal antibodies with geminiviral replicon vectors in lettuce. Plant Biotechnology Journal. 2012;10:95–104. doi: 10.1111/j.1467-7652.2011.00649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Santi L, Batchelor L, Huang Z, Hjelm B, Kilbourne J, Arntzen CJ, et al. An efficient plant viral expression system generating orally immunogenic Norwalk virus-like particles. Vaccine. 2008;26:1846–54. doi: 10.1016/j.vaccine.2008.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dent M, Hurtado J, Paul AM, Sun H, Lai H, Yang M, et al. Plant-produced anti-dengue virus monoclonal antibodies exhibit reduced antibody-dependent enhancement of infection activity. J Gen Virol. 2016 doi: 10.1099/jgv.0.000635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dent M, Hurtado J, Paul AM, Sun H, Lai H, Yang M, et al. Plant-produced anti-dengue virus monoclonal antibodies exhibit reduced antibody-dependent enhancement of infection activity. J Gen Virol. 2016;97:3280–90. doi: 10.1099/jgv.0.000635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jungblut M, Oeltze K, Zehnter I, Hasselmann D, Bosio A. Preparation of Single-Cell Suspensions from Mouse Spleen with the gentleMACS Dissociator. 2008:e1029. doi: 10.3791/1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lai H, Engle M, Fuchs A, Keller T, Johnson S, Gorlatov S, et al. Monoclonal antibody produced in plants efficiently treats West Nile virus infection in mice. Proc Natl Acad Sci U S A. 2010;107:2419–24. doi: 10.1073/pnas.0914503107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crill WD, Chang GJ. Localization and characterization of flavivirus envelope glycoprotein cross-reactive epitopes. J Virol. 2004;78:13975–86. doi: 10.1128/JVI.78.24.13975-13986.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larocca RA, Abbink P, Peron JPS, de Zanotto APM, Iampietro MJ, Badamchi-Zadeh A, et al. Vaccine protection against Zika virus from Brazil. Nature. 2016;536:474–8. doi: 10.1038/nature18952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abbink P, Larocca RA, De La Barrera RA, Bricault CA, Moseley ET, Boyd M, et al. Protective efficacy of multiple vaccine platforms against Zika virus challenge in rhesus monkeys. Science. 2016;353:1129. doi: 10.1126/science.aah6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hombach J, Solomon T, Kurane I, Jacobson J, Wood D. Report on a WHO consultation on immunological endpoints for evaluation of new Japanese encephalitis vaccines, WHO, Geneva, 2–3 September, 2004. Vaccine. 2005;23:5205–11. doi: 10.1016/j.vaccine.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Kreil TR, Burger I, Bachmann M, Fraiss S, Eibl MM. Antibodies protect mice against challenge with tick-borne encephalitis virus (TBEV)-infected macrophages. Clinical & Experimental Immunology. 1997;110:358–61. doi: 10.1046/j.1365-2249.1997.4311446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mason RA, Tauraso NM, Spertzel RO, Ginn RK. Yellow fever vaccine: direct challenge of monkeys given graded doses of 17D vaccine. Appl Microbiol. 1973;25:539–44. doi: 10.1128/am.25.4.539-544.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Demento SL, Bonafe N, Cui W, Kaech SM, Caplan MJ, Fikrig E, et al. TLR9-Targeted Biodegradable Nanoparticles as Immunization Vectors Protect against West Nile Encephalitis. The Journal of Immunology. 2010;185:2989–97. doi: 10.4049/jimmunol.1000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foged C. Subunit vaccines of the future: the need for safe, customized and optimized particulate delivery systems. Therapeutic delivery. 2011;2:1057–77. doi: 10.4155/tde.11.68. [DOI] [PubMed] [Google Scholar]
- 27.Mayer A, Sharma SK, Tolner B, Minton NP, Purdy D, Amlot P, et al. Modifying an immunogenic epitope on a therapeutic protein: a step towards an improved system for antibody-directed enzyme prodrug therapy (ADEPT) Br J Cancer. 2004;90:2402–10. doi: 10.1038/sj.bjc.6601888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.BALLOU WR, AREVALO-HERRERA M, CARUCCI D, RICHIE TL, CORRADIN G, DIGGS C, et al. UPDATE ON THE CLINICAL DEVELOPMENT OF CANDIDATE MALARIA VACCINES. The American Journal of Tropical Medicine and Hygiene. 2004;71:239–47. [PubMed] [Google Scholar]
- 29.Morens DM. Antibody-dependent of enhancement of infection and the pathogenesis of viral disease. Clin Inf Dis. 1994;19:500–12. doi: 10.1093/clinids/19.3.500. [DOI] [PubMed] [Google Scholar]
- 30.Halstead SB. Dengue Antibody-Dependent Enhancement: Knowns and Unknowns. Microbiology spectrum. 2014:2. doi: 10.1128/microbiolspec.AID-0022-2014. [DOI] [PubMed] [Google Scholar]
- 31.Dejnirattisai W, Supasa P, Wongwiwat W, Rouvinski A, Barba-Spaeth G, Duangchinda T, et al. Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with zika virus. Nat Immunol. 2016 doi: 10.1038/ni.3515. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barba-Spaeth G, Dejnirattisai W, Rouvinski A, Vaney M-C, Medits I, Sharma A, et al. Structural basis of potent Zika–dengue virus antibody cross-neutralization. Nature. 2016;536:48–53. doi: 10.1038/nature18938. [DOI] [PubMed] [Google Scholar]
- 33.Stettler K, Beltramello M, Espinosa DA, Graham V, Cassotta A, Bianchi S, et al. Specificity, cross-reactivity, and function of antibodies elicited by Zika virus infection. Science. 2016;353:823. doi: 10.1126/science.aaf8505. [DOI] [PubMed] [Google Scholar]
- 34.Sirohi D, Chen Z, Sun L, Klose T, Pierson TC, Rossmann MG, et al. The 3.8 Å resolution cryo-EM structure of Zika virus. Science. 2016;352:467–70. doi: 10.1126/science.aaf5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beltramello M, Williams KL, Simmons CP, Macagno A, Simonelli L, Quyen Nguyen Than H, et al. The Human Immune Response to Dengue Virus Is Dominated by Highly Cross-Reactive Antibodies Endowed with Neutralizing and Enhancing Activity. Cell host & microbe. 2010;8:271–83. doi: 10.1016/j.chom.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin HE, Tsai WY, Liu IJ, Li PC, Liao MY, Tsai JJ, et al. Analysis of epitopes on dengue virus envelope protein recognized by monoclonal antibodies and polyclonal human sera by a high throughput assay. PLoS Negl Trop Dis. 2012;6:e1447. doi: 10.1371/journal.pntd.0001447. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.