Abstract
Rates of major depressive disorder (MDD) have steadily increased over the past 50 years. Many factors have been implicated in the etiology of depressive disorders and environmental influences are being increasingly recognized. The increase in depression rates has coincided with increased artificial nighttime lighting. Exposure to light at night (LAN) has been associated with increased depressive-like behavior in rodents and decreased mood in humans. However, relatively little is known on the multigenerational effects of dLAN on affect. In this study, we exposed adult male and female Siberian hamsters (Phodopus sungorus) to either DARK (0 lux) or dim LAN (5 lux) for 9 weeks, then paired animals in a full factorial design; all animals were thereafter housed in dark nights. Offspring were gestated and reared in dark nights, then tested in adulthood for depressive-like behaviors and hippocampal expression of glucocorticoid (GR) and melatonin (MT1) receptor expression. Maternal exposure to dLAN decreased sucrose preference, time to first float bout in the Porsolt swim test, and GR expression in the hippocampus. Paternal exposure to dLAN increased time spent floating, and increased hippocampal GR expression. Overall, our results suggest that chronic exposure of parents to light at night has multigenerational effects on offspring depressive-like behavior. If these results pertain to humans, then our data suggest that LAN may contribute to the rapidly rising rates of major depressive disorder in industrialized and developing countries.
Keywords: Light at night, transgenerational, depression, circadian disruption, light pollution
Introduction
The early life environment can both protect or predispose individuals to mental and physiological disorders later in life (Heim and Binder, 2012; Pryce et al., 2005). Stressful environmental factors including exposure to drugs during early life has long lasting effects on gene expression and neural development (Baram et al., 2012; Murgatroyd et al., 2009; Wolstenholme et al., 2012). Conversely, early life environment and maternal care can positively influence behavior later in life (Liu et al., 1997). Recent studies have described similar behavioral outcomes in the offspring of parents that experienced stressors despite never having experienced them themselves (Bale et al., 2010). These effects are often attributed to epigenetic changes in the genome in response to physical and emotional stressors, and environment- driven epigenetic changes are credited to toxins.
Over the past century, there has been a dramatic increase in nighttime illumination (Cinzano et al., 2001; Falchi et al., 2016); we are also exposed to significant light at night (LAN) in our living and work spaces (Rajaratnam and Arendt, 2001). The master biological clock, the suprachiasmatic nucleus (SCN), requires environmental light to synchronize internal physiology to the solar day. LAN provides extraneous lighting information, disrupts the day-night distinction, and flattens amplitude of molecular clock gene expression (Fonken et al., 2013). Disruption of clock gene expression has downstream effects on behavioral and endocrine systems. Specifically, exposure to LAN increases depressive-like behaviors in rodents (Bedrosian et al., 2011; Bedrosian and Nelson, 2017; Fonken et al., 2009).
The prevalence of major depression has increased dramatically over the past several decades. Some of the increase can be attributed to better diagnosis and reporting of mood disorders, however, environmental factors could be driving the increase in mood disorders as well. Coincident with the increased incidence of light at night has been an increase in the incidence of depression disorders (Lambert et al., 2015). As countries improve their economies and embrace artificial LAN, a correlation of increased mood disorders is observed (Bedrosian and Nelson, 2017). In the US, rates of depression have increased from less than 1% in the 1960's to nearly 6% in recent years (Compton et al., 2006; Horwitz, 2010). Notably, the incidence of major depressive disorder among the Amish, who are not exposed to light at night, remains 1-2% (Egeland and Hostetter, 1983).
One putative link between LAN and affect are the disruption to endocrine organization caused by LAN (Bedrosian et al., 2015). Two endocrine ‘hands’ of the biological clock are melatonin (secreted at night) and glucocorticoids (secreted prior to activity). Light at night suppresses nightly melatonin secretion (Brainard et al., 1982) and melatonin receptor agonists improve mood in humans and decrease depressive-like behaviors in rodents (Crupi et al., 2010; Hickie and Rogers, 2011). Dysregulation of glucocorticoids underlies many cases of depression (Caspi et al., 2003; McEwen, 2005). Prenatal stress and modulations in maternal care are known to affect offspring behavioral and hypothalamus-pituitary-adrenal (HPA) responses to stress, as well as epigenetic modification of the hippocampal glucocorticoid receptor (Liu et al., 1997; Weaver et al., 2004, 2005; Weinstock et al., 1992).
Although light at night has been reported to increase depressive-like behaviors in rodents (reviewed in Bedrosian and Nelson, 2017), the extent to which light at night affects the offspring of exposed individuals remains unspecified. To our knowledge only one study, and investigation of offspring immune responses, has evaluated the multigenerational effects of LAN (Cissé et al., 2017). In the present study, we addressed the possibility of multigenerational effects of dim LAN (dLAN; 5 lux) on depressive-like behaviors and hippocampal gene expression. We provide evidence that parental exposure to dLAN increases offspring depressive-like behavioral responses. We further show that these alterations in behavior are accompanied by changes in hippocampal glucocorticoid and melatonin receptor expression. These results suggest that dLAN has multigenerational effects on the neuroendocrine system and behavior.
Methods
Animals
Parents
Male (n= 23) and female (n= 23) Siberian hamsters (Phodopus sungorus) were obtained from our in-house breeding colony at The Ohio State University. Hamsters were maintained on a standard 16h: 8h light/dark cycle (DARK; 150 lux: 0 lux) with lights on at 22.00 h and off at 14.00 h EST, in polypropylene cages (30 × 15 × 14cm) on static racks in a temperature- and humidity-controlled vivarium. All animals were provided access to food ad libitum (Harlan Teklad 8640; Madison, WI, USA) and filtered tap water. All experiments were approved by the Ohio State University Institutional Animals Care and Use Committee, and animals were maintained in accordance with the recommendations of the National Institutes of Health and The Guide for the Care and Use of Laboratory Animals.
Generation of F1
Adult (> 8 week) Siberian hamsters were individually housed and randomly assigned to a lighting condition: DARK or dLAN (light (150 lux):dim (5 lux)). Hamsters were maintained in respective lighting conditions for 9 weeks at which point all animals were paired and mated as previously described (Cissé et al., 2017), and thereafter housed in standard DARK conditions. Pairings resulted in four parental groups: DARK-DARK (Male/Female; n=5), DARK-dLAN (n=6), dLAN-DARK (n= 5), and dLAN-dLAN (n=7). Males were removed one week after pairing. Five mating pairs did not successfully mate within this time window (1 DARK/DARK, 2 DARK/dLAN, and 2 dLAN/dLAN) and one dam cannibalized her pups (1 dLAN/dLAN). Of the remaining litters, there was an average of 5 pups per litter in each group, with no difference in litter size between groups (p > 0.05). The sex ratio was slightly shifted towards males (DARK-DARK, 0.69; DARK-dLAN, 0.68; dLAN-DARK, 0.53; dLAN-dLAN, 0.60), with no statistical difference among groups. Pups (n= 86) were weaned at 21 days of age and group-housed with same sex siblings. At 7 weeks of age hamsters were individually housed. All experimental manipulations occurred after offspring reached adulthood (> 8 weeks of age).
Sucrose Anhedonia
Hamsters were acclimated to the removal of their food and water, and the addition of two water bottles in their cage for the first 6 h of the active phase (14.00-20.00 h). Acclimation lasted for three days prior to starting the test. On the fourth day, one water bottle was randomly switched out for one containing a 3% sucrose solution (in water) for two days. To account for possible side preferences, placement of the bottles in each cage was counterbalanced. Rodents typically prefer sweetened water, and lack of a preference for sucrose is considered a reliable indicator of anhedonia (Willner et al., 1992)
Porsolt Swim Test
Hamsters were placed in 14 cm of 25 ± 1°C water in a cylindrical container (24 cm in diameter) and recorded for a duration of 10 min. Recordings were later analyzed by a condition blinded observer. The behaviors scored were as follows: (1) climbing (that is, vigorous swimming or scratching directed at the wall of the tank), (2) swimming (horizontal movement in the tank) and (3) floating/immobility (that is, minimal movement necessary to keep head elevated above water surface). Increased time spent floating is indicative of behavioral despair (Porsolt et al., 1977).
Quantitative PCR (qPCR)
Hamsters were deeply anesthetized with isoflurane vapors and rapidly decapitated; brains were removed and flash frozen on dry ice for subsequent qPCR analysis. Tissues were collected during the mid-dark and mid-light phase (ZT 8 and ZT 20), to assess diurnal variations in gene expression. Total RNA from hippocampi was extracted using Trizol reagent (Qiagen). Samples were then DNased using DNase 1 Amplification grade (Invitrogen, Carlsbad, CA) to eliminate non-specific binding of SYBR Green master mix. RNA quality was assessed using a Nanodrop One Spectrophotometer (Thermo Scientific) and quantity was normalized to 200 ng/μL, reverse transcribed into cDNA using MMLV Reverse Transcriptase enzyme (Invitrogen, Carlsbad, CA) per the manufacturer's instructions. Hippocampal GR and MT1 were assessed, using primers previously described for Siberian hamster GR (Walton et al., 2012) and MT1 and 18S(Ikeno et al., 2014) on an ABI 7500 Fast Real Time PCR system using SyBR Green PCR Master Mix. Cycling conditions were: 95 °C for 5 min, followed by 40 cycles of 95°C for 15 sec and 60 °C for 1 min for GR and MT1. Relative gene expression was calculated using the delta-delta CT method, normalized to 18s rRNA signal.
Statistical Analyses
Statistical analyses were conducted using SPSS Statistics v 24 (IBM; Armonk, NY). Comparisons of sucrose anhedonia, time spent floating, and time to first float were assessed using two way ANOVAs assessing maternal and paternal lighting conditions, offspring sex, and interactions. If data did not meet the assumptions of normality or equal variance, they were first log transformed (in the case of MT1); if this transformation did not resolve unequal variance, non-parametric Mann Whitney and Kruskal Wallis tests were conducted (sucrose anhedonia and GR gene expression). Post-hoc analyses were conducted using Tukey's HSD or Dunnet's test for parametric data and a Dunn's test for non-parametric data. Litter effects were evaluated using Kruskal Wallis due to unequal variance between groups in all measures except MT1 expression. After removal of one two-pup litter, no litter effects were identified in sucrose intake, floating time, time to first float, and hippocampal MT1 expression. There was a statistically significant litter effect in hippocampal GR expression, but post-hoc analyses revealed that this effect was driven by litters in different treatment groups, reflecting a treatment effect (Supplemental Fig. 1). Outliers were determined using Z-score analysis. Differences between means were considered statistically significant when p ≤ 0.05 for all analyses.
Results
Sucrose Anhedonia
Parental lighting altered offspring sucrose consumption (X2 = 7.97, p < 0.05), such that offspring of studs in DARK and dams in dLAN (DARK-dLAN) decreased sucrose intake relative to DARK-DARK controls (Dunn's p < 0.05; Fig 1A). Males consumed more sucrose solution than females (U= 432.00, p < 0.01). Sex interacted with maternal lighting condition such that females of dLAN mothers consumed the least amount of sucrose (Dunn's p < 0.05).
Figure 1.
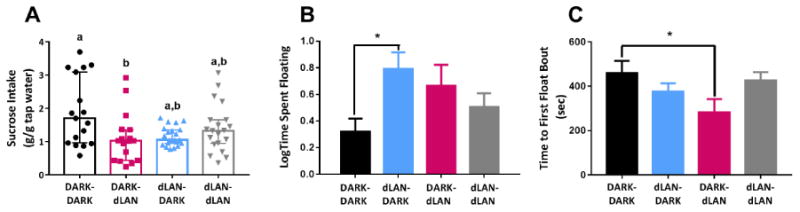
Single parental exposure to dLAN increases offspring depressive-like behavior. Offspring with a mother in dLAN (DARK-dLAN) decrease sucrose intake in adulthood relative counterparts (A). Offspring with a father in dLAN (dLAN-DARK) increase time spent floating in a Porsolt swim test (B). Offspring with a mother in dLAN (DARK-dLAN) float sooner than DARK-DARK offspring (C). Data presented as mean ± SEM or median with a 95% confidence interval for non-parametric data. Different letters indicate statistical differences of p < 0.05.
Forced Swim
Maternal and paternal lighting conditions interacted (F1,73 = 5.94, p < 0.05), such that dLAN-DARK offspring increased floating time relative to DARK-DARK offspring (Dunn's p < 0.05; Fig 1B). Maternal and paternal lighting conditions also interacted (F1,73 = 4.40, p < 0.05), such that offspring of studs in dLAN and dams in DARK (dLAN-DARK) floated sooner than their counterparts in DARK-DARK (Dunnet's, p < 0.05; Fig 1C).
Hippocampal Gene Expression
Maternal exposure to dLAN decreased offspring hippocampal GR whereas paternal exposure to dLAN increased hippocampal GR (U= 345.00, p < 0.001 and U= 433.00, p < 0.01, Fig 2A and 2B respectively). Parental lighting altered hippocampal glucocorticoid receptor (GR) expression (X2 = 23.73, p < 0.05), such that offspring of dLAN-housed studs and DARK-housed dams (dLAN-DARK) increased hippocampal GR expression relative to dLAN-dLAN and DARK-dLAN offspring (Dunn's, p < 0.05; Fig 2C). Maternal lighting and time of day interacted (X2 = 15.64, p < 0.05), such that offspring of mothers in dLAN had lower nighttime GR expression than both daytime and nighttime offspring of DARK-housed mothers (Dunn's p < 0.05).
Figure 2.
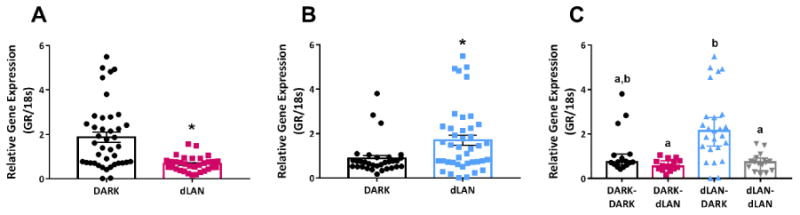
Parental exposure to dLAN alters offspring hippocampal GR expression. Maternal exposure to dLAN decreases hippocampal GR (A), whereas paternal exposure to dLAN increases hippocampal GR (B) in adult offspring. Offspring of dLAN-DARK heritage increase hippocampal GR expression relative to all other groups (C). Data presented as mean ± SEM or median with a 95% confidence interval for non-parametric data. *, p < 0.05 dLAN vs DARK. Different letters indicate statistical differences of p < 0.05.
Discussion
Parental exposure to dLAN was sufficient to increase offspring depressive-like behaviors in a parental sex-dependent manner (Fig 1). Hippocampal endocrine receptor expression, viz., melatonin and glucocorticoid receptor gene expression, was altered by parental dLAN (Fig 2). These changes occurred despite offspring being conceived, gestated, and reared under standard dark night conditions. Maternal dLAN exposure decreased offspring sucrose intake, marginally decreased time to first float, decreased hippocampal GR gene expression, but did not affect MT1 gene expression in offspring. Paternal dLAN exposure increased offspring time spent floating in a forced swim paradigm, and increased GR in the hippocampus of offspring. These data suggest that dim light exposure has multigenerational effects on affective behavior and the neuroendocrine system.
Aberrant circadian rhythms are a hallmark of major depression. Light exposure during the day is the primary entraining cue for the circadian system, but light at night disrupts molecular and behavioral circadian rhythms (Fonken et al., 2013; Fonken and Nelson, 2014; Ohta et al., 2005). Indeed, adult exposure to light at night increases time spent floating and decreases sucrose preference (Bedrosian et al., 2013). Similar to adult exposure, offspring of parents exposed to dLAN exhibit sucrose anhedonia and increased behavioral despair (Fig 1).
In this study, studs were removed one week after pairing to limit breeding to the first week out of lighting conditions. Hamsters are not biparental (Wynne-Edwards, 1995). Despite being removed prior to the birth of their offspring, paternal exposure to dLAN increased behavioral despair, sucrose anhedonia, and hippocampal GR expression (Fig 1A-B and 2). Transmission of paternal anxiety-like behaviors in the absence of postnatal contact has been previously reported in mice (Alter et al., 2009). In the absence of postnatal interaction, paternal effects can be transmitted either by altering maternal prenatal or postnatal investment, or by germline inheritance (Curley et al., 2011).
Offspring affective behavior can be altered by the quality of maternal care experienced early in life. Adult female Siberian hamsters exposed to dLAN are behaviorally and physiologically indistinguishable from their dark night counterparts after returning to dark nights for one week (Bedrosian et al., 2013). Specifically, the increase in time spent floating and decrease in sucrose preference, hippocampal BDNF expression, and CA1 apical spine density observed in dLAN-exposed hamsters are no longer present after one week back in dark nights. In our study, by the time offspring are born, mothers have been out of dLAN for three weeks, suggesting that dLAN-induced physiological and behavioral alterations not likely be present. Although this study did not assess quality of maternal care, subsequent studies have identified no overt changes in maternal care (Cisse and Nelson, unpublished data). The absence of differences in maternal care suggests that postnatal investment is not altered, but prenatal investments, altering maternal physiology/endocrine function or germline epigenetic effects may be responsible for transmission.
In addition to disruptions of the central clock, exposure to LAN also alters endocrine rhythms. Light levels of greater than 1 lux inhibit the production of pineal melatonin in nocturnal rodents (Brainard et al., 1982). Although the major association between melatonin and affective disorders focuses on seasonal affective disorder, melatonin also has general anti-depressive effects in rodents, with varying results in humans (Hansen et al., 2014; Haridas et al., 2013; Hickie and Rogers, 2011). MT1 knockout mice and mice treated with a melatonin receptor antagonist increase time spent floating in a forced swim paradigm, supporting the role of melatonin signaling in depressive-like behavior (Micale et al., 2006; Weil et al., 2006). Indeed, offspring of fathers exposed to dLAN decreased MT1 expression and increased time spent floating (Fig 1C and 2A).
Stressful life events, as well as genetics, have long been known to predispose to depressive illnesses (Caspi et al., 2003). Stress hormones, such as cortisol, alters neurogenesis and dendritic arborization in brain regions involved in depressive-like behaviors (McEwen, 1999, 2005). Previous studies have demonstrated that dLAN does not elicit a stress response in this species (Bedrosian et al., 2011). However, offspring of parents exposed to dLAN exhibit altered GR expression in the hippocampus, suggesting altered glucocorticoid signaling. Specifically, paternal exposure to dLAN increases hippocampal GR, whereas maternal exposure decreases hippocampal GR. This GR gene expression pattern coincides with the increase in behavioral despair observed in offspring of solely fathers, but not mothers exposed to dLAN.
The behavioral effects demonstrated in this study occur in groups in which only one parent was exposed to dLAN. In offspring of both DARK-DARK and dLAN-dLAN experimental groups, parental effects on GR appear to cancel one another. In dLAN-DARK offspring, GR is increased and MT1 is decreased, suggestive of increased depressive-like behavior reflected in the forced swim test results. DARK-dLAN offspring have comparable expression of GR to both DARK-DARK and dLAN-dLAN offspring, but have relatively increased MT1 expression. Corticosterone can negate the positive effects of melatonin on forced swim test performance (Hill et al., 2003), possibly contributing to the differential depressive-like behavior in DARK-dLAN offspring.
Overall, our results suggest that chronic exposure of parents to light at night is sufficient to alter offspring depressive-like behavior. If these results pertain to humans, then given the growing prevalence of electrical lighting, this suggests that LAN may be contributing to the rapidly rising rates of major depressive disorder in industrialized and developing countries. This study indicates that additional research on the transgenerational effects of circadian disruption is warranted.
Supplementary Material
Highlights.
Parents are exposed to DARK or dLAN for 9 weeks prior to pairing. Mating, gestation, and offspring rearing occurred under DARK nights.
Maternal exposure to dLAN decreased offspring sucrose intake, time to first float bout, and hippocampal GR expression in adult offspring.
Paternal exposure to dLAN increased time spent floating and hippocampal GR in adult offspring.
Light at night has transgenerational effects on offspring behavioral and neuroendocrine system.
Acknowledgments
The authors thank Megan Fleming and the University Laboratory Animals Resources staff at the Ohio State University for providing excellent care of the animals in this study. We thank Elise Lemanski, Rachel Pinchot, Adam Weiss, and Ryan Beatty for technical assistance. This work was supported by the National Science Foundation Grant IOS 11-18792 (to R.J.N). Y.M.C was supported by F31 ES026890-02.
Footnotes
Conflicts of Interest: The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alter MD, Gilani AI, Champagne FA, Curley JP, Turner JB, Hen R. Paternal transmission of complex phenotypes in inbred mice. Biol Psychiatry. 2009;66:1061–1066. doi: 10.1016/j.biopsych.2009.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Baram TZ, Brown AS, Goldstein JM, Insel TR, McCarthy MM, et al. Early life programming and neurodevelopmental disorders. Biol Psychiatry. 2010;68:314–319. doi: 10.1016/j.biopsych.2010.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baram TZ, Davis EP, Obenaus A, Sandman CA, Small SL, Solodkin A, et al. Fragmentation and unpredictability of early-life experience in mental disorders. Am J Psychiatry. 2012;169:907–915. doi: 10.1176/appi.ajp.2012.11091347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedrosian TA, Fonken LK, Nelson RJ. Endocrine effects of circadian disruption. Annu Rev Physiol. 2015;78:109–131. doi: 10.1146/annurev-physiol-021115-105102. [DOI] [PubMed] [Google Scholar]
- Bedrosian TA, Fonken LK, Walton JC, Haim A, Nelson RJ. Dim light at night provokes depression-like behaviors and reduces CA1 dendritic spine density in female hamsters. Psychoneuroendocrinology. 2011;36:1062–1069. doi: 10.1016/j.psyneuen.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Bedrosian TA, Nelson RJ. Timing of light exposure affects mood and brain circuits. Transl Psychiatry. 2017;7:e1017. doi: 10.1038/tp.2016.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedrosian TA, Weil ZM, Nelson RJ. Chronic dim light at night provokes reversible depression-like phenotype: possible role for TNF. Mol Psychiatry. 2013;18:930–6. doi: 10.1038/mp.2012.96. [DOI] [PubMed] [Google Scholar]
- Brainard GC, Richardson BA, Petterborg LJ, Reiter RJ. The effect of different light intensities on pineal melatonin content. Brain Res. 1982;233:75–81. doi: 10.1016/0006-8993(82)90931-3. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science (80-) 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Cinzano P, Falchi F, Elvidge CD. The first World Atlas of the artificial night sky brightness. Mon Not R Astron Soc. 2001;328:689–707. doi: 10.1046/j.1365-8711.2001.04882.x. [DOI] [Google Scholar]
- Compton WM, Conway KP, Stinson FS, Grant BF. Changes in the prevalence of major depression and comorbid substance use disorders in the United States between 1991–1992 and 2001–2002. Am J Psychiatry. 2006;163:2141–2147. doi: 10.1176/ajp.2006.163.12.2141. [DOI] [PubMed] [Google Scholar]
- Crupi R, Mazzon E, Marino A, La Spada G, Bramanti P, Cuzzocrea S, et al. Melatonin treatment mimics the antidepressant action in chronic corticosterone-treated mice. J Pineal Res. 2010;49 doi: 10.1111/j.1600-079X.2010.00775.x. no-no. [DOI] [PubMed] [Google Scholar]
- Curley JP, Mashoodh R, Champagne FA. Epigenetics and the origins of paternal effects. Horm Behav. 2011;59:306–314. doi: 10.1016/j.yhbeh.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egeland JA, Hostetter AM. Amish Study, I: Affective disorders among the Amish, 1976-1980. Am J Psychiatry. 1983;140:56–61. doi: 10.1176/ajp.140.1.56. [DOI] [PubMed] [Google Scholar]
- Falchi F, Cinzano P, Duriscoe D, Kyba CCM, Elvidge CD, Baugh K, et al. The new world atlas of artificial night sky brightness. Sci Adv. 2016;2:e1600377. doi: 10.1126/sciadv.1600377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonken LK, Aubrecht TG, Meléndez-Fernández OH, Weil ZM, Nelson RJ. Dim light at night disrupts molecular circadian rhythms and increases body weight. J Biol Rhythms. 2013;28:262–71. doi: 10.1177/0748730413493862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonken LK, Finy MS, Walton JC, Weil ZM, Workman JL, Ross J, et al. Influence of light at night on murine anxiety- and depressive-like responses. Behav Brain Res. 2009;205:349–54. doi: 10.1016/j.bbr.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Fonken LK, Nelson RJ. The effects of light at night on circadian clocks and metabolism. Endocr Rev. 2014:er20131051. doi: 10.1210/er.2013-1051. [DOI] [PubMed] [Google Scholar]
- Hansen MV, Danielsen AK, Hageman I, Rosenberg J, Gögenur I. The therapeutic or prophylactic effect of exogenous melatonin against depression and depressive symptoms: A systematic review and meta-analysis. Eur Neuropsychopharmacol. 2014;24:1719–1728. doi: 10.1016/j.euroneuro.2014.08.008. [DOI] [PubMed] [Google Scholar]
- Haridas S, Kumar M, Manda K. Melatonin ameliorates chronic mild stress induced behavioral dysfunctions in mice. Physiol Behav. 2013;119:201–207. doi: 10.1016/j.physbeh.2013.06.015. [DOI] [PubMed] [Google Scholar]
- Heim C, Binder EB. Current research trends in early life stress and depression: Review of human studies on sensitive periods, gene–environment interactions, and epigenetics. Exp Neurol. 2012;233:102–111. doi: 10.1016/j.expneurol.2011.10.032. [DOI] [PubMed] [Google Scholar]
- Hickie IB, Rogers NL. Novel melatonin-based therapies: potential advances in the treatment of major depression. Lancet. 2011;378:621–631. doi: 10.1016/S0140-6736(11)60095-0. [DOI] [PubMed] [Google Scholar]
- Hill MN, Brotto LA, Lee TTY, Gorzalka BB. Corticosterone attenuates the antidepressant-like effects elicited by melatonin in the forced swim test in both male and female rats. Prog Neuro-Psychopharmacology Biol Psychiatry. 2003;27:905–911. doi: 10.1016/S0278-5846(03)00149-0. [DOI] [PubMed] [Google Scholar]
- Horwitz AV. How an age of anxiety became an age of depression. Milbank Q. 2010;88:112–38. doi: 10.1111/j.1468-0009.2010.00591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeno T, Weil ZM, Nelson RJ. Dim light at night disrupts the short-day response in Siberian hamsters. Gen Comp Endocrinol. 2014;197:56–64. doi: 10.1016/j.ygcen.2013.12.005. [DOI] [PubMed] [Google Scholar]
- Lambert KG, Nelson RJ, Jovanovic T, Cerdá M. Brains in the city: Neurobiological effects of urbanization. Neurosci Biobehav Rev. 2015;58:107–122. doi: 10.1016/j.neubiorev.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, et al. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–62. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–22. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Glucocorticoids, depression, and mood disorders: structural remodeling in the brain. Metabolism. 2005;54:20–23. doi: 10.1016/j.metabol.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Micale V, Arezzi A, Rampello L, Drago F. Melatonin affects the immobility time of rats in the forced swim test: The role of serotonin neurotransmission. Eur Neuropsychopharmacol. 2006;16:538–545. doi: 10.1016/j.euroneuro.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Murgatroyd C, Patchev AV, Wu Y, Micale V, Bockmühl Y, Fischer D, et al. Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nat Neurosci. 2009;12:1559–1566. doi: 10.1038/nn.2436. [DOI] [PubMed] [Google Scholar]
- Ohta H, Yamazaki S, McMahon DG. Constant light desynchronizes mammalian clock neurons. Nat Neurosci. 2005;8:267–9. doi: 10.1038/nn1395. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229:327–36. [PubMed] [Google Scholar]
- Pryce CR, Rüedi-Bettschen D, Dettling AC, Weston A, Russig H, Ferger B, et al. Long-term effects of early-life environmental manipulations in rodents and primates: Potential animal models in depression research. Neurosci Biobehav Rev. 2005;29:649–674. doi: 10.1016/j.neubiorev.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Rajaratnam SM, Arendt J. Health in a 24-h society. Lancet. 2001;358:999–1005. doi: 10.1016/S0140-6736(01)06108-6. [DOI] [PubMed] [Google Scholar]
- Walton JC, Grier AJ, Weil ZM, Nelson RJ. Photoperiod and stress regulation of corticosteroid receptor, brain-derived neurotrophic factor, and glucose transporter GLUT3 mRNA in the hippocampus of male Siberian hamsters (Phodopus sungorus) Neuroscience. 2012;213:106–11. doi: 10.1016/j.neuroscience.2012.03.043. [DOI] [PubMed] [Google Scholar]
- Weaver ICG, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–54. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Weaver ICG, Champagne FA, Brown SE, Dymov S, Sharma S, Meaney MJ, et al. Reversal of maternal programming of stress responses in adult offspring through methyl supplementation: Altering epigenetic marking later in life. J Neurosci. 2005;25:11045–11054. doi: 10.1523/JNEUROSCI.3652-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil ZM, Hotchkiss AK, Gatien ML, Pieke-Dahl S, Nelson RJ. Melatonin receptor (MT1) knockout mice display depression-like behaviors and deficits in sensorimotor gating. Brain Res Bull. 2006;68:425–429. doi: 10.1016/j.brainresbull.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Weinstock M, Matlina E, Maor GI, Rosen H, McEwen BS. Prenatal stress selectively alters the reactivity of the hypothalamic-pituitary adrenal system in the female rat. Brain Res. 1992;595:195–200. doi: 10.1016/0006-8993(92)91049-K. [DOI] [PubMed] [Google Scholar]
- Willner P, Muscat R, Papp M. An animal model of anhedonia. Clin Neuropharmacol. 1992:550A–551A. doi: 10.1097/00002826-199201001-00286. [DOI] [PubMed] [Google Scholar]
- Wolstenholme JT, Edwards M, Shetty SRJ, Gatewood JD, Taylor JA, Rissman EF, et al. Gestational exposure to Bisphenol A produces transgenerational changes in behaviors and gene expression. Endocrinology. 2012;153:3828–3838. doi: 10.1210/en.2012-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynne-Edwards KE. Biparental care in Djungarian but not Siberian dwarf hamsters (Phodopus) Anim Behav. 1995;50:1571–1585. doi: 10.1016/0003-3472(95)80012-3. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


