The musculoskeletal system generates, absorbs and transmits force, enabling functional movement. Given this mechanical role, it follows that musculoskeletal tissues adapt to mechanical demands. Mechanical forces direct musculoskeletal cellular activities, altering tissue mass, structure and/or quality (figure 1).1 The net result is altered tissue-level stresses and strains to applied loads and, subsequently, altered injury risk.
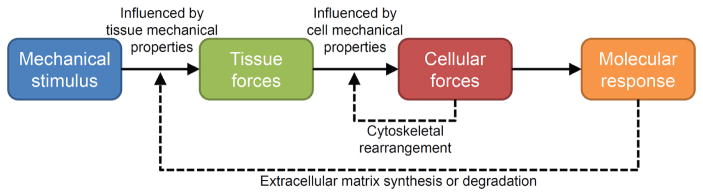
Figure 1.
Mechanical forces direct cellular activities to induce tissue adaptation. Extrinsically and intrinsically generated mechanical forces load musculoskeletal tissues, with the characteristics of the resultant tissue forces being dependent on the ability of the tissue to resist those forces. Tissue forces are transmitted to the micromechanical environment of resident cells, with cellular mechanical properties influencing the characteristics of the cellular forces. Cells can modify their micromechanical environment via cytoskeletal rearrangement, which feedbacks to alter cellular sensitivity to incoming forces. When cellular forces are sufficient, the cell initiates a molecular response, which ultimately alters synthesis and/or degradation of the extracellular matrix. The latter alters tissue mechanical properties, which feeds back to influence tissue forces. (Reprinted from Thompson et al,1 by permission of Oxford University Press and the American Physical Therapy Association.)
Therapists have long identified the therapeutic potential of mechanical forces. Nearly every rehabilitation intervention introduces mechanical forces, irrespective of whether they are generated extrinsically (eg, via external modalities) or intrinsically (eg, via physical activity). These ‘mechanotherapies’ harness musculoskeletal mechanosensitivity to induce adaptation; however, how the adaptation occurs remains underappreciated.
Khan and Scott2 previously introduced readers of BJSM to the concept of mechanotransduction, whereby biophysical forces are converted into cellular and molecular responses. For musculoskeletal tissues to adapt to loading, the mechanical signal must be transmitted to the cell microenvironment and for the cell to possess sensory machinery (mechanocoupling) (figure 2A). The signal must subsequently be converted into a biochemical response (biochemical coupling), which is propagated to other nearby effector cells via the release of stored molecules or transmitted to the nucleus to induce expression of mechanosensitive genes (figure 2B). The effector cells ultimately bring about adaptation by altering extracellular matrix (ECM) synthesis and/or degradation (effector response).
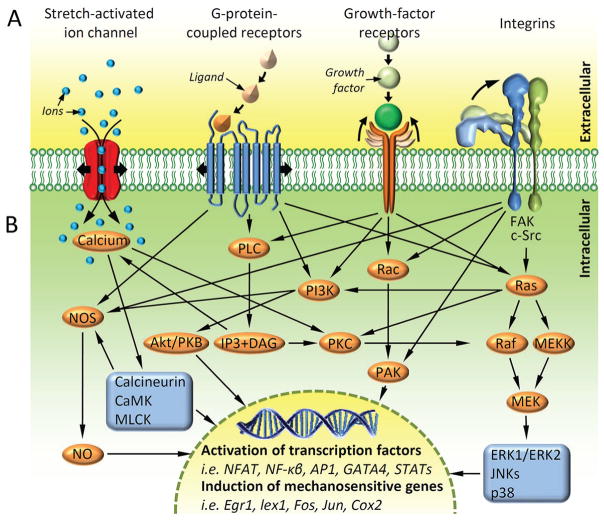
Figure 2.
A variety of extracellular receptors activate an overlapping network of mechanosensitive pathways. (A) Musculoskeletal cells can sense incoming mechanical signals using a diverse group of transmembrane mechanosensitive proteins (‘mechanosensors’), including stretch-activated ion channels, cell-membrane spanning G protein-coupled receptors, growth-factor receptors and integrins. The mechanical stimulation of these proteins can lead to changes in their affinity to binding partners or ion conductivity. (B) Mechanical stimulation of the mechanosensors and alteration in their binding capacity or ion conductivity converts the mechanical signal into a biochemical signal (‘biochemical coupling’) triggering intracellular signalling cascades. Many of the pathways overlap sharing signalling molecules. The convergence of the pathways results in the activation of select transcription factors, including nuclear factor of activated T cells, nuclear factor-κβ, activator protein 1, GATA4 (a member of the transcription factor family characterised by the ability to bind the DNA sequence ‘GATA’) and signal transducer and activator of transcription factors. The transcription factors translocate to the nucleus and modulate the expression of a panel of mechanosensitive genes, including early growth response 1, lex1, Fos, Jun and cyclooxygenase-2. Ultimately, the net sum of gene-expression reprogramming determines the functional response of the cell to a mechanical stimulus. Akt/PKB, protein kinase B; AP1, activator protein 1; CaMK, calcium/calmodulin-dependent kinase; Cox2, cyclooxygenase-2; DAG, diacyl-glycerol; Egr1, early growth response 1; ERK, extracellular signal-regulated kinase; FAK, focal adhesion kinase; IP3, inositol triphosphate; JNKs, c-Jun N-terminal kinases; MEK, mitogen-activated protein kinase; MEKK, mitogen-activated protein kinase kinase; MLCK, myosin light-chain kinase; NFAT, nuclear factor of activated T cells; NF-κβ, nuclear factor-κβ; NO, nitric oxide; NOS, nitric oxide synthase; PAK, p21-activated kinase; PI3K, phosphoinositide 3-kinase; PKC, protein kinase C; PLC, phospholipase C; Raf, rapidly accelerated fibrosarcoma kinase; Ras, rat sarcoma small GTPase; STATs, signal transducer and activator of transcription factors. (Reprinted from Thompson et al,1 by permission of Oxford University Press and the American Physical Therapy Association.)
Understanding mechanobiology and decoding mechanotransduction pathways extends beyond scientific curiosity. Altered mechanotransduction contributes to musculoskeletal disorders, ranging from osteoporosis and osteoarthritis to muscular dystrophies and sarcopenia.3 Altered mechanoadaptation in sports medicine contributes to injury risk, with athletes exhibiting less adaptation being at greater risk. The ability to identify athletes exhibiting factors that alter musculoskeletal mechanosensitivity is important. While not yet a clinical reality, the application of omic-based technologies (eg, genomics, proteomics, metabolomics) may permit personalised medicine approaches to identifying and manipulating mechanosensitivity issues in individual athletes so as to optimise adaptation.
While we await the evolution of personalised medicine approaches, there are ways wherein knowledge of mechanotransduction can be presently applied. By understanding the forces to which musculoskeletal cells respond, it is possible to develop novel means of introducing those forces. This has been the premise of pre-existing mechanotherapies, such as therapeutic ultrasound and low-intensity vibration therapy. Although the efficacy of the latter modalities remains questioned, they provide examples of how microscopic forces induce cellular and tissue adaptation. As the stimuli to which cells respond are further defined, new mechanotherapies will be developed. These may particularly benefit emerging regenerative medicine techniques wherein cellular stimulation may be desired in the absence of tissue deformation (eg, in scenarios of restricted load bearing).
Appreciation of the machinery via which cells sense mechanical signals (mechano-coupling) informs load dosing parameters, among other things. The mechanosensor mechanism/s within musculoskeletal cells desensitise or become ‘deaf’ to persistent stimulation. Part of this is attributed to transmembrane receptors called integrins, some of which connect extracellularly to ECM proteins and intracellularly to the cytoskeleton. In response to mechanical loading, the ECM-integrin cytoskeletal axis induces cytoskeletal remodelling to stiffen musculoskeletal cells and temporarily reduce mechanosensitivity. Understanding factors contributing to cellular desensitisation and subsequent resensitisation, it is possible to design better loading regimes. This has best been illustrated in the skeleton where repetitive loading yielded diminishing bone mass gains, but inclusion of rest periods on orders of magnitude from seconds, hours and weeks between loading cycles and bouts enhanced gains.4 Preliminary evidence suggests the desensitisation phenomenon also occurs in tendon.5
Biochemical coupling of mechanical stimuli into molecular responses represents the most exciting target for manipulating mechanotransduction. Identifying molecules involved in mechanotransduction may reveal novel targets for therapeutic intervention that independently induce adaptation or have additive effects when combined with mechanotherapies. More interestingly, molecular targeting may sensitise mechanotransductive pathways such that superimposed loading results in synergistic adaptation. As an example, parathyroid hormone (PTH) therapy and mechanical stimuli both act through the PTH type 1 receptor. By introducing load to coincide with peak PTH receptor sensitisation, greater than summative skeletal adaptation is induced.6
Therapists should also consider potential negative interactions between mechanotherapies and biologically active compounds. An example of one such interaction is the combined effects of non-steroidal anti-inflammatory drugs (NSAIDs) and loading. Loading results in rapid cyclooxygenase-2 expression and release of prostaglandins, culminating in collagen (ie, ECM) synthesis. NSAIDs administered before mechanical loading inhibit cyclooxygenase expression and prostaglandin release to blunt bone, muscle and tendon adaptive responses.7,8
As biological advancements continue, there is a need for therapists to understand the principals of mechanobiology and how mechanotherapies augment tissue responses at the cellular and molecular levels. By understanding the mechanical stimuli to which musculoskeletal cells best respond, and the mechanisms these cells use to convert mechanical signals into molecular responses, therapists may be able to use the force to augment the response of musculo-skeletal cells to mechanical stimuli. The net result will be additive or synergistic facilitation of tissue adaptation and a subsequent reduction in injury.
Acknowledgments
The present contribution was made facilitated by funding from the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01 AR057740 (to SJW) and R15 AR069943 (to WRT)).
Footnotes
Contributors SJW and WRT provided initial writing, manuscript review and final approval.
Competing interests None declared.
Provenance and peer review Commissioned; externally peer reviewed.
References
- 1.Thompson WR, Scott A, Loghmani MT, et al. Understanding Mechanobiology: physical therapists as a force in Mechanotherapy and musculoskeletal regenerative Rehabilitation. Phys Ther. 2016;96:560–9. doi: 10.2522/ptj.20150224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khan KM, Scott A. Mechanotherapy: how physical therapists’ prescription of exercise promotes tissue repair. Br J Sports Med. 2009;43:247–52. doi: 10.1136/bjsm.2008.054239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ingber DE. Mechanobiology and diseases of mechanotransduction. Ann Med. 2003;35:564–77. doi: 10.1080/07853890310016333. [DOI] [PubMed] [Google Scholar]
- 4.Robling AG, Castillo AB, Turner CH. Biomechanical and molecular regulation of bone remodeling. Annu Rev Biomed Eng. 2006;8:455–98. doi: 10.1146/annurev.bioeng.8.061505.095721. [DOI] [PubMed] [Google Scholar]
- 5.Huisman E, Lu A, McCormack RG, et al. Enhanced collagen type I synthesis by human tenocytes subjected to periodic in vitro mechanical stimulation. BMC Musculoskelet Disord. 2014;15:386. doi: 10.1186/1471-2474-15-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fuchs RK, Warden SJ. Combination therapy using exercise and Pharmaceutical Agents to optimize Bone Health. Clin Rev Bone Miner Metab. 2008;6:37–45. [Google Scholar]
- 7.Christensen B, Dandanell S, Kjaer M, et al. Effect of anti-inflammatory medication on the running-induced rise in Patella tendon collagen synthesis in humans. J Appl Physiol. 2011;110:137–41. doi: 10.1152/japplphysiol.00942.2010. [DOI] [PubMed] [Google Scholar]
- 8.Warden SJ. Prophylactic use of NSAIDs by athletes: a risk/benefit assessment. Phys Sportsmed. 2010;38:132–8. doi: 10.3810/psm.2010.04.1770. [DOI] [PubMed] [Google Scholar]