Abstract
Status Epilepticus is common in neonates and infants, and is associated with neuronal injury and adverse developmental outcomes. GABAergic drugs, the standard treatment for neonatal seizures, can have excitatory effects in the neonatal brain, which may worsen the seizures and their effects. Using a recently developed model of status epilepticus in P7 rat pups that results in widespread neuronal injury, we found that the GABAA agonists phenobarbital and midazolam significantly increased Status Epilepticus-associated neuronal injury in various brain regions. Our results suggest that more research is needed into the possible deleterious effects of GABAergic drugs on neonatal seizures and on excitotoxic neuronal injury in the immature brain.
Introduction
In the neonatal rodent brain, GABAergic drugs, which are the standard treatment for neonatal seizures, can have excitatory effects, which might aggravate seizures, and their long-term consequences.1–4 In clinical use and in some experimental models, GABAergic drugs appear to stop behavioral seizures,5 but adverse effects might be hard to detect if they occurred in a subpopulation of immature neurons which have little behavioral expression at that age.6 We recently developed a model of SE in P7 rat pups that resulted in high survival rates and widespread neuronal injury7 and for the first time offered us the opportunity to test the effect of GABAergic drugs on SE-associated neuronal injury. We found that both phenobarbital and midazolam treatment of SE increased acute neuronal injury in several brain regions, raising questions about the safety of their clinical use.
Materials and Methods
Male and female Sprague-Dawley albino rats (Charles River Laboratories, San Diego, CA) (P7, n=66) were used at postnatal day 7 (P7) with the day of birth considered as day 0, as previously reported.7 Lithium chloride (LiCl, 5 mEq/kg) was administered intraperitoneally (i.p.) at P6 and the next day SE was induced with subcutaneous pilocarpine hydrochloride (Pilo, 320 mg/kg, s.c.) together with scopolamine methyl chloride (1mg/kg, i.p.) to block the peripheral effect of pilocarpine. All experiments were conducted with the approval of and in accordance with the regulations of the Institutional Animal Care and Use Committee of West Los Angeles VA Medical Center, and with the United States Public Health Service’s Policy on Humane Care and Use of Laboratory Animals.
In preliminary experiments, we found that high doses of midazolam (6 mg/kg, but not 3 mg/kg) and phenobarbital (25mg/kg, but not 10 mg/kg) induce apoptosis in control (no-seizure) pups. Ten minutes after the development of stage 3 seizures, some pups were treated with midazolam (3 mg/kg, i.p., SE+Mz group, n=36) and others with phenobarbital (10mg/kg, i.p., SE+Ph group, n=26). Some pups (SE group, n=30) were kept untreated as SE controls and only received saline (i.p.), while other pups receiving only midazolam (3mg/kg, i.p., Mz group, n=6) or phenobarbital (10mg/kg, i.p., Ph group, n=8) were kept as no-seizure controls for injury comparison. An untreated, no-seizure group was also included (Sham, n=5). All animals were rehydrated with saline approximately 4 h after SE (10% of body weight, s.c.) and sacrificed 24 h after SE onset.
Preparation of tissue for histology and active caspase-3 (caspase-3a) immunohistochemistry was performed as previously reported.7 The quantification of neuronal injury using Fluoro-Jade B (FJB) staining was performed by manual counting by an observer blinded to the animal condition using a grid to reduce the possibility of over-counting. Three adjacent coronal sections per animal were taken for each brain region at the following anatomical locations: caudate putamen, septal nuclei, nucleus accumbens (Bregma +1.0 mm) 8–10, globus pallidus (Bregma −1.0 mm), dorsal hippocampus, parietal cortex, piriform cortex, thalamus, hypothalamus, amygdala (Bregma −2 mm), and ventral hippocampus, lateral entorhinal cortex, substantia nigra (Bregma −4.2 mm). Counting of the entire brain structure was performed using a Leica 40x objective and the number of FJB-positive (FJB+) cells for each of the three coronal sections were averaged for each region. To compensate for minor anatomical variations in hippocampus, FJB+ cells were expressed per mm length of each cell layer. Parietal cortex was counted from the top of the cingulum down to the rhinal fissue. Only cells that had visible nuclei were counted. FJB+ cell counts were corrected for cell size by a modified Abercrombie factor11, in which population cell size was estimated by averaging the diameter of the counted cells’ nuclei in the section per neuronal population. Three animals per group were chosen at random and a total of 70–100 profile measurements for experimental groups (SE, SE+Mz, SE+Ph) and 15–30 for control groups (Sham, Mz only, Ph only) per brain region were used for this average. The average correction factors ranged from 0.73 to 0.79 (table available by request) and were not significantly different among groups, suggesting that differential swelling of the tissue did not occur.
For a subset of SE+Mz and SE+Ph animals, we first stained 3 adjacent sections (Bregma −2 mm) for caspase-3a, counted the cells in thalamus, then removed the coverslip, performed FJB staining and counted the FJB+ cells. With this data we obtained a percentage of FJB+ cells that expressed caspase-3a in thalamus.
Experimental groups were analyzed with non-parametric statistical methods: Kruskal–Wallis test followed by Dunn’s test for multiple comparisons. Statistical significances were considered when p<0.05.
Results
The course of SE in P7 pups
The combination of high-dose lithium (5 mEq/kg, i.p.) and pilocarpine (320 mg/kg, s.c.) induced SE in 66 out of 66 pups (100%), an incidence much higher than we previously reported with administering pilocarpine intraperitoneally.7 Shortly after pilocarpine injection, pups became hyperactive, showing running seizures with vocalization (stage 3). After midazolam or phenobarbital treatment, however, this behavioral component was reduced as the pups became sedated. Sedation was transient and was more severe after midazolam (pups unresponsive) than after phenobarbital (pups moving and responsive). Animal survival at 24 h post-SE was 64% (30/47) in the untreated SE group, 77% (36/47) in the SE+Mz group (not significant), and 65% (26/40) in the SE+Ph group (not significant). As shown in Table 1, neither midazolam nor phenobarbital alone caused a significant increase in neuronal injury compared to Sham (p>0.05, Kruskal-Wallis analysis).
Table 1.
Distribution of neuronal injury among various brain regions in Sham, Mz only (Mz), Ph only (Ph), SE, SE+Mz, and SE+Ph pups. Neuronal injury median values are shown in bold with interquartile range in parenthesis. Hippocampal regions show FJB+ cells/mm while other region values represent the total number of FJB+ cells per field (see Materials and Methods).
| Region | Sham | Mz | Ph | SE | SE + Mz | SE + Ph |
|---|---|---|---|---|---|---|
|
| ||||||
| Hippocampus | ||||||
| Dorsal CA1/Sub | 0.2 (0.2, 0.3) | 0.3 (0.2, 0.4) | 0.2 (0.2, 0.3) | 8 (4, 16) | 8 (4, 34)***, ### | 11 (5, 19)**, $$$$ |
| CA3 | 0.2 (0.2, 0.3) | 0.2 (0.1, 0.3) | 0.2 (0.2, 0.4) | 5 (2, 8) | 4 (2, 7)**, ### |
2 (1, 3)**, $$,

|
| DG | 0.1 (0.1, 0.2) | 0.2 (0.1, 0.2) | 0.1 (0, 0.2) | 0.8 (0.4, 2) |
2 (1, 2)****, ####,

|
1 (0.4, 2)**, $$$ |
| Ventral CA1/Sub | 0.5 (0.4, 0.5) | 0.6 (0.4, 0.7) | 0.3 (0.2, 0.6) | 11 (7, 20) |
20 (11, 32)****, ####,

|
13 (7, 20)**, $$$$ |
|
| ||||||
| Other Limbic Regions | ||||||
| Amygdala | 12 (11, 15) | 11 (9, 12) | 11 (9, 13) | 126 (50, 221) | 140 (63, 241)**, #### | 118 (61, 240)**, $$$$ |
| Hypothalamus | 4 (3, 5) | 76 (4, 6) | 5 (3, 5) | 20 (15, 30) | 32 (19, 76)****, ### |
206 (102, 300)****, $$$$,
|
| Septal Nuclei | 9 (7, 11) | 7 (5, 8) | 6 (6, 7) | 21 (12, 24) |
29 (23, 101)***, ####,
|
81 (60, 160)****, $$$$,
|
|
| ||||||
| Thalamus | 17 (15, 20) | 22 (20, 25) | 17 (12, 22) | 174 (121, 238) |
408 (185, 782)****, ####,

|
410 (279, 649)****, $$$$,

|
|
| ||||||
| Cortical Regions | ||||||
| Parietal Ctx | 16 (15, 18) | 19 (18, 20) | 17 (12, 22) | 290 (158, 366) |
434 (227, 909)****, ####,

|
148 (90, 243)*, $$ |
| Piriform Ctx | 4 (3, 5) | 7 (6, 8) | 6 (4, 8) | 14 (8, 20) | 19 (11, 37)***, ## | 18 (12, 27)***, $$$ |
| Lateral Entorhinal Ctx | 6 (3, 7) | 7 (3, 10) | 5 (4, 6) | 12 (5, 14) |
24 (20, 37)***, ###,
|
23 (12, 41)**, $$$$,

|
|
| ||||||
| Basal Ganglia | ||||||
| Caudate Putamen | 25 (20, 27) | 20 (19, 25) | 23 (21, 25) | 151 (113, 373) |
569 (394, 918)****, ####,
|
460 (183, 870)****, $$$$,

|
| Nucleus Accumbens | 8 (7, 11) | 10 (8, 12) | 6 (6, 8) | 118 (93, 228) |
249 (153, 340)****, ####,

|
192 (129, 366)***, $$$$ |
| Globus Pallidus | 4 (2, 5) | 4 (4, 6) | 5 (3, 6) | 11 (7, 13) |
25 (15, 32)****, ####,
|
28 (20, 32)****, $$$$,
|
| Substantia Nigra | 2 (1, 3) | 3 (2, 4) | 2 (0.4, 4) | 3 (2, 6) |
14 (10, 18)**, ##,
|
28 (21, 43)**, $$$$,
|
Result of statistical analysis of each treatment group (SE+Mz vs. Sham, Mz, and SE, or SE+Ph vs. Sham, Ph, and SE) is indicated by the following symbols:
p<0.05,
p<0.01,
p<0.001,
p<0.0001 vs. Sham;
p<0.05,
p<0.01,
p<0.001,
p<0.0001 vs. Mz;
p<0.05,
p<0.01,
p<0.001,
p<0.0001 vs. Ph;
p<0.05,
p<0.01,
p<0.001,
p<0.0001 vs. SE (shown in red).
Abbreviations: ns=not significant, sub=subiculum, ctx=cortex.
Effect of treatment on distribution of SE-associated neuronal injury
Thalamus
Both midazolam and phenobarbital treatment significantly increased SE-associated neuronal injury in the thalamus (+135%, p<0.01; +136%, p<0.001). Neuronal injury varied among thalamic nuclei, predominating in dorsolateral nuclei in some pups and ventromedial or other nuclei in others (Fig 1).
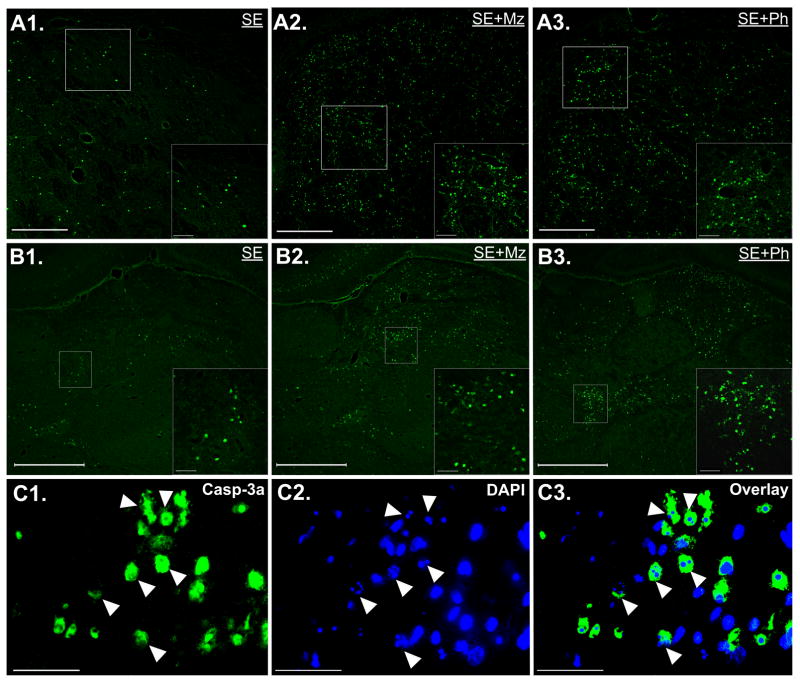
Figure 1. Effect of midazolam or phenobarbital treatment on SE-associated neuronal injury in caudate putamen and thalamus, and mechanism of thalamic injury.
(A) Images of FJB staining in caudate putamen of (A1) SE, (A2) SE+Mz, and (A3) SE+Ph pups with a higher magnification of the boxed area on the bottom right of each image. SE induces neuronal injury throughout caudate and this injury is exasperated following midazolam or phenobarbital treatment by 24hrs post-SE. (B) Images of FJB staining in thalamus of (B1) SE, (B2) SE+Mz, and (B3) SE+Ph pups. Both midazolam and phenobarbital treatment increase FJB cell distribution throughout various thalamic nuclei to approximately the same extent. Scale bars: (A) long bars = 200 μm and short bars = 20 μm (B) long bars = 500 μm and short bars = 50 μm. (C) Images of overlay of caspase-3a immunoreactivity (IR) (yellow) and DAPI (blue) staining in thalamus of SE+Mz pups. On left, high magnification shows distribution of caspase-3a IR cells in ventromedial thalamus of a SE+Mz pup; in middle, high magnification images show that caspase-3a IR cells have fragmented nuclei indicative of neuronal cell death; on the right, this overlay shows that many of these caspase-3a IR cells have fragmented nuclei, suggesting a caspase-dependent form of cell death. Scale bars: 50 μm.
Basal ganglia
Both midazolam and phenobarbital treatment significantly increased neuronal injury in caudate putamen (+277%, p<0.0001; +205%, p<0.01), globus pallidus (+127%, p<0.0001; +155%, p<0.0001), and the substantia nigra (+367%, p<0.0001; +833%, p<0.0001) compared to the untreated SE group (Fig 1). However, only midazolam treatment significantly increased neuronal injury in the nucleus accumbens (+111%, p<0.05).
Hippocampus
Neuronal injury in the stratum pyramidale of dorsal and ventral CA1/subiculum was examined in SE, SE+Mz, and SE+Ph animals at 24 h after SE induction. As shown in Table 1, midazolam treatment significantly increased neuronal injury in DG (+150%, p<0.05) and ventral CA1/Subiculum (+82%, p<0.05) (Fig 2). Interestingly, phenobarbital treatment significantly decreased injury in dorsal CA3 (−60%, p<0.05). The distribution of hippocampal injury was similar to that previously reported.7
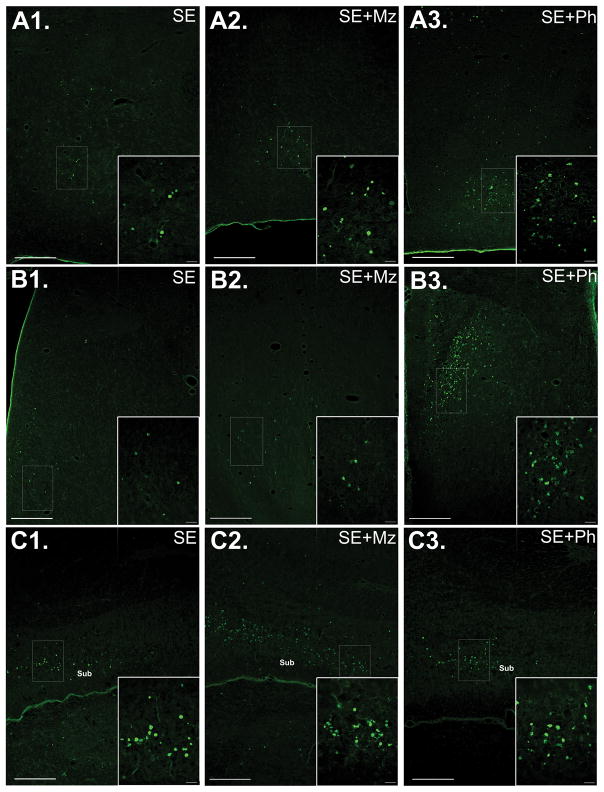
Figure 2. Midazolam or phenobarbital treatment increases SE-associated neuronal injury in some limbic regions.
(A) Images of FJB staining in hypothalamus of (A1) SE, (A2) SE+Mz, and (A3) SE+Ph pups, with a higher magnification of the boxed area on the bottom right of each image. Phenobarbital treatment significantly increased FJB+ cells compared to untreated SE. (B) Images of FJB staining in septal nuclei of (B1) SE, (B2) SE+Mz, and (B3) SE+Ph pups. Both midazolam and (to a greater extent) phenobarbital treatment significantly increase neuronal injury in septum. (C) Images of FJB staining in Ventral CA1/Subiculum of (C1) SE, (C2) SE+Mz, and (C3) SE+Ph P7 pups. As shown, SE (C1) induces neuronal injury in Ventral CA1/Subiculum by 24 hours post-SE and this injury is enhanced following midazolam treatment (C2), although no significant difference is seen after phenobarbital treatment (C3). Abbreviations: Sub=Subiculum. Scale bars: (A, B, C) long bars = 200 μm and short bars = 20 μm.
Other Limbic regions
Phenobarbital treatment significantly increased neuronal injury in hypothalamus and septal nuclei (+988%, p<0.0001; +293%, p<0.0001), but midazolam increased it only in septal nuclei (+37%, p<0.0001) (Fig 2). As shown in Table 1, amygdala neuronal injury was not affected by midazolam or by phenobarbital treatment. Injury among amygdaloid nuclei varied and, therefore, the structure was counted as a whole.
Neocortex
Both midazolam and phenobarbital treatment significantly increased neuronal injury in lateral entorhinal cortex (+171%, p<0.0001; +114%, p<0.01), but there was no significant change in piriform cortex. Neuronal injury in parietal cortex was significantly increased by midazolam (+54%, p<0.05), but not phenobarbital. Injury was consistently found along layer 2, although some FJB+ cells were spread throughout layer 4 and other cortical layers.
SE triggers an active form of cell death
The contribution of caspase-3 to SE-associated neuronal death was assessed by immunohistochemistry using an antibody which recognizes caspase-3a, the active form of that enzyme. As shown previously in this model,7 SE resulted in a significant increase in caspase-3a immunoreactivity (IR) compared to sham treatment. In a subset of SE+Mz and SE+Ph animals, we found that the percentage of FJB+ cells expressing caspase-3a in the thalamus was 79 ± 10% and 73 ± 16%, respectively. As shown in Fig 1C, these caspase-3a IR cells had distinct changes in nuclear morphology, such as fragmented nuclei, suggesting that the neuronal injury resulting from midazolam and phenobarbital treatment is irreversible.
Discussion
This is the first reproducible model showing evidence that GABAergic drugs, a standard treatment for neonatal status epilepticus, may aggravate SE-associated neuronal injury in some regions of the neonatal rodent brain. We used drug dosages too low to cause apoptosis by themselves, and found that early treatment (10 minutes post-SE induction) with the barbiturate phenobarbital or the benzodiazepine midazolam significantly increased seizure-associated neuronal injury in thalamus, basal ganglia, hypothalamus, septal nuclei, ventral CA1/subiculum, parietal cortex, and entorhinal cortex in P7 rat pups 24 hrs after SE induction. Neither treatment significantly increased pup survival, discarding the possibility that the increase of neuronal injury is due to the survival of severely injured animals that may have otherwise died. These drugs seemed to stop behavioral seizures, but the possibility exists that they hyperpolarized relatively mature neurons that have behavioral expression,12–14 therefore stopping the behavioral seizures, while depolarizing less mature neurons that have immature connections and little behavioral expression.15 This might increase neuronal injury in relatively silent areas, and the putative cognitive or behavioral sequellae of that injury might not be expressed until much later in development (weeks in rats, possibly years in humans). In mice, flurothyl-induced seizures beginning at P7 result in long-term deficits in social behavior, social interaction, and learning16 and exposure to phenobarbital at P6 results in acute and long-term changes to cerebral cortex.17 In rats, exposure to phenobarbital at P7 results in increased anxiety-like behavior and deficits in long-term learning and memory.18 In a mouse model of inflammation-induced SE, midazolam treatment induced an abnormal increase in hyperactivity by the chronic phase,19 indicating that benzodiazepine treatment may have increased the behavioral sequellae of seizures, but SE occurred at P15, when GABA is no longer excitatory in most cells.
Midazolam allosterically enhances chloride flux by binding to the benzodiazepine binding site, between the α1 and the γ2 subunits of the GABAA receptor, and other actions are only seen at higher concentrations. Phenobarbital binds to the barbiturate binding site, but has several other actions at pharmacologically relevant doses.20 Although these two drugs have different mechanisms of action, the fact that both cause widespread increases in SE-associated neuronal injury suggests that this increase may involve GABAergic mechanisms.
The clinical significance of our results is uncertain. They cannot be blindly extrapolated to clinical situations, which deal with a different type of seizures in a much larger brain, but they suggest that more research is needed in animal models as well as in clinical neonatal SE. The possibility that the use of GABAergic drugs for the treatment of neonatal seizures could be deleterious in brain areas which have little behavioral expression at that age raises important questions, since behavioral and clinical expression of the damage could be delayed. Physiological studies of the GABAA system in rodent neonates provide a potential conceptual framework for that concept, but their clinical significance and their possible developmental effects need to be better understood.
Supplementary Material
Table 2.
Abercrombie Correction:
| Sham | Mz only | Ph only | SE | SE+Mz | SE+Ph | |
|---|---|---|---|---|---|---|
| Dorsal CA1/Subiculum | 0.757 | 0.760 | 0.753 | 0.755 | 0.765 | 0.751 |
| CA3 | 0.741 | 0.748 | 0.733 | 0.737 | 0.759 | 0.728 |
| DG | 0.752 | 0.779 | 0.769 | 0.766 | 0.792 | 0.773 |
| Ventral CA1/Subiculum | 0.760 | 0.764 | 0.759 | 0.765 | 0.762 | 0.753 |
| Amygdala | 0.767 | 0.772 | 0.766 | 0.776 | 0.768 | 0.757 |
| Hypothalamus | 0.772 | 0.779 | 0.768 | 0.782 | 0.775 | 0.758 |
| Septal Nuclei | 0.764 | 0.766 | 0.759 | 0.759 | 0.774 | 0.760 |
| Thalamus | 0.752 | 0.764 | 0.753 | 0.755 | 0.773 | 0.766 |
| Parietal Ctx | 0.754 | 0.773 | 0.752 | 0.782 | 0.764 | 0.764 |
| Piriform Ctx | 0.768 | 0.771 | 0.767 | 0.772 | 0.771 | 0.762 |
| Lateral Entorhinal Ctx | 0.758 | 0.754 | 0.761 | 0.755 | 0.753 | 0.767 |
| Caudate Putamen | 0.779 | 0.783 | 0.778 | 0.786 | 0.781 | 0.770 |
| Nucleus Accumbens | 0.782 | 0.787 | 0.779 | 0.784 | 0.789 | 0.775 |
| Globus Pallidus | 0.787 | 0.787 | 0.784 | 0.781 | 0.794 | 0.786 |
| Substantia Nigra | 0.746 | 0.744 | 0.740 | 0.729 | 0.759 | 0.752 |
Acknowledgments
This work was supported in part by Merit Review Award # I01 BX000273-07 from the Department of Veterans Health Affairs by NIH/ NINDS (grant UO1 NS074926) and by the James and Debbie Cho Foundation.
Footnotes
Author Contributions
D.T., L.S., J.N., and C.G.W. were responsible for conception and design of the study. D.T., L.S., and J.N were responsible for acquisition and analysis of data. D.T. and C.G.W. were responsible for drafting the manuscript or figures.
Conflicts of Interest
No authors have any conflict of interest to disclose.
References
- 1.Ben-Ari Y. The GABA excitatory/inhibitory developmental sequence: a personal journey. Neuroscience. 2014 Oct 24;279:187–219. doi: 10.1016/j.neuroscience.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Dzhala VI, Staley KJ. Excitatory actions of endogenously released GABA contribute to initiation of ictal epileptiform activity in the developing hippocampus. J Neurosci. 2003 Mar 01;23(5):1840–6. doi: 10.1523/JNEUROSCI.23-05-01840.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dzhala VI, Talos DM, Sdrulla DA, et al. NKCC1 transporter facilitates seizures in the developing brain. Nat Med. 2005 Nov;11(11):1205–13. doi: 10.1038/nm1301. [DOI] [PubMed] [Google Scholar]
- 4.Staley K. Enhancement of the excitatory actions of GABA by barbiturates and benzodiazepines. Neurosci Lett. 1992 Oct 26;146(1):105–7. doi: 10.1016/0304-3940(92)90183-8. [DOI] [PubMed] [Google Scholar]
- 5.Mares P, Ticha K, Mikulecka A. Anticonvulsant and behavioral effects of muscimol in immature rats. Brain Res. 2014 Sep 25;1582:227–36. doi: 10.1016/j.brainres.2014.07.038. [DOI] [PubMed] [Google Scholar]
- 6.Glykys J, Dzhala VI, Kuchibhotla KV, et al. Differences in cortical versus subcortical GABAergic signaling: a candidate mechanism of electroclinical uncoupling of neonatal seizures. Neuron. 2009 Sep 10;63(5):657–72. doi: 10.1016/j.neuron.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torolira D, Suchomelova L, Wasterlain CG, Niquet J. Widespread neuronal injury in a model of cholinergic status epilepticus in postnatal day 7 rat pups. Epilepsy Res. 2016 Feb;120:47–54. doi: 10.1016/j.eplepsyres.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramachandra R, Subramanian T. Atlas of the Neonatal Rat Brain. Boca Raton, FL: CRC Press; 2011. [Google Scholar]
- 9.Khazipov R, Zaynutdinova D, Ogievetsky E, et al. Atlas of the Postnatal Rat Brain in Stereotaxic Coordinates. Front Neuroanat. 2015;9:161. doi: 10.3389/fnana.2015.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sherwood N, Timiras P. A Stereotaxic Atlas of the Developing Rat Brain. Berkeley, CA: Berkeley: University of California Press; 1970. [Google Scholar]
- 11.Abercrombie M. Estimation of nuclear population from microtome sections. Anat Rec. 1946 Feb;94:239–47. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- 12.Blumenfeld H, Varghese GI, Purcaro MJ, et al. Cortical and subcortical networks in human secondarily generalized tonic-clonic seizures. Brain. 2009 Apr;132(Pt 4):999–1012. doi: 10.1093/brain/awp028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gale K. GABA and epilepsy: basic concepts from preclinical research. Epilepsia. 1992;33( Suppl 5):S3–12. [PubMed] [Google Scholar]
- 14.White LE, Price JL. The functional anatomy of limbic status epilepticus in the rat. II. The effects of focal deactivation. J Neurosci. 1993 Nov;13(11):4810–30. doi: 10.1523/JNEUROSCI.13-11-04810.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kahle KT, Staley KJ. The bumetanide-sensitive Na-K-2Cl cotransporter NKCC1 as a potential target of a novel mechanism-based treatment strategy for neonatal seizures. Neurosurg Focus. 2008 Sep;25(3):E22. doi: 10.3171/FOC/2008/25/9/E22. [DOI] [PubMed] [Google Scholar]
- 16.Lugo JN, Swann JW, Anderson AE. Early-life seizures result in deficits in social behavior and learning. Exp Neurol. 2014 Jun;256:74–80. doi: 10.1016/j.expneurol.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaindl AM, Koppelstaetter A, Nebrich G, et al. Brief alteration of NMDA or GABAA receptor-mediated neurotransmission has long term effects on the developing cerebral cortex. Mol Cell Proteomics. 2008 Dec;7(12):2293–310. doi: 10.1074/mcp.M800030-MCP200. [DOI] [PubMed] [Google Scholar]
- 18.Frankel S, Medvedeva N, Gutherz S, Kulick C, Kondratyev A, Forcelli PA. Comparison of the long-term behavioral effects of neonatal exposure to retigabine or phenobarbital in rats. Epilepsy Behav. 2016 Apr;57(Pt A):34–40. doi: 10.1016/j.yebeh.2016.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakajima K, Hirai S, Morio T, Okado H. Benzodiazepines induce sequelae in immature mice with inflammation-induced status epilepticus. Epilepsy Behav. 2015 Nov;52(Pt A):180–6. doi: 10.1016/j.yebeh.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 20.Loscher W, Rogawski MA. How theories evolved concerning the mechanism of action of barbiturates. Epilepsia. 2012 Dec;53( Suppl 8):12–25. doi: 10.1111/epi.12025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.