Abstract
A small-molecule inducer of beta-cell proliferation in human islets represents a potential regeneration strategy for treating type 1 diabetes. However, the lack of suitable human beta cell lines makes such a discovery a challenge. Here, we adapted an islet cell culture system to high-throughput screening to identify such small molecules. We prepared microtiter plates containing extracellular matrix from a human bladder carcinoma cell line. Dissociated human islets were seeded onto these plates, cultured for up to 7 days, and assessed for proliferation by simultaneous Ki67 and C-peptide immunofluorescence. Importantly, this environment preserved beta-cell physiological function, as measured by glucose-stimulated insulin secretion. Adenoviral overexpression of cdk-6 and cyclin D1, known inducers of human beta cell proliferation, was used as a positive control in our assay. This induction was inhibited by cotreatment with rapamycin, an immunosuppressant often used in islet transplantation. We then performed a pilot screen of 1280 compounds, observing some phenotypic effects on cells. This high-throughput human islet cell culture method can be used to assess various aspects of beta-cell biology on a relatively large number of compounds.
Keywords: human islet, assay development, high-throughput screening, beta-cell proliferation
Introduction
Small-molecule-induced beta-cell proliferation in humans could be of therapeutic importance to type 1 diabetes. Type 1 diabetes involves the autoimmune destruction of pancreatic beta cells, resulting in absolute dependence on injected insulin for survival. There is evidence that beta-cell replacement can be at least temporarily therapeutic for the treatment of type 1 diabetes. The Edmonton protocol for islet transplantation1 demonstrated that patients could achieve insulin independence up to about 1 year after the procedure. However, patients required islets from at least two donors, with the result that the demand for islets exceeds organ supply. Furthermore, in a follow-up study of 36 islet recipients treated at nine transplantation centers,2 only one-third of patients were insulin-independent after 2 years. These results demonstrate that beta-cell supplementation can result in insulin independence but that islet transplantation itself may be most appropriate for patients with very poor glycemic control given the limited supply of islets.
Type 1 diabetic patients with long-standing disease exhibit residual beta-cell mass. Meier et al.3 examined 42 samples from type 1 diabetic donors and found that 88% of them had at least some beta cells, which was not correlated with the duration of disease. The high frequency of apoptosis observed suggested that the beta cells were continuing to regenerate. Accordingly, a later study found a 100-fold increase in beta-cell proliferation in an elderly patient with recent-onset type 1 diabetes.4 Importantly, a recent study of Joslin Medalists (patients with type 1 diabetes for >50 years) showed that all postmortem pancreata contained some insulin-positive cells, a subset of which were also terminal dUTP nick-end labeling (TUNEL)–positive and therefore apoptotic.5 Identification of a residual beta-cell population in type 1 diabetic pancreata means that these cells could be a potential source of new beta cells and suggests that stimulation of beta-cell proliferation may be a feasible approach in type 1 diabetes.
Beta-cell proliferation is the primary means of beta-cell replacement in rodents. Seminal work in 2004 determined that new beta cells in the mouse arise from cell division of existing beta cells and not from a stem-cell population.6 It is unclear whether this principle applies to humans. A recent effort to study pancreatic samples from young human donors showed that replication is indeed responsible for beta-cell expansion after birth but that it drops off dramatically to negligible levels by late adolescence.7 A follow-up study of beta-cell turnover by BrdU staining confirmed that although some replicating beta cells could be found in donors younger than 20 years, none were observed in patients older than 30 years.8 However, an analysis of donors between 7 and 66 years of age found cells positive for the proliferative marker Ki67 in every sample tested.5 Although many reports have documented conditions to induce the proliferation of rodent beta cells in culture, far fewer have confirmed human beta-cell proliferation. Recent work in human islets using in vitro lineage-tracing techniques detected human beta-cell proliferation in cell culture.9 In this case, however, the proliferating cells dedifferentiated and showed lower insulin expression, suggesting that redifferentiation to a beta-cell state may be necessary.10 These studies illustrate the ambiguity of whether adult human beta cells replicate, and they demonstrate the need for small-molecule probes to modulate and better understand this process.
Overexpression of key genes can induce human beta-cell proliferation. For example, overexpression of cyclin-dependent kinase 6, in the presence or absence of cyclin D1, caused an increase in human beta cell proliferation.11,12 Importantly, these effects also extended to in vivo models of islet transplantation. Similarly, overexpression of the developmental transcription factor Nkx6.1 increased beta cell proliferation in human islets, as measured by thymidine incorporation, with a preservation of insulin secretion.13 These examples suggest that proliferation of human beta cells, although rare in normal adults, is inducible under the right conditions.
The general lack of a suitable human beta cell line14 has led to reliance on cadaveric donor islets for study. To enable identification of small molecules capable of inducing human beta-cell proliferation, we report here the development of a system to culture dissociated human islet cells, by adapting existing islet culture techniques to a 384-well format, for high-throughput screening (HTS). This cell culture method preserves insulin expression and secretion, and beta cells remain responsive to a known genetic stimulus for proliferation. We used this system to perform a pilot screen on 1280 compounds. This cell culture system lays the groundwork for the identification of novel small molecules that affect human beta-cell biology.
Materials and Methods
Ethics Statement
The use of discarded human islet tissue for basic research was exempted from institutional review board approval.
Preparation of Extracellular Matrix Cell Culture Plates
HTB-9 human bladder carcinoma cells (American Type Culture Collection [ATCC], Manassas, VA) were cultured in 96-well and 384-well plates with 100 µL/well and 50 µL/well, respectively, of RPMI media containing 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 µg/mL streptomycin. We modified the methods developed by Beattie et al.15 to high-density cell culture. Cells were grown to confluence and cultured for an additional 3 days to allow the deposition of more extracellular matrix (ECM). To denude (or decellularize) the surface, in which cells are removed while leaving behind the intact ECM, media were aspirated and incubated for 6 min at 37 °C with 100 µL/well (96) of 20 mM NH4OH in phosphate-buffered saline (PBS). The NH4OH was then triturated four to five times with a multichannel pipettor and aspirated. Plates were washed four to five times with fresh PBS and inspected to ensure that cells were removed. Denuded plates were stored for up to 3 weeks in a 37 °C incubator in PBS for future use. For 384-well plates, we used a CyBio Vario (CyBio, Jena, Germany) to add 50 µL/well of 20 mM NH4OH in PBS. Cultures were left at room temperature for 8 min. The NH4OH solution was triturated six times, then washed and triturated with fresh PBS four times, with plates stored in a fresh round of PBS. Plates were left hydrated in PBS to store for approximately 3 weeks in a 37 °C incubator.
Human Islet Cell Culture
Human islets were obtained through the Integrated Islet Distribution Program (IIDP; http://iidp.coh.org/), the National Disease Research Interchange (http://www.ndriresource.org/), and Beta-Pro (http://betaprollc.com). The purity and viability of human islets are reported to be 70% to 93% and 70% to 98%, respectively, and the average age of cadaveric donors was 41.4 ± 11.5 years (range, 12–58 years; n = 18). Specific data on individual donors are reported in Supplemental Table S1. Islets were washed with PBS and incubated in CMRL medium (Cellgro; Mediatech, Manassas, VA) supplemented with 10% FBS, 2 mM glutamine, 100 U/mL penicillin, and 100 µg/mL streptomycin. Intact islets were stored in 60-mm Petri dishes in a 37 °C incubator at approximately 5000 islet equivalents (IEQ) per 10 mL media.
Islet Dissociation
To dissociate tissue, islets were pelleted and washed in PBS to remove any additional media/serum that might interfere with dissociation process. Islets were centrifuged at 1000 rpm for 5 min at room temperature. Pelleted islets were incubated at 5000 IEQ/mL in Accutase (Innovative Cell Technologies, San Diego, CA) at 37 °C for 20 min. The islet suspension was triturated five times, the dissociated material was diluted with fresh CMRL complete media and centrifuged at 1000 rpm for 5 min, and supernatant was aspirated. The pellet was resuspended in CMRL complete media, and an aliquot was removed for cell counting with a hemacytometer. Trypan blue was used to determine viability. We seeded 30 000 cells per well in 96-well plates (100 µL/well) and 8000 cells per well in 384-well plates (50 µL/well) with a Multidrop Combi automated liquid dispenser (Thermo Scientific, Waltham, MA).
Immunofluorescence
Cultures were fixed for 15 min at room temperature using freshly prepared 3% paraformaldehyde and washed twice with PBS. Cells were permeabilized for 20 min at room temperature in PBS containing 0.2% Triton-X 100 and blocked overnight at 4 °C with 2% bovine serum albumin (BSA) in PBS. We used a FITC-conjugated anti-Ki67 antibody. The C-peptide antibody, developed by Ole D. Madsen (Hagedorn Research Institute, Copenhagen, Denmark), was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development (NICHD) and maintained by the University of Iowa, Department of Biology (Iowa City, IA). Primary antibodies were diluted in antibody dilution buffer (ADB; 1% BSA in PBS) and incubated overnight at 4 °C, followed by three washes in ADB. Cultures were incubated in secondary antibody (Alexa Fluor-conjugated anti-mouse; Invitrogen, Carlsbad, CA) diluted in ADB for 1 h at room temperature, followed by five washes with PBS. Plates were stored foil-sealed at 4 °C with 100 µL/well PBS. Plates were imaged with an ImageXpress Micro automated microscope (Molecular Devices, Sunnyvale, CA), and automated image analysis was performed using the Multiwavelength Cell Scoring Application Module in MetaXpress software (Molecular Devices).
Results
All cells in the body are surrounded by an ECM that provides structural support and plays a role in cell adhesion, migration, and differentiation. This environment can be re-created in vitro by a variety of methods to develop a more physiological cell culture system. Attachment of islet cells to tissue culture–treated plates has historically proven difficult, so reports in the literature discussed the development of methods to deposit ECM on plates before culturing islets. In particular, the ECM secreted by the human HTB-9 bladder carcinoma cell line has been shown to preserve beta-cell function, with glucose-stimulated insulin secretion (GSIS) preserved to a large extent.15
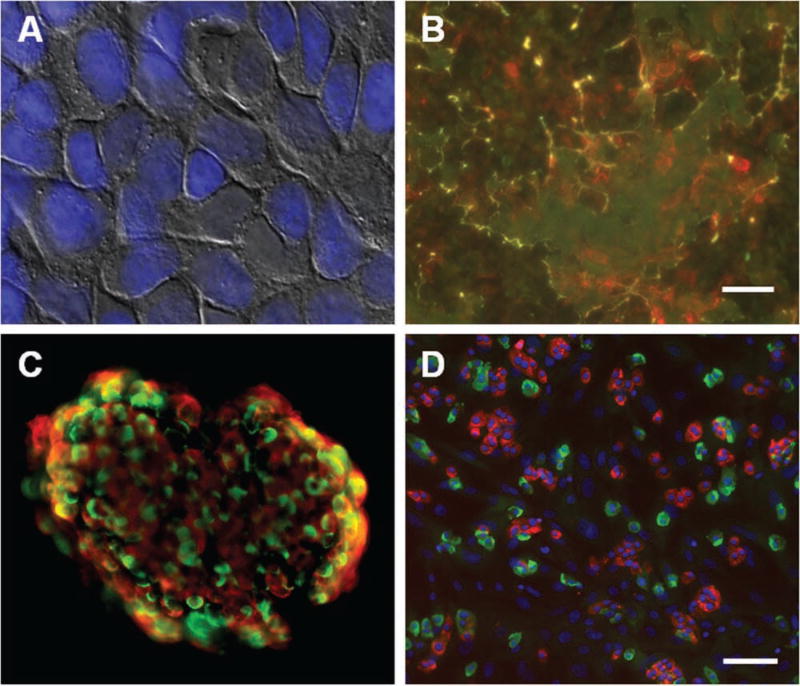
We have developed an automated method of creating this optimized microenvironment in 96- and 384-well plates. HTB-9 cells are cultured to confluence in standard tissue culture–treated plates (Fig. 1A), allowing them time to secrete various matrix factors, such as collagen IV, laminin, and fibronectin, on the surface of the plate (Fig. 1B). The cells are then detached from the tightly associated matrix by brief treatment with ammonium hydroxide, followed by extensive washing with PBS. This method results in the preservation of the ECM on the surface of the plate (Suppl. Fig. S1), into which islet cells can be subsequently seeded for attachment.
Figure 1.
Preparation of surface for islet cell culture system. (A) Human bladder carcinoma HTB-9 cells grown to confluence on 96- and 384-well plates. Overlay of bright-field and fluorescent Hoechst nuclear dye. (B) After denuding the cells from the plate, the plate surface was stained for fibronectin (red) and laminin (green) levels. Scale bar = 10 µm. (C) Immunofluorescence on an intact human islet, revealing cellular architecture and distribution of cells. Red, insulin; green, glucagon. (D) Immunofluorescence on dissociated islet cells seeded in a 384-well plate. Red, C-peptide; green, glucagon; blue, Hoechst nuclear dye. Scale bar = 100 µm.
To ensure that our methods preserve beta-cell function and identity in high-density culture, we gently dissociated human islets into small cell clusters and compared insulin and glucagon expression to that of intact islets. A fairly even distribution of endocrine cells was observed in the intact islets (Fig. 1C), consistent with an examination of the differences in islet cytoarchitecture between different species.16 When we dissociated human islets and plated them in our system, we observed that 20% to 50% of the cells were C-peptide-positive beta cells, depending on the purity of the islet preparation (Fig. 1D). We also detected 10% to 40% glucagon-positive alpha cells (Fig. 1D). We moved from staining beta cells with an insulin antibody to a C-peptide antibody. C-peptide is a cleavage product of proinsulin, and we used this marker because of concerns that insulin present in the media can be taken up by cells and detected incorrectly as beta cells. Both antibodies worked well in our culture system and identified the same cell population (Suppl. Fig. S2).
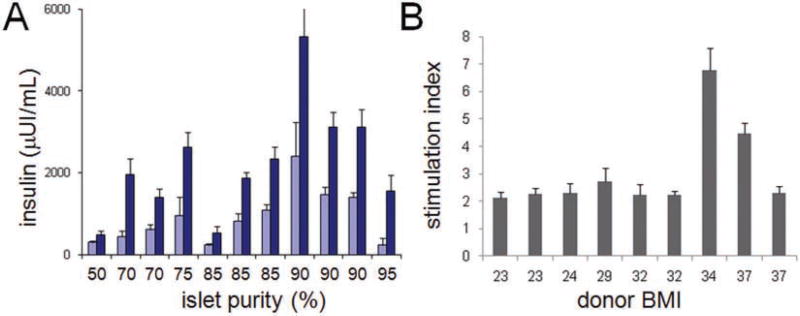
Cells remained viable for at least 7 days in culture, which was the maximum duration we tested (Suppl. Fig. S3). To confirm beta-cell function, we measured GSIS in dissociated culture. We consistently observed a stimulation index of at least twofold, with this value partially dependent on islet purity and donor body mass index (BMI). In general, the purity of the islet preparation was important to GSIS; above ~85% purity, islet cells secreted higher levels of insulin reliably (Fig. 2A). Most islet preparations resulted in an insulin stimulation index of ~2, although a few high-BMI samples showed greater insulin secretion (Fig. 2B), reflecting not only the high donor-to-donor variability we observed throughout the process but also the variation in islet isolation, as determination of islet purity is a subjective measure. Overall, these results indicate that this high-throughput cell culture system is capable of preserving beta-cell identity and function.
Figure 2.
Cell culture system preserves glucose-stimulated insulin secretion (GSIS). (A) Islets from preparations with the indicated purity of isolation were dissociated, seeded into 96-well plates, and incubated for 1 h with either 1.67 mM (light blue bars) or 16.7 mM (dark blue bars) glucose to assess GSIS. (B) GSIS data are plotted relative to the body mass index (BMI) of the donor and, as the stimulation index, relative to 1.67 mM glucose. Data represent the mean ± standard deviation of four to eight independent wells.
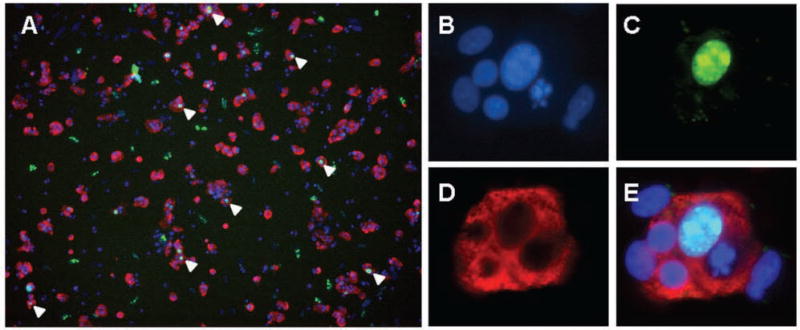
We observed that 5% to 10% of all islet cells are proliferating, as measured by Ki67 immunofluorescence.17 However, by morphology, the Ki67-positive cells generally appear to be fibroblasts (large nuclei not surrounded by C-peptide-positive cytoplasm) and not beta cells (small nuclei surrounded by C-peptide-positive cytoplasm) (Suppl. Fig. S4). We found that Bay K8644, a small molecule reported to induce human beta-cell proliferation,18 was more likely increasing the proliferation of supporting cells, such as fibroblasts, and not beta cells (Suppl. Fig. S5). To identify a positive-control condition for HTS, we infected dissociated islets in our culture system with adenoviruses encoding cdk-6 and cyclin D1.11,12 After 3 days of overexpression, we observed a visible increase in beta cell proliferation (Fig. 3A). Because the cells were dissociated and cultured as a single layer of cells on ECM, we could clearly distinguish individual replicating cells and therefore could be certain that Ki67-positive nuclei indeed belonged to beta cells (Fig. 3B – E). This is not the case when identifying individual replicating cells within intact islets, for which 3D imaging is very challenging. Previous work to overexpress these genes in human islet cells showed that TUNEL staining was not increased.11,12 Furthermore, we observed no apparent visual differences in replicating beta cells; the morphology of replicating beta cells was similar to those beta cells that were healthy and quiescent, consistent with published reports.11,12
Figure 3.
Beta cell proliferation induced by cdk-6 and cyclin D1 in high-density culture. (A) Dissociated islet cells were cultured on extracellular matrix (ECM) and infected for 24 h with adenovirus vectors encoding cdk-6 and cyclin D1, incubated for 3 days in cell culture, and then fixed and assessed for C-peptide (a cleavage product of proinsulin) and Ki67 expression. Cells simultaneously positive for Ki67 and C-peptide are indicated by white arrowheads. To ensure that individual cells positive for Ki67 were expressing C-peptide, higher magnification images were acquired: (B) Hoechst nuclear dye, (C) Ki67, (D) C-peptide, and (E) image overlay.
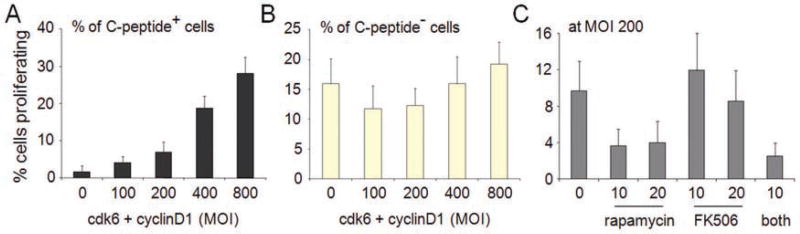
In addition to examining the islet cells visually, we quantified the number of beta cells in each well and the percentage of those that were also Ki67-positive. Proliferating beta cells increased in proportion to the total multiplicity of infection (MOI) used for infection, proceeding from less than 1% in the uninfected state (Fig. 4A, column 1) to nearly 30% at an MOI of 800 (Fig. 4A, column 5). These MOI values are consistent with previous efforts to infect dissociated islet cells.11,12 The low but nonzero background value in the uninfected state does not, in fact, represent proliferating beta cells but is an artifact of the automated image analysis (Suppl. Fig. S6). The effects of cdk-6 and cyclin D1 appeared to be specific to beta cells, as the percentage of proliferation within the heterogeneous population of insulin-negative cells did not change with increasing MOI (Fig. 4B). Finally, we tested whether rapamycin or FK506 could inhibit the proliferation induced by cdk-6 and cyclin D1. These immunosuppressants are used to treat islet transplant recipients, and it has been shown that these treatments are deleterious to beta cells.19 We observed a strong decrease in the percentage of proliferating beta cells in the presence of 10 nM and 20 nM rapamycin but not in the presence of 10 nM or 20 nM FK506 (Fig. 4C). These results demonstrate that we can reliably detect beta-cell proliferation in this system and that this phenomenon is inhibited by rapamycin cotreatment.
Figure 4.
Quantification of proliferation in response to cdk-6 and cyclin D1 . Dissociated islet cells infected for 24 h with increasing total multiplicity of infection (MOI) of cdk6 and cyclin D1 and incubated for 3 days were imaged and quantified for cell identity and proliferation status. (A) The percentage of proliferating C-peptide-expressing beta cells was quantified in each well of a 96-well plate. (B) The percentage of proliferating C-peptide-negative cells (including other islet endocrine cell types, endothelial cells, and fibroblasts) was quantified in each well of a 96-well plate. (C) Islet cells were infected with cdk-6 and cyclin D1 at an MOI of 200 for each gene in the absence or presence of indicated concentrations of the immunosuppressants rapamycin, FK506, or both. After a 3-day incubation, cells were fixed, stained, and quantified in the same manner as above. Data represent the mean ± standard deviations of 32 independent wells.
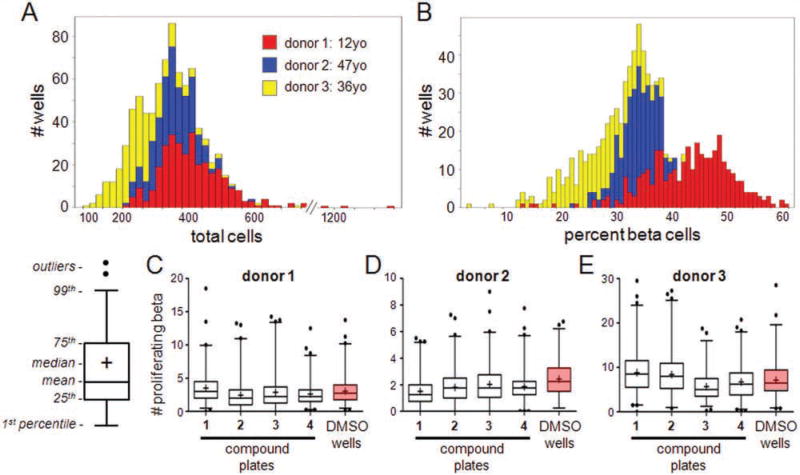
We then sought to determine the suitability of this system for small-molecule screening and the effects of donor variation on screening outcomes. This aspect is important to establish reproducibility and reliability during HTS. We screened a small collection of 1280 compounds, representing a subset of G-protein-coupled receptor (GPCR)–biased compounds and a subset from a structurally diverse internally synthesized library, in islet samples from three donors. Sixty-four DMSO wells present on each 384-well plate were used as vehicle controls. Comparing the control wells across donors, we observed that two of the samples, from relatively similar donors (Suppl. Table S1), were very similar in the total number of cells per well and the percent of beta cells present in each well (Fig. 5A,B). However, a sample of islets from a very young (12 years old) donor had a higher percentage of beta cells in each well (Fig. 5B), perhaps reflecting a greater starting beta-cell mass in each islet.
Figure 5.
Pilot screen of 1280 compounds across three batches of islets. Islets from three organ donors were dissociated and cells seeded into extracellular matrix (ECM)–prepared 384-well plates. Cells were treated with individual members of a small-molecule collection, one compound per well, and incubated for 3 days. (A) Histogram of total cells per well across the three samples. (B) Histogram of the percentage of C-peptide-positive beta cells per well across the three samples. (C–E) Screening outcomes. The absolute number of proliferating beta cells was quantified in each well. Data are represented as box-whisker plots summarizing the wells of each compound plate (“1–4”) and all DMSO control wells across the plates (“DMSO wells,” boxed in pink). For the box-whisker plots, the mean value of the data set is represented by the central line of the box and the median by “+.” The box size represents the 25th and 75th percentiles of the data and the whiskers the 1st and 99th percentiles. Further outliers are represented by dots. Graphical schematic is shown. Box-whisker plots of screening outcomes for (C) donor 1, (D) donor 2, and (E) donor 3.
We used the DMSO wells to calculate the averages, standard deviations, and coefficients of variation (CVs) for each of the parameters output by image analysis (Suppl. Table S2). In general, the detection of Ki67-positive cells was more variable than the detection of total cells. Interestingly, the lowest CVs were observed in parameters involving detecting beta cells, either as counting the total number or as a percentage of each field of view (Suppl. Table S2). Again, there were nonzero values reported for the number of cells positive for all three stains, but these generally corresponded to false positives, which were unavoidable with our current image analysis but do not affect the overall outcome. Analysis of each compound-treated well for proliferating beta cells revealed that none of the compounds were positive outliers compared to the DMSO control wells (Fig. 5C – E) and that all compound treatments fell within the background of the DMSO distribution.
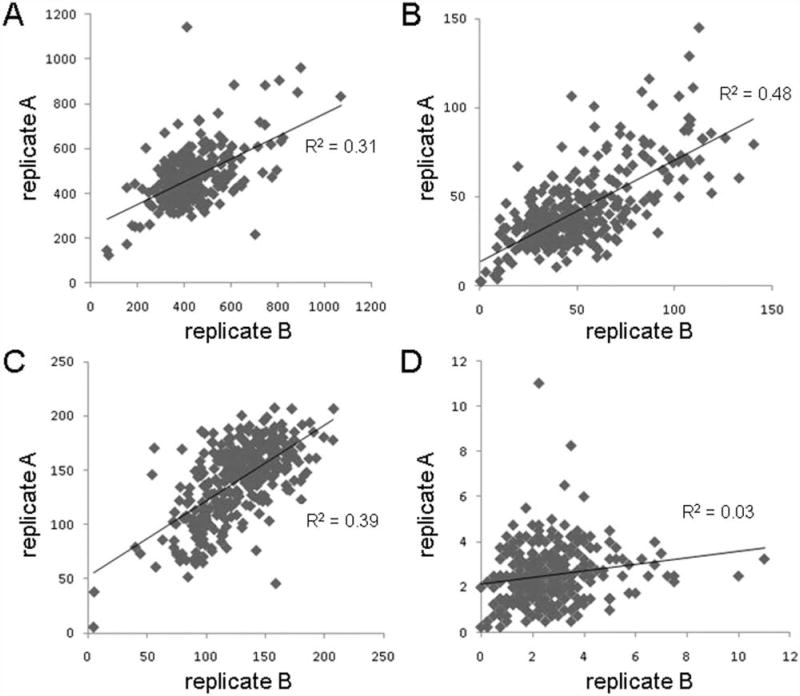
Each of the three pilot screens was performed in a single replicate. To assess intradonor reproducibility of the assay, we tested each donor with one compound plate in duplicate and analyzed the compound treatments within several per-well statistics. There was quite a bit of variability in the donors (Suppl. Fig. S7). In one donor sample, we observed that some measurements had modest reproducibility, such as total number of cells (Fig. 6A; R2 = 0.31), number of proliferating insulin-negative cells (Fig. 6B; R2 = 0.48), and number of quiescent beta cells (Fig. 6C; R2 = 0.39). However, the number of proliferating beta cells was essentially random (Fig. 6D; R2 = 0.03). Knowing that these compounds have no effect on beta-cell proliferation, it was not particularly surprising that these measurements, all essentially within the noise of the assay, do not reliably reproduce. This islet sample was high purity (90%) and from a donor with relatively average biometrics (Suppl. Table S1), suggesting that these biometrics might best be optimized for screening.
Figure 6.
Analysis of screening performance reproducibility. Islet cells were dissociated and treated for 3 days in duplicate with 1280 compounds. The two replicates are plotted against each other examining (A) total cells, (B) proliferating C-peptide-negative cells, (C) quiescent C-peptide-positive beta cells, and (D) proliferating beta cells. The correlations of each data set to a linear fit are shown.
Discussion
To identify small molecules capable of inducing human beta-cell proliferation, we needed an in vitro system in which dissociated human islet cells can be maintained in culture for HTS. Human beta cells do not proliferate as readily as rat or mouse cells, so results obtained using rodent cell lines would presumably not translate well to human cells. Furthermore, the lack of suitable human beta cell lines14 for high-throughput culture necessitates the use of primary tissue. To achieve this goal, we adapted a culture method using the ECM secreted by the human HTB-9 bladder carcinoma cell line (Fig. 1).15 Dissociated islet cells cultured on ECM remained viable for at least 7 days in culture. To confirm beta-cell function, we measured GSIS in dissociated culture (Fig. 2); our results show that the dissociated islet cells not only express insulin protein but also secrete it in a glucose-dependent manner.
Human pancreatic islets require specific culture conditions in terms of media composition and supplements,10,20,21 but it is becoming increasingly clear that the preservation of beta-cell function also requires the maintenance of interactions between beta cells and the ECM.22,23 These interactions have important roles in regulating cell survival,24 insulin secretion,25 and proliferation.26 The peripheral ECM of mature human islets is composed essentially of laminin and collagen IV,27 although fibronectin and collagens III, V, and VI have been also reported.23,28 It is possible that the laminin component of the ECM from the HTB-9 cell line is more similar to that of intact islets in vivo and is thus conducive to beta cell attachment and function. In vitro adhesion studies, for example, show that human beta cells adhere well to surfaces coated with purified laminin-511.29 Human islet isolation leads to a loss of the ECM that can contribute to eventual apoptosis and dedifferentiation in vitro. We believe that one advantage of our cell culture system is that it may more faithfully replicate the physiological conditions of the human pancreas.
We used adenoviral overexpression of cdk-6 and cyclin D1 as a positive control to induce beta-cell proliferation (Fig. 3). These conditions were previously used to demonstrate the importance of modulating cell-cycle activity in beta cells.11 Although this treatment would not be suitable therapeutically, our results confirm the ability of the assay to detect beta-cell proliferation under these conditions. We also show that this induced proliferation is inhibited by cotreatment with 10 nM rapamycin (Fig. 4), consistent with a report showing impairment of beta-cell regeneration in mice by a combination of rapamycin and FK506.19 Rapamycin and FK506 are immunosuppressive drugs used in organ transplants to prevent rejection of islet transplants, but these drugs are deleterious to beta cells. Interestingly, we observed that rapamycin inhibited proliferation to a much greater extent than FK506. An intriguing use of this cell culture system involves testing novel immunosuppressants for their effects on beta-cell proliferation. Because human islet transplantation for the treatment of type 1 diabetes is accompanied by immunosuppressant therapy, the identification of new therapies that spare beta cells would represent a clinically important advance.
We performed a pilot screen of 1280 compounds across three donor islet samples (Fig. 5). We found that the variability of readouts was lowest for all three donors in the detection of numbers of beta cells per well. Furthermore, we observed that reproducibility of readouts was greater in the donor in which biometrics were more average (age, BMI) than others (Fig. 6). We did observe high donor-to-donor variation, even among donors with similar islet purity and/or BMI. Given the rarity of proliferating beta cells, larger scale screening with replicate donors will be necessary to increase the opportunity to identify small molecules with this activity, and additional statistical modeling of rare events will be appropriate to such screens. Our results validate this cell culture system and indicate that it is a practical and useful system for HTS of human islet cells.
Supplementary Material
Acknowledgments
We thank T. Gilbert, V. Dančík, S. Burns, J. Hecksher-Sørensen, and S. Kubicek for technical assistance and discussion.
Funding
The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: This work was funded by the Juvenile Diabetes Research Foundation (17-2008-1030 to S.L.S. and B.K.W.; 1-2008-39 and 34-2008-630 to A.F.S.), a Type 1 Diabetes Pathfinder Award to B.K.W. (DP2-DK083048, NIH-NIDDK), and NIH U-01 DK0089538 (to A.F.S.). D.F.Y. acknowledges support from an MCO training grant at Harvard University. S.L.S. is an Investigator at the Howard Hughes Medical Institute.
Footnotes
Supplementary material for this article is available on the Journal of Biomolecular Screening Web site at http://jbx.sagepub.com/supplemental.
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet Transplantation in Seven Patients with Type 1 Diabetes Mellitus Using a Glucocorticoid-Free Immunosuppressive Regimen. N. Engl. J. Med. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, Secchi A, Brendel MD, Berney T, Brennan DC, et al. International Trial of the Edmonton Protocol for Islet Transplantation. N. Engl. J. Med. 2006;355:1318–1330. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 3.Meier JJ, Bhushan A, Butler AE, Rizza RA, Butler PC. Sustained Beta Cell Apoptosis in Patients with Long-Standing Type 1 Diabetes: Indirect Evidence for Islet Regeneration? Diabetologia. 2005;48:2221–2228. doi: 10.1007/s00125-005-1949-2. [DOI] [PubMed] [Google Scholar]
- 4.Meier JJ, Lin JC, Butler AE, Galasso R, Martinez DS, Butler PC. Direct Evidence of Attempted Beta Cell Regeneration in an 89-Year-Old Patient with Recent-Onset Type 1 Diabetes. Diabetologia. 2006;49:1838–1844. doi: 10.1007/s00125-006-0308-2. [DOI] [PubMed] [Google Scholar]
- 5.Keenan HA, Sun JK, Levine J, Doria A, Aiello LP, Eisenbarth G, Bonner-Weir S, King GL. Residual Insulin Production and Pancreatic Beta-Cell Turnover after 50 Years of Diabetes: Joslin Medalist Study. Diabetes. 2010;59:2846–2853. doi: 10.2337/db10-0676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dor Y, Brown J, Martinez OI, Melton DA. Adult Pancreatic Beta-Cells Are Formed by Self-Duplication Rather Than Stem-Cell Differentiation. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 7.Meier JJ, Butler AE, Saisho Y, Monchamp T, Galasso R, Bhushan A, Rizza RA, Butler PC. Beta-Cell Replication Is the Primary Mechanism Subserving the Postnatal Expansion of Beta-Cell Mass in Humans. Diabetes. 2008;57:1584–1594. doi: 10.2337/db07-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perl S, Kushner JA, Buchholz BA, Meeker AK, Stein GM, Hsieh M, Kirby M, Pechhold S, Liu EH, Harlan DM, et al. Significant Human Beta-Cell Turnover Is Limited to the First Three Decades of Life as Determined by In Vivo Thymidine Analog Incorporation and Radiocarbon Dating. J. Clin. Endocrinol. Metab. 2010;95:E234–E239. doi: 10.1210/jc.2010-0932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Russ HA, Bar Y, Ravassard P, Efrat S. In Vitro Proliferation of Cells Derived from Adult Human Beta-Cells Revealed by Cell-Lineage Tracing. Diabetes. 2008;57:1575–1583. doi: 10.2337/db07-1283. [DOI] [PubMed] [Google Scholar]
- 10.Ouziel-Yahalom L, Zalzman M, Anker-Kitai L, Knoller S, Bar Y, Glandt M, Herold K, Efrat S. Expansion and Redifferentiation of Adult Human Pancreatic Islet Cells. Bio-chem. Biophys. Res. Commun. 2006;341:291–298. doi: 10.1016/j.bbrc.2005.12.187. [DOI] [PubMed] [Google Scholar]
- 11.Fiaschi-Taesch N, Bigatel TA, Sicari B, Takane KK, Salim F, Velazquez-Garcia S, Harb G, Selk K, Cozar-Castellano I, Stewart AF. Survey of the Human Pancreatic Beta Cell G1/S Proteome Reveals a Potential Therapeutic Role for cdk-6 and Cyclin D1 in Enhancing Human Beta Cell Replication and Function In Vivo. Diabetes. 2009;58:882–893. doi: 10.2337/db08-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fiaschi-Taesch NM, Salim F, Kleinberger J, Troxell R, Cozar-Castellano I, Selk K, Cherok E, Takane KK, Scott DK, Stewart AF. Induction of Human Beta-Cell Proliferation and Engraftment Using a Single G1/S Regulatory Molecule cdk6. Diabetes. 2010;59:1926–1936. doi: 10.2337/db09-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schisler JC, Fueger PT, Babu DA, Hohmeier HE, Tessem JS, Lu D, Becker TC, Naziruddin B, Levy M, Mirmira RG, Newgard CB. Stimulation of Human and Rat Islet Beta-Cell Proliferation with Retention of Function by the Homeodomain Transcription Factor Nkx6.1. Mol. Cell. Biol. 2008;28:3465–3476. doi: 10.1128/MCB.01791-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hohmeier HE, Newgard CB. Cell Lines Derived from Pancreatic Islets. Mol. Cell. Endocrinol. 2004;228:121–128. doi: 10.1016/j.mce.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 15.Beattie GM, Cirulli V, Lopez AD, Hayek A. Ex Vivo Expansion of Human Pancreatic Endocrine Cells. J. Clin. Endocrinol. Metab. 1997;82:1852–1856. doi: 10.1210/jcem.82.6.4009. [DOI] [PubMed] [Google Scholar]
- 16.Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren PO, Caicedo A. The Unique Cytoarchitecture of Human Pancreatic Islets Has Implications for Islet Cell Function. Proc. Natl. Acad. Sci. U. S. A. 2006;103:2334–2339. doi: 10.1073/pnas.0510790103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scholzen T, Gerdes J. The Ki-67 Protein: From the Known and the Unknown. J. Cell. Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 18.Wang W, Walker JR, Wang X, Tremblay MS, Lee JW, Wu X, Schultz PG. Identification of Small-Molecule Inducers of Pancreatic Beta-Cell Expansion. Proc. Natl. Acad. Sci. U. S. A. 2009;106:1427–1432. doi: 10.1073/pnas.0811848106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nir T, Melton DA, Dor Y. Recovery from Diabetes in Mice by Beta Cell Regeneration. J. Clin. Invest. 2007;11 7:2553–2561. doi: 10.1172/JCI32959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Efrat S. Ex-vivo Expansion of Adult Human Pancreatic Beta-Cells. Rev. Diabetes Stud. 2008;5:116–122. doi: 10.1900/RDS.2008.5.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murdoch TB, McGhee-Wilson D, Shapiro AM, Lakey JR. Methods of Human Islet Culture for Transplantation. Cell Transplant. 2004;13:605–617. [PubMed] [Google Scholar]
- 22.Daoud J, Petropavlovskaia M, Rosenberg L, Tabrizian M. The Effect of Extracellular Matrix Components on the Preservation of Human Islet Function In Vitro. Biomaterials. 2010;31:1676–1682. doi: 10.1016/j.biomaterials.2009.11.057. [DOI] [PubMed] [Google Scholar]
- 23.Stendahl JC, Kaufman DB, Stupp SI. Extracellular Matrix in Pancreatic Islets: Relevance to Scaffold Design and Transplantation. Cell Transplant. 2009;18:1–12. doi: 10.3727/096368909788237195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Navarro-Alvarez N, Rivas-Carrillo JD, Soto-Gutierrez A, Yuasa T, Okitsu T, Noguchi H, Matsumoto S, Takei J, Tanaka N, Kobayashi N. Reestablishment of Microenvi-ronment Is Necessary to Maintain In Vitro and In Vivo Human Islet Function. Cell Transplant. 2008;17:111–119. doi: 10.3727/000000008783907125. [DOI] [PubMed] [Google Scholar]
- 25.Nagata N, Gu Y, Hori H, Balamurugan AN, Touma M, Kawakami Y, Wang W, Baba TT, Satake A, Nozawa M, et al. Evaluation of Insulin Secretion of Isolated Rat Islets Cultured in Extracellular Matrix. Cell Transplant. 2001;10:447–451. [PubMed] [Google Scholar]
- 26.Beattie GM, Montgomery AM, Lopez AD, Hao E, Perez B, Just ML, Lakey JRT, Hart ME, Hayek A. A Novel Approach to Increase Human Islet Cell Mass While Preserving Beta-Cell Function. Diabetes. 2002;51 doi: 10.2337/diabetes.51.12.3435. [DOI] [PubMed] [Google Scholar]
- 27.Virtanen I, Banerjee M, Palgi J, Korsgren O, Lukinius A, Thornell LE, Kikkawa Y, Sekiguchi K, Hukkanen M, Konttinen YT, et al. Blood Vessels of Human Islets of Langerhans Are Surrounded by a Double Basement Membrane. Diabetologia. 2008;51:1181–1191. doi: 10.1007/s00125-008-0997-9. [DOI] [PubMed] [Google Scholar]
- 28.Hughes SJ, Clark A, McShane P, Contractor HH, Gray DW, Johnson PR. Characterisation of Collagen VI within the Islet-Exocrine Interface of the Human Pancreas: Implications for Clinical Islet Isolation? Transplantation. 2006;81:423–426. doi: 10.1097/01.tp.0000197482.91227.df. [DOI] [PubMed] [Google Scholar]
- 29.Otonkoski T, Banerjee M, Korsgren O, Thornell LE, Virtanen I. Unique Basement Membrane Structure of Human Pancreatic Islets: Implications for Beta-Cell Growth and Differentiation. Diabetes Obes. Metab. 2008;10(suppl 4):119–127. doi: 10.1111/j.1463-1326.2008.00955.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.