Abstract
Objectives
To investigate the tumor-suppressive properties of enzalutamide in androgen-driven ovarian cancer.
Methods
Mice were implanted subcutaneously with OVCAR-3 cells and treated with dihydrotestosterone in combination with enzalutamide or vehicle control. Tumor volumes were measured twice weekly until day 56.
Results
Dihydrotestosterone exposure led to a significant increase in tumor growth, while concomitant treatment with enzalutamide led to significant reductions in tumor volume compared to the androgen-exposed groups.
Conclusions
We present the first evidence that the second-generation antiandrogen enzalutamide may possess efficacy in the treatment of ovarian cancer, paving the way for future clinical trials.
Keywords: Enzalutamide, androgen receptor, epithelial ovarian cancer, OVCAR-3, xenograft
Introduction
The second-generation antiandrogen enzalutamide is currently FDA approved for castration-resistant prostate cancer (1). Previous preclinical data has demonstrated that enzalutamide may also be effective in estrogen receptor-expressing breast cancer (2), and more recently, the in vitro efficacy of enzalutamide against triple negative breast cancer was demonstrated (3), In light of these developments, we present data from an in vivo xenograft model of ovarian cancer, in which we investigated the tumor-suppressive properties of enzalutamide on androgen-driven ovarian cancer. Our results demonstrate that the potential applications of enzalutamide may extend to an even broader range of androgen receptor (AR)-expressing cancers than previously thought, including ovarian cancer.
Materials and Methods
Drugs, reagents and animals
Dihydrotestosterone (DHT) was purchased from Isosciences (King of Prussia, PA, USA). PG-21 anti-AR antibody was purchased from Merck Millipore (Temecula, CA, USA). Enzalutamide was provided by Medivation Corporation (San Francisco, CA, USA). Female NOD scid gamma mice, aged 6–8 weeks on arrival, were purchased from The Jackson Laboratory (Bar Harbor, ME, USA) and housed under standard conditions in the Memorial Sloan Kettering Cancer Center (MSKCC) vivarium. All experiments were conducted under an Institutional Animal Care and Use Committee (IACUC)-approved protocol.
Cell culture
The human ovarian adenocarcinoma cell line OVCAR-3 was obtained from a specimen library within the David Spriggs laboratory at MSKCC Zuckerman Research Center (New York, NY, USA). The cells were cultured in RPMI-1640 medium with 10% fetal bovine serum and insulin, and kept at 37°C in a humidified 5% CO2 atmosphere.
In vivo tumor growth assessment
Mice were implanted subcutaneously with approximately 10×106 OVCAR-3 cells along with Matrigel (BD Biosciences), divided into 4 groups (N = 6 per group) and treated with either enzalutamide (30 mg/kg); DHT (12.5 mg/60 days); enzalutamide + DHT, or vehicle control for 7 weeks. The same experiment was repeated using a higher dose of enzalutamide (50 mg/kg) and a larger cohort size (N = 15 per group). Enzalutamide was administered daily PO once the mean tumor size reached 100 mm3 in each cohort. DHT was administered as pellets set to release 12.5 mg DHT over 60 days (approximately 0.21 mg/day), and was implanted 5 days prior to the start of the experiment, according to the manufacturer's instructions (Innovative Research of America, Sarasota, FL, USA). Tumors were measured twice weekly until day 56, and tumor volumes were calculated using the formula length × width2 × 0.52.
Immunoblot analysis to confirm the presence of ARs
Approximately 3.0×106 cells from the OVCAR-3 were obtained and lysed using EDTA-containing buffer [1mM EDTA, 1% Triton-X, 0.5% sodium deoxycholate]. In addition to the OVCAR-3 cells, LnCAP and OVCA-429 cells were cultured as above and used as positive and negative controls, respectively. Membranes were incubated overnight at 4°C with rabbit polyclonal anti-AR antibody (clone PG-21; Millipore Sigma), at a concentration of 1:500. Immune blots were visualized using a 1:5000 dilution of horseradish peroxidase (HRP)-conjugated anti-rabbit secondary antibody, using enhanced chemiluminescence Western blotting substrate (Roche Applied Science, Penzberg, Germany).
Statistics
Statistical analysis was performed using either the tumor volume at day 56 or the area under the curve (AUC) of the tumor volume until day 56. The Wilcoxon Rank Sum Test was used for between-group comparisons.
Results
Enzalutamide-induced reduction in tumor growth
The median volume of tumors on day 56 in the 30mg/kg cohort was 1031 mm3 (mean 1108.7, range 502–2838) and 318 mm3 (mean 346.4, range 46–749) in the 50 mg/kg cohort. Using tumor volume AUC as the measurement, androgen exposure led to a significant increase in tumor growth in both cohorts (p = 0.025 [30 mg] and p = 0.009 [50mg]). Concomitant treatment with enzalutamide led to significant reductions in tumor volume, compared to the androgen-exposed groups in both cohorts (p = 0.045 [30 mg/kg] and p = 0.004 [50 mg/kg]). Using tumor volume at day 56, concomitant treatment with enzalutamide significantly reduced the tumor volume, compared to the androgen-exposed group in the 50 mg/kg cohort (p = 0.011) (Figure 1).
Figure 1.
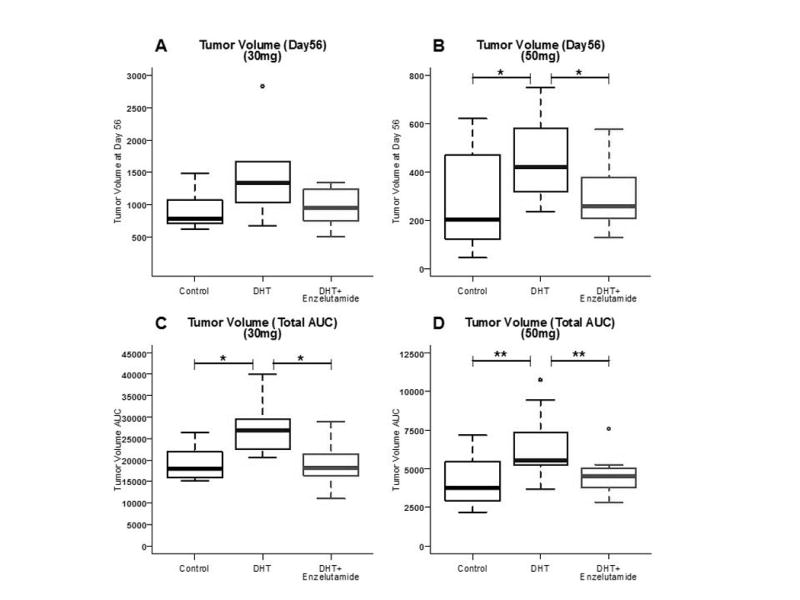
The effect of enzalutamide at 30 mg/kg (A and C) and 50 mg/kg (B and D) on median tumor volume at day 56 (A and B) and on total tumor volume AUC (C and D). Significant reductions were seen in tumor volume AUC both at 30mg/kg (0.045) and 50 mg/kg(0.004), and median tumor volume at 50mg/kg (p=0. 011)
Expression of ARs in the OVCA-3 cell line
Western blotting, performed to confirm the presence of ARs in the OVCAR-3 cell line, revealed the presence of the standard 110 kD AR isoform in the OVCAR-3 cell and the LnCaP positive control, but not in the OVCAR-429 negative control (Figure 2).
Figure 2.
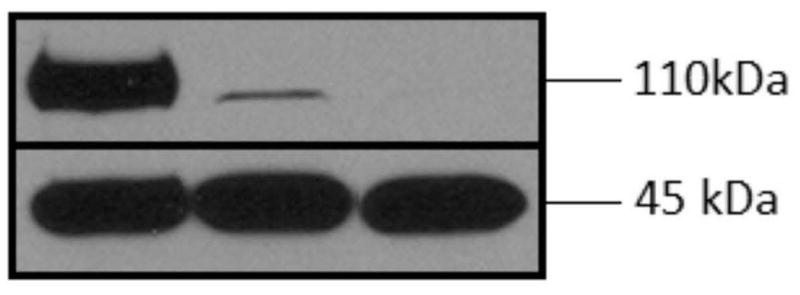
Top: Western blot with anti-AR antibody PG-21 at 110 kDa reveals expression of androgen receptor (AR) in OVCAR-3 but not the OVCA-429 cell line. From left to right: LnCaP (positive control), OVCAR-3 and VCA-429 (blank). Bottom: corresponding β-actins at 45 kDa.
Discussion
Here we show that enzalutamide blocks in vivo androgen-driven growth of OVCAR-3 tumors in a mouse xenograft model of ovarian cancer. The presence of ARs in the OVCA-3 cell line, expected based on previous descriptions of the cell line (4), was confirmed by immunoblot.
Ovarian cancer is an extremely serious condition; with a 5-year survival of less than 50%, it is the fifth leading cause of cancer-related deaths among women. Most patients initially diagnosed with advanced disease will recur, and require chronic treatment. These women are often treated with multiple lines of chemotherapy. The development of new therapies with tolerable toxicity is greatly needed (5, 6). Enzalutamide is a well-tolerated oral AR antagonist with minimal long-term toxicity (1).
There is mounting evidence supporting the use of AR antagonists like enzalutamide to target AR-positive cancers, such as ovarian cancers. Tumor biopsies from patients with epithelial ovarian cancer show the presence of ARs in up to 90% of cases (7). AR signaling may promote ovarian cancer progression, in part, by decreasing transforming growth factor β (TGFB) levels and allowing the cancer cells to escape TGFB1 growth inhibition (4). Based on the results presented here, we believe that clinical trials aimed at investigating the efficacy of enzalutamide in ovarian cancer are warranted. The use of enzalutamide as a single agent in AR-positive ovarian cancer is currently being investigated in an ongoing phase II study (NCT01974765).
In conclusion, the data presented here provides first evidence that the second-generation antiandrogen enzalutamide may possess efficacy in the treatment of ovarian cancer. These results also suggest that enzalutamide may be efficacious in a wide range of AR-expressing cancers, paving the way for future clinical trials.
Acknowledgments
The authors thank Esa R. Korpi for his advice and assistance with the manuscript.
Funding: This study was funded in part through the NIH/NCI Support Grant P30 CA008748.
Footnotes
Declaration of Interests: Carol Aghajanian: Consulting or Advisory Role: AstraZeneca; Travel, Accommodations, and Expenses: AstraZeneca, AbbVie. No other authors declare any conflicts of interest.
References
- 1.Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371:424–33. doi: 10.1056/NEJMoa1405095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cochrane DR, Bernales S, Jacobsen BM, et al. Role of the androgen receptor in breast cancer and preclinical analysis of enzalutamide. Breast Cancer Res. 2014;16:R7. doi: 10.1186/bcr3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caiazza F, Murray A, Madden SF, et al. Endocr Relat Cancer. 2016. Pre-clinical evaluation of the AR inhibitor enzalutamide in triple negative breast cancer cells. [DOI] [PubMed] [Google Scholar]
- 4.Evangelou A, Jindal SK, Brown TJ, Letarte M. Down-regulation of transforming growth factor beta receptors by androgen in ovarian cancer cells. Cancer Res. 2000;60:929–35. [PubMed] [Google Scholar]
- 5.Liu J, Matulonis UA. New strategies in ovarian cancer: translating the molecular complexity of ovarian cancer into treatment advances. Clin Cancer Res. 2014;20:5150–6. doi: 10.1158/1078-0432.CCR-14-1312. [DOI] [PubMed] [Google Scholar]
- 6.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2013 National Cancer Institute based on November 2015 SEER data submission. Bethesda, MD: 2016. [Google Scholar]
- 7.Kuhnel R, de Graaff J, Rao BR, Stolk JG. Androgen receptor predominance in human ovarian carcinoma. J Steroid Biochem. 1987;26:393–7. doi: 10.1016/0022-4731(87)90106-3. [DOI] [PubMed] [Google Scholar]


