Abstract
Obsessive–compulsive disorder (OCD) is considered to be associated with atypical brain asymmetry. However, no study has examined the asymmetry in OCD from the perspective of cortical morphometry. This study is aimed to describe the characteristics of cortical asymmetry in OCD patients, and to investigate whether these features exist in their unaffected siblings – a vital step in identifying putative endophenotypes for OCD. A total of 48 subjects (16 OCD patients, 16 unaffected siblings, and 16 matched controls) were recruited who had complete magnetic resonance imaging scans. Left–right hemispheric asymmetries of cortical thickness were measured using a surface-based threshold-free cluster enhancement method. OCD patients and siblings both showed leftward asymmetries of cortical thickness in the anterior cingulate cortex (ACC), which showed a significant positive correlation with compulsive subscale scores. In addition, siblings and healthy controls showed significantly decreased leftward asymmetries in the orbitofrontal cortex (OFC), and the decreased leftward bias in the OFC was accompanied by lower scales on the Yale–Brown Obsessive–Compulsive Scale. To sum up, leftward asymmetries of cortical thickness in the ACC may represent an endophenotype of increased hereditary risk for OCD, while decreased leftward asymmetries of cortical thickness in the OFC may represent a protective factor.
Keywords: Cortical thickness, Obsessive–compulsive disorder, Endophenotype, Hemispheric asymmetry, Siblings
1. Introduction
Obsessive–compulsive disorder (OCD) is characterized with persistent intrusive thoughts (obsessions), repetitive behaviors (compulsions) or both (Weissman et al., 1994). OCD is highly familial, with the first-degree relatives of OCD patients having an almost 3–12 times increased risk of developing the disorder compared with risk in the general population (Hanna et al., 2002; Nestadt et al., 2000; Pauls et al., 1995).
Hemispheric structural asymmetry in the human brain has been observed, and is attributed to evolutionary, developmental, hereditary, experiential and pathological factors (Toga and Thompson, 2003). Handedness and language dominance are the most prominent examples of extensive brain asymmetry. About 70–90 percent of the world's population is right-handed, and about 97 percent of right-handers show left-hemisphere speech and language localization (Toga and Thompson, 2003). Brain asymmetry is related to functional specialization and lateralization. Abnormal brain asymmetry could result in functional imbalance (Rilling and Insel, 1999). Disruption of brain asymmetry has implicated in the pathogenesis of several neuropsychiatric disorders, such as schizophrenia (Narr et al., 2001), autism (Herbert et al., 2005), and attention-deficit/hyperactivity disorder (ADHD) (Shaw et al., 2009). Similarly, empirical neurocognitive and neuroimaging studies have frequently found anomalous brain asymmetries in OCD patients. Regarding neurocognition, Maril et al. explored the spatial attention asymmetry of OCD patients and matched control groups with the Posner task (Maril et al., 2007). Their findings indicated that OCD patients had lost the normal spatial asymmetry, and had a faster response to left visual field targets than to right; furthermore, more anomalous asymmetry was correlated with more serious obsessional symptoms. In the area of neuroimaging, Shin and colleagues found that OCD patients' regional alterations of cortical thickness were only located in the left-hemisphere, including the inferior frontal, the middle frontal, the precentral, the superior temporal, the parahippocampal, the orbitofrontal, and the lingual cortices (Shin et al., 2007). Kim et al. reported that the regional alterations of gray matter volume in OCD patients were mainly located in the left hemisphere, including the orbitofrontal cortex, superior temporal gyrus, inferior parietal lobule, cuneus, thalamus and cerebellum, while only some areas of regional brain abnormality were located in the right hemisphere, such as the insula, inferior occipital cortex, and middle temporal gyrus (Kim et al., 2001). As to the white matter, most regional alterations in OCD patients were also located in the left hemisphere (Ha et al., 2009; Fan et al., 2012). Nevertheless, several studies reported that the alterations of structural asymmetry in OCD patients were mostly distributed to the right hemisphere (Menzies et al., 2008; Narayan et al., 2008). For instance, Menzies et al. found that OCD patients and their first-degree relatives both had alterations of white matter in the right inferior parietal and the medial frontal cortex, while no region of abnormality was found in the left hemisphere (Menzies et al., 2008). Although previous findings using whole-brain or region-of-interest analysis methods were inconsistent, they indicated that OCD patients exhibited atypical structural brain asymmetry. To date, however, no published work investigated the structural asymmetry in OCD patients by comparing their left and right hemispheres, which would elucidate the structural asymmetry straightforwardly.
Because OCD is highly hereditary, OCD patients' unaffected siblings might share a similar genetic profile that might be associated with vulnerability to OCD. Thus, the siblings might show some OCD traits even if they do not fully meet diagnostic criteria for OCD (Nestadt et al., 2000). Herein, studies on the unaffected siblings can offer rich genetic information, which may prove helpful in disentangling state-vs.-trait markers of this disorder.
According to the studies cited above, we hypothesized that OCD patients should have atypical cortical asymmetries and that the anatomical substrate of brain asymmetry would likely pertain to brain regions within the cortico-striato-thalamo-cortical circuitry, such as the OFC and the ACC, which have been widely posited to be involved in the pathophysiological mechanisms of OCD (Baxter et al., 1992). In addition, due to high vulnerability to OCD, we hypothesized that the siblings should share similar atypical cortical asymmetries as OCD patients. To test our hypotheses, cortical thickness of each hemisphere of every subject was extracted. Then, the cortical surfaces of each hemisphere were mapped to a hybrid atlas with its left and right hemispheres being in a precise geographic correspondence. Lastly, left–right hemispheric asymmetries of cortical thickness were measured by the surface-based threshold-free cluster enhancement (TFCE) method (Hill et al., 2010). We expected that OCD patients should show atypical cortical asymmetries and that their unaffected siblings should also show similar anomalous patterns.
2. Methods
2.1. Participants
Nineteen OCD patients and their unaffected siblings were recruited. Two siblings and one OCD patient were excluded due to the misalignment of left–right hemispheres. (Here, “misalignment of left–right hemispheres” meant that their cortical folding patterns were incorrectly aligned, e.g., the left pre-central gyrus was aligned onto the right post-central gyrus, which would lead to meaningless statistical analysis). In order to eliminate the effect of undesirable variances, the remaining unpaired two OCD patients and one sibling were also excluded in the final analysis. In this way, 16 OCD patients, 16 siblings, and 16 healthy controls were included in this study (see Table 1 for demographic information). All subjects were right-handed. The OCD patients and their unaffected siblings were recruited through Guangzhou Psychiatric Hospital, and the healthy controls were recruited from local communities and internet advertisements.
Table 1.
Treatment details of OCD patients.
| Treatment | Number of cases | Average dosage (mg) |
|---|---|---|
| Citalopram | 2 | 40 |
| Clomipramine | 2 | 100 |
| Fluoxetine | 1 | 46.7 |
| Fluvoxamine | 2 | 250 |
| Mirtazapine | 1 | 30 |
| Paroxetine | 3 | 60 |
| Sertraline | 2 | 162.5 |
| Paroxetine+sodium valproate | 1 | 40+666.7 |
| Paroxetine+quetiapine | 2 | 40+200 |
OCD patients were evaluated by the Structured Clinical Interview (SCID) for DSM-IV Axis I disorders (First et al., 1996). All patients had a primary diagnosis of OCD, excluding hoarding subtypes. Five of them were comorbid with major depressive disorder (three recurrent and two with a single episode, without psychotic features), and their depressive symptoms were in partial or full remission during clinical evaluation. Three of them had social phobia, one of them had an eating disorder, and all others had OCD as their sole diagnosis. All patients received stable drug treatment, consisting of selective serotonin reuptake inhibitors for the majority of subjects (see Table 1).
The unaffected siblings and healthy controls were screened by the SCID for the DSM-IV-TR Axis I disorders, Research Version, and Non-Patient edition (SCID- I/NP) (First et al., 2002). Healthy controls with a family history of Axis I or II psychiatric disorders were excluded. This research was approved by the ethics committee of Guangzhou Psychiatric Hospital, following the principles set forth by the Declaration of Helsinki. All subjects provided the written consent.
2.2. Clinical assessments
Obsessive–compulsive (OC) symptom severity was assessed with Yale–Brown Obsessive–Compulsive Scale (Y–BOCS) (Goodman et al., 1989), and the Obsessive–Compulsive Inventory-Revised (OCI-R) was used to evaluate obsessive and compulsive (OC) symptom substyles (Foa et al., 2002; Peng et al., 2011). Symptoms of depression and anxiety were quantified using the Beck Depression Inventory (BDI) (Beck and Steer, 1984) and the State-Trait Anxiety Inventory (STAI) (Spielberger, 1983), respectively. The Annett Handedness Inventory was used to assess handedness (Spreen and Strauss, 1991). Estimated IQ was assessed with the short form of the Chinese version of the Wechsler Adult Intelligence Scale-Revised (WAIS-R), including four subscales, i.e., information, arithmetic, similarity and digit span (Gong, 1992). Any subject with a total score of less than 80 was excluded.
2.3. Magnetic resonance imaging (MRI) procedures
MRI images were obtained on a Signa HDe 1.5-T GE scanner (GE Medical Systems, Milwaukee, WI, USA), which was equipped with an eight-channel phased-array head coil, at the First Affiliated Hospital of Jinan University. A high-resolution T1-weighted anatomical image was acquired by a three-dimensional fast spoiled gradient recalled (FSPGR) sequence with 128 contiguous slices (repetition time=8 ms; echo time=1.7 ms; flip angle=20° field of view=240 × 240 mm2; matrix=256 × 256; slice thickness=1.0 mm). Magnetic field uniformity was investigated before every scan. No gross abnormalities were observed for the included subjects, when the images were visually inspected by an experienced radiologist (C.Z.S.) before the experiment.
2.4. Image analysis
Freesurfer (v5.3.0) was used to reconstruct the cortical surfaces and measure cortical thickness from MR images (http://surfer.nmr.mgh.harvard.edu/). The details were described in our previous study (Li et al., 2014). In brief, the procedure included the following steps: (1) resample all images into isotropic voxel of 1 × 1 × 1 mm3; (2) conduct both intensity inhomogeneity correction and skull stripping; (3) segment white matter with a hybrid watershed classifier; (4) reconstruct the white matter surface of each hemisphere and search for the corresponding pial surface with a deformable surface algorithm; (5) measure local cortical thickness as the distance between the inner and the outer cortical surfaces at each vertex (Fischl et al., 1999); (6) map the cortical surface of each hemisphere onto a standard sphere, and register each spherical surface into a hemisphere-specific atlas space; and (7) map left and right spherical surfaces in the hemisphere-specific atlas space into a hybrid atlas with the left and right hemispheres being in precise geographic correspondence (Van Essen et al., 2012).
2.5. Statistical analyses
To identify statistical significant clusters of left–right hemispheric asymmetries of the cortical thickness and local surface area, we utilized a surface-based threshold-free cluster enhancement (TFCE) method with 2500 times permutations (p<0.01) (Hill et al., 2010).
We analyzed demographic and clinical data using SPSS 14.0 for Windows, and set statistical significance at p<0.05. Clinical assessments were analyzed with one-way analysis of variance (ANOVA), and post-hoc analysis showed the results of between-group comparisons. The Pearson correlation coefficient was used to calculate the correlation between the clinical assessments and difference values of (left–right) hemisphere.
3. Results
3.1. Demographic and clinical characteristics
The three groups are well matched in age, gender, IQ, education and handedness. There are significant differences in clinical measures (Y-BOCS, OCI-R, and BDI) among them. Post-hoc tests found that OCD patients had higher scores in total for the Y–BOCS, OCI-R and BDI than those of the healthy controls or the siblings. Siblings significantly differ from healthy controls in both Y–BOCS total scores (p<0.01) and compulsive subscale scores (p<0.01). Table 2 shows the demographic and clinical characteristics of the participants.
Table 2.
Demographic and clinical characteristics of study subjects.
| Measure | OCD (N=16) | SIB (N=16) | CON (N=16) | f-Value | p-Value |
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | |||
| Age (years) | 25.8 (6.0) | 27.1 (7.2) | 23.3 (5.8) | 1.85 | 0.17 |
| Male/female | 11/5 | 9/7 | 12/4 | 0.52 | |
| Education (years) | 13.0 (2.5) | 14.8 (2.5) | 12.8 (4.0) | 2.07 | 0.14 |
| IQ estimate | 102.4 (12.4) | 108.6 (13.5) | 110.3 (9.8) | 1.92 | 0.16 |
| Age at onset of OCD (years) | 18.5 (4.1) | ||||
| Duration of illness (years) | 7.4 (6.4) | ||||
| Y–BOCS (total) | 29.8 (6.4) | 9.1 (3.6) | 2.7 (2.1) | 164.06 | <0.01a,b,c |
| Y–BOCS (obsessions) | 15.9 (7.3) | 4.4 (2.1) | 1.7 (1.7) | 44.81 | <0.01a,c |
| Y–BOCS (compulsions) | 14.0 (3.5) | 4.6 (2.0) | 1.0 (1.1) | 123.80 | <0.01a,b,c |
| OCI-R | 20.1 (6.8) | 8.8 (3.7) | 12.3 (6.5) | 15.67 | <0.01a,c |
| BDI | 11.6 (12.9) | 5.9 (4.2) | 4.2 (3.6) | 3.63 | 0.04a |
| STAI (state) | 39.8 (7.3) | 37.8 (15.2) | 42.3 (4.7) | 0.82 | 0.45 |
| STAI (trait) | 40.4 (6.2) | 37.5 (15.6) | 39.9 (5.2) | 0.38 | 0.69 |
Abbreviations: BDI, Beck Depression Inventory; CON, healthy control subjects; OCD, patients with obsessive–compulsive disorder; OCI-R, Obsessive–Compulsive Inventory-Revised; SD, standard deviation; SIB, siblings of individuals with OCD; STAI, State-Trait Anxiety Inventory; Y–BOCS, Yale–Brown Obsessive–Compulsive Scale. a–c: Statistically significant values after Tukey post-hoc test between
OCD × CON.
SIB × CON.
OCD × SIB.
3.2. Cortical thickness and surface area asymmetries
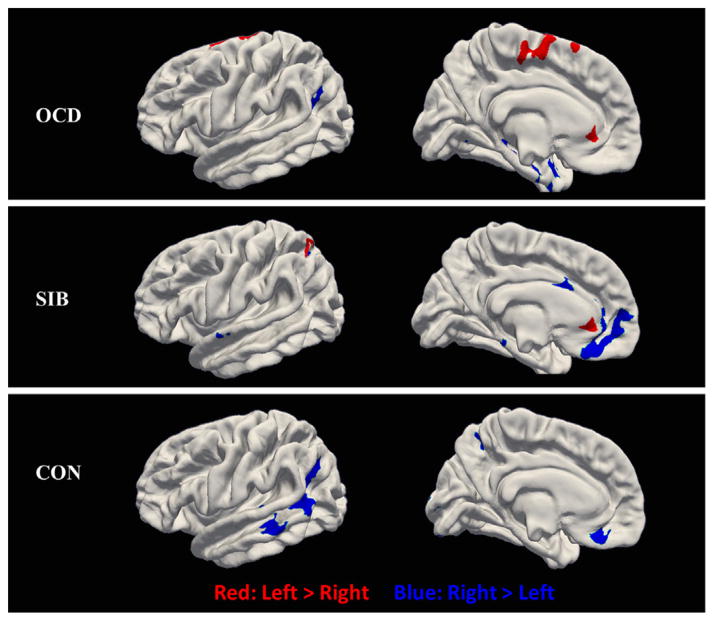
OCD patients demonstrate leftward asymmetries in the ACC, the superior frontal lobe, and the paracentral lobule. Decreased leftward asymmetries are found in the supramarginal gyrus and some clusters in the temporal lobe. Siblings show decreased leftward asymmetries in the OFC, the superior frontal lobe, and a small cluster in the cingulate cortex. Leftward asymmetries are found in the ACC and the superior parietal lobe. The healthy controls exhibit greater cortical thickness in the right hemisphere than in the left, mainly including the middle temporal gyrus (MT), OFC, and supramarginal gyrus. Different from the right hemisphere, no increased regional cortical thickness was found in the left. Fig. 1 shows the distinctive cortical asymmetries in every group. Intriguingly, OCD patients and their siblings both have leftward asymmetries in the ACC. The values of cortical thickness asymmetry in each group are shown in Fig. 1S. The regional surface area asymmetries of the rightward lateralization in the frontal lobe and also the leftward lateralization in the planum temporale existed in each group, with the detailed patterns shown in Fig. 2S.
Fig. 1.
Cortical thickness asymmetries in OCD patients, siblings, and healthy controls (p<0.01). The red indicates that the cortical thickness is thicker in left hemisphere than that in the right hemisphere (left<right), while the blue indicates that the cortical thickness is thinner in the left hemisphere than that in the right hemisphere (left<right). SIB=Siblings; CON=healthy controls. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.3. Correlations between cortical thickness asymmetries and clinical evaluations
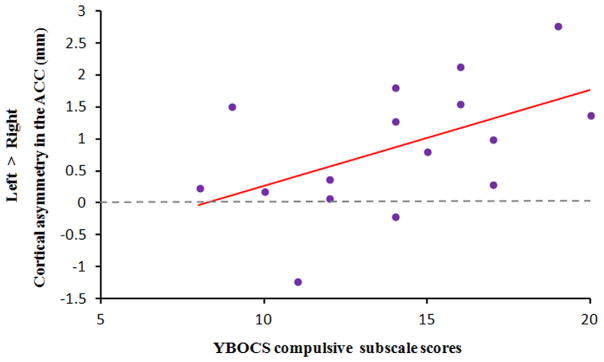
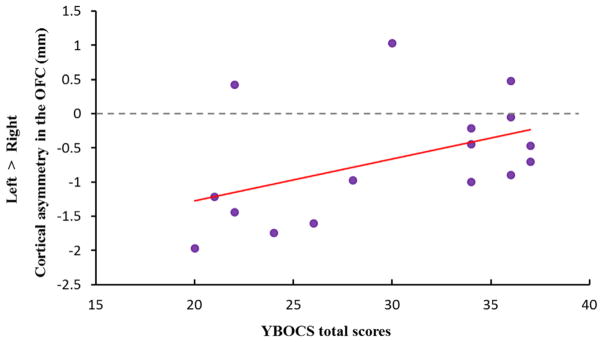
We used the overlapping region of cortical thickness asymmetries in the ACC and OFC to calculate the correlation figures between cortical-thickness difference (left hemisphere – right hemisphere) and clinical scores in the group of OCD patients. A significant positive correlation existed between leftward asymmetry in the ACC and the compulsive subscale scores in the YBOCS (r=0.52, p=0.039). The correlation plot shown in Fig. 2 suggests that more leftward asymmetry in the ACC is accompanied by more serious compulsive symptoms. The decreased leftward asymmetry in the OFC has a trend of positive correlation with the YBOCS scores (r=0.46, p=0.073), indicating that less leftward asymmetry in the OFC is correlated with more OC symptoms (see Fig. 3).
Fig. 2.
The scatter plot for the correlation between leftward asymmetry in ACC and the YBOCS compulsive scores in OCD patients (r=0.52, p=0.039).
Fig. 3.
The scatter plot for the correlation between decreased leftward asymmetry in OFC and the YBOCS (total) scores in OCD patients (r=0.46, p=0.073).
4. Discussion
The current study is the first to investigate cortical asymmetries of cortical thickness and surface area in OCD patients and their unaffected siblings. We have two notable findings. First, OCD patients and their unaffected siblings both showed leftward asymmetry in the ACC, which is considered as a potential hereditary risk indicator of OCD. Second, the siblings and the healthy controls showed decreased leftward asymmetry in the OFC, and decreased leftward asymmetry in the OFC was accompanied by lower Y–BOCS scores. Our findings provide new insights into the understanding of asymmetrical structural substrates in OCD.
4.1. Cortical thickness asymmetries in the ACC
Our results identified that both OCD patients and their siblings have leftward asymmetry of cortical thickness in the ACC. Previous imaging studies found that OCD patients had a morphological leftward bias in the ACC. For example, Shim and colleagues found that cortical folding in the ACC had leftward asymmetry in OCD patients, and their findings suggest that morphologic anomalies in the ACC reflected disrupted neuronal connectivities in the early life of OCD patients (Shim et al., 2009). The evidence from comparative studies has indicated that larger brain volume is associated with increased specialization and lateralization of function (Rilling and Insel, 1999). In addition, larger volumes of the right ACC were found to be associated with better performance on an attentional task (Casey et al., 1997). So, the lower cortical thickness in the right ACC of OCD patients and siblings may be related to attentional bias, suggesting that OCD patients and their siblings are apt to fixate on threatening stimuli. Then, repetitive behaviors accrue as a mechanism to relieve the anxiety resulting from the threatening information. The ACC has been implicated consistently in the pathophysiology of OCD. This region is linked to cognitive interference characterized by competing information-processing demands, characteristics that have been frequently reported in OCD (Penades et al., 2007). Furthermore, a neurocognitive study found that regional N-acetyl aspartate (NAA) in the right ACC, but not the left ACC, which is a neuronal marker relating to specific cognitive activity, was highly correlated with the cognitive interference. This fact implies that only the right ACC was involved in the cognitive interference (Jang et al., 2006). Thus, based on the existing literature, we could speculate confidently that the leftward bias of cortical thickness in the ACC is related to the anomalous cognitive interference in OCD patients. On the other hand, some researchers have argued that the ACC may underlie the development of self-regulatory capacities, and the abnormal development of the ACC may lead to deficits in the self-regulation of intrusive thoughts and images or the well-documented deficits in inhibitory control in OCD (Posner et al., 2007). However, more studies are needed to further investigate whether the leftward asymmetry in the ACC is associated with impaired cognitive interference in OCD patients. Intriguingly, their siblings also have leftward bias in the ACC. Thus, the anomalous leftward bias in the ACC appears to reflect a trait marker for OCD that can exist in the absence of clinically significant symptoms. This trait is in accordance with the endophenotype principle that a heritable quantitative trait is associated with the increased genetic risk for a disorder and is presented both in patients and their unaffected relatives (Gottesman and Gould, 2003). In addition, the leftward asymmetry in the ACC is positively correlated with YBOCS compulsive subscale scores, which means that more serious leftward asymmetry in the ACC is associated with more serious compulsive manifestations. Our findings suggest that the leftward asymmetry in the ACC may constitute an endophenotype for OCD.
4.2. Cortical thickness asymmetries in the OFC
The siblings group and the healthy controls group both have decreased leftward asymmetries in the OFC. Previous studies have demonstrated that the OFC is the most replicated brain region associating with OCD (Rotge et al., 2009). Recently, a meta-analysis of ROI volume studies on OCD reported that OCD patients had a smaller volume in the OFC than the matched control group (Rotge et al., 2009). Functionally, the OFC hyperactivations may be involved in obsessions and compulsive actions. For instance, Stern et al. found that OCD patients had shown greater activation in the OFC as identified with an event-related functional MRI paradigm to investigate neural function of uncertainty (Stern et al., 2013). Moreover, their findings demonstrated that the activations in the OFC were negatively associated with OC symptom severity, although the mechanism underlying the hyperactivation in the OFC of OCD patients is still unclear. Some researchers speculated that the overactivations in the OFC may reflect patients' effort to inhibit their symptoms during OC symptom provocation because greater activations of the OFC associate with a smaller increase in reported symptoms during the provocation (Adler et al., 2000). Most importantly, the OFC and ACC are intimately inter-connected via cortico-striato-thalamo-cortical circuitry (Alexander et al., 1986). These two regions have been widely implicated in OCD from the findings of symptom-provocation studies (Saxena and Rauch, 2000; Saxena et al., 2001). OCD patients were found to be hyper-active in the OFC and ACC under symptom provocation, and to show less activity after treatment, which was interpreted as evidence that overactivation in these two regions generates the symptoms of OCD (Saxena and Rauch, 2000; Saxena et al., 2001). Of note, the decreased leftward asymmetries in the OFC are only found in the siblings group and the healthy controls group, not in the OCD patients group. As mentioned above, the leftward asymmetry in the ACC is served as a susceptibility factor for OCD. Based on that fact that the interactions between the OFC and the ACC jointly exert a powerful influence on OC symptom provocation, one possible interpretation of these findings is that the decreased leftward asymmetries in the OFC may act as a protective factor for OCD. This speculation is consistent with the phenomenon that the siblings have the high risk factor (the leftward asymmetry in the ACC), but they do not fully meet the diagnostic criteria for OCD, which suggests that the protective factor (the decreased leftward asymmetry in the OFC) might prevent the provocation of OCD symptoms in siblings by interacting with the ACC. Further studies on the interaction between the leftward asymmetry in the ACC and the decreased leftward asymmetry in the OFC are needed to assess the validity of this speculation. Our findings further provide substantial evidence that both the OFC and the ACC play a predominant role in the pathology of OCD, and we strongly suggest that the regions in the left and right hemispheres may have different effects on the mechanism of OC symptom provocation.
4.3. Cortical thickness asymmetries in uncommon regions
Healthy controls show thicker cortical thickness in the middle temporal gyrus of the right hemisphere than the left hemisphere, which is consistent with a recent study suggesting that a clearly decreased leftward asymmetry with respect to cortical thickness is salient with both the primary and the secondary auditory cortex (Meyer et al., 2014). Furthermore, healthy controls and OCD patients both exhibit decreased leftward asymmetry in the inferior parietal gyrus (angular part), which is in accordance with the findings that healthy adults show decreased leftward asymmetry of cortical volume in the angular gyrus (Goldberg et al., 2013). Decreased leftward asymmetry in the angular gyrus was not observed in siblings, which may result from the fact that there were more females in the siblings group; females are more proficient at verbal tasks while males are more proficient at visuospatial tasks (Plessen et al., 2014).
Several limitations exist in our study. First, we have a relatively small sample, and thus it is impractical to perform subgroup analysis by dividing the OCD patient samples into different subtypes. We suggest different OCD subtypes to be underpinned by different neural mechanisms, so the study on different OCD subgroups in a large sample would be interesting. Second, our study is restricted to cortical regions, not including subcortical regions. Previous studies found that some subcortical regions, such as the caudate nucleus, had a vital role in OCD. Future studies are needed to measure structural symmetry both in cortical and subcortical regions in an attempt to explore the gross morphological characteristics in OCD. Third, OCD patients in this study were receiving medications when scanned, so the effects of medication cannot be completely ruled out in our results. The issue of medication use needs to be addressed in future work by using the samples of drug-naïve patients with OCD. Fourth, most OCD participants in this study had significantly above-normal anxiety, and the potential effect of anxiety on asymmetry of cortical thickness cannot yet be determined. Future research should directly investigate the asymmetry of cortical thickness in OCD patients without obvious anxiety.
4.4. Conclusion
This study investigated both the cortical thickness and the surface area asymmetry in OCD patients, their unaffected siblings, and healthy controls by comparing their left–right hemisphere differences. Our results show that OCD patients and their siblings both had leftward asymmetries of cortical thickness in the ACC, while their siblings and the healthy controls had decreased leftward asymmetries of cortical thickness in the OFC. According to the definition of an endophenotype, the leftward asymmetry in the ACC may serve as an endophenotype representing the increased genetic risk of OCD while the decreased leftward asymmetry in the OFC may constitute a protective factor. Our findings provide novel evidence that both the OFC and the ACC play vital roles in the pathophysiology of OCD and, most importantly, the left–right differences of these two regions may have a distinctive impact on the pathogenesis of OCD.
Supplementary Material
Acknowledgments
This study was partly supported by National Institutes of Health (EB006733, EB008374, EB009634, AG041721), the National Science Fund China Young Investigator Award (81088001), the National Key Technologies R&D Program (2012BAI36B01), National Natural Science Foundation of China (81201049), The Knowledge Innovation Project of the Chinese Academy of Sciences (KSCX2-EW-J-8), and also a Grant from the initiation fund of the CAS/SAFEA International Partnership Programme for Creative Research Teams to Raymond Chan.
Appendix A. Supplementary material
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.pscychresns.2015.10.005.
Footnotes
Competing interest
The authors declare no conflict of interest.
References
- Adler CM, McDonough-Ryan P, Sax KW, Holland SK, Arndt S, Strakowski SM. fMRI of neuronal activation with symptom provocation in unmedicated patients with obsessive compulsive disorder. J Psychiatr Res. 2000;34:317–324. doi: 10.1016/s0022-3956(00)00022-4. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Ann Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Baxter LR, Jr, Schwartz JM, Bergman KS, Szuba MP, Guze BH, Mazziotta JC, Alazraki A, Selin CE, Ferng HK, Munford P, et al. Caudate glucose metabolic rate changes with both drug and behavior therapy for obsessive–compulsive disorder. Arch Gen Psychiatry. 1992;49:681–689. doi: 10.1001/archpsyc.1992.01820090009002. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA. Internal consistencies of the original and revised Beck Depression Inventory. J Clin Psychol. 1984;40:1365–1367. doi: 10.1002/1097-4679(198411)40:6<1365::aid-jclp2270400615>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Trainor R, Giedd J, Vauss Y, Vaituzis CK, Hamburger S, Kozuch P, Rapoport JL. The role of the anterior cingulate in automatic and controlled processes: a developmental neuroanatomical study. Dev Psychobiol. 1997;30:61–69. [PubMed] [Google Scholar]
- Fan Q, Yan X, Wang J, Chen Y, Wang X, Li C, Tan L, You C, Zhang T, Zuo S, Xu D, Chen K, Finlayson-Burden JM, Xiao Z. Abnormalities of white matter microstructure in unmedicated obsessive–compulsive disorder and changes after medication. PLoS One. 2012;7:e35889. doi: 10.1371/journal.pone.0035889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV-TR Axis I disorders, Research Version, Non-patient Edition (SCID-I/NP) New York State Psychiatric Institute, Biometrics Research Department; New York: 2002. [Google Scholar]
- First MB, Spitzer RL, Williams JBW, Gibbon M. Structured Clinical Interview for DSM-IV (SCID) APA Press; Washington, DC: 1996. [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical Surface-Based Analysis. II: Inflation, Flattening, and a Surface-Based Coordinate System. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Foa EB, Huppert JD, Leiberg S, Langner R, Kichic R, Hajcak G, Salkovskis PM. The obsessive–compulsive inventory: development and validation of a short version. Psychol Assess. 2002;14:485–496. [PubMed] [Google Scholar]
- Goldberg E, Roediger D, Kucukboyaci NE, Carlson C, Devinsky O, Kuzniecky R, Halgren E, Thesen T. Hemispheric asymmetries of cortical volume in the human brain. Cortex. 2013;49:200–210. doi: 10.1016/j.cortex.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Gong YX. Manual of Wechsler Adult Intelligence Scale: Chinese Version. Chinese Map Press; Changsha: 1992. [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, Heninger GR, Charney DS. The Yale–Brown Obsessive Compulsive Scale. I Development, use, and reliability. Arch Gen Psychiatry. 1989;46:1006–1011. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Ha TH, Kang DH, Park JS, Jang JH, Jung WH, Choi JS, Park JY, Jung MH, Choi CH, Lee JM, Ha K, Kwon JS. White matter alterations in male patients with obsessive–compulsive disorder. Neuroreport. 2009;20:735–739. doi: 10.1097/WNR.0b013e32832ad3da. [DOI] [PubMed] [Google Scholar]
- Hanna GL, Veenstra-VanderWeele J, Cox NJ, Boehnke M, Himle JA, Curtis GC, Leventhal BL, Cook EH., Jr Genome-wide linkage analysis of families with obsessive–compulsive disorder ascertained through pediatric probands. Am J Med Genet. 2002;114:541–552. doi: 10.1002/ajmg.10519. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Ziegler DA, Deutsch CK, O’Brien LM, Kennedy DN, Filipek PA, Bakardjiev AI, Hodgson J, Takeoka M, Makris N, Caviness VS., Jr Brain asymmetries in autism and developmental language disorder: a nested whole-brain analysis. Brain. 2005;128:213–226. doi: 10.1093/brain/awh330. [DOI] [PubMed] [Google Scholar]
- Hill J, Dierker D, Neil J, Inder T, Knutsen A, Harwell J, Coalson T, Van Essen D. A surface-based analysis of hemispheric asymmetries and folding of cerebral cortex in term-born human infants. J Neurosci. 2010;30:2268–2276. doi: 10.1523/JNEUROSCI.4682-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang JH, Kwon JS, Jang DP, Moon WJ, Lee JM, Ha TH, Chung EC, Kim IY, Kim SI. A proton MRSI study of brain N-acetylaspartate level after 12 weeks of citalopram treatment in drug-naive patients with obsessive–compulsive disorder. Am J Psychiatry. 2006;163:1202–1207. doi: 10.1176/ajp.2006.163.7.1202. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Lee MC, Kim J, Kim IY, Kim SI, Han MH, Chang KH, Kwon JS. Grey matter abnormalities in obsessive–compulsive disorder: statistical parametric mapping of segmented magnetic resonance images. Br J Psychiatry. 2001;179:330–334. doi: 10.1192/bjp.179.4.330. [DOI] [PubMed] [Google Scholar]
- Li G, Nie J, Wang L, Shi F, Lyall AE, Lin W, Gilmore JH, Shen D. Mapping longitudinal hemispheric structural asymmetries of the human cerebral cortex from birth to 2 years of age. Cereb Cortex. 2014;24(5):1289–1300. doi: 10.1093/cercor/bhs413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maril S, Hermesh H, Gross-Isseroff R, Tomer R. Spatial attention and neural asymmetry in obsessive–compulsive disorder. Psychiatry Res: Neuroimaging. 2007;153:189–193. doi: 10.1016/j.psychres.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Menzies L, Williams GB, Chamberlain SR, Ooi C, Fineberg N, Suckling J, Sahakian BJ, Robbins TW, Bullmore ET. White matter abnormalities in patients with obsessive–compulsive disorder and their first-degree relatives. Am J Psychiatry. 2008;165:1308–1315. doi: 10.1176/appi.ajp.2008.07101677. [DOI] [PubMed] [Google Scholar]
- Meyer M, Liem F, Hirsiger S, Jancke L, Hanggi J. Cortical surface area and cortical thickness demonstrate differential structural asymmetry in auditory-related areas of the human cortex. Cereb Cortex. 2014;24:2541–2552. doi: 10.1093/cercor/bht094. [DOI] [PubMed] [Google Scholar]
- Narayan VM, Narr KL, Phillips OR, Thompson PM, Toga AW, Szeszko PR. Greater regional cortical gray matter thickness in obsessive–compulsive disorder. Neuroreport. 2008;19:1551–1555. doi: 10.1097/WNR.0b013e3283112720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narr K, Thompson P, Sharma T, Moussai J, Zoumalan C, Rayman J, Toga A. Three-dimensional mapping of gyral shape and cortical surface asymmetries in schizophrenia: gender effects. Am J Psychiatry. 2001;158:244–255. doi: 10.1176/appi.ajp.158.2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestadt G, Samuels J, Riddle M, Bienvenu OJ, 3rd, Liang KY, LaBuda M, Walkup J, Grados M, Hoehn-Saric R. A family study of obsessive–compulsive disorder. Arch Gen Psychiatry. 2000;57:358–363. doi: 10.1001/archpsyc.57.4.358. [DOI] [PubMed] [Google Scholar]
- Pauls DL, Alsobrook JP, 2nd, Goodman W, Rasmussen S, Leckman JF. A family study of obsessive–compulsive disorder. Am J Psychiatry. 1995;152:76–84. doi: 10.1176/ajp.152.1.76. [DOI] [PubMed] [Google Scholar]
- Penades R, Catalan R, Rubia K, Andres S, Salamero M, Gasto C. Impaired response inhibition in obsessive compulsive disorder. Eur Psychiatry. 2007;22:404–410. doi: 10.1016/j.eurpsy.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Peng ZW, Yang WH, Miao GD, Jing J, Chan RC. The Chinese version of the Obsessive–Compulsive Inventory-Revised scale: replication and extension to non-clinical and clinical individuals with OCD symptoms. BMC Psychiatry. 2011;11:129. doi: 10.1186/1471-244X-11-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plessen KJ, Hugdahl K, Bansal R, Hao X, Peterson BS. Sex, age, and cognitive correlates of asymmetries in thickness of the cortical mantle across the life span. J Neurosci. 2014;34:6294–6302. doi: 10.1523/JNEUROSCI.3692-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK, Sheese BE, Tang Y. The anterior cingulate gyrus and the mechanism of self-regulation. Cogn Affect Behav Neurosci. 2007;7:391–395. doi: 10.3758/cabn.7.4.391. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Insel TR. The primate neocortex in comparative perspective using magnetic resonance imaging. J Hum Evol. 1999;37:191–223. doi: 10.1006/jhev.1999.0313. [DOI] [PubMed] [Google Scholar]
- Rotge JY, Guehl D, Dilharreguy B, Tignol J, Bioulac B, Allard M, Burbaud P, Aouizerate B. Meta-analysis of brain volume changes in obsessive–compulsive disorder. Biol Psychiatry. 2009;65:75–83. doi: 10.1016/j.biopsych.2008.06.019. [DOI] [PubMed] [Google Scholar]
- Saxena S, Bota RG, Brody AL. Brain-behavior relationships in obsessive–compulsive disorder. Semin Clin Neuropsychiatry. 2001;6:82–101. doi: 10.1053/scnp.2001.21833. [DOI] [PubMed] [Google Scholar]
- Saxena S, Rauch SL. Functional neuroimaging and the neuroanatomy of obsessive–compulsive disorder. Psychiatr Clin North Am. 2000;23:563–586. doi: 10.1016/s0193-953x(05)70181-7. [DOI] [PubMed] [Google Scholar]
- Shaw P, Lalonde F, Lepage C, Rabin C, Eckstrand K, Sharp W, Greenstein D, Evans A, Giedd JN, Rapoport J. Development of cortical asymmetry in typically developing children and its disruption in attention-deficit/hyper-activity disorder. Arch Gen Psychiatry. 2009;66:888–896. doi: 10.1001/archgenpsychiatry.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim G, Jung WH, Choi JS, Jung MH, Jang JH, Park JY, Choi CH, Kang DH, Kwon JS. Reduced cortical folding of the anterior cingulate cortex in obsessive–compulsive disorder. J Psychiatr Res. 2009;34:443–449. [PMC free article] [PubMed] [Google Scholar]
- Shin YW, Yoo SY, Lee JK, Ha TH, Lee KJ, Lee JM, Kim IY, Kim SI, Kwon JS. Cortical thinning in obsessive compulsive disorder. Hum Brain Mapp. 2007;28:1128–1135. doi: 10.1002/hbm.20338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger DC. Manual for the State-Trait Anxiety Inventory (STAI) Consulting Psychologists Press; Palo Alto, CA: 1983. [Google Scholar]
- Spreen O, Strauss E. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. Oxford University Press; New York: 1991. [Google Scholar]
- Stern ER, Welsh RC, Gonzalez R, Fitzgerald KD, Abelson JL, Taylor SF. Subjective uncertainty and limbic hyperactivation in obsessive–compulsive disorder. Hum Brain Mapp. 2013;34(8):1956–1970. doi: 10.1002/hbm.22038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toga AW, Thompson PM. Mapping brain asymmetry. Nat Rev Neurosci. 2003;4:37–48. doi: 10.1038/nrn1009. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Glasser MF, Dierker DL, Harwell J, Coalson T. Parcellations and hemispheric asymmetries of human cerebral cortex analyzed on surface-based atlases. Cereb Cortex. 2012;22:2241–2262. doi: 10.1093/cercor/bhr291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman MM, Bland RC, Canino GJ, Greenwald S, Hwu HG, Lee CK, Newman SC, Oakley-Browne MA, Rubio-Stipec M, Wickramaratne PJ, et al. The cross national epidemiology of obsessive compulsive disorder. The Cross National Collaborative Group. J Clin Psychiatry. 1994;55:S5–S10. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.