Abstract
Objective
To provide a scientific basis for the prevention and treatment of cervical intraepithelial neoplasia grade 1 (CIN1). This study evaluated the impact of human papillomavirus (HPV) infection on the natural history of CIN1.
Methods
Electronic databases of Cochrane Library, EMBASE, PubMed, CNKI, CBM, and Wanfang were searched in April 2016. The eligibility criteria were documented by Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). We used the Newcastle-Ottawa scale (NOS) to assess study quality.
Results
Thirty-eight studies out of 3,246 identified papers were eligible for inclusion. The risk of CIN1 progression (relative risk [RR]: 3.04; 95% confidence interval [CI]: 2.41–3.83; P < 0.00001) and persistence (RR: 1.48; 95% CI: 1.17–1.87; P = 0.001) was higher in the HPV-positive group than HPV-negative group. Specifically, the risk of CIN1 progression (RR: 13.91; 95% CI: 3.46–55.90; P = 0.000) was higher among persistent high-risk HPV-positive patients and the ratio of CIN1 regression (RR: 0.65; 95% CI: 0.59–0.71; P < 0.00001) was lower in the HPV-positive group than HPV-negative group.
Conclusion
HPV infection resulted in an increased risk of CIN1 progression and decreased disease reversibility. Persistent high-risk HPV infection resulted in a further increased risk of CIN1 progression.
1. Introduction
Cervical intraepithelial neoplasia grade 1 (CIN1) is a precancerous lesion closely related to cervical cancer and characterized by a shorter and less observable clinical course. There is no consensus for intervention and treatment of CIN1, and there are currently no clear markers to predict disease progression and regression [1]. Human papillomavirus (HPV) is a major causative pathogen of reproductive tract infections and can induce the immortalization of normal cells, which precedes their malignant transformation. Approximately 90% of CIN cases and over 99% of cervical cancer cases occur in HPV-positive patients [2]; therefore, HPV testing has become a major component of cervical disease screening, diagnosis, and follow-up. The association between CIN1 and HPV remains controversial. The results of several studies suggest that CIN1 is mainly caused by low-risk HPV infection [3–5]. However, there is also evidence that high-risk HPV is strongly associated with CIN1 [6, 7]. Differences in the risk of HPV infection and CIN1 disease outcome [8, 9] may be due to regional differences in populations. Furthermore, there are scarce independent systematic reviews on the effects of HPV infection and CIN1. This study evaluated the impact of HPV infection on the natural history of CIN1 by conducting a literature review in order to provide a scientific basis for the prevention and treatment of CIN1.
2. Methods
2.1. Electronic Literature Databases
A systematic search was conducted using the Cochrane Library, Excerpta Medica database (EMBASE), PubMed, China National Knowledge Infrastructure (CNKI), Chinese Biomedical Literature Database (CBM), and the Wan fang Data. The literature search was performed on April 20, 2016. The PICOS items were identified (see Appendix 1 in Supplementary Material available online at https://doi.org/10.1155/2017/8971059) in this study as follows: P, cervical intraepithelial neoplasia grade 1 (CIN1); I, HPV positivity; C, HPV negative; O, the relative risk (RR) of progression, persistence, and regression of CIN1 in HPV-positive and HPV-negative patients being compared; S, retrospective studies and prospective studies. The search strategies were determined (the specific search strategy is described in Appendix 2) before the study. The MESH search terms for PubMed included the following: (“Squamous Intraepithelial Lesions of the Cervix”[MeSH] OR low-grade squamous intraepithelial lesion OR mild cervical dysplasia OR CIN1 OR mild Cervical Intraepithelial Neoplasia) AND (“Human Papillomavirus DNA Tests”[MeSH] OR human papillomavirus detected OR human papillomavirus test OR human papillomavirus infection) AND (Cohort Study OR follow up).
2.2. Inclusion and Exclusion Criteria
We systematically reviewed published studies according to the following inclusion criteria: studies examining the impact of HPV infection on the natural history of CIN1 disease; studies including at least HPV-negative and HPV-positive; results at the start and end of follow-up including cervical histology or cytology, a diagnosis consistent with the CIN classification system or atypical hyperplasia (dysplasia) and the carcinoma in situ (CIS) classification system; patients diagnosed with CIN1 who did not undergo interventions including cryosurgery, electrocoagulation therapy, laser therapy, microwave therapy, cold knife conization, loop electrosurgical excision procedure, and trachelectomy; follow-up observation for at least 6 months; complete information so that each document contained sufficient information to calculate statistical indicators of relative risk (RR) or 95% confidence intervals (CIs). The exclusion criteria were as follows: studies that did not meet the inclusion criteria, literature reviews, the absence of a control group, and duplicate publications. We also excluded papers with incomplete initial data.
2.3. Quality Assessment
The Newcastle-Ottawa scale (NOS), recognized as a good study quality assessment tool, was used to assess the quality of the studies identified in our literature search (see Appendix 3). The evaluation system included eight literature evaluation entries for a total of nine possible points [44], including the selection of the study population, comparability, exposure assessment, and the results of the evaluation. The NOS scale validity rating criteria are as follows: 8-9, high quality; 6-7, medium quality; <5, low quality.
2.4. Data Collection
Two authors (Mingzhu Liu and Xiaolong Yan) independently extracted data and crosschecked their data after aggregating the results. Disagreements were resolved by discussion with Professor Mingxia Jing. Data were collected at the start of the study, including basic information, background and characteristics of the research object, and disease diagnosis and evolution. This information is presented in Table 1.
Table 1.
Basic characteristics and quality assessment of included studies.
| Study | Design | Age (years) |
Region (subgroup) |
HPV type | HPV infection time | Indexes | Follow-up time (months) | Quality rating |
|---|---|---|---|---|---|---|---|---|
| Sagasta et al. (2016) [10] | Prospective | 33 ± 10 | Spain (Europe) | HR-HPV | NR | ①②③ | 28 | 8 |
| Veijalainen et al. (2015) [11] | Retrospective | 40.4 | Finland (Europe) | HR-HPV | NR | ① | 96 | 8 |
| He et al. (2015) [12] | Retrospective | 35 ± 16.93 | China (Asia) | HR-HPV | NR | ①②③ | 15 (8–24) | 9 |
| Zhou et al. (2015) [13] | Prospective | 37.57 ± 9.12 | China (Asia) | HR-HPV | NR | ③ | 24 | 7 |
| Mou et al. (2014) [14] | Retrospective | 38.18 ± 4.26 | China (Asia) | NR | NR | ①②③ | 36 | 7 |
| Siriaunkgul et al. (2014) [15] | Prospective | 46.6 | Thailand (Asia) | NR | NR | ①②③ | 24 | 8 |
| Hu et al. (2014) [16] | Prospective | 30–59 | China (Asia) | HR-HPV | Transient | ①②③ | 24 | 9 |
| Persistent | ①②③ | |||||||
| Jiang (2013) [17] | Retrospective | 39.16 ± 8.97 | China (Asia) | HR-HPV | NR | ①②③ | 24 | 8 |
| Waldstrøm et al. (2013) [18] | Prospective | 32.3 | Denmark (Europe) | NR | NR | ①②③ | 60 | 8 |
| Katki et al. (2013) [19] | Prospective | 30–64 | United States (America) | NR | NR | ①②③ | 60 | 8 |
| Byun et al. (2013) [20] | Prospective | 46 | Korea (Asia) | HR-HPV | NR | ① | 8 | 8 |
| Liao et al. (2013) [21] | Prospective | 30–49 | China (Asia) | HR-HPV | NR | ① | 36 | 8 |
| Li et al. (2013) [22] | Prospective | 38 | China (Asia) | HR-HPV | NR | ③ | 6 | 8 |
| Wang et al. (2012) [6] | Retrospective | 35.4 (20–53) | China (Asia) | HR-HPV | Persistent | ① | 18.6 (8–24) | 9 |
| Huang et al. (2012) [23] | Retrospective | 30 (22–70) | China (Asia) | HR-HPV | NR | ① | 24 | 7 |
| Bowring et al. (2012) [24] | Prospective | 36.8 ± 10.2 | Britain (Europe) | HR-HPV | NR | ①②③ | 12 | 8 |
| Jakobsson et al. (2012) [25] | Retrospective | 34 | Finland (Europe) | HR-HPV | NR | ① | 6 | 8 |
| Ozaki et al. (2011) [26] | Prospective | 39 | Japan (Asia) | NR | NR | ① | 17 | 7 |
| Li and Yang (2011) [5] | Prospective | 30 ± 2.32 | China (Asia) | HR-HPV | NR | ①②③ | 6 | 8 |
| LR-HPV | ②③ | |||||||
| Gonzalez-Bosquet et al. (2010) [27] | Prospective | 32.25 | Germany (Europe) | HR-HPV | NR | ① | 25 | 7 |
| Waldstrøm and Ømskov (2010) [28] | Retrospective | 32 | Denmark (Europe) | NR | NR | ①②③ | 36 | 8 |
| Heider et al. (2010) [29] | Retrospective | 33 | United States (America) | HR-HPV | NR | ①②③ | 34 | 9 |
| Cotton et al. (2010) [30] | Prospective | 20–59 | Britain (Europe) | HR-HPV | NR | ① | 36 | 8 |
| Thrall et al. (2009) [7] | Prospective | ≥30 | United States (America) | HR-HPV | NR | ①②③ | 24 | 9 |
| Liao (2008) [31] | Prospective | 30–49 | China (Asia) | HR-HPV | NR | ③ | 24 | 7 |
| Gong (2007) [1] | Prospective | 38.37 ± 5.26 | China (Asia) | HR-HPV | Transient | ①②③ | 24 | 7 |
| Persistent | ①②③ | |||||||
| Luis Ferreira Santos et al. (2006) [32] | Prospective | 31 (16–63) | United States (America) | NR | NR | ①②③ | 12 | 8 |
| Tarkkanen et al. (2006) [33] | Prospective | 35 (20–60) | Finland (Europe) | NR | NR | ① | 6 | 7 |
| Song et al. (2006) [34] | Retrospective | 38 | Korea (Asia) | NR | NR | ①②③ | 24 | 8 |
| Clavel et al. (2005) [35] | Retrospective | 30 | France (Europe) | HR-HPV | NR | ①②③ | 24 | 8 |
| Massad et al. (2004) [36] | Prospective | 37.4 | United States (America) | HR-HPV | NR | ①②③ | 90 | 8 |
| LR-HPV | NR | ①②③ | ||||||
| Sastre-Garau et al. (2004) [37] | Retrospective | 31 | France (Europe) | HR-HPV | NR | ①③ | 24 | 8 |
| Alameda et al. (2004) [38] | Retrospective | 25–45 | Spain (Europe) | HPV | NR | ②③ | 24 | 7 |
| Schlecht et al. (2003) [39] | Retrospective | 16–65 | Brazil (America) | HR-HPV | NR | ① | 53.3 | 8 |
| LR-HPV | NR | ① | ||||||
| Denise Zielinski et al. (2001) [40] | Retrospective | 40.5 (20–76) | Holland (Europe) | HR-HPV | NR | ①②③ | 16.8 (0–54) | 8 |
| Matsuura et al. (1997) [41] | Prospective | NR | United States (America) | NR | NR | ①②③ | 89.2 ± 25.2 | 8 |
| Kaufman et al. (1997) [42] | Retrospective | NR | United States (America) | HR-HPV | NR | ①③ | 6 | 7 |
| Campion et al. (1986) [43] | Prospective | <30 | Britain (Europe) | NR | NR | ①②③ | 22.4 (19–30) | 7 |
Note. ①: the relative risk (RR) of progression of CIN1 patients of HPV-positive compared with the HPV-negative; ②: the relative risk (RR) of persistence of CIN1 patients of HPV-positive compared with the HPV-negative; ③: the relative risk (RR) of regression of CIN1 patients of HPV-positive compared with the HPV-negative. HPV, human papillomavirus; HR-HPV, high-risk HPV; LR-HPV, low-risk HPV; HPV(+), HPV-positive; NR, not reported.
2.5. Data Analysis
Thirty-eight articles were analyzed using RevMan 5.0 (Cochrane systems IMS) and Stata 12.0 (Stata Corp, College Station, Texas, TX, USA). To assess the heterogeneity among studies, we calculated the I2 index. Low and high levels of heterogeneity were considered as I2 ≤ 50% and >50%, respectively. We use a fixed model on the conditions of P > 0.05 and I2 ≤ 50%. We use a randomized model on the conditions of P < 0.05 or I2 > 50%. Combined effects were estimated as relative risk (RR) values with 95% confidence intervals (CIs). All reported P values were two-sided, and a significance level of 0.05 was used. Subgroup analyses were also performed by HPV type (high-risk HPV and low-risk HPV), study design (retrospective and prospective studies), regional population distribution (Asian, European, and American populations), sample size (<100 cases, 100–500 cases, and >500 cases), and follow-up time (6–18 months, 18–24 months, and >24 months). Sensitivity analyses were performed using Stata 12.0.
3. Results
3.1. Search Result
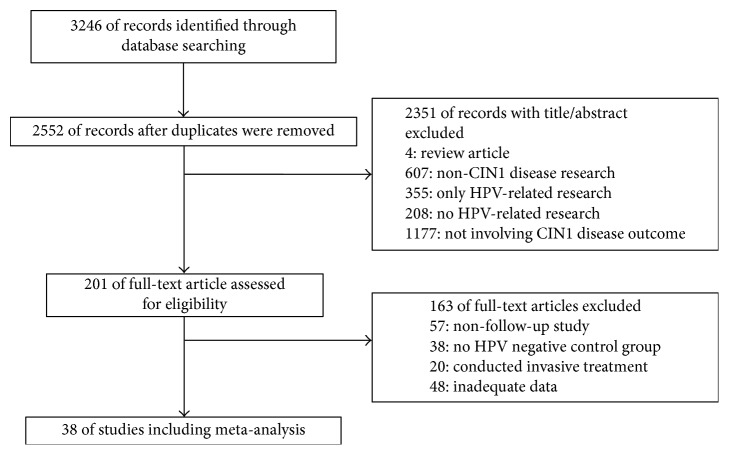
Figure 1 shows the study selection process. Initially, 3,246 articles were included in our search strategy. A total of 38 articles, including 9,758 patient cases, were finally included in the analysis, based on the inclusion and exclusion criteria. A total of 27 studies assessed regression of CIN1 to a normal status. Twenty-five studies examined persistent CIN1 and 36 articles evaluated the progression from CIN1 to high-grade cervical intraepithelial neoplasia and cervical cancer (CIN2+).
Figure 1.
Flowchart of identifying and including studies.
3.2. Basic Characteristics and Quality Assessment of the Included Studies
The basic features of the 38 studies included in this meta-analysis are listed in Table 1. The studies spanned a period of 30 years (1986 to 2016) and included 22 prospective and 16 retrospective studies. The study sample sizes ranged from 29 [41] to 2,009 [30] cases. Twenty-seven studies reported median/mean ages, ranging from 16 to 76 years. The mean follow-up time ranged from 6 to 96 months. The studies included Asian (16 studies), European (14 studies), and American (8 studies) populations. Thirty-eight studies had quality ratings between 7 and 9 points, with an average of 7.84 points. Twenty-eight articles had quality scores ≥8 points, corresponding to high-quality research, while 10 articles were of medium quality.
4. Results of the Meta-Analysis
4.1. The Influence of HPV Infection on the Outcome of CIN1 Lesions
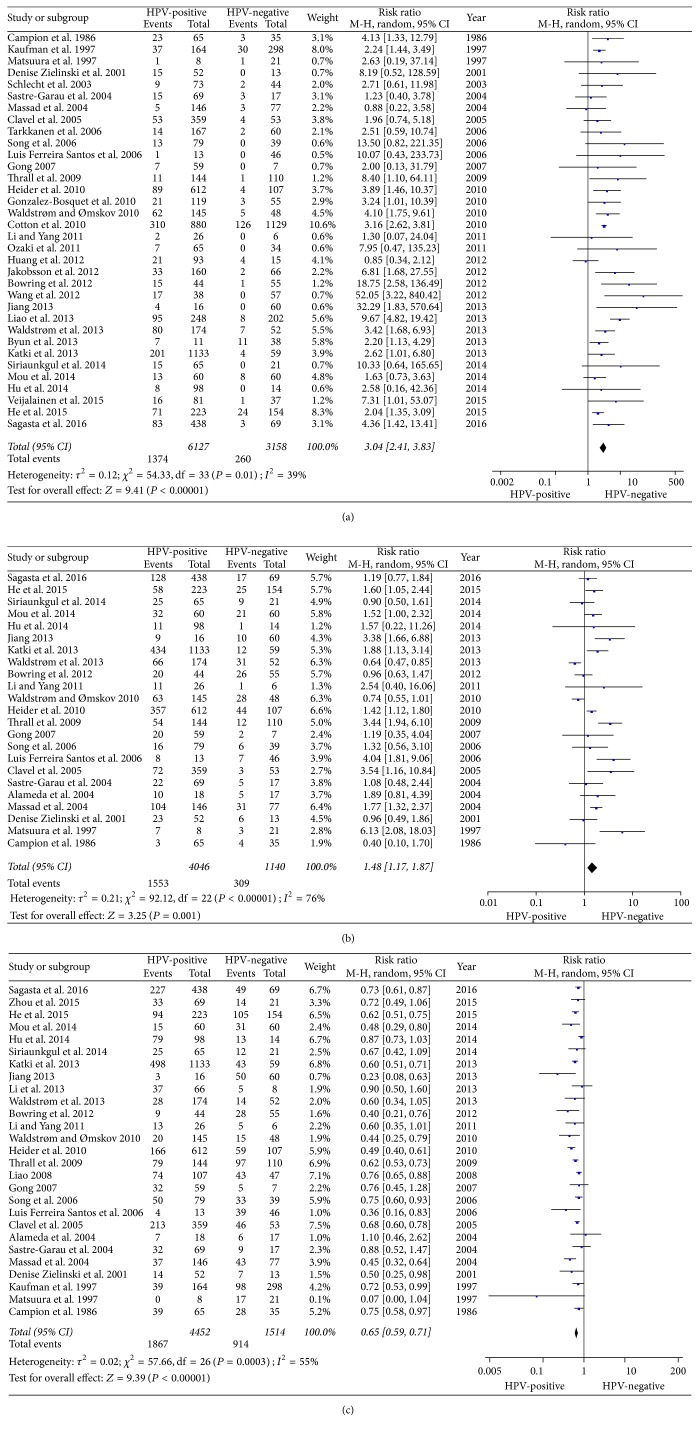
A total of 38 studies were included in this study, and of these, 34 estimated the impact of HPV infection on the progression of CIN1 lesions; pooled analysis showed that the risk of CIN1 disease progression was 3.04-fold higher in the HPV-positive group than in the HPV-negative group (95% CI: 2.41–3.83; Z = 6.28; P < 0.00001), with low heterogeneity (P = 0.01; I2 = 39%; Figure 2(a)). Twenty-three studies estimated the impact of HPV infection on the persistence of CIN1 lesions; pooled analysis showed that the risk of CIN1 disease persistence was 1.48-fold higher in the HPV-positive group than in the HPV-negative group (95% CI: 1.17–1.87; Z = 3.25; P = 0.001), with significant heterogeneity (P < 0.00001; I2 = 76%; Figure 2(b)). Twenty-seven studies estimated the impact of HPV infection on the regression of CIN1 lesions; pooled analysis showed that the ratio of CIN1 disease regression was 0.65-fold lower in the HPV-positive group than in the HPV-negative group (95% CI: 0.59–0.71; Z = 9.39; P < 0.00001), with high heterogeneity (P = 0.0003; I2 = 55%; Figure 2(c)).
Figure 2.
Forest plot of HPV-positive patients and CIN1 disease outcomes. HPV positivity in the exposed group and HPV negativity in the control group. (a) Forest plot of HPV positivity and CIN1 disease progression; (b) Forest plot of HPV positivity and CIN1 disease persistence; (c) Forest plot of HPV positivity and CIN1 disease regression.
In subgroup analyses, the risk of the persistence of CIN1 was higher in American than European or Asian populations (RRAsian = 1.54, 95% CI: 1.17–2.02; RREuropean = 0.97, 95%CI: 0.73–1.30; RRAmerican = 2.29, 95%CI: 1.59–3.28; P = 0.001). The ratio of regression of CIN1 was higher in patients followed up for 18–24 months than in those followed up for 6–18 months or >24 months (RR6–18 = 0.61, 95% CI: 0.53–0.70; RR18–24 = 0.73, 95% CI: 0.67–0.80; RR>24 = 0.55, 95% CI: 0.46–0.66; P = 0.007). Significant differences in HPV type, study design, and sample size were not detected (see Appendix 4).
4.2. The Influence of HR-HPV Infection Time on CIN1 Lesions
Long or short HR-HPV infection times had different effects on CIN1 lesion history. Persistent HR-HPV infection means that, in two or more times, the HR-HPV detected was positive and transient HR-HPV infection means that, in only one time, the HR-HPV detected was positive [6]. The risk of CIN1 disease progression was 13.91-fold higher in the persistent HR-HPV infection group than in the HPV-negative group (95% CI: 3.46–55.90; P = 0.000); the ratio of CIN1 disease regression was 0.61-fold lower in the persistent HR-HPV infection group than in the HPV-negative group (95% CI: 0.47–0.80; P = 0.000). The impact of transient HR-HPV infection on CIN1 disease progression and regression was not statistically significant. Furthermore, persistent and transient HPV infection did not have a significant impact on CIN1 persistence (Table 2).
Table 2.
The influence of HR-HPV infection time on CIN1 lesions.
| Disease outcome | Infection time | Number of studies | Heterogeneity test results | Merged effect RR value (95% CI) | P value | ||
|---|---|---|---|---|---|---|---|
| Q Value | P Value | I 2(%) | |||||
| Progression | Persistent | 3 | 2.49 | 0.290 | 20 | 13.91 (3.46, 55.90) | 0.000 |
| Transient | 2 | 0.05 | 0.820 | 0 | 1.06 (0.12, 9.01) | 0.960 | |
| Persistent | Persistent | 2 | 0.22 | 0.640 | 0 | 2.15 (0.75, 6.18) | 0.160 |
| Transient | 2 | 0.02 | 0.890 | 0 | 0.57 (0.17, 1.92) | 0.360 | |
| Regression | Persistent | 2 | 1.86 | 0.170 | 46 | 0.61 (0.47, 0.80) | 0.000 |
| Transient | 2 | 0.22 | 0.640 | 0 | 1.03 (0.86, 1.24) | 0.750 | |
RR, relative risk; CI, confidence interval.
4.3. Sensitivity Analysis
We conducted a sensitivity analysis for the progression, persistence, and regression of CIN1 disease, respectively (see Appendix 5). All of the included studies were distributed evenly from the central line, with no significant deviation. Therefore, no individual study affected the pooled effect results.
5. Discussion
A total of 38 studies were included in the current study. Of these, 23 studies examined HR-HPV infections and three of these also considered LR-HPV infection. Three studies assessed HPV infection times. Studies have shown that HPV infections are associated with an extended disease course in CIN1, increasing the risk of disease progression and hampering the reversal of CIN1. Persistent HR-HPV infection was a major factor associated with CIN1 progression. This finding provides important data for the clinical management of CIN1 disease, in order to avoid excessive or inadequate treatment. Regional population distribution and follow-up time were also associated with CIN1 disease outcome.
In the HPV-positive group, the risks of CIN1 progression, persistence, and regression, respectively, were 3.02, 1.45, and 0.65, compared to the HPV-negative group. A randomized controlled study from the atypical squamous cells of undetermined significance-low-grade squamous intraepithelial lesion (ASCUS-LSIL) (ALTS) group [45] reported higher risks of CIN1 progression and persistence (12.34 and 2.41, resp.) in the HPV-positive group than that observed in our study. The ratio of CIN1 regression in the HPV-positive group was 0.19, which is lower than that found in this study. These findings may be explained by the fact that 34.2% of the included studies involved population-based screening and identification of patients with CIN1 was the object of study. Compared to the ALTS study, with subjects from four large clinical centers, the patient's condition is relatively severe. However, this study reached a comparable conclusion. HPV positivity was associated with hampered CIN1 lesion regression and increased risk of disease progression and persistence.
Our research on the impact of HPV type and infection duration on CIN1 disease history found that persistent HR-HPV infection was a major risk factor for CIN1 progression to CIN2+, while LR-HPV and transient infections were not significantly associated with increased risk of CIN1 progression. The results are corroborated in a large prospective study by Dalstein et al. [46]. Furthermore, only Huang et al. [23] explored the relationship between human papillomavirus type 16 (HPV16), HPV18, and other oncogenic HPV and CIN1 disease, reporting that the risk of CIN1 progression to CIN2/3 in patients with HPV16 infection was 2.51 and 6.95 times that in patients with HPV18 infection and other oncogenic forms, respectively. HPV16 is considered the major risk factor for CIN1 disease progression.
Regional population distribution and follow-up time were the two main factors influencing CIN1 disease history. The risk of disease progression in HPV-positive CIN1 patients in the Americas was lower than that of patients in Asian and European countries (RRAsian = 3.94; RREuropean = 3.10; RRAmerican = 2.31; P = 0.380), and the ratios of disease regression (RRAsian = 1.54; RREuropean = 0.97; RRAmerican = 2.29; P = 0.001) and persistence (RRAsian = 0.70; RREuropean = 0.69; RRAmerican = 0.56; P = 0.060) were higher than those in Asia and European countries. In these Americas countries, 87.5% are North Americas countries (seven US and one Brazilian states). Previous studies have shown that a screening strategy of ThinPrep cytologic tests (TCTs) combined with HPV tests has gained popularity [43]. CIN1 had a lower probability of progression to invasive cervical cancer (ICC) and a higher probability of regression. In some Asian and European countries, screening is performed mainly by visual inspection with acetic acid or iodine and Pap smears. These methods are less costly than TCT and HPV DNA tests [47]. Therefore, the probability of CIN1 progression was relatively high and the probability of disease reversal was relatively low. Numerous studies have shown gradual CIN1 regression with clearance of HPV infections, and our study found that natural clearance of HPV infections may take longer than 24 months. This is consistent with the interval of two years or longer for HPV-based cervical cancer screening [48–50].
This study has the following advantages. First, the well-designed studies provided strong evidence for the analysis of the influence of HPV infection on CIN1 disease history. Furthermore, the full search was relatively comprehensive and included a large number of studies, significantly increasing the sample size compared with using the single original research study criteria; therefore, the combined effect size was more accurate. Second, subgroup analyses were performed according to HPV type, study design, regional population distribution, sample size, and follow-up time, in order to explore potential confounders. Some limitations should be considered when interpreting the results of this study. First, CIN1 is affected by many factors and we were unable to control for parameters such as age at first sexual intercourse, number of pregnancies and delivery times, and individual immune status. Second, most studies included a larger age range; therefore, we did not conduct subgroup analysis by patient age. Third, more papers from China were included in the present study, which would limit the significance. This conclusion needs to be further testified in the people from other countries. Finally, there are few studies on low-risk HPV infection potentially affecting the results of subgroup analysis of HPV type. Therefore, we will research low-risk HPV infection in future studies to improve the accuracy of these results.
6. Conclusion
HPV infection resulted in an increased risk of CIN1 progression and reducing disease reversibility. Persistent high-risk HPV infection resulted in a further increased risk of CIN1 progression. Furthermore, regional population distribution and follow-up times influenced CIN1 disease history.
Supplementary Material
Appendix 1: The PICOS Principles in the PRISMA Statement. Appendix 2: Full Search strategy. Appendix 3: Newcastle-Ottawa Quality Assessment Scale: Cohort Studied. Appendix 4: Subgroup Analyses. Appendix 5: Sensitivity Analysis.
Acknowledgments
The authors acknowledge funding from the Science and Technology Plan Project of Xinjiang Production and Construction Corps (no. 2013BB015).
Disclosure
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of Interest
The authors have declared that no conflicts of interest exist.
Authors' Contributions
Mingzhu Liu performed the data analysis and drafted the manuscript. Mingxia Jing and Shugang Li conceived and designed the study and led the writing of the paper. Mingzhu Liu and Xiaolong Yan jointly developed the search strategy for this study and assessed the titles and abstracts for their relevance to this study. Mingzhu Liu, Xiaolong Yan, Mei Zhang, and Xiaoju Li assessed full articles for inclusion. Mingxia Jing resolved disagreements between authors. All authors read and approved the final manuscript. Mingzhu Liu and Xiaolong Yan contributed equally to this work and should be considered co-first authors.
References
- 1.Gong C. H. The Study of Multi-Monitor Methods on the Natural Prognosis and Intervention Treatment of Cervical Intraepithelial Neoplasiai. Medical School of Nanchang University, Nanchang University; 2007. [Google Scholar]
- 2.Xie X., Gou W. L. Obstetrics and Gynecology. 8 version. Beijing, China: People's Health Publishing House; 2013. [Google Scholar]
- 3.Feng Y. J. Cervical intraepithelial neoplasia. Journal of Practical Oncology. 2003;18(3):169–171. [Google Scholar]
- 4.Rong X., Wu L. N., Gao J. Y., et al. Study of natural history of cervical intraepithelial neoplasia gradeI. Progress in Obstetrics and Gynecology. 2012;21(5):365–368. [Google Scholar]
- 5.Li R. L., Yang Y. Clinical study of cervical HPV infection and the prognosis of cervical intraepithelial neoplasia I. Health Research. 2011;31(2):97–99. [Google Scholar]
- 6.Wang H. H., Chen L., Chen M., Wu L. L., Li J. P. Significance of high risk human papillomavirus monitoring in follow-up of cervical intraepithelial neoplasia? Maternal and Child Health Care of China. 2012;27(6):820–822. [Google Scholar]
- 7.Thrall M. J., Smith D. A., Mody D. R. Women ≥30 years of age with low grade squamous intraepithelial lesion (LSIL) have low positivity rates when cotested for high-risk human papillomavirus: Should we reconsider HPV triage for LSIL in older women? Diagnostic Cytopathology. 2010;38(6):407–412. doi: 10.1002/dc.21209. [DOI] [PubMed] [Google Scholar]
- 8.Mill A. B. Genevao World Health Organization Geneve. Cervical cancer screening programmes. 1992. The natural history of cervical cancer and the implications for screening policy; pp. 6–14. [Google Scholar]
- 9.Kjaer S. K. Risk factors for cervical neoplasia in Denmark. APMIS. 1998;80:1–41. doi: 10.1111/j.1600-0463.1998.tb05622.x. [DOI] [PubMed] [Google Scholar]
- 10.Sagasta A., Castillo P., Saco A., et al. P16 staining has limited value in predicting the outcome of histological low-grade squamous intraepithelial lesions of the cervix. Modern Pathology. 2016;29(1):51–59. doi: 10.1038/modpathol.2015.126. [DOI] [PubMed] [Google Scholar]
- 11.Veijalainen O., Tuomisaari S., Luukkaala T., Mäenpää J. High risk HPV testing in the triage of repeat ASC-US and LSIL. Acta Obstetricia et Gynecologica Scandinavica. 2015;94(9):931–936. doi: 10.1111/aogs.12686. [DOI] [PubMed] [Google Scholar]
- 12.He X., Tao H. C., Wang S. Z., et al. An analysis of high-risk human papillomavirus DNA-negative cervical intraepithelial neoplasia in atypical squamous cells of undetermined significance and low-grade squamous intraepithelial lesion smears: a retrospective study. Journal of Capital Medical University. 2015;36(2):205–211. [Google Scholar]
- 13.Zhou Y. H., Fan W. F., Huang Y. Q., Dong J. J., Guo H. Y. Combined detection of high-risk human papillomavirus and telomerase gene amplification in a mild cervical intraepithelial neoplasia shunt management role. Journal of Xinxiang Medical University. 2015;32(11):1032–1035. [Google Scholar]
- 14.Mou L., Hu Y., Lan Y., Lv F. L. Correlative research of HPV infection and the disease progression of cervical intraepithelial neoplasia. Chongqing Medicine. 2014;43(29):3895–3896. [Google Scholar]
- 15.Siriaunkgul S., Settakorn J., Sukpan K., et al. Population-based cervical cancer screening using high-risk HPV DNA test and liquid-based cytology in northern Thailand. Asian Pacific Journal of Cancer Prevention. 2014;15(16):6837–6842. doi: 10.7314/apjcp.2014.15.16.6837. [DOI] [PubMed] [Google Scholar]
- 16.Hu S. Y., Zhao F. H., Ma J. F., et al. A prospective study on the prognosis of biopsy·confirmed cervical intraepithelial neoplasia grade 1 and the relationship witlI higII-risk human papiliomavirus. Chinese Journal of Preventive Medicine. 2014;48(5):361–365. [PubMed] [Google Scholar]
- 17.Jiang H. The impact of high-risk HPV infection and follow-up on the prognosis of CINI. International Journal of Gynecology & Obstetrics. 2013;40(4):324–326. [Google Scholar]
- 18.Waldstrøm M., Christensen R. K., Ornskov D. Evaluation of p16INK4a/Ki-67 dual stain in comparison with an mRNA human papillomavirus test on liquid-based cytology samples with low-grade squamous intraepithelial lesion. Cancer Cytopathology. 2013;121(3):136–145. doi: 10.1002/cncy.21233. [DOI] [PubMed] [Google Scholar]
- 19.Katki H. A., Schiffman M., Castle P. E., et al. Five-year risks of CIN 3+ and cervical cancer among women who test pap-negative but are HPV-positive. Journal of Lower Genital Tract Disease. 2013;17(5, supplement 1):S56–S63. doi: 10.1097/LGT.0b013e318285437b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Byun S. W., Lee A., Kim S., Choi Y. J., Lee Y. S., Park J. S. Immunostaining of p16INK4a/Ki-67 and L1 capsid protein on liquid-based cytology specimens obtained from ASC-H and LSIL-H cases. International Journal of Medical Sciences. 2013;10(12):1602–1607. doi: 10.7150/ijms.6526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liao G.-D., Sellors J. W., Sun H.-K., et al. P16INK4A immunohistochemical staining and predictive value for progression of cervical intraepithelial neoplasia grade 1: a prospective study in China. International Journal of Cancer. 2014;134(7):1715–1724. doi: 10.1002/ijc.28485. [DOI] [PubMed] [Google Scholar]
- 22.Li L., Jiang W., Zeng S. Y., Li L. Y. Prospective study of human telomerase ribonucleic acid component gene detection by fluorescence in situ hybridization in predicting natural prognosis of cervical intraepithelial neoplasia 1. Chinese Journal of Clinical Oncology. 2013;40(1):25–28. [Google Scholar]
- 23.Huang L.-W., Lin Y.-H., Pan H.-S., Seow K.-M., Lin C.-Y. Human papillomavirus genotyping as a predictor of high-grade cervical dysplasia in women with mildly cytologic abnormalities: a two-year follow-up report. Diagnostic Cytopathology. 2012;40(8):673–677. doi: 10.1002/dc.21591. [DOI] [PubMed] [Google Scholar]
- 24.Bowring J., Albrow R., Fisher A., et al. A prospective study of human papillomavirus (HPV) testing to resolve uncertainty in colposcopy. Cytopathology. 2013;24(5):309–313. doi: 10.1111/j.1365-2303.2012.01003.x. [DOI] [PubMed] [Google Scholar]
- 25.Jakobsson M., Tarkkanen J., Auvinen E., Häkkinen R., Laurila P., Tapper A. M. Colposcopy referral rate can be reduced by high-risk human papillomavirus triage in the management of recurrent atypical squamous cells of undetermined significance or low-grade squamous intraepithelial lesion cytology in Finland. International Journal of STD and AIDS. 2012;23(7):485–489. doi: 10.1258/ijsa.2011.011336. [DOI] [PubMed] [Google Scholar]
- 26.Ozaki S., Zen Y., Inoue M. Biomarker expression in cervical intraepithelial neoplasia: Potential progression predictive factors for low-grade lesions. Human Pathology. 2011;42(7):1007–1012. doi: 10.1016/j.humpath.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 27.Gonzalez-Bosquet E., Selva L., Sabria J. Predictive factors for the detection of CINII-III in the follow-up of women with CIN I. European Journal of Gynaecological Oncology. 2010;31(4):369–71. [PubMed] [Google Scholar]
- 28.Waldstrøm M., Ømskov D. Clinical performance of a human papillomavirus messenger RNA test (Aptima HPV Assay)on residual material from archived 3-year-old PreservCyt samples with low-grade squamous intraepithelial lesion. Archives of Pathology and Laboratory Medicine. 2011;135(8):1052–1056. doi: 10.5858/2010-0411-OAR. [DOI] [PubMed] [Google Scholar]
- 29.Heider A., Austin R. M., Zhao C. HPV test results stratify risk for histopathologic follow-up findings of high-grade cervical intra-epithelial neoplasia in women with low-grade squamous intra-epithelial lesion pap results. Acta Cytologica. 2010;55(1):48–53. doi: 10.1159/000320877. [DOI] [PubMed] [Google Scholar]
- 30.Cotton S., Sharp L., Little J., et al. The role of human papillomavirus testing in the management of women with low-grade abnormalities: multicentre randomised controlled trial. BJOG. 2010;117(6):645–659. doi: 10.1111/j.1471-0528.2010.02519.x. [DOI] [PubMed] [Google Scholar]
- 31.Liao G. D. Prevalence of P161NK4a expression in cervical histology and predictive validity of P161NK4a in cervical intraepithelial neoplasia grade l transformation. Journal of Chongqing Medical University. 2008 [Google Scholar]
- 32.Luis Ferreira Santos A., Françoise Mauricette Derchain S., Otávio Sarian L., Roberto Martins M., Siani Morais S., Juhani Syrjänen K. Performance of Pap smear and human papilloma virus testing in the follow-up of women with cervical intraepithelial neoplasia grade 1 managed conservatively. Acta Obstetricia et Gynecologica Scandinavica. 2006;85(4):444–450. doi: 10.1080/00016340600604682. [DOI] [PubMed] [Google Scholar]
- 33.Tarkkanen J., Auvinen E., Nieminen P., et al. HPV DNA testing as an adjunct in the management of patients with low grade cytological lesions in Finland. Acta Obstetricia et Gynecologica Scandinavica. 2007;86(3):367–372. doi: 10.1080/00016340601185343. [DOI] [PubMed] [Google Scholar]
- 34.Song S.-H., Lee J.-K., Oh M.-J., Hur J.-Y., Park Y.-K., Saw H.-S. Risk factors for the progression or persistence of untreated mild dysplasia of the uterine cervix. International Journal of Gynecological Cancer. 2006;16(4):1608–1613. doi: 10.1111/j.1525-1438.2006.00634.x. [DOI] [PubMed] [Google Scholar]
- 35.Clavel C., Bory J.-P., Caudroy S., et al. Usefulness of HPV testing in the follow-up of untreated cervical low grade lesions. Histology and Histopathology. 2005;20(4):1085–1091. doi: 10.14670/HH-20.1085. [DOI] [PubMed] [Google Scholar]
- 36.Massad L. S., Evans C. T., Minkoff H., et al. Natural history of grade 1 cervical intraepithelial neoplasia in women with human immunodeficiency virus. Obstetrics and Gynecology. 2004;104(5, part 1):1077–1085. doi: 10.1097/01.AOG.0000143256.63961.c0. [DOI] [PubMed] [Google Scholar]
- 37.Sastre-Garau X., Cartier I., Jourdan-Da Silva N., De Crémoux P., Lepage V., Charron D. Regression of low-grade cervical intraepithelial neoplasia in patients with HLA-DRB1∗13 genotype. Obstetrics and Gynecology. 2004;104(4):751–755. doi: 10.1097/01.AOG.0000139834.84628.61. [DOI] [PubMed] [Google Scholar]
- 38.Alameda F., Fuste P., Boluda S., et al. The Ki-67 labeling index is not a useful predictor for the follow-up of cervical intraepithelial neoplasia 1. Journal of Lower Genital Tract Disease. 2004;8(4):313–316. doi: 10.1097/00128360-200410000-00010. [DOI] [PubMed] [Google Scholar]
- 39.Schlecht N. F., Platt R. W., Duarte-Franco E., et al. Human papillomavirus infection and time to progression and regression of cervical intraepithelial neoplasia. Journal of the National Cancer Institute. 2003;95(17):1336–1343. doi: 10.1093/jnci/djg037. [DOI] [PubMed] [Google Scholar]
- 40.Denise Zielinski G., Snijders P. J. F., Rozendaal L., et al. High-risk HPV testing in women with borderline and mild dyskaryosis: long-term follow-up data and clinical relevance. Journal of Pathology. 2001;195(3):300–306. doi: 10.1002/path.981. [DOI] [PubMed] [Google Scholar]
- 41.Matsuura Y., Kawagoe T., Toki N., Sugihara K., Kashimura M. Low grade cervical intraepithelial neoplasia associated with human papillomavirus infection. Long-term follow-up. Acta Cytologica. 1998;42(3):625–630. doi: 10.1159/000331818. [DOI] [PubMed] [Google Scholar]
- 42.Kaufman R. H., Adam E., Icenogle J., Reeves W. C. Human papillomavirus testing as triage for atypical squamous cells of undetermined significance and low-grade squamous intraepithelial lesions: sensitivity, specificity, and cost-effectiveness. American Journal of Obstetrics and Gynecology. 1997;177(4):930–936. doi: 10.1016/S0002-9378(97)70296-5. [DOI] [PubMed] [Google Scholar]
- 43.Campion M. J., Cuzick J., McCance D. J., Singer A. Progressive potential of mild cervical atypia: prospective cytological, colposcopic, and virological study. The Lancet. 1986;328(8501):237–240. doi: 10.1016/S0140-6736(86)92067-2. [DOI] [PubMed] [Google Scholar]
- 44.Zeng X. T., Liu H., Chen X., Leng W. D. Meta analysis series IV: quality assessment tool for observational research. Chinese Journal of Evidence-Based Cardiovascular Medicine. 2012;4(4):297–299. [Google Scholar]
- 45.ASCUS-LSIL Triage Study (ALTS) Group. A randomized trial on the management of low-grade squamous intraepithelial lesion cytology interpretations. American Journal of Obstetrics and Gynecology. 2003;188(6):1393–1400. doi: 10.1067/mob.2003.462. [DOI] [PubMed] [Google Scholar]
- 46.Dalstein V., Riethmuller D., Préteti J.-L., et al. Persistence and load of high-risk HPV are predictors for development of high-grade cervical lesions: a longitudinal French cohort study. International Journal of Cancer. 2003;106(3):396–403. doi: 10.1002/ijc.11222. [DOI] [PubMed] [Google Scholar]
- 47.Mendes D., Bains I., Vanni T., Jit M. Systematic review of model-based cervical screening evaluations. BMC Cancer. 2015;15(1, article 334) doi: 10.1186/s12885-015-1332-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sroczynski G., Schnell-Inderst P., Mühlberger N., et al. Cost-effectiveness of primary HPV screening for cervical cancer in Germany—a decision analysis. European Journal of Cancer. 2011;47(11):1633–1646. doi: 10.1016/j.ejca.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 49.Goldie S. J., Kim J. J., Wright T. C. Cost-effectiveness of human papillomavirus DNA testing for cervical cancer screening in women aged 30 years or more. Obstetrics and Gynecology. 2004;103(4):619–631. doi: 10.1097/01.AOG.0000120143.50098.c7. [DOI] [PubMed] [Google Scholar]
- 50.Kim J. J., Brisson M., Edmunds W. J., Goldie S. J. Modeling cervical cancer prevention in developed countries. Vaccine. 2008;26(supplement 10):K76–K86. doi: 10.1016/j.vaccine.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1: The PICOS Principles in the PRISMA Statement. Appendix 2: Full Search strategy. Appendix 3: Newcastle-Ottawa Quality Assessment Scale: Cohort Studied. Appendix 4: Subgroup Analyses. Appendix 5: Sensitivity Analysis.