Abstract
Background
Inorganic arsenic exposure from naturally contaminated groundwater is related to vascular disease. No prospective studies have evaluated the association between arsenic and carotid atherosclerosis at low-moderate levels. We examined the association of long-term, low-to-moderate inorganic arsenic exposure with carotid arterial disease.
Methods
American Indians, 45 to 74 years old, in Arizona, Oklahoma, and North and South Dakota had arsenic concentrations (sum of inorganic and methylated species, μg/g urine creatinine) measured from baseline urine samples (1989-1991). Carotid artery ultrasound was performed in 1998-1999. Vascular disease was assessed by the carotid intima media thickness (CIMT), the presence of atherosclerotic plaque in the carotid, and by the number of segments containing plaque (plaque score).
Results
2402 participants (mean age 55.3 years, 63.1% female, mean body mass index 31.0kg/m2, diabetes 45.7%, hypertension 34.2%) had a median (interquintile range) urine arsenic concentration of 9.2 (5.00, 17.06) μg/g creatinine. The mean CIMT was 0.75 mm. 64.7% had carotid artery plaque (3% with >50% stenosis). In fully adjusted models comparing participants in the 80th vs. 20th percentile in arsenic concentrations, the mean difference in CIMT was 0.01 (95% confidence interval (95%CI): 0.00, 0.02) mm, the relative risk of plaque presence was 1.04 (95%CI: 0.99, 1.09), and the geometric mean ratio of plaque score was 1.05 (95%CI: 1.01, 1.09).
Conclusions
Urine arsenic was positively associated with CIMT and increased plaque score later in life although the association was small. The relationship between urinary arsenic and the presence of plaque was not statistically significant when adjusted for other risk factors. Arsenic exposure may play a role in increasing the severity of carotid vascular disease.
Keywords: carotid stenosis, risk factors for stroke, atherosclerosis, arsenic, vascular disease
Introduction
Arsenic is a chemical of major public health concern.1 An established carcinogen, inorganic arsenic is most commonly ingested through drinking naturally contaminated groundwater, although exposure may also occur via food (rice and other grains), air pollution, smelting operations, and some occupational settings. In the United States, inorganic arsenic exposure through consumption of naturally contaminated groundwater has been a long-term concern in many rural and suburban communities, especially for those with private water wells.2, 3
Increasing evidence supports a role of inorganic arsenic in a broad range of vascular diseases, particularly among populations exposed to levels above the World Health Organization and U.S. Environmental Protection Agency (EPA)'s recommended upper limit in drinking water (10μg/liter).4, 5 Arsenic exposure may be a risk factor for vascular disease through its putative role in potentiating atherosclerosis.6 A common etiology of stroke is carotid atherosclerosis leading to artery-to-artery thromboembolism into the cerebral circulation. Chronic arsenic exposure has been linked to stroke in other studies,4, 7 but the mechanism has not been well studied. Arsenic may therefore have a subclinical relationship to carotid artery disease that is poorly recognized.
In southwestern Taiwan, a region with historically high arsenic levels, a cross-sectional study found a dose-dependent relationship between the number of years exposed to arsenic-containing well water and a higher degree of carotid atherosclerosis, but not with discrete carotid plaque.8 Others have found a relationship with carotid intima media thickness (CIMT) but have used varying definitions of carotid vascular disease.9, 10, 11 Studies at low-moderate levels of arsenic exposure, more similar to those occurring in U.S. populations, are lacking. In large cohort studies in Denmark12 and Italy,13 low-moderate levels of arsenic exposure were associated with higher lifetime risk of myocardial infarction and deaths from chronic diseases respectively. We are aware of no prospective studies evaluating the association between arsenic and carotid vascular disease. New data from cohort studies providing individual level arsenic measurements of community dwellers with chronic low-moderate levels of exposure and prospective, long term follow up are needed to measure this potential association.
We aim to examine the prospective association of arsenic exposure with CIMT and atherosclerotic plaque in the Strong Heart Study (SHS) cohort of American Indians from the Southwestern and Central USA. A prior study of carotid artery disease in the SHS found that diabetes and hypertension each statistically significantly increased both the CIMT and carotid plaque score in this cohort.14 A separate study found higher levels of low-moderate arsenic exposure in the SHS were related to increased cardiovascular, coronary heart disease, and stroke mortality as well as a higher incidence of each of these conditions.4 Given these findings, we hypothesized that inorganic arsenic exposure is a risk factor for subclinical carotid atherosclerosis.
Methods
Study Population
The SHS prospective cohort study of American Indians, begun in the 1980s, in whom vascular diseases were a leading cause of death but little was reported on their vascular risk factors. Targeted enrolment was 1,500 participants, ages 45 to 74 years old, in each of Oklahoma, Arizona, and North and South Dakota.15, 16 Thirteen tribes were included. Cluster sampling was used for participant enrolment in North and South Dakota, whereas all tribal members in the selected communities in Oklahoma and Arizona were invited, either by telephone or letter. Final enrolment in the Strong Heart Study was 4,549 participants with a participation rate of 62%.17 The Indian Health Service, institutional review boards, and the participating tribes approved the study protocol. Each participant provided individuated consent to participate in the Strong Heart Study.
Each participant underwent a structured interview, physical examination, anthropometric measurements, and collection of blood and urine specimens.15, 16 The study observation period began at the date of the participant's baseline study examination (1989-1992) when urinary samples and data to assess vascular risk factors were collected. For the present study, the follow up was until study visit three (1998-1999) when 88% of all surviving cohort participants were re-examined, including carotid ultrasound measurements. We used data from 3,974 participants with sufficient urine available for arsenic measurements. We then excluded 1,494 participants without available carotid ultrasound data due to death (n=787), non-participation in the 3rd Strong Heart Study examination (n=372), and lack of technically adequate carotid imaging (n=335, including 69 with only a left side measurement and 40 with only a right side measurement). Living subjects who did not undergo carotid ultrasound measurements were not different in terms of baseline risk factors for atherosclerosis compared to those who had the procedure performed (data not shown). After further excluding 78 additional participants due to missing data on other covariates of interest, the final sample size for this study was 2,402. Participants in the SHS were followed for vascular events, as defined previously, ending in 2008.2
Arsenic Measurements
Spot urine arsenic level was measured at the baseline study visit, from one urine sample, and used as a proxy for arsenic exposure and arsenic internal dose. Urine was collected in polypropylene tubes, frozen within 1-2 hours of collection, and transported on dry ice to long-term storage at -70 degrees Celsius at the Penn Medical Laboratory (MedStar Research Institute, Washington DC, USA). Urine arsenic measurements were performed on thawed samples, using up to 1mL of urine in 2009 (Trace Element Laboratory, Graz University, Austria). Urinary concentrations of inorganic arsenic (arsenite, arsenate), monomethylarsonate (MMA), dimethylarseninate (DMA) and arsenobetaine and other arsenic cations were measured using high-performance liquid chromatography inductively coupled plasma-mass spectrometry (Agilent 1100 HPLC and Agilent 7700× ICP-MS, Agilent Technologies, Waldbronn Germany).18 The concentration of arsenobetaine - a measure of seafood arsenicals - was very low, confirming that seafood consumption was low in this population.4 In a previously reported analysis from a random sample of 380 adults in the Strong Heart Study, the interclass coefficient of combined inorganic and methylated arsenic species was 0.64 (95% confidence interval (CI) 0.60, 0.69), and the average change in urine arsenic concentration between study visits spanning ten years was -0.8 μg/g urine creatinine.2 In general, DMA, MMA, and inorganic arsenic have half-lives of approximately 2, 9, and 38 days respectively.19, 20 To assess inorganic arsenic exposure, the sum of inorganic (arsenite plus arsenate, i.e. iAs) and methylated arsenic species was used, hereafter referred to as “arsenic.”
Other Risk Factors
Smoking was defined as current, past, or never in one's lifetime. Past smoking was defined as having smoked at least 100 cigarettes but not currently smoking. Current smoking includes smoking currently and having smoked at least 100 cigarettes. Current alcohol use was defined as drinking regularly and having 12 alcoholic drinks or more in a lifetime. Past alcohol use was defined as drinking regularly in the past and not drinking alcohol within the previous year. Body mass index was measured in the standard way (kg/m2). The seventh report of the Joint National Committee on Prevention, Detection, and Evaluation, and Treatment of Hypertension definition of hypertension was employed: systolic blood pressure ≥140mmHg, diastolic blood pressure ≥90mmHg, and/or the use of antihypertensive medications to treat blood pressure.21 Diabetes was defined as fasting glucose ≥7.0mmol/L (126mg/dL), post-oral glucose challenge glucose measurement of ≥11.1 mmol/L (200mg/dL), the use of oral hypoglycemic medications or insulin to treat diabetes and/or glycated hemoglobin ≥6.5%.22 Serum high density lipoprotein (HDL), cholesterol and triglycerides were measured in the fasting state at study baseline and treated as continuous variables.23 Plasma creatinine was measured by an alkaline-picrate rate method. Estimated glomerular filtration rate (eGFR) was calculated from recalibrated creatinine, age and sex using the Chronic Kidney Disease Epidemiology Collaboration formula.24, 25
Carotid Ultrasonography
Imaging of the extracranial carotid arteries was performed using standardized protocols with centralized training of field sonographers.26 Three vascular measurements were assessed in this study: (1) The presence of atherosclerotic plaque in the common carotid artery, which was defined as focal carotid arterial wall thickening >50% compared to the thickness of the surrounding wall.27 (2) A carotid plaque score, which was calculated by the number of segments containing plaque, combining left and right common carotid, carotid bulb, and external and internal carotid arteries.28 Carotid plaque scores ranged from zero (no plaque in any segment in either artery) to eight affected segments. (3) Thickness of the far wall of the common carotid artery (CIMT), which was measured at end-diastole using electronic calipers, on several cycles and averaged.14 Wall thickness was not measured at the level of a plaque. Left and right wall thicknesses were averaged and the mean thickness of the two (in mm) was calculated.
Statistical Analysis
Urine arsenic and arsenic species concentrations were divided by urinary creatinine to account for dilution in the spot samples and log-transformed for the analyses. Plaque score was analyzed as log-transformed after adding 1. After graphical display, we decided that it was appropriate to model CIMT in the original scale. We estimated the mean difference of CIMT, the relative risk of presence of atherosclerotic plaque and the geometric mean ratio of plaque score based on differences in arsenic levels and others risk factors, including arsenic methylation capacity (assessed as the relative proportions of iAs, MMA and DMA over the sum of the three). Arsenic was modeled as continuous (comparing participants in the 80th vs. 20th percentile), as quartiles (comparing quartiles 2, 3, and 4 to the lowest one), and as restricted quadratic splines with knots at the 10th, 50th, and 90th percentiles. The relative risk for the presence of atherosclerotic plaque were estimated from robust Poisson regression models.29 The geometric mean ratios of plaque score and the mean differences of carotid intima media thickness and the corresponding 95% confidence intervals were estimated using linear regression models. All models were progressively adjusted as follows: model 1 was unadjusted. Model 2 was adjusted for age and sex. Model 3 was additionally adjusted for smoking status and education. Model 4 was adjusted for all prior variables as well as BMI, eGFR, hypertension and diabetes.
In sensitivity analyses, we further adjusted for study community, cigarette pack-years, alcohol drinking status, systolic blood pressure, and fasting glucose levels with similar findings (data not shown). We evaluated the associations between no-creatinine-corrected arsenic and the three vascular measurements, treating creatinine as a covariate (Supplemental Material). We conducted models for CIMT, presence of plaque and plaque score, evaluating the effect modification between age groups, sex, smoking status, hypertension and diabetes status by adding the interaction term of arsenic levels and these covariates in the models. We found no evidence of effect modification for any of those variables (data not shown). Finally, we evaluated the associations between arsenic and the three vascular measurements in men, women, never smokers, former smokers, current smokers, non-diabetic and diabetic participants, separately, with essentially consistent results (Supplemental Material). All analyses were performed with Stata version 13.1 (College Station, TX, USA) and R software version 3.3.1 (Vienna, Austria).
Results
The overall mean (SD) of CIMT was 0.75 (0.15) mm and the prevalence of plaque in one or more carotid arterial segments was 64.7% (plaque score 1=21.5%, 2=19.9%, ≥3=23.3%) (Table 1). Most participants had <50% carotid artery stenosis (61.7%). An additional 1.8% of participants had between 50-74% stenosis and 0.9% had >75% stenosis. The remainder had no stenosis. The prevalence of traditional vascular risk factors was high (mean BMI 31.0 kg/m2, diabetes 45.7%, hypertension 34.2%, and past or present smokers 66.7%). Participants with plaque were older, more often male and current smokers and more likely to have hypertension, diabetes, higher total cholesterol, triglycerides and LDL-cholesterol and lower eGFR levels (Table 1). Average BMI was higher among participants without carotid plaque, although the average BMI of those with and without plaque were both in the obese category (30.2 vs 32.5 kg/m2, p<0.001). These same patterns in participants' characteristics were observed among participants with plaque score > 1 and participants with CIMT above the median (Supplemental Table 1). The overall median (interquartile range) concentration for the sum of inorganic and methylated arsenic species was 9.2 (5.6, 14.7) μg/g creatinine.
Table 1. Participants' characteristics overall and by presence of plaque in common carotid artery.
| Characteristic | Overall | No Carotid Plaque | Carotid Plaque |
|---|---|---|---|
| N | 2,402 | 852 | 1,550 |
| Age, years | 55.3 (7.6) | 52.6 (6.5) | 56.8 (7.8) |
| Women (%) | 63.1 | 70.1 | 59.2 |
| Region (%) | |||
| Arizona | 32.9 | 40.5 | 28.7 |
| Oklahoma | 34.4 | 35.9 | 33.6 |
| North/South Dakota | 32.7 | 23.6 | 37.7 |
| Educational attainment, years | 11.3 (3.0) | 11.6 (2.9) | 11.2 (3.0) |
| Smoking (%) | |||
| Current | 31.9 | 25.2 | 35.6 |
| Former | 34.8 | 36.2 | 34.1 |
| Never | 33.3 | 38.6 | 30.3 |
| Cigarette pack-years | 15.3 (19.3) | 12.1 (17.0) | 16.8 (20.2) |
| Alcohol use (%) | |||
| Current | 41.3 | 40.7 | 41.6 |
| Former | 42.4 | 42.6 | 42.3 |
| Never | 16.3 | 16.7 | 16.1 |
| Body mass index, kg/m2 | 31.0 (6.1) | 32.5 (6.6) | 30.2 (5.6) |
| Waist-to-hip ratio | 0.95 (0.07) | 0.94 (0.07) | 0.95 (0.07) |
| Hypertension (%) | 34.2 | 29.3 | 36.9 |
| Systolic blood pressure, mmHg | 125.5 (18.5) | 122.7 (16.1) | 127.1 (19.5) |
| Diabetes (%) | 45.7 | 38.4 | 49.7 |
| Fasting blood glucose, ng/mL | 145.3 (72.1) | 136.9 (67.3) | 150.0 (74.2) |
| Hemoglobin A1c, % | 6.56 (2.31) | 6.30 (2.24) | 6.70 (2.34) |
| Serum total cholesterol, mg/dL | 191.6 (37.3) | 183.4 (36.2) | 196.0 (37.1) |
| Serum triglycerides, mg/dL | 145.2 (113.3) | 135.7 (117.1) | 150.3 (110.9) |
| Serum HDL cholesterol, mg/dL | 46.0 (13.1) | 46.5 (12.7) | 45.8 (13.3) |
| Serum LDL cholesterol, mg/dL | 117.0 (32.7) | 110.2 (31.2) | 120.8 (32.9) |
| eGFR, mL/min/1.73m2 | 99.5 (15.9) | 102.5 (14.2) | 97.8 (16.5) |
| Cardiovascular disease (%) | 5.1 | 3.4 | 6.1 |
| Arsenic, μg/g creatinine | 12.1 (10.4) | 11.5 (8.8) | 12.4 (11.2) |
| Arsenic, median (25th, 75th percentile), μg/g | 9.2 (5.6, 14.7) | 9.1 (5.6, 14.6) | 9.3 (5.7, 14.9) |
| iAs, median (25th, 75th percentile), μg/g | 0.69 (0.33, 1.37) | 0.67 (0.33, 1.32) | 0.7 (0.34, 1.4) |
| MMA, median (25th, 75th percentile), μg/g | 1.24 (0.73, 2.10) | 1.15 (0.70, 1.93) | 1.29 (0.75, 2.19) |
| DMA, median (25th, 75th percentile), μg/g | 7.02 (4.34, 11.6) | 6.92 (4.32, 11.5) | 7.09 (4.35, 11.6) |
| iAs%, median (25th, 75th percentile) | 7.8 (5.6, 10.8) | 7.8 (5.5, 10.8) | 7.8 (5.6, 10.9) |
| MMA%, median (25th, 75th percentile) | 13.8 (10.8, 17.3) | 13.4 (10.3, 16.8) | 14.1 (11.0, 17.7) |
| DMA%, median (25th, 75th percentile) | 77.9 (72.0, 82.7) | 78.4 (72.7, 83.1) | 77.6 (71.7, 82.5) |
| CIMT, mm | 0.75 (0.15) | 0.70 (0.13) | 0.77 (0.16) |
| Plaque score, median (25th, 75th percentile) | 1 (0, 2) | ---- | 2 (1, 3) |
Data are mean (SD) unless otherwise indicated. HDL: high density lipoprotein. Abbreviations: eGFR, estimated glomerular filtration rate; CIMT, carotid intima media thickness.
After multivariable adjustment, the mean difference (95% CI) in CIMT comparing participants in the 80th vs. 20th percentile in urine arsenic concentrations was 0.01 (0.00, 0.02 mm) (p=0.008) (Table 2, Model 4). The corresponding difference comparing the highest to the lowest quartile of urine arsenic concentrations was 0.02 mm (95% CI: (0.00, 0.04), p=0.03) (Table 2, Model 4). The magnitude of the association for arsenic with CIMT was similar to that of an interquintile range of LDL-cholesterol levels (difference in CIMT 0.01mm (0.00, 0.02, p=0.03)). Flexible dose-response modeling (Figure 1, left panel) shows that the mean difference of CIMT levels increased with increasing arsenic levels (p-value for linearity 0.001 and p-value for non-linearity 0.48).
Table 2. Crude and adjusted mean difference (95%confidence interval) in common carotid intima media thickness (mm) by arsenic levels and traditional vascular risk factors (N=2,402).
| Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|
| Arsenic, p80 vs. p20 | 0.01 (0.00, 0.02) | 0.02 (0.01, 0.03) | 0.02 (0.01, 0.03) | 0.01 (0.00, 0.02) |
| Arsenic quartiles | ||||
| < 5.64 μg/g | 0.00 (reference) | 0.00 (reference) | 0.00 (reference) | 0.00 (reference) |
| 5.65-9.24 | 0.01 (-0.01, 0.02) | 0.01 (0.00, 0.03) | 0.01 (0.00, 0.03) | 0.01 (-0.01, 0.02) |
| 9.25-14.75 | 0.01 (0.00, 0.03) | 0.02 (0.00, 0.03) | 0.02 (0.00, 0.03) | 0.01 (0.00, 0.03) |
| 14.76-123.61 | 0.02 (0.00, 0.03) | 0.03 (0.01, 0.04) | 0.03 (0.01, 0.04) | 0.02 (0.00, 0.04) |
| iAs, p80 vs. p20 | 0.01 (0.00, 0.02) | 0.02 (0.01, 0.03) | 0.02 (0.01, 0.03) | 0.01 (0.00, 0.02) |
| MMA, p80 vs. p20 | 0.02 (0.01, 0.03) | 0.02 (0.01, 0.02) | 0.02 (0.01, 0.03) | 0.02 (0.01, 0.03) |
| DMA, p80 vs. p20 | 0.01 (0.00, 0.02) | 0.02 (0.01, 0.03) | 0.02 (0.01, 0.03) | 0.01 (0.00, 0.02) |
| iAs%, 5% increase | 0.00 (-0.01, 0.01) | 0.00 (-0.01, 0.00) | 0.00 (-0.01, 0.01) | 0.00 (-0.01, 0.01) |
| MMA%, 5% increase | 0.00 (0.00, 0.01) | 0.00 (-0.01, 0.00) | 0.00 (-0.01, 0.00) | 0.00 (0.00, 0.01) |
| DMA%, 5% increase | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.01) | 0.00 (0.00, 0.00) | 0.00 (-0.01, 0.00) |
| Age, per 10 years | 0.07 (0.06, 0.08) | 0.07 (0.06, 0.08) | 0.07 (0.06, 0.08) | 0.08 (0.07, 0.09) |
| Female vs. male | -0.04 (-0.05, -0.02) | -0.04 (-0.05, -0.03) | -0.04 (-0.05, -0.02) | -0.04 (-0.05, -0.03) |
| Education | ||||
| Some vs. no HS | 0.00 (-0.02, 0.01) | 0.00 (-0.01, 0.02) | 0.00 (-0.02, 0.02) | 0.00 (-0.02, 0.02) |
| HS vs. no HS | -0.03 (-0.05, -0.02) | -0.01 (-0.02, 0.01) | -0.01 (-0.02, 0.01) | -0.01 (-0.02, 0.01) |
| Smoking | ||||
| Former vs. never | 0.02 (0.01, 0.04) | 0.02 (0.00, 0.03) | 0.02 (0.00, 0.03) | 0.02 (0.00, 0.03) |
| Current vs. never | -0.01 (-0.02, 0.01) | 0.00 (-0.01, 0.02) | 0.00 (-0.01, 0.02) | 0.01 (0.00, 0.03) |
| Body mass index | ||||
| 25-29 vs. <25 kg/m2 | 0.03 (0.02, 0.05) | 0.04 (0.02, 0.05) | 0.04 (0.02, 0.05) | 0.03 (0.01, 0.05) |
| ≥30 vs. <25 kg/m2 | 0.03 (0.01, 0.05) | 0.04 (0.02, 0.06) | 0.04 (0.02, 0.06) | 0.03 (0.01, 0.05) |
| Hypertension (yes vs. no) | 0.04 (0.02, 0.05) | 0.01 (0.00, 0.03) | 0.01 (0.00, 0.03) | 0.01 (0.00, 0.02) |
| Diabetes (yes vs. no) | 0.04 (0.02, 0.05) | 0.03 (0.02, 0.05) | 0.03 (0.02, 0.05) | 0.03 (0.02, 0.04) |
| LDL cholesterol, p80 vs. p20 | 0.01 (-0.01, 0.02) | 0.00 (-0.01, 0.01) | 0.01 (-0.01, 0.01) | 0.01 (0.00, 0.02) |
| eGFR ≤60 vs. > 60 mL/min/1.73m2 | 0.01 (-0.03, 0.05) | -0.02 (-0.06, 0.01) | -0.02 (-0.06, 0.01) | -0.03 (-0.07, 0.01) |
eGFR: estimated glomerular filtration rate, HS: high school, LDL: low density lipoprotein
Arsenic is measured as the sum of inorganic and methylated arsenic species in urine and it was log-transformed for the analyses. The 20th and 80th percentiles (p20, p80) of arsenic were 5.00 and 17.06 μg/g creatinine, respectively.
20th and 80th percentiles (p20, p80) of iAs were 0.28 and 1.59 μg/g creatinine, respectively.
20th and 80th percentiles (p20, p80) of MMA were 0.64 and 2.38 μg/g creatinine, respectively.
20th and 80th percentiles (p20, p80) of DMA were 3.83 and 12.80 μg/g creatinine, respectively.
The 20th and 80th percentiles of LDL cholesterol were 90 and 143 mg/dL, respectively.
Model 1: Crude. Model 2: Adjusted for age (years) and sex. Model 3: Further adjusted for education (no high school/some high school/high school graduate) and smoking (never/former/current). Model 4: Further adjusted for body mass index (<25, 25-29, ≥30 kg/m2), hypertension, diabetes, LDL cholesterol (mg/dL) and eGFR (mL/min/1.73m2).
Figure 1.
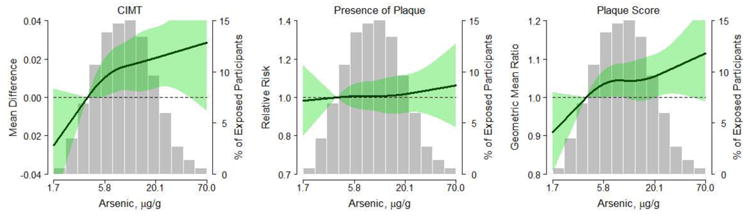
Dose-response relationship of arsenic with CIMT, presence of plaque and plaque score using restricted cubic splines. Solid lines represent adjusted estimates based on restricted quadratic splines for log transformed arsenic with knots at the 5th, 50th, and 95th percentiles. The green shade areas represent the corresponding 95th confidence intervals. The reference is set at the 10th percentile of arsenic distribution (3.76 μg/g). Adjustment factors are the same as in Tables 2 to 4 (Model 4). Vertical bars represent a histogram of urine arsenic distribution among the study participants. Nine participants in the extreme tail of the distribution are not shown in the histogram.
The relative risk (95% CI) for the presence of plaque in the carotid artery comparing participants in the 80th vs. 20th percentile in arsenic concentrations was 1.06 (1.01, 1.12) in models adjusted for age and sex (Table 3, Model 2), but was attenuated and only borderline statistically significant after adjustment for additional vascular disease risk factors (relative risk 1.04, 95% CI: 0.99, 1.09) (Table 3, Model 4). Flexible dose-response modeling graphically shows how the relative risk of the presence of plaque increased with increasing urine arsenic concentrations, although the association was not significant (Figure 1, central panel; p-value for linearity 0.14, p-value for non-linearity 0.63). Regarding the relative risk of presence of plaque based on other risk factors, age, sex, smoking, diabetes, and LDL-cholesterol remained strongly associated with presence of plaque even in multi-adjusted models, while the association with hypertension and reduced eGFR was in the expected direction but not statistically significant (Table 3, Model 4). Body mass index was inversely associated with the presence of plaque.
Table 3. Crude and adjusted relative risk (95% confidence interval) of presence of plaque in the common carotid by arsenic levels and traditional vascular risk factors (N=2,402).
| Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|
| Arsenic, p80 vs p20 | 1.04 (0.98, 1.09) | 1.06 (1.01, 1.12) | 1.05 (1.00, 1.10) | 1.04 (0.99, 1.09) |
| Arsenic quartiles | ||||
| < 5.64 μg/g | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 5.65-9.24 | 1.02 (0.93, 1.11) | 1.05 (0.97, 1.14) | 1.04 (0.96, 1.13) | 1.05 (0.97, 1.13) |
| 9.25-14.75 | 1.02 (0.94, 1.11) | 1.04 (0.95, 1.13) | 1.03 (0.94, 1.11) | 1.04 (0.96, 1.13) |
| 14.76-123.61 | 1.03 (0.95, 1.12) | 1.07 (0.98, 1.16) | 1.05 (0.97, 1.14) | 1.03 (0.95, 1.12) |
| iAs, p80 vs. p20 | 1.01 (0.99, 1.03) | 1.02 (1.00, 1.04) | 1.01 (0.99, 1.03) | 1.01 (0.99, 1.03) |
| MMA, p80 vs. p20 | 1.03 (1.01, 1.05) | 1.03 (1.01, 1.05) | 1.02 (1.00, 1.04) | 1.02 (1.00, 1.03) |
| DMA, p80 vs. p20 | 1.01 (0.99, 1.03) | 1.02 (1.00, 1.04) | 1.02 (1.00, 1.04) | 1.01 (1.00, 1.03) |
| iAs%, 5% increase | 1.00 (0.99, 1.02) | 1.00 (0.99, 1.01) | 1.00 (0.98, 1.01) | 1.00 (0.99, 1.01) |
| MMA%, 5% increase | 1.03 (1.01, 1.04) | 1.02 (1.00, 1.03) | 1.01 (1.00, 1.02) | 1.01 (0.99, 1.02) |
| DMA%, 5% increase | 0.99 (0.98, 1.00) | 0.99 (0.99, 1.00) | 1.00 (0.99, 1.00) | 1.00 (0.99, 1.01) |
| Age, per 10 years | 1.32 (1.27, 1.39) | 1.33 (1.27, 1.39) | 1.34 (1.28, 1.41) | 1.29 (1.23, 1.36) |
| Female vs. male | 0.85 (0.80, 0.90) | 0.84 (0.79, 0.89) | 0.86 (0.82, 0.92) | 0.88 (0.84, 0.94) |
| Education | ||||
| Some vs. no HS | 0.99 (0.91, 1.07) | 1.02 (0.93, 1.11) | 1.01 (0.93, 1.10) | 1.01 (0.93, 1.10) |
| HS vs. no HS | 0.87 (0.81, 0.94) | 0.96 (0.89, 1.03) | 0.96 (0.89, 1.03) | 0.94 (0.88, 1.01) |
| Smoking | ||||
| Former vs. never | 1.07 (0.99, 1.16) | 1.06 (0.98, 1.15) | 1.07 (0.99, 1.16) | 1.08 (1.00, 1.16) |
| Current vs. never | 1.22 (1.14, 1.32) | 1.26 (1.17, 1.35) | 1.26 (1.18, 1.36) | 1.26 (1.17, 1.35) |
| Body mass index | ||||
| 25-29 vs. <25 kg/m2 | 0.99 (0.91, 1.07) | 0.99 (0.91, 1.07) | 1.02 (0.94, 1.11) | 0.98 (0.91, 1.06) |
| ≥30 vs. <25 kg/m2 | 0.80 (0.74, 0.87) | 0.84 (0.77, 0.91) | 0.87 (0.80, 0.95) | 0.83 (0.77, 0.90) |
| Hypertension (yes vs. no) | 1.12 (1.06, 1.19) | 1.03 (0.97, 1.10) | 1.06 (1.00, 1.12) | 1.05 (0.99, 1.11) |
| Diabetes (yes vs. no) | 1.18 (1.11, 1.25) | 1.17 (1.10, 1.23) | 1.20 (1.13, 1.27) | 1.26 (1.19, 1.33) |
| LDL cholesterol, p80 vs. p20 | 1.21 (1.15, 1.28) | 1.20 (1.14, 1.26) | 1.20 (1.14, 1.26) | 1.21 (1.15, 1.27) |
| eGFR ≤60 vs. > 60 mL/min/1.73m2 | 1.28 (1.13, 1.45) | 1.19 (1.01, 1.39) | 1.18 (1.01, 1.39) | 1.11 (0.93, 1.33) |
eGFR: estimated glomerular filtration rate, HS: high school, LDL: low density lipoprotein
Arsenic is measured as the sum of inorganic and methylated arsenic species in urine and it was log-transformed for the analyses. The 20th and 80th percentiles (p20, p80) of arsenic were 5.00 and 17.06 μg/g creatinine, respectively
20th and 80th percentiles (p20, p80) of iAs were 0.28 and 1.59 μg/g creatinine, respectively.
20th and 80th percentiles (p20, p80) of MMA were 0.64 and 2.38 μg/g creatinine, respectively.
20th and 80th percentiles (p20, p80) of DMA were 3.83 and 12.80 μg/g creatinine, respectively.
The 20th and 80th percentiles of LDL cholesterol were 90 and 143 mg/dL, respectively.
Model 1: Crude. Model 2: Adjusted for age (years) and sex. Model 3: Further adjusted for education (no high school/some high school/high school graduate) and smoking (never/former/current). Model 4: Further adjusted for body mass index (<25, 25-29, ≥30 kg/m2), hypertension, diabetes, LDL cholesterol (mg/dL) and eGFR (mL/min/1.73m2).
The geometric mean ratio (95% CI) for plaque score comparing participants in the 80th vs. 20th percentile in arsenic levels was 1.07 (1.03, 1.12) in models adjusted for age and sex (Table 4, Model 2). This ratio attenuated to 1.05 (1.01, 1.09) in the fully adjusted model, but remained statistically significant (Table 4, Model 4). In models adjusted for age and sex the geometric mean ratios (95% CI) of plaque score comparing arsenic quartiles 2, 3 and 4 to the lowest quartile were 1.08 (1.01, 1.16), 1.07 (1.00, 1.14), and 1.09 (1.02, 1.17) respectively. These results were slightly attenuated to 1.08 (1.01, 1.15), 1.07 (1.00, 1.14), and 1.05 (0.98, 1.13) in the fully adjusted model. In flexible dose-response analysis (Figure 1, right panel), the geometric mean ratio of plaque score increased with increasing urine arsenic concentrations (p-value for linearity 0.02, p-value for non-linearity 0.43).
Table 4. Crude and adjusted geometric mean ratio (95% confidence interval) of plaque score by arsenic levels and traditional vascular risk factors (N=2,402).
| Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|
| Arsenic, p80 vs p20 | 1.05 (1.00, 1.10) | 1.07 (1.03, 1.12) | 1.06 (1.01, 1.10) | 1.05 (1.01, 1.09) |
| Arsenic quartiles | ||||
| < 5.64 μg/g | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 5.65-9.24 | 1.05 (0.98, 1.13) | 1.08 (1.01, 1.16) | 1.08 (1.01, 1.15) | 1.08 (1.01, 1.15) |
| 9.25-14.75 | 1.05 (0.98, 1.13) | 1.07 (1.00, 1.14) | 1.06 (0.99, 1.13) | 1.07 (1.00, 1.14) |
| 14.76-123.61 | 1.06 (0.98, 1.13) | 1.09 (1.02, 1.17) | 1.07 (1.00, 1.15) | 1.05 (0.98, 1.13) |
| iAs, p80 vs. p20 | 1.04 (1.00, 1.08) | 1.06 (1.02, 1.10) | 1.03 (0.99, 1.08) | 1.04 (0.99, 1.08) |
| MMA, p80 vs. p20 | 1.08 (1.04, 1.13) | 1.08 (1.04, 1.13) | 1.06 (1.02, 1.10) | 1.05 (1.01, 1.09) |
| DMA, p80 vs. p20 | 1.04 (0.99, 1.08) | 1.07 (1.02, 1.11) | 1.05 (1.01, 1.10) | 1.05 (1.00, 1.09) |
| iAs%, 5% increase | 1.01 (0.98, 1.04) | 1.01 (0.99, 1.04) | 1.00 (0.97, 1.02) | 1.01 (0.98, 1.03) |
| MMA%, 5% increase | 1.05 (1.03, 1.08) | 1.03 (1.01, 1.06) | 1.02 (0.99, 1.04) | 1.01 (0.99, 1.03) |
| DMA%, 5% increase | 0.98 (0.96, 0.99) | 0.98 (0.97, 1.00) | 0.99 (0.98, 1.01) | 0.99 (0.98, 1.01) |
| Age, per 10 years | 1.30 (1.26, 1.34) | 1.30 (1.26, 1.34) | 1.32 (1.28, 1.36) | 1.26 (1.22, 1.30) |
| Female vs. male | 0.87 (0.83, 0.92) | 0.86 (0.82, 0.91) | 0.89 (0.85, 0.94) | 0.91 (0.86, 0.95) |
| Education | ||||
| Some vs. no HS | 0.95 (0.88, 1.03) | 0.98 (0.91, 1.05) | 0.98 (0.91, 1.05) | 0.97 (0.91, 1.04) |
| HS vs. no HS | 0.85 (0.80, 0.91) | 0.94 (0.88, 1.00) | 0.94 (0.88, 1.00) | 0.93 (0.87, 0.98) |
| Smoking | ||||
| Former vs. never | 1.06 (1.00, 1.13) | 1.06 (1.00, 1.12) | 1.07 (1.01, 1.13) | 1.07 (1.01, 1.13) |
| Current vs. never | 1.21 (1.14, 1.29) | 1.27 (1.20, 1.35) | 1.27 (1.20, 1.35) | 1.28 (1.20, 1.35) |
| Body mass index | ||||
| 25-29 vs. <25 kg/m2 | 0.99 (0.91, 1.07) | 0.99 (0.92, 1.07) | 1.03 (0.95, 1.10) | 0.98 (0.91, 1.05) |
| ≥30 vs. <25 kg/m2 | 0.82 (0.76, 0.88) | 0.86 (0.80, 0.92) | 0.90 (0.84, 0.97) | 0.84 (0.79, 0.90) |
| Hypertension (yes vs. no) | 1.18 (1.12, 1.24) | 1.09 (1.03, 1.14) | 1.11 (1.06, 1.17) | 1.10 (1.04, 1.15) |
| Diabetes (yes vs. no) | 1.19 (1.14, 1.25) | 1.18 (1.13, 1.24) | 1.21 (1.16, 1.27) | 1.27 (1.21, 1.33) |
| LDL cholesterol, p80 vs. p20 | 1.18 (1.14, 1.23) | 1.17 (1.13, 1.22) | 1.17 (1.13, 1.22) | 1.18 (1.14, 1.23) |
| eGFR ≤60 vs. > 60 mL/min/1.73m2 | 1.59 (1.35, 1.88) | 1.41 (1.20, 1.64) | 1.41 (1.21, 1.64) | 1.30 (1.12, 1.51) |
eGFR: estimated glomerular filtration rate, HS: high school, LDL: low density lipoprotein
Arsenic is measured as the sum of inorganic and methylated arsenic species in urine and it was log-transformed for the analyses. The 20th and 80th percentiles (p20, p80) of arsenic were 5.00 and 17.06 μg/g creatinine, respectively
20th and 80th percentiles (p20, p80) of iAs were 0.28 and 1.59 μg/g creatinine, respectively.
20th and 80th percentiles (p20, p80) of MMA were 0.64 and 2.38 μg/g creatinine, respectively.
20th and 80th percentiles (p20, p80) of DMA were 3.83 and 12.80 μg/g creatinine, respectively.
The 20th and 80th percentiles of LDL cholesterol were 90 and 143 mg/dL, respectively.
Model 1: Crude. Model 2: Adjusted for age (years) and sex. Model 3: Further adjusted for education (no high school/some high school/high school graduate) and smoking (never/former/current). Model 4: Further adjusted for body mass index (<25, 25 -29, ≥30 kg/m2), hypertension, diabetes, LDL cholesterol (mg/dL) and eGFR (mL/min/1.73m2).
The associations of iAs, MMA and DMA concentrations with CIMT, presence of plaque and plaque score were consistently positive, small and not always statistically significant (Tables 2, 3 and 4). Arsenic methylation capacity was not associated with any of the three vascular measurements. In sensitivity analyses controlling for urine creatinine as a covariate in the models, the association of arsenic concentration was stronger for CIMT and weaker for presence of plaque and plaque score (Supplemental Tables 2, 3 and 4).
Discussion
In this prospective study of 2,402 adults with low-to-moderate chronic arsenic exposure, we found a small positive association of arsenic levels in individuals' urine with mean CIMT and the extent of carotid atherosclerosis as measured by plaque score. The association of arsenic with the presence of plaque was positive but not statistically significant in the fully adjusted model.
Although other cohort studies reported an association between arsenic exposure and CIMT,9, 10, 30 or included plaque in their definition of carotid atherosclerosis,11, 31 only one other study,8 in Taiwan, has reported the association of arsenic and discrete carotid artery plaque. The odds ratio of carotid atherosclerosis (defined as either CIMT ≥1.0mm or plaque ≥50% of CIMT compared to adjacent areas) was 3.1 (95% CI 1.3 to 7.4) among Taiwanese with a cumulative arsenic exposure of ≥20mg/L-years versus those without arsenic exposure from drinking well water, after adjustment for other vascular risk factors. The relationship between high arsenic exposure and discrete carotid plaque was not statistically significant (odds ratio 2.3, 95% CI 0.8-6.4). Notably, that population was exposed to very high levels of arsenic in drinking water (>150μg/L) and arsenic was measured in drinking water at the community level. In a recent systematic review of arsenic and vascular disease,7 nine studies specifically reported the relationship between arsenic exposure and stroke, all of which lacked individuated arsenic measurements. Studies of Mexican children and Bangladeshi adults measured urinary arsenic concentrations in individuals, but did not include the presence or extent of atherosclerosis.9, 30
The prevalence of diabetes mellitus, smoking, obesity, and hypertension was high in the Strong Heart Study. This cohort was at high risk for developing carotid atherosclerosis. CIMT levels in the Strong Heart Study were higher than in other studies.32 For example, the mean CIMT in the US Atherosclerosis Risk in Communities (vascular disease free participants, mean age 57 years)33 was 0.60 mm in women and 0.66 mm in men. In the Vascular Aging Study (mean age 65 years), the mean CIMT was 0.65 mm in women and 0.69 mm in men.34 Higher CIMTs are generally reported in the oldest age groups, including the Seven Countries Study (mean 1.5 mm, age range 70 to 89 years).35 Plaque prevalence is also high here, given a range of 24% in the MONICA Project in Germany (ages 25-65 years)36 to 93% in Finland in the Seven Countries Study.35 We are uncertain how the high prevalence of established vascular risk factors affected the inference on the relationship between arsenic exposure and carotid artery disease, but assume that this cohort serves as an enriched sample in whom to observe any putative relationships on carotid vascular disease.
CIMT increase and presence of plaque are correlated and may both predict the long-term risk of stroke and coronary heart disease. CIMT and plaque represent different steps in the biological development and progression of carotid arterial disease. Atherosclerotic plaques result from the deposition of cholesterol, activated macrophages, and other inflammatory cells on the endothelial layer. CIMT thickening may represent medial hypertrophy or hyperplasia of the carotid artery due to smooth muscle proliferation from shear and tensile stress. Thickening of the medial layer is more reflective of chronic high stress on the smooth muscle wall and thus more strongly related to hypertension. As such, CIMT may reflect effects of higher blood pressure and aging independent of atherosclerosis. We found no effect modification between arsenic and age or arsenic and the presence of hypertension. Arsenic has been associated with hypertension and increased blood pressure levels in several studies, although the number of studies is relatively small, especially at low-moderate exposure levels.37, 38 A critical threshold may exist for arsenic to etiologically affect both atherosclerosis and thickening of the carotid artery wall. Gene-arsenic interactions may also increase the risk of blood pressure in certain individuals.39 It is also possible that arsenic in low-to-moderate versus high levels has a less potent or less immediate effect on the development of carotid atherosclerosis.
This study differs from other reports on the relationship between arsenic and carotid artery disease in important methodological, environmental, and clinical ways. We were able to estimate the individual's exposure using urinary arsenic, a well-established biomarker of exposure and internal dose as utilized by the U.S. EPA. Many studies in low-income populations have depended upon ecological assessment of arsenic exposure, including levels in the groundwater at the community level. The potential for unmeasured confounders is high in ecological studies,40 and retrospective determination of duration of residence is subject to recall bias. Other studies are histopathological. For example, in Antofagasta, Chile, autopsy studies of children and young adults exposed to high arsenic levels in drinking water in utero and in early life (∼600μg/L) showed fibrous intimal thickening of small and medium-sized arteries, and plaque was not described.41, 42, 43
The Strong Heart Study cohort is prospective, allowing for the consideration of relevant behavioral and biological risk factors at study baseline. This large, well-characterized cohort is ideally suited to study carotid atherosclerosis. Our results show that the traditional risk factors for higher CIMT and carotid atherosclerosis, including age, sex, systolic blood pressure, diabetes, smoking, and waist-to-hip ratio, are also important risk factors in American Indians. While confirming these recognized risk factors, we identified a dose dependent association of arsenic with CIMT and burden of atherosclerotic plaque. Our study is also the first to report on the risk of carotid disease in the setting of lower than toxic levels of arsenic, an exposure much more relevant to most populations.
Our study also had several limitations. Carotid atherosclerosis measures were not available at baseline. Our follow up period was 6 to 9 years. While the number of educational years attained is used here as a proxy for socioeconomic status, it is an imperfect measure for addressing all of the various exposures and behaviors that accompany low income. Arsenic exposure itself could act as a proxy for other exposures and behaviors related to atherosclerosis. At the same time, adjustment for the community or education or both could result in over adjustment, since arsenic exposure tracks with location and socioeconomic status. Conversely, there is a possibility of residual confounding. In sensitivity analyses, adjusting for study community, the results were similar. We cannot rule out survival bias since some participants may have had fatal diseases that were related to arsenic exposure, such as fatal vascular events, cancer, or other illnesses. Only participants who survived to the point of the follow up study visit could thus be observed in our study sample. In this scenario, it is possible that the associations that we were able to observe are attenuated compared to the actual ones.
In summary, we observed a positive although small association between low-to-moderate exposure to inorganic arsenic on CIMT and extent of atherosclerosis. The association with presence of plaque was positive but not statistically significant. However, we cannot discard an attenuation of the associations due to survival bias. Overall, our results improve the quality of evidence for the relationship between carotid atherosclerosis and arsenic. Since the assignment of arsenic exposure can never be ethically assigned in a randomized experimental setting, the level of evidence for arsenic exposure and health risk necessarily rests upon high quality prospective data. Although this study involved the carotid arteries, atherosclerosis is a diffuse, generalized process in the body, and imaging findings of the carotid arteries may be considered an in vivo biomarker of general vascular health.
Supplementary Material
Highlights.
We evaluated the long-term association of arsenic with carotid vascular disease.
2402 American Indians, 45 to 74 years old, with urine arsenic measurements.
Arsenic was associated with increased CIMT and carotid segments containing plaque.
The associations could be attenuated due to survival bias.
Results improve the evidence for the relationship of carotid atherosclerosis and arsenic.
Acknowledgments
Study Funding: The Strong Heart Study was funded by the National Heart, Lung, and Blood Institute (NHLBI grant HL090863, HL41642, HL41654, HL65521) and by the National Institute of Environmental Health Sciences (R01ES021367, R01ES025216). Dr. Mateen received salary support from a fellowship grant from the Canadian Institute of Health Research. Dr. Katherine Moon was supported by NHLBI training grant 5T32HL007024.
Footnotes
Disclosures: All authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.World Health Organization. International Programme on Chemical Safety. Ten Chemicals of Major Public Health Concern. 2015 Available from: http://www.who.int/ipcs/assessment/public_health/chemicals_phc/en/
- 2.Navas-Acien A, Umans JG, Howard BV, Goessler W, Francesconi KA, Crainiceanu CM, Silbergeld EK, Guallar E. Urine Arsenic Concentrations and Species Excretion Patterns in American Indian Communities over a 10-Year Period: The Strong Heart Study. Environ Health Perspect. 2009;117:1428–33. doi: 10.1289/ehp.0800509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmidt CW. Low-Dose Arsenic: In Search of a Risk Threshold. Environ Health Perspect. 2014;122:A130–4. doi: 10.1289/ehp.122-A130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moon KA, Guallar E, Umans JG, Devereux RB, Best LG, Francesconi KA, Goessler W, Pollak J, Silbergeld EK, Howard BV, Navas-Acien A. Association between Exposure to Low to Moderate Arsenic Levels and Incident Cardiovascular Disease. A Prospective Cohort Study. Ann Intern Med. 2013;159:649–59. doi: 10.7326/0003-4819-159-10-201311190-00719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y, Graziano JH, Parvez F, Liu M, Slavkovich V, Kalra T, Argos M, Islam T, Ahmed A, Rakibuz-Zaman M, Hasan R, Sarwar G, Levy D, van Geen A, Ahsan H. Arsenic Exposure from Drinking Water and Mortality from Cardiovascular Disease in Bangladesh: Prospective Cohort Study. BMJ. 2011;342:d2431. doi: 10.1136/bmj.d2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu F, Molinaro P, Chen Y. Arsenic Exposure and Subclinical Endpoints of Cardiovascular Diseases. Curr Environ Health Rep. 2014;1:148–162. doi: 10.1007/s40572-014-0011-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moon K, Guallar E, Navas-Acien A. Arsenic Exposure and Cardiovascular Disease: An Updated Systematic Review. Curr Atheroscler Rep. 2012;14:542–55. doi: 10.1007/s11883-012-0280-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang CH, Jeng JS, Yip PK, Chen CL, Hsu LI, Hsueh YM, Chiou HY, Wu MM, Chen CJ. Biological Gradient between Long-Term Arsenic Exposure and Carotid Atherosclerosis. Circulation. 2002;105:1804–9. doi: 10.1161/01.cir.0000015862.64816.b2. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y, Wu F, Graziano JH, Parvez F, Liu M, Paul RR, Shaheen I, Sarwar G, Ahmed A, Islam T, Slavkovich V, Rundek T, Demmer RT, Desvarieux M, Ahsan H. Arsenic Exposure from Drinking Water, Arsenic Methylation Capacity, and Carotid Intima-Media Thickness in Bangladesh. Am J Epidemiol. 2013;178:372–81. doi: 10.1093/aje/kwt001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li WF, Sun CW, Cheng TJ, Chang KH, Chen CJ, Wang SL. Risk of Carotid Atherosclerosis Is Associated with Low Serum Paraoxonase (Pon1) Activity among Arsenic Exposed Residents in Southwestern Taiwan. Toxicol Appl Pharmacol. 2009;236:246–53. doi: 10.1016/j.taap.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 11.Huang YL, Hsueh YM, Huang YK, Yip PK, Yang MH, Chen CJ. Urinary Arsenic Methylation Capability and Carotid Atherosclerosis Risk in Subjects Living in Arsenicosis-Hyperendemic Areas in Southwestern Taiwan. Sci Total Environ. 2009;407:2608–14. doi: 10.1016/j.scitotenv.2008.12.061. [DOI] [PubMed] [Google Scholar]
- 12.Monrad M, Ersboll AK, Sorensen M, Baastrup R, Hansen B, Gammelmark A, Tjonneland A, Overvad K, Raaschou-Nielsen O. Low-Level Arsenic in Drinking Water and Risk of Incident Myocardial Infarction: A Cohort Study. Environ Res. 2017;154:318–324. doi: 10.1016/j.envres.2017.01.028. [DOI] [PubMed] [Google Scholar]
- 13.D'Ippoliti D, Santelli E, De Sario M, Scortichini M, Davoli M, Michelozzi P. Arsenic in Drinking Water and Mortality for Cancer and Chronic Diseases in Central Italy, 1990-2010. PLoS One. 2015;10:e0138182. doi: 10.1371/journal.pone.0138182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roman MJ, Kizer JR, Best LG, Lee ET, Howard BV, Shara NM, Devereux RB. Vascular Biomarkers in the Prediction of Clinical Cardiovascular Disease: The Strong Heart Study. Hypertension. 2012;59:29–35. doi: 10.1161/HYPERTENSIONAHA.111.181925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Welty TK, Lee ET, Yeh J, Cowan LD, Go O, Fabsitz RR, Le NA, Oopik AJ, Robbins DC, Howard BV. Cardiovascular Disease Risk Factors among American Indians. The Strong Heart Study. Am J Epidemiol. 1995;142:269–87. doi: 10.1093/oxfordjournals.aje.a117633. [DOI] [PubMed] [Google Scholar]
- 16.Lee ET, Welty TK, Fabsitz R, Cowan LD, Le NA, Oopik AJ, Cucchiara AJ, Savage PJ, Howard BV. The Strong Heart Study. A Study of Cardiovascular Disease in American Indians: Design and Methods. Am J Epidemiol. 1990;132:1141–55. doi: 10.1093/oxfordjournals.aje.a115757. [DOI] [PubMed] [Google Scholar]
- 17.Stoddart ML, Jarvis B, Blake B, Fabsitz RR, Howard BV, Lee ET, Welty TK. Recruitment of American Indians in Epidemiologic Research: The Strong Heart Study. Am Indian Alsk Native Ment Health Res. 2000;9:20–37. doi: 10.5820/aian.0903.2000.20. [DOI] [PubMed] [Google Scholar]
- 18.Scheer J, Findenig S, Goessler W, Francesconi KA, Howard B, Umans JG, Pollak J, Tellez-Plaza M, Silbergeld EK, Guallar E, Navas-Acien A. Arsenic Species and Selected Metals in Human Urine: Validation of Hplc/Icpms and Icpms Procedures for a Long-Term Population-Based Epidemiological Study. Anal Methods. 2012;4:406–413. doi: 10.1039/C2AY05638K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pomroy C, Charbonneau SM, McCullough RS, Tam GK. Human Retention Studies with 74as. Toxicol Appl Pharmacol. 1980;53:550–6. doi: 10.1016/0041-008x(80)90368-3. [DOI] [PubMed] [Google Scholar]
- 20.Cullen W, Reimer K. Arsenic Speciation in the Environment. 1989:713–764. [Google Scholar]
- 21.The Seventh Report of the Joint National Committee. prevention, Detection, Evaluation, and Treatment of High Blood Pressure - Complete Report. 2004 [Google Scholar]
- 22.Alberti KG, Zimmet PZ. Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications. Part 1: Diagnosis and Classification of Diabetes Mellitus Provisional Report of a Who Consultation. Diabet Med. 1998;15:539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 23.National Cholesterol Education Program (NCEP) Expert Panel on Detection E, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (Ncep) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel Iii) Final Report. 2002:3143–421. [PubMed] [Google Scholar]
- 24.Shara NM, Wang H, Mete M, Al-Balha YR, Azalddin N, Lee ET, Franceschini N, Jolly SE, Howard BV, Umans JG. Estimated Gfr and Incident Cardiovascular Disease Events in American Indians: The Strong Heart Study. Am J Kidney Dis. 2012;60:795–803. doi: 10.1053/j.ajkd.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A New Equation to Estimate Glomerular Filtration Rate. Ann Intern Med. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tahmasebpour HR, Buckley AR, Cooperberg PL, Fix CH. Sonographic Examination of the Carotid Arteries. RadioGraphics. 2005;25:1561–1575. doi: 10.1148/rg.256045013. [DOI] [PubMed] [Google Scholar]
- 27.Salonen R, Seppänen K, Rauramaa R, Salonen JT. Prevalence of Carotid Atherosclerosis and Serum Cholesterol Levels in Eastern Finland. Arteriosclerosis. 1988;8:788–92. doi: 10.1161/01.atv.8.6.788. [DOI] [PubMed] [Google Scholar]
- 28.Hollander M, Bots ML, Del Sol AI, Koudstaal PJ, Witteman JC, Grobbee DE, Hofman A, Breteler MM. Carotid Plaques Increase the Risk of Stroke and Subtypes of Cerebral Infarction in Asymptomatic Elderly: The Rotterdam Study. Circulation. 2002;105:2872–7. doi: 10.1161/01.cir.0000018650.58984.75. [DOI] [PubMed] [Google Scholar]
- 29.Zou G. A Modified Poisson Regression Approach to Prospective Studies with Binary Data. Am J Epidemiol. 2004;159:702–6. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 30.Osorio-Yáñez C, Ayllon-Vergara JC, Aguilar-Madrid G, Arreola-Mendoza L, Hernández-Castellanos E, Barrera-Hernández A, De Vizcaya-Ruiz A, Del Razo LM. Carotid Intima-Media Thickness and Plasma Asymmetric Dimethylarginine in Mexican Children Exposed to Inorganic Arsenic. Environ Health Perspect. 2013;121:1090–6. doi: 10.1289/ehp.1205994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsieh YC, Hsieh FI, Lien LM, Chou YL, Chiou HY, Chen CJ. Risk of Carotid Atherosclerosis Associated with Genetic Polymorphisms of Apolipoprotein E and Inflammatory Genes among Arsenic Exposed Residents in Taiwan. Toxicol Appl Pharmacol. 2008;227:1–7. doi: 10.1016/j.taap.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 32.Ebrahim S, Papacosta O, Whincup P, Wannamethee G, Walker M, Nicolaides AN, Dhanjil S, Griffin M, Belcaro G, Rumley A, Lowe GD. Carotid Plaque, Intima Media Thickness, Cardiovascular Risk Factors, and Prevalent Cardiovascular Disease in Men and Women: The British Regional Heart Study. Stroke. 1999;30:841–50. doi: 10.1161/01.str.30.4.841. [DOI] [PubMed] [Google Scholar]
- 33.Heiss G, Sharrett AR, Barnes R, Chambless LE, Szklo M, Alzola C. Carotid Atherosclerosis Measured by B-Mode Ultrasound in Populations: Associations with Cardiovascular Risk Factors in the Aric Study. Am J Epidemiol. 1991;134:250–6. doi: 10.1093/oxfordjournals.aje.a116078. [DOI] [PubMed] [Google Scholar]
- 34.Bonithon-Kopp C, Touboul PJ, Berr C, Leroux C, Mainard F, Courbon D, Ducimetière P. Relation of Intima-Media Thickness to Atherosclerotic Plaques in Carotid Arteries. The Vascular Aging (Eva) Study. Arterioscler Thromb Vasc Biol. 1996;16:310–6. doi: 10.1161/01.atv.16.2.310. [DOI] [PubMed] [Google Scholar]
- 35.Salonen R, Tervahauta M, Salonen JT, Pekkanen J, Nissinen A, Karvonen MJ. Ultrasonographic Manifestations of Common Carotid Atherosclerosis in Elderly Eastern Finnish Men. Prevalence and Associations with Cardiovascular Diseases and Risk Factors. Arterioscler Thromb. 1994;14:1631–40. doi: 10.1161/01.atv.14.10.1631. [DOI] [PubMed] [Google Scholar]
- 36.Gostomzyk JG, Heller WD, Gerhardt P, Lee PN, Keil U. B-Scan Ultrasound Examination of the Carotid Arteries within a Representative Population (Monica Project Augsburg) Klin Wochenschr. 1988;66(11):58–65. [PubMed] [Google Scholar]
- 37.Abhyankar LN, Jones MR, Guallar E, Navas-Acien A. Arsenic Exposure and Hypertension: A Systematic Review. Environ Health Perspect. 2012;120:494–500. doi: 10.1289/ehp.1103988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang J, Liu M, Parvez F, Wang B, Wu F, Eunus M, Bangalore S, Newman JD, Ahmed A, Islam T, Rakibuz-Zaman M, Hasan R, Sarwar G, Levy D, Slavkovich V, Argos M, Bryan MS, Farzan SF, Hayes RB, Graziano JH, Ahsan H, Chen Y. Association between Arsenic Exposure from Drinking Water and Longitudinal Change in Blood Pressure among Heals Cohort Participants. Environ Health Perspect. 2015;123:806–12. doi: 10.1289/ehp.1409004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Farzan SF, Karagas MR, Jiang J, Wu F, Liu M, Newman JD, Jasmine F, Kibriya MG, Paul-Brutus R, Parvez F, Argos M, Bryan MS, Eunus M, Ahmed A, Islam T, Rakibuz-Zaman M, Hasan R, Sarwar G, Slavkovich V, Graziano J, Ahsan H, Chen Y. Gene-Arsenic Interaction in Longitudinal Changes of Blood Pressure: Findings from the Health Effects of Arsenic Longitudinal Study (Heals) in Bangladesh. Toxicol Appl Pharmacol. 2015;288:95–105. doi: 10.1016/j.taap.2015.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greenland S, Morgenstern H. Ecological Bias, Confounding, and Effect Modification. Int J Epidemiol. 1989;18:269–74. doi: 10.1093/ije/18.1.269. [DOI] [PubMed] [Google Scholar]
- 41.Zaldivar R. A Morbid Condition Involving Cardio-Vascular, Broncho-Pulmonary, Digestive and Neural Lesions in Children and Young Adults after Dietary Arsenic Exposure. Zentralbl Bakteriol B. 1980;170:44–56. [PubMed] [Google Scholar]
- 42.Rosenberg HG. Systemic Arterial Disease and Chronic Arsenicism in Infants. Arch Pathol. 1974;97:360–5. [PubMed] [Google Scholar]
- 43.Moran S, Maturana G, Rosenberg H, Casanegra P, Dubernet J. Coronary Occlusions Associated with Chronic Arsenic Poisoning. Apropos of 2 Operated Cases. Arch Mal Coeur Vaiss. 1977;70:1115–20. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


