Abstract
Many countries recommend combined tetanus toxoid, reduced diphtheria toxoid and acellular pertussis immunization (Tdap) during pregnancy to stimulate transplacental transmission of pertussis antibodies to newborns. The immune system can be altered during pregnancy, potentially resulting in differing immunization risks in pregnant women. The safety of widespread Tdap immunization during pregnancy needs to be established. Our objective was to assess whether prenatal Tdap immunization was associated with adverse birth outcomes, and to evaluate the effect of timing of Tdap administration on these outcomes.
We identified pregnancies at delivery in a large insurance claims database (2010–2014). Tdap immunization was categorized as optimal prenatal (27+ weeks), early prenatal (<27 weeks), postpartum (≤7 days post-delivery), or none. Medical claims were searched to identify maternal adverse immunization reactions (e.g. anaphylaxis, fever, Guillian-Barre syndrome [GBS]), adverse birth outcomes (e.g. preeclampsia/eclampsia, premature rupture or membranes, chorioamnionitis) and newborn outcomes (e.g. respiratory distress, pulmonary hypertension, neonatal jaundice). Women with optimal or early prenatal Tdap were compared to those not immunized in pregnancy, using propensity score-weighted log-binomial regression and Cox proportional hazards models to estimate risk ratios (RR) and hazard ratios (HR). We identified 1,079,034 deliveries and 677,075 linked newborns; 11.5% were immunized optimally and 2.3% immunized early. There were 1 case of post-immunization anaphylaxis, and 12 cases of maternal encephalopathy (all post-delivery); there were no cases of GBS. Optimally-timed immunization was associated with small increased relative risks of: chorioamnionitis [RR=1.11, (95% CI: 1.07–1.15), overall risk=2.8%], and postpartum hemorrhage [RR=1.23 (95% DI: 1.18–1.28), overall risk=2.4%]; however, these relative increases corresponded to low absolute risk increases. Tdap was not associated with increased risk of any adverse newborn outcome. Overall, prenatal Tdap immunization was not associated with newborn adverse events, but potential associations with chorioamnionitis consistent with one previous study and postpartum hemorrhage require further investigation.
Keywords: Tdap, pregnancy, safety, epidemiology, pertussis, whooping cough, pregnancy, mother-child linkage, pharmacoepidemiology
Introduction
Tetanus toxoid, reduced diphtheria toxoid and acellular pertussis immunization (Tdap) administered during pregnancy conveys passive pertussis immunity to newborns via transplacental transmission of maternal pertussis antibodies;[1–6] thus, several countries recommend Tdap during every pregnancy, including closely spaced pregnancies.[7–9] Despite international efforts to prevent infant pertussis through maternal Tdap, prenatal uptake remains sub-optimal,[10–14] due in part to concerns about safety.[15, 16] While generally accepted as safe, widespread prenatal Tdap requires careful scrutiny to establish the safety for the mother and newborn.
Tdap has not been associated with clinically significant harms for the fetus or neonate such as preterm birth, small for gestational age, stillbirth, low birth weight, or congenital anomalies.[17] Similarly, there is no evidence of increased risk of serious adverse events in pregnancy, with the exception of unreplicated findings of increased chorioamnionitis risk from one retrospective study.[17, 18] Previous non-experimental studies have been limited by small sample size, unclear immunization timing, and confounding due to comparing guideline-adherent immunized versus non-adherent unimmunized populations. Given that a strong understanding of the safety of Tdap during pregnancy may be central to increasing uptake, additional studies are needed.
Furthermore, considerable uncertainty persists about the optimal timing of prenatal Tdap administration. The CDC recommends administration at any time during pregnancy, though preferably between gestational weeks 27–36 [19] to maximize maternal antibody response and passive antibody transfer; other countries recommend administration during the second [8] or third trimesters [9] to improve immunization coverage for preterm deliveries. Safety studies which account for the timing of immunization are limited.[17]
To examine the safety of Tdap administration during pregnancy, we conducted a large-scale, cohort study comparing the risks of adverse outcomes in the infant and mother associated with Tdap and examined the impact of timing of Tdap administration on safety.
Methods
This administrative insurance claims-based cohort study was approved by the Institutional Review Board of the University of North Carolina at Chapel Hill. Analyses were performed using SAS 9.4 (SAS Institute, Cary NC).
Data source
We utilized the MarketScan Commercial Claims and Encounters (Truven Health Analytics) insurance claims databases for the years 2010–2014 which spanned CDC’s changing Tdap recommendations. These databases contain individual-level health insurance enrollment and billing information, including inpatient and outpatient procedures and diagnoses, and pharmacy-dispensed medications for those with employer-based commercial insurance, spouses, and dependents for tens of millions of enrollees across the U.S.
Study population
We identified females with livebirth or stillbirth deliveries using International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) diagnosis codes (ICD-9-CM V27). Where possible, we linked mothers to offspring by identifying newborns within the same insurance plan family grouping with birth coding (ICD-9-CM codes V30-V37) within 7 days of the mother’s delivery code to account for slight differences in billing and enrollment timing between the mother and child[14, 20, 21]; linkage was not possible if the mothers and newborns were covered by different insurance plans. We estimated gestational age at delivery by imputing standard ages based on delivery prematurity or post-term delivery diagnoses codes from the mother and linked child.[22–24] To capture complete prenatal and postpartum immunization and clinical information, we required continuous health plan enrollment from the estimated onset of pregnancy to seven days post-delivery (Figure 1).
Figure 1.
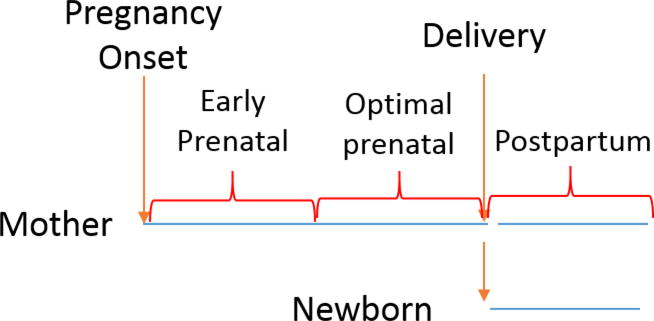
Schematic showing Tdap immunization timing by delivering women and linked newborns
We included only the first observed pregnancy per woman, and excluded pregnancies with twins or multiples due to increased complication risk and hindered ability to accurately estimate gestational age. We excluded women with estimated gestational ages at delivery ≤26 weeks as the CDC recommendation for optimal Tdap is at 27+ weeks. We also excluded women ≤18 years in 13 states with universal childhood immunization policies,[25] as immunization claims would be unavailable.
Tdap Exposure
We searched insurance claims from pregnancy onset to 7 days post-delivery for Tdap administration using billing procedure codes (Current Procedural Terminology [CPT] code 90715, or ICD-9-CM procedure codes, 99.37, 99.39, V06.1). Tdap timing was categorized as optimal (≥27 weeks gestational age according to the 2012 CDC recommendation), early (prior to 27 weeks), or postpartum (from delivery to 7 days afterward) (Figure 1).
Maternal and Newborn Characteristics
We identified clinical and demographic characteristics of women and newborns to account for confounding by differences between Tdap groups using insurance enrollment data and diagnosis or procedure claims. Maternal demographic characteristics were assessed at estimated pregnancy onset and included: age; U.S. geographic region; residence in a metropolitan statistical area (MSA) as a marker of urbanicity; other covered children on health plan; and delivery year. Clinical characteristics assessed from conception to 140 days gestational age included: markers of complicated pregnancies, such as maternal hospitalizations and emergency department visits; health care utilization, including prenatal blood panel and ultrasound receipt. Maternal comorbidities assessed from conception to delivery included hypertension, diabetes, gestational diabetes, kidney dysfunction, lupus, and use of antihypertensive, antidiabetic agents, antidepressants, or antibiotics. Newborn characteristics assessed at delivery included: sex, premature or post-dates birth, maternal preeclampsia/eclampsia, delivery by cesarean section, and meconium aspiration.
Outcomes and Analysis
We considered 3 categories of safety outcomes: maternal adverse immunization reactions, maternal birth outcomes, and newborn outcomes.
Maternal Adverse Immunization Reactions
Among immunized women, we evaluated adverse reactions following prenatal or postpartum Tdap administration. We searched claims post-immunization for: 2 days for anaphylaxis; 3 days for fever[26]; 7 days for cellulitis, and pain in limb[26, 27]; and 42 days for encephalopathy[26] and Guillan-Barre syndrome (GBS)[26, 27], We considered either inpatient or outpatient diagnoses of all outcomes except for encephalopathy(which required an inpatient diagnosis) and GBS (which required a diagnosis in the primary position [28]) to ensure accurate identification of cases. To ensure identification of new-onset cases, those experiencing encephalopathy or GBS at any time from conception to immunization, or the other acute outcomes in the 7 days prior to immunization were excluded.
We reported counts and risks of post-immunization events. Women were censored due to disenrollment from the insurance plan. Given substantial increase in healthcare utilization and other medical events occurring immediately post-partum, we did not directly compare the pre- and post-partum periods.
Maternal Birth Outcomes
Among all delivering women, insurance records were searched for pregnancy complications identified by diagnoses or procedures (eTable 1). Various time windows before, during, or after delivery were searched depending on the outcome: placental abruption, premature rupture of membranes, and caesarian section were identified on the delivery date; chorioamnionitis was identified during the delivery hospitalization; post-partum hemorrhage was identified from the delivery date through seven days post-delivery; preeclampsia/eclampsia was identified from seven days pre-delivery through 30 days post-delivery. We separately compared women with optimally-timed and early Tdap to those not immunized prior to delivery with multivariable log-binomial models to estimate adjusted risk ratios (RR) and 95% confidence intervals (CI). Models were adjusted for a priori identified maternal demographics and clinical characteristics. Propensity score (PS) methods were also used to control for confounding. A PS for Tdap receipt was estimated with logistic regression using maternal characteristics and was then transformed into stabilized inverse-probability of treatment weights (IPTW). The analysis was repeated in an IPTW-weighted population to estimate the average treatment effect in the population.[29, 30] We trimmed individuals with PS below the 0.5th and above the 99.5th percentiles of the PS distributions to reduce the influence of confounding concentrated in the tails of the PS distribution.[31] IPTW results are presented as our primary results, with multivariable adjusted models also presented for comparison.
Since preeclampsia/eclampsia required the longest follow-up (30 days post-delivery), we compared immunization groups with Cox proportional hazards models allowing for censoring due to health plan disenrollment, and estimated hazard ratios (HR) and 95% CI with follow-up beginning 7 days before delivery. The proportional hazards assumption for all Cox models was tested for all cox models by plotting Kaplan-Meier curves.
Newborn Outcomes
In the linked maternal-newborn pairs, we followed newborns for up to 30 days post-delivery for neonatal intensive care unit (NICU) admissions, respiratory distress, pulmonary hypertension, inpatient encephalopathy, seizures, neonatal sepsis, and inpatient neonatal jaundice. Follow-up began at delivery, and newborns could be censored due to health plan disenrollment. We estimated IPTW-weighted HRs and 95% CIs for newborns with optimally- or early-immunized mothers compared with newborns whose mothers were not vaccinated during pregnancy adjusted for maternal and newborn characteristics.
Sensitivity Analyses
As there may be misclassification of “early” and “optimal” timing categorizations, we evaluated any Tdap in pregnancy without respect to timing. To reduce confounding by differences in healthcare access, behaviors, and attitudes between immunization receivers and non-receivers,[32–34] we repeated all analyses restricting the cohort to women who received influenza immunization during pregnancy.
Results
We identified 1,079,034 women (mean age=29.2 years, SD 5.4 years) with deliveries meeting our study criteria (eFigure 1). Of these women, 148,817 (13.8%) received Tdap during pregnancy, and an additional 59,040 (5.5%) women received Tdap postpartum. The percentage of pregnant women receiving Tdap increased over time; cohort characteristics are shown in Table 1.
Table 1.
Characteristics of the cohort of women with deliveries after 26 weeks gestational age by Tdap immunization status
| Total N=1,079,034 |
Optimal Prenatal N=123,780 |
Early Prenatal N=25,037 |
Postpartum N=59,040 |
None N=871,177 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean Age, mean (SD) | 29.2 | 5.4 | 29.6 | 5.1 | 29.4 | 5.2 | 28.9 | 5.3 | 29.2 | 5.4 |
| Year | ||||||||||
| 2010 n, % | 182,828 | 16.9 | 1,189 | 1.0 | 462 | 1.9 | 5,942 | 10.1 | 175,235 | 20.1 |
| 2011 n, % | 236,606 | 21.9 | 2,512 | 2.0 | 2,673 | 10.7 | 14,906 | 25.3 | 216,515 | 24.9 |
| 2012 n, % | 254,731 | 23.6 | 12,094 | 9.8 | 4,963 | 19.8 | 17,630 | 29.9 | 220,044 | 25.3 |
| 2013 n, % | 188,043 | 17.4 | 34,714 | 28.0 | 7,610 | 30.4 | 10,342 | 17.5 | 135,377 | 15.5 |
| 2014 n, % | 216,826 | 20.1 | 73,271 | 59.2 | 9,329 | 37.3 | 10,2 20 | 17.3 | 124,006 | 14.2 |
| Preterm n, % | 79,677 | 7.4 | 6,154 | 5.0 | 2,593 | 10.4 | 3,962 | 6.7 | 66,968 | 7.7 |
| Other covered children on plan, mean (SD) | 1.6 | 1.2 | 1.5 | 1.1 | 1.5 | 1.1 | 1.6 | 1.1 | 1.7 | 1.2 |
| Received obstetric blood panel* n, % | 587,342 | 54.4 | 68,887 | 55.7 | 14,168 | 56.6 | 35,906 | 60.8 | 468,381 | 53.8 |
| Received ultrasound* n, % | 853,745 | 79.1 | 109,157 | 88.2 | 22,517 | 89.9 | 48,400 | 82.0 | 673,671 | 77.3 |
| Received flu immunization n, % | 239,593 | 22.2 | 59,932 | 48.4 | 12,919 | 51.6 | 12,384 | 21.0 | 154,358 | 17.7 |
| Hospitalizations, mean (SD)* | 0.01 | 0.01 | 0.01 | 0.09 | 0.01 | 0.11 | 0.01 | 0.11 | 0.01 | 0.11 |
| Emergency department visits, mean (SD)* | 0.21 | 0.67 | 0.19 | 0.61 | 0.22 | 0.71 | 0.22 | 0.67 | 0.21 | 0.67 |
| Lives in MSA n, % | 917,056 | 85.0 | 107,713 | 87.0 | 21,784 | 87.0 | 50,285 | 85.2 | 737,274 | 84.6 |
| Missing n, % | 28,150 | 2.6 | 3,942 | 3.2 | 796 | 3.2 | 1,573 | 2.7 | 21,839 | 2.5 |
| Region | ||||||||||
| Northeast n, % | 169,749 | 15.7 | 19,300 | 15.6 | 3,634 | 14.5 | 7,943 | 13.5 | 138,872 | 15.9 |
| Midwest n, % | 257,559 | 23.9 | 32,881 | 26.6 | 5,790 | 23.1 | 12,951 | 21.9 | 205,937 | 23.6 |
| South n, % | 392,581 | 36.4 | 35,264 | 28.5 | 6,509 | 26.0 | 23,531 | 39.9 | 327,277 | 37.6 |
| West n, % | 230,952 | 21.4 | 32,392 | 26.2 | 8,308 | 33.2 | 13,041 | 22.1 | 177,211 | 20.3 |
| Unknown n, % | 28,193 | 2.6 | 3,943 | 3.2 | 796 | 3.2 | 1,574 | 2.7 | 21,880 | 2.5 |
| Maternal hypertension n, % | 151,135 | 14.0 | 17,927 | 14.5 | 3,489 | 13.9 | 8,070 | 13.7 | 121,649 | 14.0 |
| Diabetes mellitus n, % | 19,751 | 1.8 | 2,060 | 1.7 | 411 | 1.6 | 952 | 1.6 | 16,328 | 1.9 |
| Gestational Diabetes n, % | 152,059 | 14.1 | 18,372 | 14.8 | 3,533 | 14.1 | 8,461 | 14.3 | 121,693 | 14.0 |
| Kidney dysfunction n, % | 2,203 | 0.2 | 266 | 0.2 | 52 | 0.2 | 75 | 0.1 | 1,810 | 0.2 |
| Lupus n, % | 2,415 | 0.2 | 271 | 0.2 | 64 | 0.3 | 104 | 0.2 | 1,976 | 0.2 |
| Antihypertensive use n, % | 36,684 | 3.4 | 3,631 | 2.9 | 769 | 3.1 | 1,902 | 3.2 | 30,382 | 3.5 |
| Antidiabetic use n, % | 30,485 | 2.8 | 4,262 | 3.4 | 850 | 3.4 | 1,469 | 2.5 | 23,904 | 2.7 |
| SSRI use n, % | 34,674 | 3.2 | 4,749 | 3.8 | 919 | 3.7 | 1,774 | 3.0 | 27,232 | 3.1 |
| Antibiotic use n, % | 298,282 | 27.6 | 34,519 | 27.9 | 6,927 | 27.7 | 17,050 | 28.9 | 239,786 | 27.5 |
| Matched to baby n, % | 677,075 | 62.8 | 80,217 | 64.8 | 16,322 | 65.2 | 36,630 | 62.0 | 543,906 | 62.4 |
assessed from pregnancy onset to 20 weeks
Abbreviations: Tdap, tetanus-diphteria-acellular pertussis immunization; SD, standard deviation; MSA, metropolitan statistical area; SSRI, serotonin selective reuptake inhibitor.
Maternal Adverse Immunization Reactions
Among the 207,857 women receiving Tdap, the most common medically-attended adverse reactions experienced were pain in limb or fever (Table 2, eTable 2 for rates). 2% of women were censored before the full 42-day follow up for GBS, yet no cases of inpatient GBS were observed. Other serious adverse reactions experienced after immunization were very rare, with only a very small number of cases occurring in the entire sample; there was 1 case of anaphylaxis in a woman vaccinated postpartum,, and 7 encephalopathy cases occurred in women vaccinated prenatally, but in all 7 cases, the 42-day follow-up overlapped the delivery, and the encephalopathy occurred during the delivery hospitalization or after.
Table 2.
Adverse immunization reactions by timing of pertussis immunization among pregnant and delivering women in the US, 2010–2014.
| Outcome | Follow-up length post-immunization | Immunization timing | Total N | Cases | Unadjusted Risks (per 10,000 women) |
|---|---|---|---|---|---|
| Anaphylaxis | 2 days | Total | 207,851 | 1 | 0.00 |
| Early | 25,035 | 0 | 0.00 | ||
| Optimal | 123,780 | 0 | 0.00 | ||
| Postpartum | 59,038 | 1 | 0.17 | ||
| Fever | 3 days | Total | 207,821 | 129 | 6.2 |
| Early | 25,033 | 13 | 5.19 | ||
| Optimal | 123,760 | 47 | 3.80 | ||
| Postpartum | 59,028 | 69 | 11.69 | ||
| Cellulitis | 7 days | Total | 207,822 | 122 | 5.87 |
| Early | 25,030 | 43 | 17.18 | ||
| Optimal | 123,763 | 27 | 2.18 | ||
| Postpartum | 59,029 | 52 | 8.81 | ||
| Pain in limb | 7 days | Total | 207,663 | 503 | 24.22 |
| Early | 25,001 | 92 | 36.80 | ||
| Optimal | 123,441 | 217 | 17.58 | ||
| Postpartum | 59,004 | 194 | 32.88 | ||
| Encephalopathy | 42 days | Total | 207,853 | 12 | 0.58 |
| Early | 25,037 | 0 | 0.00 | ||
| Optimal | 123,777 | 7 | 0.57 | ||
| Postpartum | 59,039 | 5 | 0.85 | ||
| Guillain-Barré Syndrome | 42 days | Total | 207,857 | 0 | 0.00 |
| Early | 25,037 | 0 | 0.00 | ||
| Optimal | 123,780 | 0 | 0.00 | ||
| Postpartum | 59,040 | 0 | 0.00 |
Adverse reaction risks tended to be higher for those vaccinated postpartum and early prenatal periods (Table 2), but it was difficult to disentangle the effects of an immunization from other medical conditions experienced in the delivery and postpartum period in a setting characterized by increased contact from the healthcare providers during this time; consequently, we did not make direct comparisons between the postpartum and prenatal periods.
Maternal Birth Outcomes
In the 1,079,034 women, the IPTW-weighted population resulted in good balance between covariates (eTables 3–4). The risks of many birth outcomes were similar between those who did and did not receive Tdap during pregnancy (see Table 3). However, optimally-timed Tdap was associated with a small increase in chorioamnionitis [IPTW-weighted risks: 3.3% optimally immunized women, 3.0% unimmunized women; RR=1.11 (95% CI: 1.07–1.15)] and post-partum hemorrhage [IPTW-weighted risks: 2.9% optimally immunized women, 2.4% unimmunized women; RR=1.23 (95% CI: 1.18–1.28), as compared to women who did not receive Tdap during pregnancy. Early Tdap receipt was also associated with chorioamnionitis [IPTW-weighted risks: 3.6% in early immunized women, 2.8% in unimmunized women; RR=1.19 (95% CI: 1.11–1.28)], and post-partum hemorrhage, [IPTW-weighted risks: 3.13% early immunized women, 2.3% unimmunized women; RR=1.34 (95% CI: 1.25–1.44)], and additionally with premature rupture of membranes [IPTW-weighted risks: 5.2% in early immunized women, 4.9% in unimmunized women; RR=1.08 (95% CI: 1.02–1.15)]. 4% of women were censored before the full 30-day post-delivery follow-up for eclampsia.
Table 3.
Birth outcomes by pertussis immunization status among delivering women in the US, 2010–2014.
| Immunization timing | Cases | % | Crude | Adjusted | IPTW | ||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| HR | (95% CI) | HR | (95% CI) | HR | (95% CI) | ||||
| Preeclampsia/Eclampsia | None in pregnancy | 40,930 | 4.40 | – | – | – | – | – | – |
| Optimal | 5,248 | 4.24 | 0.96 | (0.94, 0.99) | 0.90 | (0.87, 0.93) | 0.96 | (0.94, 0.99) | |
| Early | 1,096 | 4.38 | 1.00 | (0.94, 1.06) | 0.99 | (0.93, 1.05) | 1.05 | (0.99, 1.12) | |
|
|
|||||||||
| RR | (95% CI) | RR | (95% CI) | RR | (95% CI) | ||||
|
|
|||||||||
| Premature rupture of membranes | None in pregnancy | 43,485 | 4.67 | – | – | – | – | – | – |
| Optimal | 7,524 | 6.08 | 1.30 | (1.27, 1.33) | 1.08 | (1.05, 1.11) | 1.03 | (1.00, 1.06) | |
| Early | 1,418 | 5.66 | 1.21 | (1.15, 1.28) | 1.04 | (0.99, 1.10) | 1.08 | (1.02, 1.15) | |
| Chorioamnionitis | None in pregnancy | 25,149 | 2.70 | – | – | – | – | – | – |
| Optimal | 4,529 | 3.66 | 1.35 | (1.31, 1.40) | 1.14 | (1.10, 1.18) | 1.11 | (1.07, 1.15) | |
| Early | 984 | 3.93 | 1.45 | (1.37, 1.55) | 1.23 | (1.16, 1.31) | 1.19 | (1.11, 1.28) | |
| Cesarean section | None in pregnancy | 305,882 | 32.88 | – | – | – | – | – | – |
| Optimal | 37,900 | 30.62 | 0.93 | (0.92, 0.94) | 0.93 | (0.92, 0.94)_ | 0.95 | (0.94, 0.96) | |
| Early | 7,889 | 31.51 | 0.96 | (0.94, 0.98) | 0.97 | (0.95, 0.98) | 0.97 | (0.95, 0.99) | |
| Placental abruption | None in pregnancy | 7,601 | 0.82 | – | – | – | – | – | – |
| Optimal | 874 | 0.71 | 0.86 | (0.81, 0.93) | 0.82 | (0.76, 0.89) | 0.86 | (0.80, 0.93) | |
| Early | 197 | 0.79 | 0.96 | (0.84, 1.11) | 0.93 | (0.80, 1.07) | 0.88 | (0.73, 1.08) | |
| Post-partum hemorrhage | None in pregnancy | 21,120 | 2.27 | – | – | – | – | – | – |
| Optimal | 3,814 | 3.08 | 1.36 | (1.31, 1.40) | 1.21 | (1.17, 1.26) | 1.23 | (1.18, 1.28) | |
| Early | 829 | 3.31 | 1.46 | (1.36, 1.56) | 1.34 | (1.25, 1.44) | 1.34 | (1.25, 1.44) | |
Abbreviations: IPTW, inverse probability of treatment weighting; HR, hazard ratio; CI, confidence interval; RR, risk ratio.
Models adjusted for: maternal age, delivery year, maternal hospitalizations and emergency department visits, other covered children on health insurance plan, US region, residence in a Metropolitan Statistical Area, receipt of prenatal obstetric blood panel, ultrasound receipt, hypertension, diabetes, gestational diabetes, kidney dysfunction, lupus, use of antihypertensives, use of antidiabetic medications, use of antidepressants, use of antibiotics. Postpartum hemorrhage also adjusted for: maternal chorioamnionitis, eclampsia, premature rupture of membranes, cesarean section, and placental abruption.
Newborn Outcomes
Of all the delivering women, 677,075 (62.8%) successfully matched to a newborn. The distributions of baseline characteristics among mothers who successfully matched to a newborn were very similar to the overall sample, but matching mothers had slightly more routine preventive care (eTable 5–6). 4% of newborns were censored before the end of the 30-day post-delivery follow-up. Some newborn events were quite common (e.g. NICU admission, respiratory distress, and neonatal jaundice all had incidence >6%). The proportional hazards assumptions were met for all models, but optimally-timed or early prenatal Tdap were not associated with increased risks for any outcome compared to non-receivers (Table 4).
Table 4.
Newborn outcomes by maternal immunization status among linked mother-newborn pairs in the US, 2010–2014.
| Immunization timing | Cases | % | Crude | Adjusted | IPTW | ||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| HR | (95% CI) | HR | (95% CI) | HR | (95% CI) | ||||
| NICU admission | None in pregnancy | 42,904 | 7.39 | – | – | – | – | – | – |
| Optimal | 6,996 | 7.25 | 0.98 | (0.96, 1.01) | 0.97 | (0.95, 1.00) | 1.00 | (0.97, 1.03) | |
| Early | 1,458 | 8.93 | 1.22 | (1.16, 1.29) | 0.93 | (0.88, 0.98) | 0.95 | (0.89, 1.01) | |
| Respiratory distress | None in pregnancy | 37,241 | 6.41 | – | – | – | – | – | – |
| Optimal | 5,739 | 5.94 | 0.93 | (0.90, 0.95) | 0.99 | (0.96, 1.02) | 0.97 | (0.95, 1.00) | |
| Early | 1,125 | 6.89 | 1.08 | (1.02, 1.14) | 0.91 | (0.86, 0.97) | 0.94 | (0.88, 1.00) | |
| Pulmonary hypertension | None in pregnancy | 1,408 | 0.24 | – | – | – | – | – | – |
| Optimal | 205 | 0.21 | 0.88 | (0.76, 1.01) | 0.85 | (0.72, 1.00) | 0.80 | (0.67, 0.94) | |
| Early | 50 | 0.31 | 1.26 | (0.95, 1.68) | 0.99 | (0.74, 1.32) | 0.96 | (0.68, 1.35) | |
| Neonatal jaundice | None in pregnancy | 90,215 | 15.54 | – | – | – | – | – | – |
| Optimal | 14,603 | 15.13 | 0.97 | (0.96, 0.99) | 1.06 | (1.04, 1.08) | 0.98 | (0.97, 1.00) | |
| Early | 2,562 | 15.70 | 1.01 | (0.97, 1.05) | 1.00 | (0.97, 1.05) | 0.94 | (0.90, 0.98) | |
| Encephalopathy | None in pregnancy | 577 | 0.10 | – | – | – | – | – | – |
| Optimal | 135 | 0.14 | 1.41 | (1.17, 1.70) | 1.20 | (0.97, 1.49) | 1.01 | (0.80, 1.28) | |
| Early | 30 | 0.18 | 1.85 | (1.28, 2.67) | 1.36 | (0.93, 1.99) | 0.94 | (0.54, 1.61) | |
| Seizures | None in pregnancy | 1,607 | 0.28 | – | – | – | – | – | – |
| Optimal | 261 | 0.27 | 0.98 | (0.86, 1.12) | 0.95 | (0.82, 1.10) | 1.06 | (0.92, 1.21) | |
| Early | 75 | 0.46 | 1.67 | (1.32, 2.10) | 1.38 | (1.08, 1.76) | 1.18 | (0.88, 1.59) | |
| Neonatal sepsis | None in pregnancy | 13,187 | 2.27 | – | – | – | – | – | – |
| Optimal | 1,774 | 1.84 | 0.81 | (0.77, 0.85) | 0.83 | (0.79, 0.88) | 0.89 | (0.84, 0.94) | |
| Early | 394 | 2.41 | 1.07 | (0.97, 1.18) | 0.89 | (0.81, 0.99) | 0.91 | (0.81, 1.02) | |
Abbreviations: IPTW, inverse probability of treatment weighting; HR, hazard ratio; CI, confidence interval; NICU, neonatal intensive care unit.
Models adjusted for: maternal age, delivery year, maternal hospitalizations and emergency department visits, other covered children on health insurance plan, US region, residence in a Metropolitan Statistical Area, receipt of prenatal obstetric blood panel, ultrasound receipt, hypertension, diabetes, gestational diabetes, kidney dysfunction, lupus, use of antihypertensives, use of antidiabetic medications, use of antidepressants, use of antibiotics, premature delivery, postmature delivery, preeclampsia/eclampsia, caesarean section, newborn sex, and meconium aspiration.
Sensitivity Analysis
When evaluating any Tdap immunization during pregnancy without regards to the timing, the overall results were consistent with the optimal and early timing results (eTables 7–9). Among a subset of women who also received influenza immunization during pregnancy (N=239,593), overall rates of most safety events were either very similar or slightly reduced compared to the overall analyses (eTables 10–12). In many cases, the observed associations between Tdap and outcomes were attenuated to the null, though several RRs remained slightly elevated: optimally-timed Tdap, postpartum hemorrhage, RR=1.09 (95% CI: 1.03–1.16); and early Tdap, premature rupture of membranes, RR=1.11 (95% CI: 1.03–1.20), and postpartum hemorrhage, RR=1.18 (95% CI: 1.07–1.30). Among the newborn outcomes, early Tdap was associated with seizures [IPTW-weighted risks: 0.4% early immunized women, 0.3% unimmunized women; RR=1.50 (95% CI: 1.04–2.16)]; however, the number of cases was relatively small, translating into very small absolute risk increases, and this increase was not observed in the primary analysis or in the optimally-vaccinated women.
Discussion
In this cohort of 1,079,034 pregnant women in the U.S., serious maternal and infant adverse reactions following immunization were rare; for example, only 1 of 207,857 immunized women experienced anaphylaxis, and we detected no cases of GBS within 42 days after Tdap receipt. Twelve prenatally-vaccinated women were later diagnosed with encephalopathy, although all cases occurred during the delivery hospitalization or following delivery, obscuring whether the encephalopathy was associated with the immunization or with complications from the delivery itself. Also, there were no increased risks in newborn outcomes associated with maternal prenatal Tdap. However, we detected an increased risk of mothers being diagnosed with chorioamnionitis and post-partum hemorrhage associated with prenatal Tdap. Overall, the risk of chorioamnionitis and postpartum hemorrhage were small, 2.8% and 2.4%, respectively, and these observed relative increases do not translate to large overall absolute increases in birth complications. While Tdap was associated with slight reductions in the risk of some outcomes, our primary purpose was to evaluate risks, and we chose not to emphasize them.
The safety of prenatal Tdap has previously been evaluated by smaller studies set in the UK or in smaller US regions. Our cross-national US was larger than previous studies; additionally, we also evaluated the timing of Tdap exposure. The current study both confirmed an earlier-reported chorioamnionitis association and reported a new association with post-partum hemorrhage. An earlier British study did not demonstrate an association between postpartum hemorrhage and prenatal Tdap,[35] and among three prior studies assessing chorioamnionitis and prenatal Tdap,[18, 36, 37] only one demonstrated an association; the largest examined 123,494 pregnancies in regional electronic health records, of which 21% received prenatal Tdap, and found RR=1.19 (95% CI: 1.13–1.26) for chorioamnionitis among women who received prenatal Tdap, with chorioamnionitis diagnosed in 6.1% of immunized women and 5.5% in unimmunized women, higher than our national, claims-based IPTW-weighted risks of 3.3% in optimally-immunized women and 3.0% of unimmunized women.[18] Our primary analysis found an association similar in magnitude, however, these findings should be interpreted with caution; in sensitivity analyses of women who also received prenatal influenza immunization and were therefore more similar in terms of healthcare access and behaviors, we did not observe an association between Tdap and chorioamnionitis, and the post-partum hemorrhage association was attenuated to RR=1.05 (95% CI: 0.99–1.10); this suggests the potential for residual confounding by behavioral or clinical factors uncaptured by claims. However, newborns whose mothers received prenatal Tdap were not more likely to experience any adverse outcomes, including NICU stays, respiratory distress, or neonatal sepsis, as compared to newborns of unvaccinated mothers. Therefore, it does not appear that these adverse birth outcomes, if indeed increased, had a clinically relevant impact on newborns.
Immunization induces a short-lived nonspecific inflammatory response, [38] however, it is difficult to identify a biological mechanism between this transient inflammatory response with maternal adverse outcomes of postpartum hemorrhage or chorioamnionitis which may occur weeks after immunization. As such, the findings in our study should lead to further investigation.
When examining safety by the timing of Tdap administration, early receipt was not associated with greater risks of the newborn outcomes than optimal timing. Birth outcomes were also similar between optimal and early Tdap receipt, but early receipt was associated with an increased postpartum hemorrhage and chorioamnionitis risks, and additionally with a slightly increased risk of premature rupture of membranes. The sensitivity analysis among influenza immunization-receiving women demonstrated attenuated associations with maternal adverse events, though the associations of early immunization with postpartum hemorrhage and premature rupture of membranes persisted. Similar to our discussion above, these results should be interpreted cautiously, as early receipt of immunization prior to the recommended timing may be indicative of atypical care or presumed fear of premature birth.
Insurance claims provide longitudinal information across providers, and this very large cohort contains women from across the US receiving care and delivering babies in a broad array of clinical settings. However, there is potential for unmeasured confounding as women receiving guideline-concordant immunizations may also be receiving more thorough surveillance, detailed diagnoses, and comprehensive care compared to women not receiving recommended Tdap; our sensitivity analysis among influenza-immunized women addressed this point, and found more attenuated or null associations with adverse outcomes. Additionally, the use of diagnosis coding for outcomes may result in misclassification, yet we would not expect differential misclassification by immunization status. The women in this study have employer-sponsored commercial insurance, and thus our results may not be generalizable to publically-insured or uninsured women. Gestational age is not available in insurance claims data; however, we estimated gestational age at delivery from multiple sources, including diagnosis information from both the mother and linked newborns, where available. However, not all mothers successfully linked to newborns who may be covered on different insurance plans; although the characteristics of matching and non-matching mothers were generally very similar, there may be residual inaccuracies in the gestational age estimation; therefore, we chose to utilize broader timing categories (’optimal’ or ‘early’) in which we have greater confidence than a continuous estimate of gestational age in weeks. For most comparisons, the IPTW and multivariable adjusted models yielded similar results; yet there were instances (typically with small numbers of outcomes) with sizable differences in estimates (e.g. newborn encephalopathy, early vs. none comparison), although the imprecision of the estimates did not cause the conclusions to differ.
In conclusion, serious adverse events after Tdap receipt are rare, and our study did not demonstrate an increased incidence of newborn adverse events associated with Tdap. We did observe an increased risk of chorioamnionitis consistent with a previous study and novel findings of increased risk of postpartum hemorrhage which warrants further investigation.
Supplementary Material
Acknowledgments
This project was funded by a grant from the US National Institute of Allergy and Infectious Disease (5R21AI115205). The database infrastructure used for this project was funded by the Department of Epidemiology, UNC Gillings School of Global Public Health; the Cecil G. Sheps Center for Health Services Research, UNC; the CER Strategic Initiative of UNC’s Clinical & Translational Science Award (UL1TR001111); and the UNC School of Medicine. S.B.-D. is supported by Grant 2015213 from the Doris Duke Charitable Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
COI: Dr. Layton receives unrestricted salary support from the Center for Pharmacoepidemiology in the UNC Department of Epidemiology; current member companies include GlaxoSmithKline, Merck, and UCB. Dr. Weber served on Speakers’ Bureaus and as a vaccine consultant (Merck, Pfizer). Dr. Becker-Dreps has received investigator-initiated research funds from Pfizer for an unrelated study on pneumococcal vaccines.
References
- 1.Healy CM, Munoz FM, Rench MA, Halasa NB, Edwards KM, Baker CJ. Prevalence of pertussis antibodies in maternal delivery, cord, and infant serum. The Journal of infectious diseases. 2004;190:335–40. doi: 10.1086/421033. [DOI] [PubMed] [Google Scholar]
- 2.Healy CM, Rench MA, Baker CJ. Importance of timing of maternal combined tetanus, diphtheria, and acellular pertussis (Tdap) immunization and protection of young infants. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2013;56:539–44. doi: 10.1093/cid/cis923. [DOI] [PubMed] [Google Scholar]
- 3.Leuridan E, Hens N, Peeters N, de Witte L, Van der Meeren O, Van Damme P. Effect of a prepregnancy pertussis booster dose on maternal antibody titers in young infants. The Pediatric infectious disease journal. 2011;30:608–10. doi: 10.1097/INF.0b013e3182093814. [DOI] [PubMed] [Google Scholar]
- 4.Winter K, Nickell S, Powell M, Harriman K. Effectiveness of Prenatal Versus Postpartum Tetanus, Diphtheria, and Acellular Pertussis Vaccination in Preventing Infant Pertussis. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2017;64:3–8. doi: 10.1093/cid/ciw634. [DOI] [PubMed] [Google Scholar]
- 5.Atkins KE, Fitzpatrick MC, Galvani AP, Townsend JP. Cost-Effectiveness of Pertussis Vaccination During Pregnancy in the United States. American journal of epidemiology. 2016;183:1159–70. doi: 10.1093/aje/kwv347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dabrera G, Amirthalingam G, Andrews N, Campbell H, Ribeiro S, Kara E, et al. A case-control study to estimate the effectiveness of maternal pertussis vaccination in protecting newborn infants in England and Wales, 2012–2013. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2015;60:333–7. doi: 10.1093/cid/ciu821. [DOI] [PubMed] [Google Scholar]
- 7.Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) in pregnant women-Advisory Committee on Immunization Practices (ACIP), 2012. MMWR Morbidity and mortality weekly report. 2013;62:131–5. [PMC free article] [PubMed] [Google Scholar]
- 8.RCOG statement: Pertussis (whooping cough) vaccination now offered from 20 weeks of pregnancy. London: Royal College of Obstetricians and Gynaecologists; 2016. [Google Scholar]
- 9.4.12 Pertussis. The Australian Immunisation Handbook 10th Edition: Australian Government Department of Health; 2016.
- 10.Kharbanda EO, Vazquez-Benitez G, Lipkind HS, Klein NP, Cheetham TC, Naleway AL, et al. Maternal Tdap vaccination: Coverage and acute safety outcomes in the vaccine safety datalink, 2007–2013. Vaccine. 2016;34:968–73. doi: 10.1016/j.vaccine.2015.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kharbanda EO, Vazquez-Benitez G, Lipkind H, Naleway AL, Klein NP, Cheetham TC, et al. Receipt of pertussis vaccine during pregnancy across 7 Vaccine Safety Datalink sites. Preventive medicine. 2014;67:316–9. doi: 10.1016/j.ypmed.2014.05.025. [DOI] [PubMed] [Google Scholar]
- 12.Koepke R, Kahn D, Petit AB, Schauer SL, Hopfensperger DJ, Conway JH, et al. Pertussis and Influenza Vaccination Among Insured Pregnant Women - Wisconsin, 2013–2014. MMWR Morbidity and mortality weekly report. 2015;64:746–50. [PMC free article] [PubMed] [Google Scholar]
- 13.Housey M, Zhang F, Miller C, Lyon-Callo S, McFadden J, Garcia E, et al. Vaccination with tetanus, diphtheria, and acellular pertussis vaccine of pregnant women enrolled in Medicaid–Michigan, 2011–2013. MMWR Morbidity and mortality weekly report. 2014;63:839–42. [PMC free article] [PubMed] [Google Scholar]
- 14.Butler AM, Layton JB, Li D, Hudgens MG, Boggess KA, McGrath LJ, et al. Predictors of Low Uptake of Prenatal Tetanus Toxoid, Reduced Diphtheria Toxoid, and Acellular Pertussis Immunization in Privately Insured Women in the United States. Obstetrics and gynecology. 2017 doi: 10.1097/AOG.0000000000001927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gesser-Edelsburg A, Shir-Raz Y, Hayek S, Aassaraf S, Lowenstein L. Despite awareness of recommendations, why do health care workers not immunize pregnant women? American journal of infection control. 2017 doi: 10.1016/j.ajic.2016.11.025. [DOI] [PubMed] [Google Scholar]
- 16.Chamberlain AT, Seib K, Ault KA, Orenstein WA, Frew PM, Malik F, et al. Factors Associated with Intention to Receive Influenza and Tetanus, Diphtheria, and Acellular Pertussis (Tdap) Vaccines during Pregnancy: A Focus on Vaccine Hesitancy and Perceptions of Disease Severity and Vaccine Safety. PLoS currents. 2015:7. doi: 10.1371/currents.outbreaks.d37b61bceebae5a7a06d40a301cfa819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McMillan M, Clarke M, Parrella A, Fell DB, Amirthalingam G, Marshall HS. Safety of Tetanus, Diphtheria, and Pertussis Vaccination During Pregnancy: A Systematic Review. Obstetrics and gynecology. 2017;129:560–73. doi: 10.1097/AOG.0000000000001888. [DOI] [PubMed] [Google Scholar]
- 18.Kharbanda EO, Vazquez-Benitez G, Lipkind HS, Klein NP, Cheetham TC, Naleway A, et al. Evaluation of the association of maternal pertussis vaccination with obstetric events and birth outcomes. Jama. 2014;312:1897–904. doi: 10.1001/jama.2014.14825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim DK, Riley LE, Harriman KH, Hunter P, Bridges CB. Advisory Committee on Immunization Practices Recommended Immunization Schedule for Adults Aged 19 Years or Older - United States, 2017. MMWR Morbidity and mortality weekly report. 2017;66:136–8. doi: 10.15585/mmwr.mm6605e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Figueroa R. Use of antidepressants during pregnancy and risk of attention-deficit/hyperactivity disorder in the offspring. Journal of developmental and behavioral pediatrics: JDBP. 2010;31:641–8. doi: 10.1097/DBP.0b013e3181e5ac93. [DOI] [PubMed] [Google Scholar]
- 21.Camelo Castillo W, Boggess K, Sturmer T, Brookhart MA, Benjamin DK, Jr, Jonsson Funk M. Association of Adverse Pregnancy Outcomes With Glyburide vs Insulin in Women With Gestational Diabetes. JAMA pediatrics. 2015;169:452–8. doi: 10.1001/jamapediatrics.2015.74. [DOI] [PubMed] [Google Scholar]
- 22.Li Q, Andrade SE, Cooper WO, Davis RL, Dublin S, Hammad TA, et al. Validation of an algorithm to estimate gestational age in electronic health plan databases. Pharmacoepidemiology and drug safety. 2013;22:524–32. doi: 10.1002/pds.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Margulis AV, Palmsten K, Andrade SE, Charlton RA, Hardy JR, Cooper WO, et al. Beginning and duration of pregnancy in automated health care databases: review of estimation methods and validation results. Pharmacoepidemiology and drug safety. 2015;24:335–42. doi: 10.1002/pds.3743. [DOI] [PubMed] [Google Scholar]
- 24.Margulis AV, Setoguchi S, Mittleman MA, Glynn RJ, Dormuth CR, Hernandez-Diaz S. Algorithms to estimate the beginning of pregnancy in administrative databases. Pharmacoepidemiology and drug safety. 2013;22:16–24. doi: 10.1002/pds.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. VFC Childhood Vaccine Supply Policy 2009. 2009 [Google Scholar]
- 26.Moro PL, Yue X, Lewis P, Haber P, Broder K. Adverse events after tetanus toxoid, reduced diphtheria toxoid and acellular pertussis (Tdap) vaccine administered to adults 65 years of age and older reported to the Vaccine Adverse Event Reporting System (VAERS), 2005–2010. Vaccine. 2011;29:9404–8. doi: 10.1016/j.vaccine.2011.05.100. [DOI] [PubMed] [Google Scholar]
- 27.Talbot EA, Brown KH, Kirkland KB, Baughman AL, Halperin SA, Broder KR. The safety of immunizing with tetanus-diphtheria-acellular pertussis vaccine (Tdap) less than 2 years following previous tetanus vaccination: Experience during a mass vaccination campaign of healthcare personnel during a respiratory illness outbreak. Vaccine. 2010;28:8001–7. doi: 10.1016/j.vaccine.2010.09.034. [DOI] [PubMed] [Google Scholar]
- 28.Funch D, Holick C, Velentgas P, Clifford R, Wahl PM, McMahill-Walraven C, et al. Algorithms for identification of Guillain-Barre Syndrome among adolescents in claims databases. Vaccine. 2013;31:2075–9. doi: 10.1016/j.vaccine.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 29.Brookhart MA, Wyss R, Layton JB, Sturmer T. Propensity score methods for confounding control in nonexperimental research. Circulation Cardiovascular quality and outcomes. 2013;6:604–11. doi: 10.1161/CIRCOUTCOMES.113.000359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cole SR, Hernan MA. Constructing inverse probability weights for marginal structural models. American journal of epidemiology. 2008;168:656–64. doi: 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sturmer T, Rothman KJ, Avorn J, Glynn RJ. Treatment effects in the presence of unmeasured confounding: dealing with observations in the tails of the propensity score distribution–a simulation study. American journal of epidemiology. 2010;172:843–54. doi: 10.1093/aje/kwq198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chamberlain AT, Berkelman RL, Ault KA, Rosenberg ES, Orenstein WA, Omer SB. Trends in reasons for non-receipt of influenza vaccination during pregnancy in Georgia, 2004–2011. Vaccine. 2016;34:1597–603. doi: 10.1016/j.vaccine.2016.01.058. [DOI] [PubMed] [Google Scholar]
- 33.Fridman D, Steinberg E, Azhar E, Weedon J, Wilson TE, Minkoff H. Predictors of H1N1 vaccination in pregnancy. American Journal of Obstetrics and Gynecology. 2011;204:S124–S7. doi: 10.1016/j.ajog.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 34.Lund JL, Richardson DB, Stürmer T. The Active Comparator, New User Study Design in Pharmacoepidemiology: Historical Foundations and Contemporary Application. Current Epidemiology Reports. 2015;2:221–8. doi: 10.1007/s40471-015-0053-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Donegan K, King B, Bryan P. Safety of pertussis vaccination in pregnant women in UK: observational study. Bmj. 2014;349:g4219. doi: 10.1136/bmj.g4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morgan JL, Baggari SR, McIntire DD, Sheffield JS. Pregnancy outcomes after antepartum tetanus, diphtheria, and acellular pertussis vaccination. Obstetrics and gynecology. 2015;125:1433–8. doi: 10.1097/AOG.0000000000000862. [DOI] [PubMed] [Google Scholar]
- 37.Berenson AB, Hirth JM, Rahman M, Laz TH, Rupp RE, Sarpong KO. Maternal and infant outcomes among women vaccinated against pertussis during pregnancy. Human vaccines & immunotherapeutics. 2016;12:1965–71. doi: 10.1080/21645515.2016.1157241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Christian LM, Iams JD, Porter K, Glaser R. Inflammatory responses to trivalent influenza virus vaccine among pregnant women. Vaccine. 2011;29:8982–7. doi: 10.1016/j.vaccine.2011.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


