Synopsis
The majority of elderly patients, particularly women, who have heart failure, have a preserved ejection fraction. Patients with this syndrome have severe symptoms of exercise intolerance, frequent hospitalizations, and increased mortality. Despite the importance of HFpEF, our understanding of its pathophysiology is incomplete, and optimal treatment remains largely undefined. Unlike the management of HFrEF, there is a paucity of large evidence-based trials demonstrating morbidity and mortality benefit for the treatment of HFpEF. There is an urgent need to understand HFpEF pathophysiology as well as focus on developing novel therapeutic targets. We present an update on information regarding pathophysiology, diagnosis, management, and future directions in this important and growing disorder.
Keywords: Heart failure, Preserved ejection fraction, Elderly, Aging, Comorbidities
Introduction
Clinical significance
There has been growing recognition over the past two decades that a substantial proportion of heart failure (HF) patients, particularly the elderly, have preserved systolic left ventricular (LV) function. An epidemiologic study from Olmstead County, Minnesota found that the prevalence of HF with preserved ejection fraction (HFpEF) relative to HF with reduced ejection fraction (HFrEF) is increasing at a rate of 1 % per year.1 Among elderly women living in the community, HFpEF comprises nearly 90% of incident HF cases.2 The annual incidence of HF in both men and women doubles with every decade after age 65, and the prevalence increases from less than 0.5% in the age group of 20–39 years to more than 10% in those 80 years and older.3 By 2020, the prevalence of HFpEF is projected to exceed 8 % of persons older than 65 years of age and because of the current pandemic of obesity, the prevalence of HFpEF in persons younger than 65 years of age is expected to rise exponentially.4
The health and economic impact of HFpEF is at least as great as that of HFrEF, with similar severity of acute hospitalization rates, and substantial mortality.1;5 Get With The Guidelines–HF, a very large, nationwide study of HF hospitalization in the United States (N>110,000), showed that the proportion of patients hospitalized with HFpEF increased from 33 % in 2005 to 39 % in 2010.6 Outcomes following hospitalization for decompensated HFpEF are poor with about 1/3 of patients rehospitalized or dead within 90 days of discharge.7 Non-cardiovascular hospital readmissions and mortality are more frequent in HFpEF than in HFrEF and the number of co-morbidities correlate with increased all-cause hospitalization and mortality.7
Diagnostic dilemma of HFpEF in older adults
Diagnosing HF in older adults poses specific challenges; false-positive clinical diagnoses are not uncommon.6 The most common symptoms of HFpEF are exertional dyspnea. However, symptoms of reduced exercise tolerance are common in the elderly and have been shown to reflect normal physiological changes related to aging or could be related to non-cardiac etiologies. Furthermore, the diagnosis of HF in the elderly may be difficult due to the presence of multiple comorbidities, some of which can mimic HF signs and further confound the diagnosis of HF. In addition, there is no universally agreed upon definition to define HFpEF. The American College of Cardiology/American Heart Association (ACC/AHA) consensus states that the diagnosis of HFpEF is based on typical symptoms and signs of HF in a patient with a normal range LV ejection fraction (EF), and no significant valvular abnormalities by echocardiography and no other obvious precipitating factors for HF or other disorders that could account for the heart failure symptoms.8 By contrast, the European Society of Cardiology (ESC) requires diastolic dysfunction for the diagnosis of HFpEF, along with symptoms and signs of HF and normal or mildly abnormal LV function.9
Why is HFpEF increasing in prevalence as the population ages?
1. Aging associated with HFpEF epidemic
There are a number of normal age-related changes in cardiovascular (CV) structure and function that are likely relevant to the development of HFpEF. These include increased arterial stiffening, increased myocardial stiffness, decreased diastolic myocardial relaxation, increased LV mass, decreased peak contractility, reduced myocardial and vascular responsiveness to β-adrenergic stimulation, decreased coronary flow reserve, and decreased mitochondrial response to increased demand for adenosine triphosphate (ATP) production.10 As observed by Borlaug et al, LV stiffness increases with normal aging, despite excellent control of blood pressure (BP) and reductions in LV mass.11 Although aging may have no effects on resting heart rate (HR), contractility, or cardiac output (CO) at rest, it blunts the capacity to enhance HR, systolic function, and CO in response to β-adrenoceptor stimulation and exercise. Aging is also associated with impaired endothelium-dependent vasodilatation.12;13 These normal age-related changes result in decreased CV reserve which contributes, along with reduced skeletal muscle mass and function, an approximately 1%/year decline in maximal exercise oxygen consumption (peak VO2).14 In addition, insults from acute myocardial ischemia/infarction, poorly controlled hypertension, atrial fibrillation (AF), iatrogenic volume overload, and pneumonia that would be tolerated in younger patients, can cause acute HF in older persons.10
Why is HFPEF so common among elderly women?
Among healthy normal subjects, older women tend to have higher LVEF, independent of their smaller chamber size, compared to men.15;16 In addition, the LV in female mammals has a distinctly different response to pressure load, such as is typical of systemic hypertension. In hypertensives in the Framingham study the predominant pattern of hypertrophic remodeling in women was concentric whereas in men it was eccentric, and this has been reported also in several other studies.17 Douglas et al18 showed the female rats developed concentric hypertrophy in response to increased afterload, and thereby maintained near-normal wall stress, and normal (or even a trend toward supranormal) contractility. In contrast, the male LV is less able to tolerate a pressure load, and in the presence of chronic systolic hypertension becomes dilated with thin walls and a depressed EF. However, the long-term cost of this female pattern of LV adaptation to a pressure load is impaired LV diastolic function. In addition, women have also been shown to have different CV physiologic responses to exercise than men, particularly in HR and stroke volume, independent of age and body size.14;19;20
Aging related body changes/skeletal muscle changes
Aging is associated with a decline in a variety of neural, hormonal and environmental trophic signals to muscle that can result in loss of muscle mass and mass-specific strength and 21–23 changes in body composition, including decreases in lean body mass and muscle strength, and increases in adiposity.24 In addition, aging is associated with a systemic pro-inflammatory state, and associated with increased levels of cytokines,25;26 that may lead to a functional decline in multiple organs even in absence of a specific disease.27
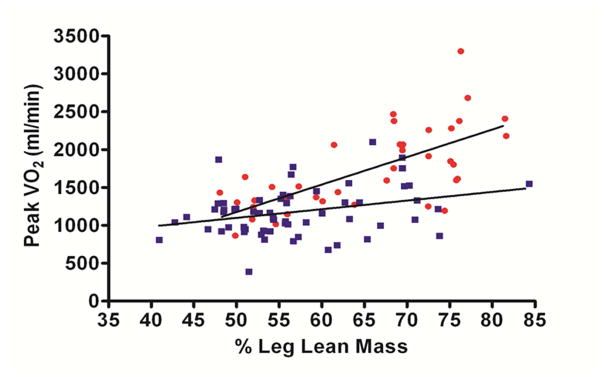
Haykowsky and colleagues found that percent body fat and percent leg fat were significantly increased, whereas percent body lean and leg lean mass were significantly reduced, in older HFpEF patients comparted to healthy controls.28 When peak VO2 was indexed to total lean body mass or leg lean mass, it remained significantly reduced, and there was a downward shift in the relationship of leg lean mass to peak VO2 in HFpEF vs healthy, age-matched controls (Figure 1).28 These data suggest that poor “quality” of skeletal muscle may contribute to the reduced peak V̇O2 found in older HFpEF patients.
Figure 1.
Relationship between peak VO2 (ml/min) and percent leg lean mass in heart failure with preserved ejection fraction (HFpEF) and healthy controls (HC) HFpEF (filled squares) and HC (filled circles)
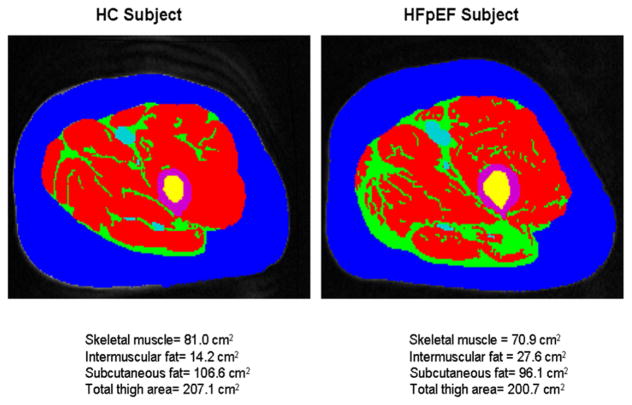
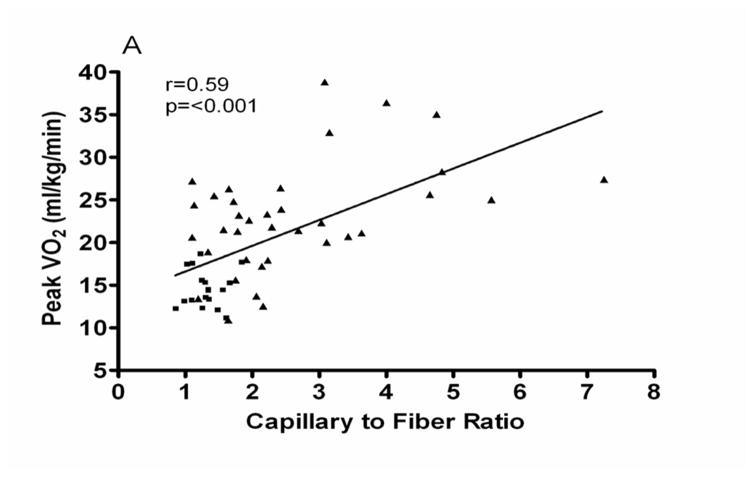
Haykowsky et al subsequently extended these results by showing that there is abnormal fat infiltration into the thigh skeletal muscle and this is associated with reduced peak exercise V̇O2 in HFpEF (Figure 2).29 Kitzman and Haykowsky also showed that compared with healthy control subjects, older HFpEF patients had a shift in skeletal muscle fiber type distribution with a reduced percentage of slow twitch type I fibers and reduced type I-to-type-II fiber ratio, as well as reduced capillary-to-fiber ratio.30 Furthermore, both the capillary-to-fiber ratio and percentage of type I fibers were significant, independent predictors of peak V̇O2 (Figure 3).30 A reduction in the percentage of type I fibers could be associated with reduced oxidative capacity and mitochondrial density and thereby contribute to the reduced peak V̇O2 in HFpEF. The same investigators subsequently reported that skeletal muscle oxidative capacity, mitochondrial content, and mitochondrial fusion are abnormal in older patients with HFpEF.31 The findings of abnormal mitochondrial function was also demonstrated by others in an animal model of HFpEF.32 In addition to this, it is known that aging results in alterations in skeletal muscle, including a reduction in the relative number of type II fibers33 and in capillary density,34 and that these are associated with a decline in physical performance. The loss of skeletal muscle and age-related alterations in skeletal muscle function are major factors in the age-associated decline in peakV̇O2.35–37 These, along with sedentary behavior as HFpEF symptoms worsen, further exacerbate exercise intolerance.38 Taken together, these findings may help explain why older HFpEF patients have such severely reduced exercise capacity, and why this has usually not improve with medications aimed solely at cardiac function in trials.39;40
Figure 2.
Magnetic resonance imaging axial image of the mid-thigh in a patient with heart failure with preserved ejection fraction (HFpEF) and healthy controls (HC).
Red = Skeletal muscle; green = Intermuscular fat (IMF); blue = Subcutaneous fat; purple = femoral cortex; yellow = femoral medulla. IMF (green) is substantially increased in the patient with HFpEF compared with the HC despite similar subcutaneous fat.
Figure 3.
Relationship of capillary-to-fiber ratio (A) and percentage of type I muscle fibers (B) with peak O2 uptake (VO2) in older patients with heart failure with preserved ejection fraction (■) and age-matched healthy control subjects (▲).
2. Marked rise in prevalence of cardiac and non-cardiac co morbidities with aging and HFpEF
Cardiac Comorbidities: Coronary Artery Disease (CAD) and Atrial Fibrillation (AF)
Although several epidemiologic and observational studies have found that CAD is less common in HFpEF compared to HFrEF,6;41 the pooled data across studies suggests that the prevalence of CAD in HFpEF is approximately 40–50 %.42 Large retrospective studies showed CAD is common in patients with HFpEF and is associated with increased risk of CV death, especially sudden death.43;44 An autopsy study recently showed epicardial CAD was frequent and extensive in HFpEF.45 In addition, with increasing life expectancy, decreased mortality and increased salvage of the myocardium with revascularization in the setting of acute coronary syndromes, patients with CAD are more likely aged and more likely to have a preserved EF. Moreover, myocardial ischemia acutely causes both systolic and diastolic dysfunction and may contribute to abnormal CV reserve with stress.46 Thus, it is not surprising that CAD has been associated with increased risk of developing HFpEF.
HFpEF and AF are inextricably linked, both to each other and to adverse CV outcomes.47;48 AF prevalence has been increasing due to an aging general population and increased longevity. AF in HFpEF associated with impaired LV systolic, diastolic function and functional reserve, larger LA with poor LA function, more severe neurohumoral activation, and impaired exercise tolerance.49,50
Non-Cardiac Comorbidities and the Epidemic of Obesity
Non-cardiac co-morbidities are highly prevalent in HFpEF and most older HFpEF patients have multiple and often severe non-cardiac comorbidities.51 The most important non-cardiac comorbidities for HFpEF are obesity, hypertension, diabetes, chronic obstructive disease (COPD), anemia and chronic kidney disease. Approximately 85% of elderly HFpEF patients are overweight or obese, and the HFpEF epidemic has largely paralleled the obesity epidemic. 52 Adiposity-induced inflammation has wide-ranging adverse effects, including endothelial dysfunction, capillary rarefaction, and mitochondrial dysfunction in both the cardiac and systemic vascular beds.53 a recent study demonstrated that body mass index was a key contributor to symptoms of breathlessness in patients with HFpEF. 54 Nearly two-thirds of HFpEF patients have COPD.55 Moreover, patients with preserved EF do not have the alternative diagnosis of low EF; they are more likely to receive a COPD diagnosis as an explanation for dyspnea.56 In addition, even in the absence of formal COPD diagnosis, patients with HFpEF have multiple pulmonary abnormalities and may contribute to their poor outcomes.57
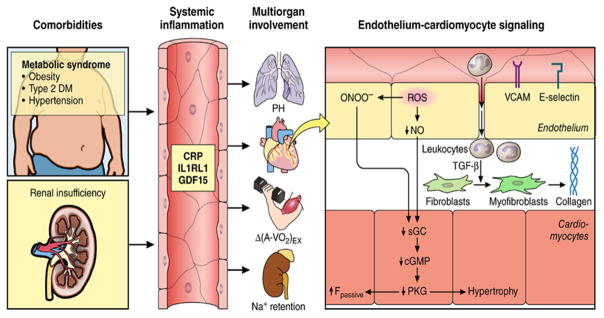
Aging and the aforementioned comorbidities may initiate and/or aggravate chronic systemic inflammation that may affect myocardial remodeling and dysfunction in HFpEF through a signaling cascade, which begins with coronary microvascular endothelial dysfunction (Figure 4).58;59 This reduces myocardial nitric oxide (NO) bioavailability and leads to reduced protein kinase G (PKG) activity in cardiomyocytes, which become stiff and hypertrophied.58 This hypothesis is supported by growing evidence, including a recent report that HFpEF patients have increased levels of tumor necrosis factor-α (TNF-α) and its type-2 receptor, and the latter was elevated even more than in HFrEF.60 Support for a systemic trigger for HFpEF came from parabiosis experiments in which hearts of young animals acquired HFpEF-like features when exposed to blood from old animals and vice versa.61
Figure 4.
Systemic and myocardial signaling in HFpEF. Comorbidities induce systemic inflammation, evident from elevated plasma levels of inflammatory biomarkers such as soluble interleukin 1 receptor-like 1 (IL1RL1), C-reactive protein (CRP), and growth differentiation factor 15 (GDF15). Chronic inflammation affects the lungs, myocardium, skeletal muscle, and kidneys leading to diverse HFpEF phenotypes with variable involvement of pulmonary hypertension (PH), myocardial remodeling, deficient skeletal muscle oxygen extraction (ΔA-Vo2) during exercise (Ex), and renal Na+ retention. Myocardial remodeling and dysfunction begins with coronary endothelial microvascular inflammation manifest from endothelial expression of adhesion molecules such as vascular cell adhesion molecule (VCAM) and E-Selectin. Expression of adhesion molecules attracts infiltrating leukocytes secreting transforming growth factor β (TGF-β), which converts fibroblasts to myofibroblasts with enhanced interstitial collagen deposition. Endothelial inflammation also results in the presence of reactive oxygen species (ROS), reduced nitric oxide (NO) bioavailability, and production of peroxynitrite (ONOO– ). This reduces soluble guanylate cyclase (sGC) activity, cyclic guanosine monophosphate (cGMP) content, and the favorable effects of protein kinase G (PKG) on cardiomyocyte stiffness and hypertrophy. HFpEF indicates heart failure with preserved ejection fraction.
Key Knowledge Gaps
What are the mechanisms whereby aging, non-cardiac comorbidities impact physical function outcomes in HFpEF?
How can we develop and test novel exercise and physical function interventions that directly address the adverse impact of multiple co-morbidities in older patients with HFpEF?
Pharmacological interventions
Summary of traditional clinical trials
Targeting the renin–angiotensin–aldosterone system (RAAS) pathway has long been considered a logical intervention for HFpEF, based on animal models as well as human hypertensives without HF and its link to LV hypertrophy, interstitial fibrosis and fluid imbalance.62–65 Angiotensin II promotes LV hypertrophy and fibrosis, both of which are contributors to HFpEF, as well as vasoconstriction and vascular remodeling.66 Aldosterone can promote interstitial collagen deposition and fibrosis, leading to ventricular stiffness and its inhibition might be expected to reduce the ventricular-vascular stiffening and diastolic dysfunction. Table I summarizes the important randomized trials. Of the three large randomized trials of angiotensin converting enzyme inhibitors (ACEI)/angiotensin receptor blockers (ARB) performed to date in HFpEF, only the CHARM-Preserved study found nominal benefit for reducing HF hospitalizations over three years of follow-up. However, most importantly, none of the trials showed benefit for their pre-planned primary endpoints (Figure 5 shows the result of I-PRESERVE trial).67–69 Similarly Kitzman et al studied a 12-month, randomized controlled trial of the ACEI enalapril in elderly patients with established HFpEF, and showed no improvement in exercise capacity or quality of life.39
Table 1.
Summary of few important randomized trials
| First Author/Trial (Ref.#) | Intervention | HFpEF Patient Type | Primary Endpoint | Trial Result |
|---|---|---|---|---|
| CHARM-Preserved 67 | Candesartan | ≥ 18 ys/NYHA class II–IV HF | CV death or HF admission | Fewer HF admissions |
| The PEP-CHF 68 | Perindopril | ≥70 ys/diagnosis of HF and treated with diuretics and an Echo-DD | All-cause mortality and HF admission | Fewer HF admissions |
| I-PRESERVE69 | Irbesartan | ≥ 60 ys/hospitalized for HF during the previous 6 months and have current NYHA class II–IV symptoms | Death from any cause or hospitalization for a CV cause | Neutral |
| Kitzman et al.39 | Enalapril | Elderly(70±1 ys), predominant female (80%) with compensated HF | Peak VO2 and 6 MWD | Neutral |
| TOPCAT70 | Spironolactone | ≥ 50 ys, Symptomatic HF. Patients had a h/o HF hospitalization within previous 12 months and elevated BNP within 60 days before randomization | CV death or aborted cardiac arrest, HF hospitalization | Neutral |
| Aldo-DHF40 | Spironolactone | ≥ 50 ys ambulatory patients/NYHA class II–III symptoms, grade1 DD and normal or near-normal BNP levels | Peak VO2, change in E/e′ | Neutral |
| RAAM-PEF71 | Eplerenone | Elderly, symptomatic NYHA class II/III, increased BNP within 60 days | 6MWD | Neutral |
| J DHF74 | Carvedilol (low-dose) | ≥ 20 ys/ambulatory patients with NYHA class II–III symptoms, grade I DD, and normal or near-normal BNP levels | Death or HF hospitalization | Neutral |
| ELANDD75 | Nebivolol | ≥ 40 ys/ambulatory patients with NYHA class II–III symptoms, grade I DD, and normal or near-normal BNP levels | 6 MWD | Neutral |
| NEAT-HFPEF trial100 | Isosorbide Mononitrate | ≥ 50 ys/ambulatory HF patients, prior hospitalization for HF within 12 months or increased invasively measured LV filling pressure or elevated BNP or echo-DD | Daily activity level, 6MWD | Neutral |
| RELAX99;100 | Sildenafil | ≥ 18 ys/elevated BNP or elevated invasively measured LV filling pressure and reduced exercise capacity | Peak VO2 | Neutral |
| DILATE -1114 | Riociguat | ≥ 18 ys/stable symptomatic HF, mean PAP≥ 25 mm of Hg and PCWP > 15 mm of Hg | Change in mean PAP | Neutral |
| Zile et al119 | Sitaxsentan | NYHA class II–III HF, Echo-DD | Change in treadmill exercise time | Positive |
| PARAMOUNT 78 | LCZ696(ARNI) | ≥ 40 ys/NYHA class II–III HF, NT-pro BNP > 400 pg/nl and be on a diuretic therapy | Change in NT-proBNP | Positive |
| Kosmala et al112 | Ivabradine | ≥ 50 ys/ambulatory patients with NYHA class II–III symptoms, grade I DD, and normal or near-normal BNP levels | Peak VO2, Peak E/e′ | Positive |
| Kitzman et al131 | Exercise training | ≥ 60ys/Ambulatory HF patients with NYHA class II–III symptoms | Peak VO2 | Positive |
| Kitzman et al 135 | Caloric restriction and exercise training | ≥ 60ys/ambulatory HF patients with NYHA class II–III symptoms | Peak VO2 and Quality of Life | Positive |
| CHAMPION127 | CardioMEMs sensor | ≥ 18 ys, NYHA class III HF, hospitalization for HF in last 12 months, | HF hospitalization | Positive |
HFpEF = heart failure with preserved ejection fraction; CV=cardiovascular; HF=heart failure; DD=diastolic dysfunction; VO2= oxygen consumption; MWD=minute walk distance; BNP=B-type natriuretic peptide; E= Mitral early diastolic velocity; e’=mitral annular velocity; ARNI = angiotensin receptor-neprilysin inhibitor; PAP=pulmonary artery pressure; PCWP=pulmonary capillary wedge pressure
Figure 5.
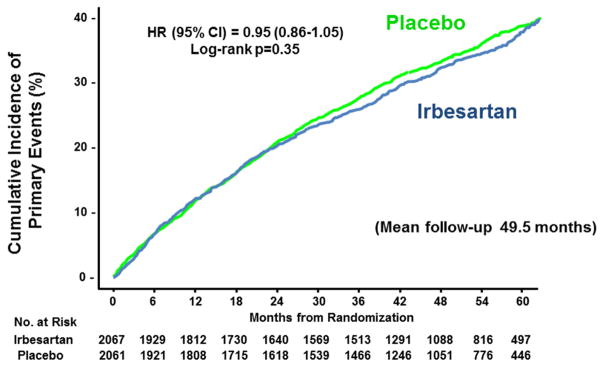
Kaplan–Meier Curves for the Primary Outcome (I-PRESERVE)
The primary outcome of death from any cause or hospitalization for prespecified cardiovascular causes (worsening heart failure, myocardial infarction, stroke, atrial or ventricular arrhythmia, and myocardial infarction or stroke occurring during hospitalization for any cause) is shown for patients receiving irbesartan and those receiving placebo.
From Massie BM, Carson PE, McMurray JJ, et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med 2008;359(23):2456–67; with permission.
Aldosterone antagonists have also been examined in HFpEF. The Aldo-DHF showed improvement in some measures of diastolic dysfunction, the RAAM-PEF trial showed reductions in circulating markers of collagen turnover and modest improvements in diastolic function and the larger TOPCAT trial showed a modest decline in hospitalizations but not mortality.40;70;71 However, a post hoc regional analysis of TOPCAT indicated that the cohort from the Americas most closely matched characteristics observed in other randomized trials and also appeared most responsive to spironolactone.72
Slowing the HR should result in an increase in the diastolic filling period in an abnormally stiff LV, thus potentially allowing greater filling of LV. As shown in Table 1, beta blockers data on HFpEF to date have not been promising. 73–76 In the Digitalis Interaction Group, there were no significant reductions in the amount of hospitalizations or mortality secondary to HF with digoxin, although trends towards decreased hospitalization and improved exercise tolerance were noted.77 Ivabridine, a novel agent for reducing HR, is discussed below.
Why have clinical pharmacological intervention trials fail to meet their primary endpoints?
Relative lack of success of prior trials has led to a re-evaluation of paradigms regarding HFpEF physiology. To date, trials have largely targeted solely targets previously thought to be specific to and universally present in HFpEF, such as LVH, diastolic dysfunction, and other features. However more recent data have challenged these assumptions. For instance, in the recently reported PARAMOUNT trial of well characterized HFpEF patients, only 8% of patients had LVH at baseline and 50% had significant/severe diastolic function at rest.78 With treatment, even though there was a positive signal on BNP, there was no difference in LV mass. Similarly Maurer and colleagues found no significantly increased LV mass in older HFpEF patients compared to controls with hypertension but not HF.79;80 The magnitude of increase in fibrosis in HFpEF patients also appears to be modest at most.81 This indicates LV hypertrophy may not be unique to, or required for diagnosis of HFpEF. This might explain the agents that had a proven ability to ameliorate LV hypertrophy, fibrosis, and other cardiac abnormalities typically found in HFpEF have failed to produce positive effect.
Studies of patients with all the clinical hallmarks of HF and an EF>50% showed that many patients appear to have modest diastolic dysfunction under resting conditions.78;82 Furthermore, similar changes can be seen in elderly patients with hypertensive heart disease with no clinical HF, and diastolic dysfunction in HFpEF patients may not be greater than age-matched sedentary controls and has not prevent a successful target for intervention.83–87 Most HFpEF trials measured diastolic or other CV measures at rest and not during exercise. Importantly, most measures used to assess diastolic function (echocardiographic or radionuclide techniques or invasive measurements) do not assess the key passive component of diastole. Furthermore, using direct invasive measurements, Kawaguchi et al show that during exercise, patients with HFpEF were able to increase preload volume with very little if any effect on the ventricular end-diastolic pressure-volume relation, despite a substantial prolongation of time constant of relaxation.88 While other studies have had varying results in this respect, these data suggest that diastolic function abnormalities may not be the sole contributor to symptoms in HFpEF.85
Across reports from a variety of sources, lower HR at peak exercise (chronotopic incompetence [CI]) has been the most consistently reported cardiac abnormality during exercise in HFpEF.46;89–91 In some studies, CI appears to be the primary mechanism accounting for reduced CO during exercise in HFpEF and the primary or sole cardiac contributor to exercise intolerance.92 In addition, there is a high prevalence of CI in HFpEF, and limitations in chronotropic reserve might be a key factor to reduce CO and exercise capacity.46;93 β-Blockers may result in pharmacologically induced CI and obscure identification of an underlying intrinsic abnormality in neural balance.94 In addition, unfavorable effect of beta-blockers on COPD and diabetes could complicate the overall effect of these drugs in HFpEF patients with such conditions.95;96
The neutral outcomes were often attributed to patient recruitment with inclusion of many HFrEF or non-cardiac patients or nonadherence to diagnostic guidelines that might have led to excessive enrollment of HF patients with eccentric LV remodeling and CAD rather than concentric remodeling and hypertension.58 For example, in TOPCAT trial, neutral outcome in the overall population has been attributed to aberrant patient enrollment in Russia/Republic of Georgia rather than to inefficacy of spironolactone.72
Perhaps most importantly, HFpEF is strongly influenced by aging, a progressive process affecting all organ systems, including the heart and arterial system, those most implicated in HFpEF. In addition, recent data, discussed above, indicates that HFpEF may be best understood as a systemic disorder, triggered by one or more circulating factors, involving virtually all organ systems, in addition to the heart, and also involves important contributions from peripheral abnormalities of vascular and skeletal muscle function that have not been addressed in trials to date. Finally, multiple comorbidities, including non-cardiovascular co-morbidities, may play a much greater role in the development of symptoms and treatment response than previously recognized. If so, they may not be addressed by agents and strategies that are primarily targeted at cardiac function. These concepts have led to the proposal of key phenotypes in HFpEF, with each phenotype having distant pathophysiological and treatment implications.97 However, past and current HFpEF studies make no or little effort to enroll specific etiologic/pathophysiological subtypes.
Novel pharmacotherapies in HFpEF
Sildenafil is an inhibitor of Phosphodiesterase 5 that increases cyclic guanosine monophosphate (GMP) levels by blocking catabolism, thus augmenting PKG activity in multiple organs relevant to HF. Increased availability of cGMP could provide benefits for both vascular and myocardial remodeling, including attenuating hypertrophy, fibrosis, and impaired cardiac relaxation.98 In the RELAX trial, sildenafil did not improve 6 minute walk distance (MWD) or quality of life.99 Nitrates: In NEAT-HFpEF trial, the isosorbide mononitrate, an organic nitrate, did not improve in 6 MWD, quality-of-life scores, or NT-pro B-type natriuretic peptide (BNP) levels compared to placebo.100 Recently two randomized study showed that intravenous or inhaled sodium nitrite, which unlike inorganic nitrate is a direct nitric oxide donor, improved CO reserve, LV stroke work and biventricular filling pressures and pulmonary artery pressures at rest and during exercise in HFpEF.101;102 These trials led to the launch of 2 clinical trials sponsored by the NHLBI (NCT02742129 and NCT02713126). A recent study with a relatively small patient sample showed that one week of daily dosing with beet root juice (supplying 6.1 mmol inorganic nitrate) significantly improved submaximal aerobic endurance and BP in elderly 20 HFpEF patients.103 Neprilysin inhibitors: Neprilysin is a zinc-dependent metalloprotease that degrades biologically active natriuretic peptides and does not affect the biologically inactive NTproBNP.78 LCZ696 is a new combination drug of the angiotensin II type-1 receptor blocker valsartan and the neprilysin inhibitor prodrug AHU377. This dual combination exerts a powerful vasodilatory and natriuresis effect by blocking angiotensin II activity on the one hand, although augmenting plasma levels of natriuretic peptides, such as BNP, on the other. In the PARAMOUNT study (table 1), the group randomized to receive LCZ696 had significantly lower NT-pro BNP levels and at 36 weeks, decreased LA size and showed a trend toward improved functional class.78 This agent also appears to reduce tumor necrosis factor-α levels, and this finding correlates with improvements in cardiac features of HFpEF.104 The promising findings of this phase-2 study led to an ongoing large, multi-center trial, PARAGON, which is comparing LCZ696 to valsartan in patients with HFpEF with the primary composite outcome of CV death or first hospitalization for HF(ClinicalTrials.gov NCT01920711). Statins: By blocking the activity of several guanosine triphosphate binding proteins and inhibiting some of the inflammatory processes, statins can suppress LV hypertrophy and decrease collagen synthesis in experimental models.105;106 Even though observational data in HFpEF patients suggest a mortality benefit with use of HMG-Co-A reductase inhibitors, definitive trials have not been performed in HFpEF patients.107;108 A recent meta-analyses suggested a potential mortality benefit with statin.109 Likewise, in a recent prospective study of HFpEF patients, statin use was associated with a higher rate of 1-year survival compared with those who were not treated.110 Ivabradine is a selective sinus node If sodium channel inhibitor that reduces HR without affecting contractility or lusiotropy. The role of ivabradine in HFpEF has not been well established. In a diabetic mouse model of HFpEF, ivabradine reduced aortic stiffness and fibrosis and improved LV contractility and diastolic function.111 In a seven-day study, ivabradine increased peak VO2 and reduced exercise E/e′ ratio in 61 patients with HFpEF.112 However in contrast, a short term, placebo-controlled, randomized, crossover study found that 2 weeks of HR reduction with ivabradine in patients with HFpEF almost uniformly exacerbated already abnormal exercise physiology.113 Riociguat is a soluble guanylate cyclase stimulator that targets the NO-soluble guanylate cyclase–cyclic GMP signaling pathway. The DILATE-1 study showed that riociguat did not impact the primary end-point of peak change in mean pulmonary artery pressure in patients with HFpEF and pulmonary hypertension.114 Other studies utilizing these agents for other endpoints are planned or underway. Ranolazine blocks inward sodium current, promotes Ca2+ extrusion through the Na+/Ca2+ exchanger and thereby improve diastolic tension and relaxation. The RALI-DHF study showed improvement in some measures of hemodynamics but no improvement in relaxation parameters.115;116 Reduction of filling pressures did occur with ranolazine but it also appeared to decrease CO.116 Alagebrium (ALT-711): Advanced glycation end products (AGEs) are formed when glucose interacts nonenzymatically with proteins. AGEs can cause increased stiffness of the extracellular matrix directly by cross-linking collagen or elastin and indirectly by stimulating the production of collagen and depleting NO, thereby increasing oxidative stress.117 A small open-label study found that administration of alagebrium chloride, was associated with slightly reduced LV mass and improved diastolic filling, however, there were no changes in EF, BP, peak VO2 and aortic distensibility (the latter 2 were the primary outcomes).118 Sitaxsentan: The effects of treatment with a selective endothelin type A (ETA) receptor antagonist on characteristics commonly found in patients with HFpEF such as pulmonary hypertension, diastolic dysfunction, and LV hypertrophy, suggest the potential for its therapeutic application in HFpEF patients. In a moderate-sized trial of HFpEF patients, 6-months treatment with sitaxsentan, a selective ETA receptor antagonist appeared to provide a modest increase in treadmill exercise time but did not improve any of secondary endpoints such as LV mass or diastolic function.119
New drugs in development or testing
Anakinra: IL-1 (alpha) and IL-1 (beta) are potent proinflammatory cytokines implicated in adverse ventricular–vascular remodeling.120 IL-1 blockade with anakinra for 14 days significantly reduced the systemic inflammatory response and improved aerobic exercise capacity in patients with HFpEF and elevated plasma CRP levels.121 The Inhibitors of sodium-glucose cotransporters type 2 (SGLT2) empagliflozin was shown to reduce HF admissions in patients with type 2 diabetes and high CV risk, with a consistent benefit in patients with and without baseline HF.122 The ongoing CANDLE trial in patients with T2DM and chronic HF (Both HFpEF and HFrEF) has the potential to evaluate the clinical safety and efficacy on HF of another SGLT2 inhibitor canagliflozin in comparison with glimepiride.123 Nifedipine and Isosorbide Dinitrate/Hydralazine: Two classic medications, nifedipine and isosorbide dinitrate/hydralazine (HISDN), are currently being tested for their potential benefit to HFpEF patients (NCT01157481 and NCT01516346, respectively). Preclinical data showed HISDN improved diastolic function, exercise capacity and reduced soluble vascular cell adhesion molecule 1 levels in mice, but there were no reductions in LV hypertrophy, cardiac fibrosis, or pulmonary congestion.124 Recently, exciting studies have revealed that microRNAs (miRNA)-34a might have an important role in cardiac aging via effects on apoptosis, DNA damage, and telomere shortening.125 The strategy of replacement of miRNAs of interest or of blockade of potentially harmful miRNAs (anti-MIRs) is currently being tested in pre-clinical studies.125 Endothelial NO synthase activators were studied in the DAHL salt–sensitive rat model of HFpEF. Diastolic dysfunction was reduced, as were both cardiac hypertrophy and fibrosis.126
Device Therapy
The CARDIOMEMS device is a wireless, implanted pulmonary artery pressure monitor implanted in the distal pulmonary artery during a right heart catheterization procedure. Patients transmit hemodynamic data daily using a wireless RF transmitter. The CHAMPION trial, a single-blind clinical trial of the CARDIOMEMs device in patients with HF of any etiology showed a significant reduction in HF hospitalizations.127 In HFpEF, CARDIOMEMS device reduced decompensation leading to hospitalization compared with standard HF management strategies.128
Given that rises in LA pressure and pulmonary venous congestion are shown to herald HF decompensation events in patients with HFpEF, creating a controlled left-to-right interatrial shunt to allow LA decompression could be a rational nonpharmacological strategy for alleviating symptoms in patients with HFpEF. Hemodynamic modelling based on clinical measurements suggested that an appropriately sized iatrogenic atrial septal defect could attenuate exercise-induced increases in LA pressure in patients with HFpEF.129 Subsequently, an open-label study demonstrated reductions in LA pressure during exercise with improvements in functional capacity and quality of life 6 months after implantation of this device.130 A prospective, multicenter, randomized, and single blinded trial is underway to confirm this finding (NCT02600234).
What treatments have worked so far?
Exercise training
Exercise intolerance is the primary manifestation of chronic HFpEF, and is a strong determinant of prognosis and of reduced quality of life. Exercise training (ET) has been shown to improve exercise intolerance in HFrEF. Kitzman and colleagues performed the first randomized, single-blinded trial comparing the effects of 16 weeks of endurance ET versus attention control in older patients with HFpEF. They found increased peak VO2, ventilatory anaerobic threshold, 6 MWD, and physical quality-of-life scores with exercise therapy.131 These results were confirmed in a subsequent multicenter, randomized trial of 3 months of combined ET and strength training in HFpEF patients.132 In a second, separate, randomized, attention-controlled, single-blind trial of 4 months upper and lower extremity endurance ET, Kitzman et al found a significant increase in peak VO2 without altering carotid arterial stiffness or brachial artery flow mediated dilation.133 Edelmann and colleagues confirmed in a multicenter trial that ET improves exercise capacity and symptoms.134 Recently, Kitzman et al further extended these results in obese older patients with HFpEF by revealing combination of diet with endurance ET training was additive and produced a relatively large increase in peak VO2. 135 In a recent pilot study, 4 week of high-intensity interval training significantly improved peak V̇o2 and left ventricular diastolic dysfunction in HFpEF patients.136 Taken together, ET is an effective non-pharmacologic therapy in clinically stable patients with HFpEF to improve exercise tolerance. Despite the increasing evidence for the benefits of ET in HFpEF and calls for additional exercise-oriented research, the Center for Medicare Services (CMS) excluded HFpEF patients from reimbursement for cardiac rehabilitation in their 2014 funding decision.137;138
How does ET improve exercise intolerance in HFpEF patients?
Aerobic ET may improve exercise capacity either by increasing exercise CO (via increased HR or stroke volume), or by increasing arterio-venous oxygen difference (A-VO2 diff) by improvement in peripheral vascular function leading to increase diffusive oxygen transport or by increased oxygen utilization by the skeletal muscle. Haykowsky et al,92 showed that an ET induced increase in A-VO2 diff was the primary contributor to improved peak VO2.92 Similarly Hundley et al.139 reported that resting and flow-mediated increases in leg blood flow in elderly HFpEF patients may not be significantly impaired; thus it is possible that in this elderly population with HFpEF muscle adaptation play a more important role, compared to vascular changes. Indeed, Bhella et al,140 showed impaired skeletal muscle oxidative metabolism in elderly patients with HFpEF at baseline, that can be favorably shifted by ET to a more efficient muscle O2 utilization. In addition, Fujimoto et al found no ET-related beneficial effect on LV diastolic function in HFpEF elderly patients, even after 1 year of exercise.141
Although the above studies support mechanisms for the beneficial effects of ET that are independent of LV systolic or diastolic function, some studies have attributed ET related improvements to exercise-induced favorable changes in LV function and CO, atrial reverse remodeling and improved LV diastolic function.142;132;136
Key Knowledge Gaps
What will be the optimal ET to improve CV and skeletal muscle function, physical functional performance in elderly HFpEF patients?
Can we develop the most cost-effective models of ET for these patients?
Can we start ET early, even shortly after a hospitalization for acute decompensated HF in elderly patients?
Dietary Caloric Restriction
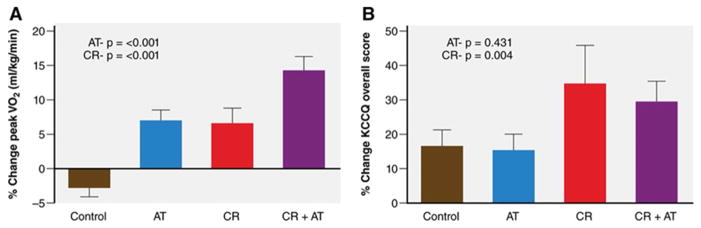
Up to 80% of older patients with HFpEF are overweight or obese, and excess adipose tissue adversely affects cardiac, arterial, and skeletal muscle function. Recently Kitzman et al showed among obese older patients with clinically stable HFpEF, caloric restriction significantly improved exercise capacity and quality of life, and the effect was additive to ET (Figure 6).135 They demonstrated that caloric restriction was feasible and appeared safe in older, obese HFpEF patients. Caloric restriction improved quality of life much more than ET. The improvements from caloric restriction appeared to be mediated by reduced total body and skeletal muscle adipose and reduced inflammation.
Figure 6.
Effects of a 20-week caloric restriction diet on exercise capacity and quality of life in HFpEF. The graph displays percent changes ± standard errors at the 20-week follow-up relative to baseline by randomized group for peak Vo2 (mL·kg–1·min–1, A), and Kansas City Cardiomyopathy Questionnaire (KCCQ) overall score (Quality of Life Score; B). P values represent effects for AT and CR. AT indicates aerobic exercise training; and CR, caloric restriction diet.
Current guidelines in HFpEF: What is the evidence?
Current guidelines for the management of HFpEF recommend management of volume status with appropriate diuretic dosing, control of BP, management of comorbidities, and dietary education.143 The 2013 ACCF/AHA HF guidelines indicate that systolic and diastolic hypertension should be controlled in accordance with published clinical practice guidelines to prevent morbidity and diuretics should be used to relieve symptoms due to volume overload (Class I with level of evidence B).143 ACCF/AHA guidelines support the use of beta-blockers, ACEI, and ARB for hypertension (IIa recommendation, level of evidence C), and recommend ARBs be considered to decrease hospitalizations (IIb recommendation, level of evidence B).143 Beta-blockers are recommended for HFpEF patients with a history of myocardial infarction, hypertension, or AF. The ESC guidelines have similar recommendations.144 To avoid the activation of the RAAS and renal insufficiency or electrolyte disturbances, lowest dose of diuretics should be utilized to maintain euvolemia. Nonsteroidal anti-inflammatory medications, frequently used in older patients, can cause relative diuretic resistance and should be discontinued if possible.
Screening for ischemic heart disease with a noninvasive stress test or coronary angiography should be considered especially in patients with chest pain and/or ‘flash pulmonary edema’ to exclude severe CAD.145 When found, manifest ischemia should be treated, including invasively if indicated (Class IIa with level of evidence C). Control of hypertension may be the single most important treatment strategy for HFpEF (Class I).146 Recently SPRINT trial demonstrated that intensive BP reduction reduced the risk of acute decompensated HF.147 The ACCF/AHA guideline recommends management of AF for symptom control for HFpEF (Class IIa with level of evidence C). Even though ESC guidelines support restoring sinus rhythm by cardioversion along with anticoagulation, strong evidence is still deficient.144 The HR control and permanent anticoagulation become mandatory in HFpEF.
Management goals in elders with HFpEF include relief of symptoms, improvement in functional capacity and quality of life, prevention of acute exacerbations and related hospital admissions, and prolongation of survival. A systematic approach should comprise several elements: diagnosis and staging of disease, search for reversible etiology, judicious use of medications, patient education, enhancement of self-management skills, coordination of care across disciplines, and effective follow-up. Elders with HF often have severe deconditioning and severe exercise intolerance and they should be encouraged to undertake regular moderate physical activity. It is likely optimal for this to be under medical supervision, at least initially, but reimbursement barriers can make this a challenge.
Recently, Shah and colleagues proposed a detailed, pheno-type specific roadmap for treatment of HFpEF patients.97 However, while informative and synthesizing our most current understanding of HFpEF, this strategy has not been prospectively evaluated.
Conclusions
Multiple lines of evidence suggest that HFpEF may be a systemic disorder with several phenotypes, influence by aging and affecting all organ systems, including the CV system principally. Moreover, the overwhelming majority of HFpEF patients have multiple comorbidities that also drive phenotypic heterogeneity and multifactorial pathophysiology. Furthermore, non-cardiovascular hospital readmissions and mortality are more frequent in HFpEF than in HFrEF. So far, only ET and calorie restriction seem to improve exercise intolerance and quality of life. Given such a multi-factorial, complex milieu, it’s not surprising that drugs and interventions aimed primarily at a central hemodynamics repeatedly failed to strongly impact overall outcomes in HFpEF. New drugs that target underlying inflammation, oxidative stress, and aging-related dysfunction may prove to be effective for improving outcomes in HFpEF, a rapidly growing disorder among older persons.
Key Points.
Heart failure with preserved ejection fraction (HFpEF) is a diverse syndrome, strongly influenced by aging, with likely systemic, multi-factorial etiologies that affect all organ systems
The overwhelming majority of HFpEF patients have multiple comorbidities that also drive phenotypic heterogeneity and multifactorial pathophysiology.
So far, only exercise training and weight loss appear to improve exercise intolerance and quality of life.
New drugs that target underlying inflammation, oxidative stress, and aging-related dysfunction may prove to be particularly effective for HFpEF.
Acknowledgments
Supported in part by NIH grants R01AG18915 and P30AG12232, and by the Kermit Glenn Phillips II Endowed Chair in Cardiovascular Medicine.
Footnotes
Potential Financial Conflicts of Interest:
Dr. Kitzman declares the following relationships: Consultant for Abbvie, Bayer, Merck, Medtronic, GSK, Relypsa, Regeneron, Merck, Corvia Medical, and Actavis, research grant funding from Novartis, and stock ownership in Gilead Sciences and Relypsa.
Dr. Upadhya has received research funding from Novarits and Corvia.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 2.Gottdiener JS, Arnold AM, Aurigemma GP, et al. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000;35:1628–1637. doi: 10.1016/s0735-1097(00)00582-9. [DOI] [PubMed] [Google Scholar]
- 3.Roger V, Go A, Lloyd-Jones D, et al. Heart Disease and Stroke Statistics - 2011 Update - A report from the American Heart Assocation Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van der Velden J, van der Wall EE, Paulus WJ. Heart failure with preserved ejection fraction: current status and challenges for the future. Neth Heart J. 2016;24:225–226. doi: 10.1007/s12471-016-0808-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunlay SM, Redfield MM, Weston SA, et al. Hospitalizations after heart failure diagnosis: a community perspective. J Am Coll Cardiol. 2009;54:1695–1702. doi: 10.1016/j.jacc.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steinberg BA, Zhao X, Heidenreich PA, et al. Trends in Patients Hospitalized With Heart Failure and Preserved Left Ventricular Ejection Fraction: Prevalence, Therapies, and Outcomes. Circulation. 2012;126:65–75. doi: 10.1161/CIRCULATIONAHA.111.080770. [DOI] [PubMed] [Google Scholar]
- 7.Fonarow GC, Stough WG, Abraham WT, et al. Characteristics, Treatments, and Outcomes of Patients With Preserved Systolic Function Hospitalized for Heart Failure: A Report From the OPTIMIZE-HF Registry. J Am Coll Cardiol. 2007;50:768–777. doi: 10.1016/j.jacc.2007.04.064. [DOI] [PubMed] [Google Scholar]
- 8.Hunt SA, Abraham WT, Chin MH, et al. 2009 Focused Update Incorporated Into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the International Society for Heart and Lung Transplantation. J Am Coll Cardiol. 2009;53:e1–90. doi: 10.1016/j.jacc.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 9.McMurray JJ, Adamopoulos S, Anker SD, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;14:803–869. doi: 10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- 10.Rich MW, Kitzman DW. Heart failure in octogenarians: A fundamentally different disease. Am J Geriatr Cardiol. 2000;9:97–104. [Google Scholar]
- 11.Borlaug B, Redfield M, Melenovsky V, et al. Longitudinal changes in left ventricular stiffness: a community-based study. Circ Heart Fail. 2013;6:944–952. doi: 10.1161/CIRCHEARTFAILURE.113.000383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeSouza CA, Shapiro LF, Clevenger CM, et al. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102:1351–1357. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- 13.Gerhard M, Roddy MA, Creager SJ, Creager MA. Aging progressively impairs endothelium-dependent vasodilation in forearm resistance vessels of humans. Hypertension. 1996;27:849–853. doi: 10.1161/01.hyp.27.4.849. [DOI] [PubMed] [Google Scholar]
- 14.Ogawa T, Spina RJ, Martin WH, Kohrt WM, Schechtman KB. Effect of aging, sex, and physical training on cardiovacular responses to exercise. Circulation. 1992;86:494–503. doi: 10.1161/01.cir.86.2.494. [DOI] [PubMed] [Google Scholar]
- 15.Gerdts E, Zabalgoitia M, Bjornstad H, Svendsen TL, Devereux RB. Gender differences in systolic left ventricular function in hypertensive patients with electrocardiographic left ventricular hypertrophy (the LIFE study) Am J Cardiol. 2001;87:980–983. doi: 10.1016/s0002-9149(01)01433-3. [DOI] [PubMed] [Google Scholar]
- 16.Kane GC, Hauser MF, Behrenbeck TR, Miller TD, Gibbons RJ, Christian TF. Impact of gender on rest Tc-99m sestamibi-gated left ventricular ejection fraction. Am J Cardiol. 2002;89:1238–1241. doi: 10.1016/s0002-9149(02)02317-2. [DOI] [PubMed] [Google Scholar]
- 17.Bella JN, Wachtell K, Palmieri V, et al. Relation of left ventricular geometry and function to systemic hemodynamics in hypertension: the LIFE Study. Losartan Intervention For Endpoint Reduction in Hypertension Study. J Hypertens. 2001;19:127–134. doi: 10.1097/00004872-200101000-00017. [DOI] [PubMed] [Google Scholar]
- 18.Douglas PS, Katz SE, Weinberg EO, Chen MH, Bishop SP, Lorell BH. Hypertrophic remodeling: gender differences in the early response to left ventricular pressure overload. J Am Coll Cardiol. 1998;32:1118–1125. doi: 10.1016/s0735-1097(98)00347-7. [DOI] [PubMed] [Google Scholar]
- 19.Spina RJ, Ogawa T, Miller TR, Kohrt WM, Ehsani AA. Effect of exercise training on left ventricular performance in older women free of cardiopulmonary disease. Am J Cardiol. 1993;71:99–194. doi: 10.1016/0002-9149(93)90718-r. [DOI] [PubMed] [Google Scholar]
- 20.Sullivan M, Cobb F, Higginbotham M. Stroke volume increases by similar mechanisms during upright exercise in normal men and women. Am J Cardiol. 1991;67:1405–1412. doi: 10.1016/0002-9149(91)90472-w. [DOI] [PubMed] [Google Scholar]
- 21.Marcell TJ. Sarcopenia: causes, consequences, and preventions. J Gerontol A Biol Sci Med Sci. 2003;58:M911–M916. doi: 10.1093/gerona/58.10.m911. [DOI] [PubMed] [Google Scholar]
- 22.Morley JE, Baumgartner RN, Roubenoff R, Mayer J, Nair KS. Sarcopenia. J Lab Clin Med. 2001;137:231–243. doi: 10.1067/mlc.2001.113504. [DOI] [PubMed] [Google Scholar]
- 23.Ronenn Roubenoff. Sarcopenia: effects on body composition and function. J Gerontol A Biol Sci Med Sci. 2003;58:1012–1017. doi: 10.1093/gerona/58.11.m1012. [DOI] [PubMed] [Google Scholar]
- 24.Forbes GB, Halloran E. The adult decline in lean body mass. Hum Biol. 1976;48:161–173. [PubMed] [Google Scholar]
- 25.Collier P, Watson C, Voon V, et al. Can emerging biomarkers of myocardial remodelling identify asymptomatic hypertensive patients at risk for diastolic dysfunction and diastolic heart failure? Eur J Heart Fail. 2011;13:1087–1095. doi: 10.1093/eurjhf/hfr079. [DOI] [PubMed] [Google Scholar]
- 26.Kalogeropoulos A, Georgiopoulou V, Psaty B, et al. Inflammatory markers and incident heart failure risk in older adults: the Health ABC (Health, Aging, and Body Composition) study. J Am Coll Cardiol. 2010;55:2129–2137. doi: 10.1016/j.jacc.2009.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franceschi C, Bonafe M, Valensin S, et al. Inflamm-aging: An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 28.Haykowsky MJ, Brubaker PH, Morgan TM, Kritchevsky SB, Eggebeen J, Kitzman DW. Impaired aerobic capacity and physical functional performance in older heart failure patients with preserved ejection fraction: role of lean body mass. J Gerontol A Biol Sci Med Sci. 2013;68:968–975. doi: 10.1093/gerona/glt011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haykowsky M, Kouba EJ, Brubaker PH, Nicklas BJ, Eggebeen J, Kitzman DW. Skeletal muscle composition and its relation to exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Cardiol. 2014;113:1211–1216. doi: 10.1016/j.amjcard.2013.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kitzman DW, Nicklas B, Kraus WE, et al. Skeletal muscle abnormalities and exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Physiol Heart Circ Physiol. 2014;306:H1364–1370. doi: 10.1152/ajpheart.00004.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Molina AJ, Bharadwaj MS, Van Horn C, et al. Skeletal Muscle Mitochondrial Content, Oxidative Capacity, and Mfn2 Expression Are Reduced in Older Patients With Heart Failure and Preserved Ejection Fraction and Are Related to Exercise Intolerance. JACC Heart Fail. 2016;4:636–645. doi: 10.1016/j.jchf.2016.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bowen TS, Rolim NP, Fischer T, et al. Heart failure with preserved ejection fraction induces molecular, mitochondrial, histological, and functional alterations in rat respiratory and limb skeletal muscle. Eur J Heart Fail. 2015;17:263–272. doi: 10.1002/ejhf.239. [DOI] [PubMed] [Google Scholar]
- 33.Larsson L, Sjodin B, Karlsson J. Histochemical and biochemical changes in human skeletal muscle with age in sedentary males, age 22–65 years. Acta Physiol Scand. 1978;103:31–39. doi: 10.1111/j.1748-1716.1978.tb06187.x. [DOI] [PubMed] [Google Scholar]
- 34.Coggan AR, Spina RJ, King DS. Histochemical and enzymatic comparison of the gastrocnemius muscle of young and elderly men and women. J Gerontol. 1992;47:B71–B76. doi: 10.1093/geronj/47.3.b71. [DOI] [PubMed] [Google Scholar]
- 35.Franssen F, Wouters E, Schols A. The contribution of starvation, deconditioning and ageing to the observed alterations in peripheral skeletal muscle in chronic organ diseases. Clinical Nutrition. 2002;21:1–14. doi: 10.1054/clnu.2001.0485. [DOI] [PubMed] [Google Scholar]
- 36.Coats A, Clark A, Piepoli M, Volterrani M, Poole-Wilson P. Symptoms and quality of life in heart failure: the muscle hypothesis. Br Heart J. 1994;72:S39. doi: 10.1136/hrt.72.2_suppl.s36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Middlekauff HR. Making the Case for Skeletal Myopathy as the Major Limitation of Exercise Capacity in Heart Failure. Circ Heart Fail. 2010;3:537–546. doi: 10.1161/CIRCHEARTFAILURE.109.903773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fried LP, Hadley EC, Walston JD, et al. From bedside to bench: research agenda for frailty. Sci Aging Knowledge Environ. 2005;2005:pe24. doi: 10.1126/sageke.2005.31.pe24. [DOI] [PubMed] [Google Scholar]
- 39.Kitzman DW, Hundley WG, Brubaker P, Stewart K, Little WC. A randomized, controlled, double-blinded trial of enalapril in older patients with heart failure and preserved ejection fraction; effects on exercise tolerance, and arterial distensibility. Circ Heart Fail. 2010;3:477–485. doi: 10.1161/CIRCHEARTFAILURE.109.898916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Edelmann F Aldo-DHF investigators. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: The aldo-dhf randomized controlled trial. JAMA. 2013;309:781–791. doi: 10.1001/jama.2013.905. [DOI] [PubMed] [Google Scholar]
- 41.Yancy C, Lopatin M, Stevenson L, De Marco T, Fonarow G ADHERE Scientific Advisory Committee and Investigators. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. J Am Coll Cardiol. 2006;47:76–84. doi: 10.1016/j.jacc.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 42.Shah SJ. Evolving approaches to the management of heart failure with preserved ejection fraction in patients with coronary artery disease. Curr Treat Options Cardiovasc Med. 2010;12:58–75. doi: 10.1007/s11936-009-0060-2. [DOI] [PubMed] [Google Scholar]
- 43.Hwang SJ, Melenovsky V, Borlaug BA. Implications of coronary artery disease in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2014;63:2817–2827. doi: 10.1016/j.jacc.2014.03.034. [DOI] [PubMed] [Google Scholar]
- 44.Rusinaru D, Houpe D, Szymanski C, Levy F, Marechaux S, Tribouilloy C. Coronary artery disease and 10-year outcome after hospital admission for heart failure with preserved and with reduced ejection fraction. Eur J Heart Fail. 2014;16:967–976. doi: 10.1002/ejhf.142. [DOI] [PubMed] [Google Scholar]
- 45.Mohammed SF, Hussain S, Mirzoyev SA, Edwards WD, Maleszewski JJ, Redfield MM. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation. 2015;131:550–559. doi: 10.1161/CIRCULATIONAHA.114.009625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Borlaug BA, Olson TP, Lam CSP, et al. Global Cardiovascular Reserve Dysfunction in Heart Failure With Preserved Ejection Fraction. J Am Coll Cardiol. 2010;56:845–854. doi: 10.1016/j.jacc.2010.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vermond RA, Geelhoed B, Verweij N, et al. Incidence of atrial fibrillation and relationship with cardiovascular events, heart failure, and mortality: a community-based study from the Netherlands. J Am Coll Cardiol. 2015;66:1000–1007. doi: 10.1016/j.jacc.2015.06.1314. [DOI] [PubMed] [Google Scholar]
- 48.Chamberlain AM, Redfield MM, Alonso A, Weston SA, Roger VL. Atrial fibrillation and mortality in heart failure: a community study. Circ Heart Fail. 2011;4:740–746. doi: 10.1161/CIRCHEARTFAILURE.111.962688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zakeri R, Borlaug BA, McNulty SE, et al. Impact of atrial fibrillation on exercise capacity in heart failure with preserved ejection fraction: a RELAX trial ancillary study. Circ Heart Fail. 2014;7:123–130. doi: 10.1161/CIRCHEARTFAILURE.113.000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lam CS, Rienstra M, Tay WT, et al. Atrial fibrillation in heart failure with preserved ejection fraction: association with exercise capacity, left ventricular filling pressures, natriuretic peptides, and left atrial volume. JACC: Heart Failure. 2016 doi: 10.1016/j.jchf.2016.10.005. (In press) [DOI] [PubMed] [Google Scholar]
- 51.Ather S, Chan W, Bozkurt B, et al. Impact of Noncardiac Comorbidities on Morbidity and Mortality in a Predominantly Male Population With Heart Failure and Preserved Versus Reduced Ejection Fraction. J Am Coll Cardiol. 2012;59:998–1005. doi: 10.1016/j.jacc.2011.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ndumele CE, Coresh J, Lazo M, et al. Obesity, subclinical myocardial injury, and incident heart failure. JACC Heart Fail. 2014;2:600–607. doi: 10.1016/j.jchf.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kitzman DW, Shah SJ. The HFpEF Obesity Phenotype: The Elephant in the Room. J Am Coll Cardiol. 2016;68:200–203. doi: 10.1016/j.jacc.2016.05.019. [DOI] [PubMed] [Google Scholar]
- 54.Dalos D, Mascherbauer J, Zotter-Tufaro C, et al. NYHA functional class is associated with diastolic pulmonary pressure and predicts outcome in patients with heart failure and preserved ejection fraction. J Am Coll Cardiol. 2016;68:189–199. doi: 10.1016/j.jacc.2016.04.052. [DOI] [PubMed] [Google Scholar]
- 55.Kitzman DW, Gardin JM, Gottdiener JS, et al. Importance of heart failure with preserved systolic function in patients > or = 65 Years of Age. CHS Research Group. Cardiovascular Health Study. Am J Cardiol. 2001;87:413–419. doi: 10.1016/s0002-9149(00)01393-x. [DOI] [PubMed] [Google Scholar]
- 56.Caruana L, Petrie MC, Davie AP, McMurray JJ. Do patients with suspected heart failure and preserved left ventricular systolic function suffer from “diastolic heart faiure” or from misdiagnosis? A prospective descriptive study. BMJ. 2000;321:215–218. doi: 10.1136/bmj.321.7255.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kitzman DW, Guazzi M. Impaired Alveolar Capillary Membrane Diffusion: A Recently Recognized Contributor to Exertional Dyspnea in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail. 2016;4:499–501. doi: 10.1016/j.jchf.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 58.Paulus W, Tschope C. A Novel Paradigm for Heart Failure with Preserved Ejection Fraction: Comorbidities Drive Myocardial Dysfunction and Remodeling Through Coronary Microvascular Endothelial Inflammation. J Am Coll Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 59.Franssen C, Chen S, Unger A, et al. Myocardial Microvascular Inflammatory Endothelial Activation in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail. 2015;4:312–324. doi: 10.1016/j.jchf.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 60.Putko BN, Wang Z, Lo J, et al. Circulating levels of tumor necrosis factor-alpha receptor 2 are increased in heart failure with preserved ejection fraction relative to heart failure with reduced ejection fraction: evidence for a divergence in pathophysiology. PLoS ONE. 2014;9:e99495. doi: 10.1371/journal.pone.0099495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Loffredo FS, Steinhauser ML, Jay SM, et al. Growth Differentiation Factor 11 Is a Circulating Factor that Reverses Age-Related Cardiac Hypertrophy. Cell. 2013;153:828–839. doi: 10.1016/j.cell.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Groban L, Pailes NA, Bennett C, et al. Growth Hormone Replacement Attenuates Diastolic Dysfunction and Cardiac Angiotensin II Expression in Senescent Rats. J Gerontol A Biol Sci Med Sci. 2006;61:28–35. doi: 10.1093/gerona/61.1.28. [DOI] [PubMed] [Google Scholar]
- 63.Groban L, Yamaleyeva LM, Westwood BM, et al. Progressive diastolic dysfunction in the female mRen(2). Lewis Rat: Influence of salt and ovarian hormones. Gerontol B Physiol Sci Sco Sci. 2008;63:3–11. doi: 10.1093/gerona/63.1.3. [DOI] [PubMed] [Google Scholar]
- 64.Lasocki S, Iglarz M, Seince PF, et al. Involvement of renin-angiotensin system in pressure-flow relationship: role of angiotensin-converting enzyme gene polymorphism. Anesthesiology. 2002;96:271–275. doi: 10.1097/00000542-200202000-00008. [DOI] [PubMed] [Google Scholar]
- 65.Little WC, Wesley-Farrington DJ, Hoyle J, et al. Effect of candesartan and verapamil on exercise tolerance in diastolic dysfunction. J Cardiovasc Pharmacol. 2004;43:288–293. doi: 10.1097/00005344-200402000-00019. [DOI] [PubMed] [Google Scholar]
- 66.Wright JW, Mizutani S, Harding JW. Pathways involved in the transition from hypertension to hypertrophy to heart failure. Treatment strategies. Heart Fail Rev. 2008;13:367–375. doi: 10.1007/s10741-007-9060-z. [DOI] [PubMed] [Google Scholar]
- 67.Yusuf S, Pfeffer MA, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362:777–781. doi: 10.1016/S0140-6736(03)14285-7. [DOI] [PubMed] [Google Scholar]
- 68.Cleland JGF, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J. 2006;27:2338–2345. doi: 10.1093/eurheartj/ehl250. [DOI] [PubMed] [Google Scholar]
- 69.Massie BM, Carson PE, McMurray JJ, et al. Irbesartan in Patients with Heart Failure and Preserved Ejection Fraction. N Engl J Med. 2008;359:2456–2467. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 70.Pitt B, Pfeffer M, Assmann S, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–1392. doi: 10.1056/NEJMoa1313731. [DOI] [PubMed] [Google Scholar]
- 71.Deswal A, Richardson P, Bozkurt B, Mann D. Results of the Randomized Aldosterone Antagonism in Heart Failure With Preserved Ejection Fraction Trial (RAAM-PEF) J Card Fail. 2011;17:634–642. doi: 10.1016/j.cardfail.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 72.Pfeffer MA, Claggett B, Assmann SF, et al. Regional variation in patients and outcomes in the treatment of preserved cardiac function heart failure with an Aldosterone Antagonist (TOPCAT) Trial. Circulation. 2015;131:34–42. doi: 10.1161/CIRCULATIONAHA.114.013255. [DOI] [PubMed] [Google Scholar]
- 73.Aronow WS, Ahn C, Kronzon I. Effect of propranolol versus no propranolol on total mortality plus nonfatal myocardial infarction in older patients with prior myocardial infarction, congestive heart failure, and left ventricular ejection fraction > or = 40% treated with diuretics plus angiotensin-converting enzyme inhibitors. Am J Cardiol. 1997;80:207–209. doi: 10.1016/s0002-9149(97)00320-2. [DOI] [PubMed] [Google Scholar]
- 74.Yamamoto K, Origasa H, Hori M J-DHF Investigators. Effects of carvedilol on heart failure with preserved ejection fraction: the Japanese Diastolic Heart Failure Study (J-DHF) Eur J Heart Fail. 2013;15:110–118. doi: 10.1093/eurjhf/hfs141. [DOI] [PubMed] [Google Scholar]
- 75.Conraads V, Metra M, Kamp O, et al. Effects of the long-term administration of nebivolol on the clinical symptoms, exercise capacity, and left ventricular function of patients with diastolic dysfunction: results of the ELANDD study. Eur J Heart Fail. 2012;14:219–225. doi: 10.1093/eurjhf/hfr161. [DOI] [PubMed] [Google Scholar]
- 76.Hernandez AF, Hammill BG, O’Connor CM, Schulman KA, Curtis LH, Fonarow GC. Clinical Effectiveness of Beta-Blockers in Heart Failure: Findings From the OPTIMIZE-HF (Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart Failure) Registry. J Am Coll Cardiol. 2009;53:184–192. doi: 10.1016/j.jacc.2008.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ahmed A, Pitt B, Rahimtoola SH, et al. Effects of digoxin at low serum concentrations on mortality and hospitalization in heart failure: A propensity-matched study of the DIG trial. Int J Cardiol. 2008;123:138–146. doi: 10.1016/j.ijcard.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Solomon S, Zile M, Pieske B, et al. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. The Lancet. 2012;380:1387–1395. doi: 10.1016/S0140-6736(12)61227-6. [DOI] [PubMed] [Google Scholar]
- 79.Maurer MS, Burkhoff D, Fried LP, Gottdiener J, King DL, Kitzman DW. Ventricular Structure and Function in Hypertensive Participants With Heart Failure and a Normal Ejection Fraction: The Cardiovascular Health Study. J Am Coll Cardiol. 2007;49:972–981. doi: 10.1016/j.jacc.2006.10.061. [DOI] [PubMed] [Google Scholar]
- 80.Solomon SD, Verma A, Desai A, et al. Effect of Intensive Versus Standard Blood Pressure Lowering on Diastolic Function in Patients With Uncontrolled Hypertension and Diastolic Dysfunction. Hypertension. 2010;55:241–248. doi: 10.1161/HYPERTENSIONAHA.109.138529. [DOI] [PubMed] [Google Scholar]
- 81.Su MY, Lin LY, Tseng YH, et al. CMR-verified diffuse myocardial fibrosis is associated with diastolic dysfunction in HFpEF. JACC Cardiovasc Imaging. 2014;7:991–997. doi: 10.1016/j.jcmg.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 82.Kitzman D, Upadhya B. Heart failure with preserved ejection fraction: a heterogenous disorder with multifactorial pathophysiology. J Am Coll Cardiol. 2014;63:457–459. doi: 10.1016/j.jacc.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zile MR, Gaasch WH, Carroll JD, et al. Heart failure with a normal ejection fraction: is measurement of diastolic function necessary to make the diagnosis of diastolic heart failure? Circulation. 2001;104:779–782. doi: 10.1161/hc3201.094226. [DOI] [PubMed] [Google Scholar]
- 84.Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure: Part I: diagnosis, prognosis, and measurements of diastolic function. Circulation. 2002;105:1387–1393. doi: 10.1161/hc1102.105289. [DOI] [PubMed] [Google Scholar]
- 85.Burkhoff D, Maurer MS, Packer M. Heart failure with a normal ejection fraction: is it really a disorder of diastolic function? Circulation. 2003;107:656–658. doi: 10.1161/01.cir.0000053947.82595.03. [DOI] [PubMed] [Google Scholar]
- 86.Melenovsky V, Borlaug BA, Rosen B, et al. Cardiovascular Features of Heart Failure With Preserved Ejection Fraction Versus Nonfailing Hypertensive Left Ventricular Hypertrophy in the Urban Baltimore Community: The Role of Atrial Remodeling/Dysfunction. J Am Coll Cardiol. 2007;49:198–207. doi: 10.1016/j.jacc.2006.08.050. [DOI] [PubMed] [Google Scholar]
- 87.Maurer MS, Hummel SL. Heart Failure With a Preserved Ejection Fraction: What Is in a Name? J Am Coll Cardiol. 2011;58:275–277. doi: 10.1016/j.jacc.2011.02.054. [DOI] [PubMed] [Google Scholar]
- 88.Kawaguchi M, Hay I, Fetics B, Kass DA. Combined Ventricular Systolic and Arterial Stiffening in Patients With Heart Failure and Preserved Ejection Fraction. Circulation. 2003;107:714–720. doi: 10.1161/01.cir.0000048123.22359.a0. [DOI] [PubMed] [Google Scholar]
- 89.Brubaker PH, Joo KC, Stewart KP, Fray B, Moore B, Kitzman DW. Chronotropic incompetence and its contribution to exercise intolerance in older heart failure patients. J Cardiopulm Rehabil. 2006;26:86–89. doi: 10.1097/00008483-200603000-00007. [DOI] [PubMed] [Google Scholar]
- 90.Borlaug BA, Melenovsky V, Russell SD, et al. Impaired Chronotropic and Vasodilator Reserves Limit Exercise Capacity in Patients With Heart Failure and a Preserved Ejection Fraction. Circulation. 2006;114:2138–2147. doi: 10.1161/CIRCULATIONAHA.106.632745. [DOI] [PubMed] [Google Scholar]
- 91.Pham PP, Balaji S, Shen I, Ungerleider R, Li X, Sahn DJ. Impact of Conventional Versus Biventricular Pacing on Hemodynamics and Tissue Doppler Imaging Indexes of Resynchronization Postoperatively in Children With Congenital Heart Disease. J Am Coll Cardiol. 2005;46:2284–2289. doi: 10.1016/j.jacc.2005.08.036. [DOI] [PubMed] [Google Scholar]
- 92.Haykowsky MJ, Brubaker PH, Stewart KP, Morgan TM, Eggebeen J, Kitzman DW. Effect of endurance training on the determinants of peak exercise oxygen consumption in elderly patients with stable compensated heart failure and preserved ejection fraction. J Am Coll Cardiol. 2012;60:120–128. doi: 10.1016/j.jacc.2012.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Phan TT, Abozguia K, Nallur Shivu G, et al. Heart Failure With Preserved Ejection Fraction Is Characterized by Dynamic Impairment of Active Relaxation and Contraction of the Left Ventricle on Exercise and Associated With Myocardial Energy Deficiency. J Am Coll Cardiol. 2009;54:402–409. doi: 10.1016/j.jacc.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 94.Witte KK, Cleland J, Clark AL. Chronic heart failure, chronotropic incompetence, and the effects of beta blockers. Heart. 2006;92:481–486. doi: 10.1136/hrt.2004.058073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hawkins NM, Petrie MC, MacDonald MR, et al. Heart failure and chronic obstructive pulmonary disease: the quandary of beta-blockers and beta-agonists. J Am Coll Cardiol. 2011;57:2127–2138. doi: 10.1016/j.jacc.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 96.Bangalore S, Parkar S, Grossman E, Messerli FH. A meta-analysis of 94,492 patients with hypertension treated with beta blockers to determine the risk of new-onset diabetes mellitus. Am J Cardiol. 2007;100:1254–1262. doi: 10.1016/j.amjcard.2007.05.057. [DOI] [PubMed] [Google Scholar]
- 97.Shah SJ, Kitzman DW, Borlaug BA, et al. Phenotype-Specific Treatment of Heart Failure With Preserved Ejection Fraction: A Multiorgan Roadmap. Circulation. 2016;134:73–90. doi: 10.1161/CIRCULATIONAHA.116.021884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kanwar M, Agarwal R, Barnes M, et al. Role of phosphodiesterase-5 inhibitors in heart failure: emerging data and concepts. Curr Heart Fail Rep. 2013;10:26–35. doi: 10.1007/s11897-012-0121-9. [DOI] [PubMed] [Google Scholar]
- 99.Redfield M, Chen H, Borlaug B, et al. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: A randomized clinical trial. JAMA. 2013;309:1268–1277. doi: 10.1001/jama.2013.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Redfield M, Anstrom K, Levine J, et al. Isosorbide Mononitrate in Heart Failure with Preserved Ejection Fraction. N Engl J Med. 2015 doi: 10.1056/NEJMoa1510774. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Borlaug BA, Melenovsky V, Koepp KE. Inhaled sodium nitrite improves rest and exercise hemodynamics in heart failure with preserved ejection fraction. Circ Res. 2016;119:880–886. doi: 10.1161/CIRCRESAHA.116.309184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Thomas GR, DiFabio JM, Gori T, Parker JD. Once daily therapy with isosorbide-5-mononitrate causes endothelial dysfunction in humans: evidence of a free-radical-mediated mechanism. J Am Coll Cardiol. 2007;49:1289–1295. doi: 10.1016/j.jacc.2006.10.074. [DOI] [PubMed] [Google Scholar]
- 103.Eggebeen J, Kim-Shapiro DB, Haykowsky MJ, et al. One week of daily dosing with beetroot juice improves submaximal endurance and blood pressure in older patients with Heart Faliure and Preserved Ejection Fraction. JACC Heart Fail. 2015;4:428–437. doi: 10.1016/j.jchf.2015.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jhund PS, Claggett BL, Voors AA, et al. Elevation in High-Sensitivity Troponin T in Heart Failure and Preserved Ejection Fraction and Influence of Treatment With the Angiotensin Receptor Neprilysin Inhibitor LCZ696. Circ Heart Fail. 2014;7:953–959. doi: 10.1161/CIRCHEARTFAILURE.114.001427. [DOI] [PubMed] [Google Scholar]
- 105.Hattori T, Shimokawa H, Higashi M, et al. Long-term inhibition of Rho-kinase suppresses left ventricular remodeling after myocardial infarction in mice. Circulation. 2004;109:2234–2239. doi: 10.1161/01.CIR.0000127939.16111.58. [DOI] [PubMed] [Google Scholar]
- 106.Martin J, Denver R, Bailey M, Krum H. In vitro inhibitory effects of atorvastatin on cardiac fibroblasts: implications for ventricular remodelling. Clin Exp Pharmacol Physiol. 2005;32:697–701. doi: 10.1111/j.1440-1681.2005.04256.x. [DOI] [PubMed] [Google Scholar]
- 107.Fukuta H, Little W. Observational studies of statins in heart failure with preserved systolic function. Heart Fail Clin. 2008;4:209–216. doi: 10.1016/j.hfc.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 108.Fukuta H, Sane DC, Brucks S, Little WC. Statin Therapy May Be Associated With Lower Mortality in Patients With Diastolic Heart Failure: A Preliminary Report. Circulation. 2005;112:357–363. doi: 10.1161/CIRCULATIONAHA.104.519876. [DOI] [PubMed] [Google Scholar]
- 109.Fukuta H, Goto T, Wakami K, Ohte N. The effect of statins on mortality in heart failure with preserved ejection fraction: a meta-analysis of propensity score analyses. Int J Cardiol. 2016;214:301–306. doi: 10.1016/j.ijcard.2016.03.186. [DOI] [PubMed] [Google Scholar]
- 110.Alehagen U, Benson L, Edner M, Dahlstrom U, Lund LH. Association between use of statins and outcomes in heart failure with reduced ejection fraction: prospective propensity score matched cohort study of 21 864 patients in the Swedish Heart Failure Registry. Circ Heart Fail. 2015;8:252–260. doi: 10.1161/CIRCHEARTFAILURE.114.001730. [DOI] [PubMed] [Google Scholar]
- 111.Reil JC, Hohl M, Reil GH, et al. Heart rate reduction by If-inhibition improves vascular stiffness and left ventricular systolic and diastolic function in a mouse model of heart failure with preserved ejection fraction. Eur Heart J. 2013;34:2839–2849. doi: 10.1093/eurheartj/ehs218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kosmala W, Holland DJ, Rojek A, Wright L, Przewlocka-Kosmala M, Marwick TH. Effect of If-Channel Inhibition on Hemodynamic Status and Exercise Tolerance in Heart Failure With Preserved Ejection Fraction: A Randomized Trial. J Am Coll Cardiol. 2013;62:1330–1338. doi: 10.1016/j.jacc.2013.06.043. [DOI] [PubMed] [Google Scholar]
- 113.Pal N, Sivaswamy N, Mahmod M, et al. Effect of Selective Heart Rate Slowing in Heart Failure With Preserved Ejection Fraction. Circulation. 2015;132:1719–1725. doi: 10.1161/CIRCULATIONAHA.115.017119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bonderman D, Pretsch I, Steringer-Mascherbauer R, et al. Acute hemodynamic effects of riociguat in patients with pulmonary hypertension associated with diastolic heart failure (dilate-1): A randomized, double-blind, placebo-controlled, single-dose study. CHEST Journal. 2014;146:1274–1285. doi: 10.1378/chest.14-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jacobshagen C, Belardinelli L, Hasenfuss G, Maier L. Ranolazine for the Treatment of Heart Failure With Preserved Ejection Fraction: Background, Aims, and Design of the RALI-DHF Study. Clin Cardiol. 2011;34:426–432. doi: 10.1002/clc.20897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Maier LS, Layug B, Karwatowska-Prokopczuk E, et al. RAnoLazIne for the treatment of diastolic heart failure in patients with preserved ejection fraction: the RALI-DHF proof-of-concept study. JACC Heart Fail. 2013;1:115–122. doi: 10.1016/j.jchf.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 117.Borbely A, Papp Z, Edes I, Paulus WJ. Molecular determinants of heart failure with normal left ventricular ejection fraction. Pharmacol Rep. 2009;61:139–145. doi: 10.1016/s1734-1140(09)70016-7. [DOI] [PubMed] [Google Scholar]
- 118.Little WC, Zile MR, Kitzman DW, Hundley WG, O’Brien TX, deGroof RC. The Effect of Alagebrium Chloride (ALT-711), a Novel Glucose Cross-Link Breaker, in the Treatment of Elderly Patients with Diastolic Heart Failure. J Card Fail. 2005;11:191–195. doi: 10.1016/j.cardfail.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 119.Zile MR, Bourge RC, Redfield MM, Zhou D, Baicu CF, Little WC. Randomized, double-blind, placebo-controlled study of sitaxsentan to improve impaired exercise tolerance in patients with heart failure and a preserved ejection fraction. JACC Heart Fail. 2014;2:123–130. doi: 10.1016/j.jchf.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 120.Bujak M, Frangogiannis NG. The role of IL-1 in the pathogenesis of heart disease. Arch Immunol Ther Exp (Warsz ) 2009;57:165–176. doi: 10.1007/s00005-009-0024-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Van Tassell BW, Arena R, Biondi-Zoccai G, et al. Effects of interleukin-1 blockade with anakinra on aerobic exercise capacity in patients with heart failure and preserved ejection fraction (from the D-HART pilot study) Am J Cardiol. 2014;113:321–327. doi: 10.1016/j.amjcard.2013.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fitchett D, Zinman B, Wanner C, et al. Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA-REG OUTCOME(R) trial. Eur Heart J. 2016;37:1526–1534. doi: 10.1093/eurheartj/ehv728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tanaka A, Inoue T, Kitakaze M, et al. Rationale and design of a randomized trial to test the safety and non-inferiority of canagliflozin in patients with diabetes with chronic heart failure: the CANDLE trial. Cardiovasc Diabetol. 2016;15:57. doi: 10.1186/s12933-016-0381-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wilson RM, De Silva DS, Sato K, Izumiya Y, Sam F. Effects of Fixed-Dose Isosorbide Dinitrate/Hydralazine on Diastolic Function and Exercise Capacity in Hypertension-Induced Diastolic Heart Failure. Hypertension. 2009;54:583–590. doi: 10.1161/HYPERTENSIONAHA.109.134932. [DOI] [PubMed] [Google Scholar]
- 125.Boon RA, Iekushi K, Lechner S, et al. MicroRNA-34a regulates cardiac ageing and function. Nature. 2013;495:107–110. doi: 10.1038/nature11919. [DOI] [PubMed] [Google Scholar]
- 126.Westermann D, Riad A, Richter U, et al. Enhancement of the endothelial NO synthase attenuates experimental diastolic heart failure. Basic Res Cardiol. 2009;104:499–509. doi: 10.1007/s00395-009-0014-6. [DOI] [PubMed] [Google Scholar]
- 127.Abraham WT, Adamson PB, Bourge RC, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377:658–666. doi: 10.1016/S0140-6736(11)60101-3. [DOI] [PubMed] [Google Scholar]
- 128.Adamson PB, Abraham WT, Bourge RC, et al. Wireless pulmonary artery pressure monitoring guides management to reduce decompensation in heart failure with preserved ejection fraction. Circ Heart Fail. 2014;7:935–944. doi: 10.1161/CIRCHEARTFAILURE.113.001229. [DOI] [PubMed] [Google Scholar]
- 129.Kaye D, Shah SJ, Borlaug BA, et al. Effects of an interatrial shunt on rest and exercise hemodynamics: results of a computer simulation in heart failure. J Card Fail. 2014;20:212–221. doi: 10.1016/j.cardfail.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 130.Hasenfuss G, Hayward C, Burkhoff D, et al. A transcatheter intracardiac shunt device for heart failure with preserved ejection fraction (REDUCE LAP-HF): a multicentre, open-label, single-arm, phase 1 trial. Lancet. 2016;387:1298–1304. doi: 10.1016/S0140-6736(16)00704-2. [DOI] [PubMed] [Google Scholar]
- 131.Kitzman D, Brubaker P, Morgan T, Stewart K, Little W. Exercise training in older patients with heart failure and preserved ejection fraction. Circ Heart Fail. 2010;3:659–667. doi: 10.1161/CIRCHEARTFAILURE.110.958785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Edelmann F, Gelbrich G, Dungen H, et al. Exercise Training Improves Exercise Capacity and Diastolic Function in Patients With Heart Failure With Preserved Ejection Fraction: Results of the Ex-DHF (Exercise training in Diastolic Heart Failure) Pilot Study. J Am Coll Cardiol. 2011;58:1780–1791. doi: 10.1016/j.jacc.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 133.Kitzman DW, Brubaker PH, Herrington DM, et al. Effect of endurance exercise training on endothelial function and arterial stiffness in older patients with heart failure and preserved ejection fraction: A randomized, controlled, single-blind trial. J Am Coll Cardiol. 2013;62:584–592. doi: 10.1016/j.jacc.2013.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Edelmann F, Gelbrich G, Dungen H, et al. Exercise Training Improves Exercise Capacity and Diastolic Function in Patients With Heart Failure With Preserved Ejection Fraction: Results of the Ex-DHF (Exercise training in Diastolic Heart Failure) Pilot Study. J Am Coll Cardiol. 2011;58:1780–1791. doi: 10.1016/j.jacc.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 135.Kitzman DW, Brubaker P, Morgan T, et al. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction: a randomised clinical trial. JAMA. 2016;315:36–46. doi: 10.1001/jama.2015.17346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Angadi SS, Mookadam F, Lee CD, Tucker WJ, Haykowsky MJ, Gaesser GA. High-intensity interval training vs. moderate-intensity continuous exercise training in heart failure with preserved ejection fraction: A pilot study. J Appl Physiol. 2014;95:15–27. doi: 10.1152/japplphysiol.00518.2014. [DOI] [PubMed] [Google Scholar]
- 137.Suchy C, Massen L, Rognmo O, et al. Optimising exercise training in prevention and treatment of diastolic heart failure (OptimEx-CLIN): rationale and design of a prospective, randomised, controlled trial. Eur J Prev Cardiol. 2014;21:18–25. doi: 10.1177/2047487314552764. [DOI] [PubMed] [Google Scholar]
- 138.Koifman E, Grossman E, Elis A, et al. Multidisciplinary rehabilitation program in recently hospitalized patients with heart failure and preserved ejection fraction: Rationale and design of a randomized controlled trial. Am Heart J. 2014;168:830–837. doi: 10.1016/j.ahj.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 139.Hundley WG, Bayram E, Hamilton CA, et al. Leg flow-mediated arterial dilation in elderly patients with heart failure and normal left ventricular ejection fraction. Am J Physiol Heart Circ Physiol. 2007;292:H1427–H1434. doi: 10.1152/ajpheart.00567.2006. [DOI] [PubMed] [Google Scholar]
- 140.Bhella PS, Prasad A, Heinicke K, et al. Abnormal haemodynamic response to exercise in heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13:1296–1304. doi: 10.1093/eurjhf/hfr133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Fujimoto N, Prasad A, Hastings JL, et al. Cardiovascular effects of 1 year of progressive endurance exercise training in patients with heart failure with preserved ejection fraction. Am Heart J. 2012;164:869–877. doi: 10.1016/j.ahj.2012.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hambrecht R, Gielen S, Linke A, et al. Effects of exercise training on left ventricular function and peripheral resistance in patients with chronic heart failure. JAMA. 2000;283:3095–3101. doi: 10.1001/jama.283.23.3095. [DOI] [PubMed] [Google Scholar]
- 143.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA Guideline for the management of heart-failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 144.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- 145.Mendes LA, Davidoff R, Cupples LA, Ryan TJ, Jacobs AK. Congestive heart failure in patients with coronary artery disease: the gender paradox. Am Heart J. 1997;134:207–212. doi: 10.1016/s0002-8703(97)70126-1. [DOI] [PubMed] [Google Scholar]
- 146.Moser M, Hebert PR. Prevention of disease progression, left ventricular hypertrophy and congestive heart failure in hypertension treatment trials. J Am Coll Cardiol. 1996;27:1214–1218. doi: 10.1016/0735-1097(95)00606-0. [DOI] [PubMed] [Google Scholar]
- 147.Wright JT, Jr, Williamson JD, Whelton PK, et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015;373:2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]