Abstract
BACKGROUND
Cardiac allograft vasculopathy (CAV) remains a leading cause of mortality after heart transplantation (HT). Angiotensin converting enzyme inhibitors (ACEIs) may retard the development of CAV, but have not been well studied after HT.
OBJECTIVES
We tested the safety and efficacy of the ACEI ramipril on the development of CAV early after HT.
METHODS
In this prospective, multicenter, randomized, double-blind, placebo-controlled trial, 96 HT recipients were randomized to ramipril or placebo. They underwent coronary angiography, endothelial function testing, measurement of fractional flow reserve (FFR), coronary flow reserve (CFR) and the index of microcirculatory resistance (IMR), and intravascular ultrasound (IVUS) of the left anterior descending coronary artery within 8 weeks of HT. At 1 year, the invasive assessment was repeated. Circulating endothelial progenitor cells (EPCs) were quantified at baseline and 1 year.
RESULTS
Plaque volume at 1 year was similar between the ramipril and placebo groups (162.1 ± 70.5 vs. 177.3 ± 94.3 mm3, p =0.73). Patients receiving ramipril had improvement in microvascular function as evidenced by a significant decrease in IMR (21.4 ± 14.7 to 14.4 ± 6.3, p =0.001) and increase in CFR (3.8 ± 1.7 to 4.8 ± 1.5, p =0.017) from baseline to 1 year. This did not occur with IMR (17.4 ± 8.4 to 21.5 ± 20.0, p =0.72) or CFR (4.1 ± 1.8 to 4.1 ± 2.2, p =0.60) in the placebo-treated patients. EPCs decreased significantly at 1 year in the placebo group but not in the ramipril group.
CONCLUSION
Ramipril does not slow development of epicardial plaque volume but does stabilize EPC levels and improve microvascular function, which have been associated with improved long-term survival after HT.
Keywords: Cardiac allograft vasculopathy, Microcirculation, Coronary physiology
Cardiac allograft vasculopathy (CAV) manifesting as progressive coronary intimal thickening remains a leading cause of morbidity and mortality after heart transplantation (HT) (1). Angiotensin converting enzyme inhibitors (ACEIs) attenuate coronary atherosclerotic events in non-transplant patients independent of antihypertensive effects, presumably by improving endothelial function, decreasing inflammation, and reducing oxidative stress, all of which stabilize and reduce plaque development (2). Previous single center and retrospective studies have suggested that ACEIs decrease coronary plaque development after cardiac transplantation, however, ACEIs have not been well studied in this setting (3–5). The goal of this prospective, multicenter, randomized, double-blind, placebo controlled trial was to test the safety and efficacy of the ACEI ramipril on the development of CAV early after HT. The primary endpoint was the difference in plaque volume at 1 year, with key secondary endpoints including the effect of ramipril on coronary physiology, endothelial function and endothelial progenitor cell levels.
METHODS
STUDY DESIGN
Consecutive HT recipients at Stanford University, the Palo Alto Veterans Affairs Health Care System, and Cedars Sinai Medical Center who were ≥12 years old and had a serum creatinine <2.0 mg/dL were included. Patients were excluded if they were undergoing more than 1 solid organ transplant or repeat HT. Within 8 weeks of HT, patients were randomized in an equal and double-blind fashion to either ramipril or to matching placebo. These were initiated 1 week after coronary angiography, coronary endothelial function testing, fractional flow reserve (FFR), coronary flow reserve (CFR), the index of microcirculatory resistance (IMR) and intravascular ultrasound (IVUS) examination of the left anterior descending (LAD) coronary artery were performed. Blood was collected to quantify circulating endothelial progenitor cells. One year after HT, the invasive and serologic tests were repeated. The primary endpoint of the study was the difference in plaque volume as assessed by intravascular ultrasound (IVUS) examination of the LAD at 1 year between those the ramipril and placebo groups. Key secondary endpoints included the safety and efficacy of ramipril administration early after HT, the effect of ramipril on coronary endothelial function, coronary physiology, and circulating endothelial progenitor cell number. This study was approved by the institutional review committee at each participating site. Informed written consent was obtained from all patients.
IMMUNOSUPPRESSIVE AND OTHER MEDICAL THERAPY
Adult patients (age ≥18 years old) received induction therapy with methylprednisolone 500 mg IV when coming off bypass and rATG at 1 mg/kg (maximum dose 125 mg) given on post-operative days 1, 2, and 3. Pediatric patients received daclizumab 1 mg.kg. Two additional doses of methylprednisolone were given over the next 24 hours. Pediatric patients received daclizumab every other week for a total of 5 doses after transplantation. The daily maintenance immunosuppressive regimen included cyclosporine (2 to 4 mg/kg/day) in children, tacrolimus (0.5 mg twice daily) in adults, and mycophenolate mofetil (1000 mg twice daily) in both adults and pediatrics patients. Dose adjustments were made as indicated by adverse effects and/or acute rejection. Prednisone was initiated at 1 mg/kg/day, and tapered to 0.5 mg/kg/day by month 6, and to 0.1 mg/kg/day by month 12. Concomitant medications were initiated as soon as the patient was able to tolerate oral intake. All patients received co-trimoxazole (1 double strength tablet daily) for pneumocystis pneumonia prophylaxis, except for patients with sulfa allergy who received atovaquone instead of co-trimoxazole.
An HMG-CoA reductase inhibitor (pravastatin) was initiated in all patients at 20 mg per day within 1 week of transplant regardless of plasma cholesterol or triglyceride concentration. The dose of pravastatin was increased to 40 mg as tolerated. Unless contraindicated by hypotension or allergic reaction, patients were placed on a calcium channel-blocking drug. The first line agent was diltiazem, commencing at a dose of 120 mg daily. Dose adjustments of the calcium channel blocker were based on routine blood pressure measurements, targeting a goal of <130/80 mmHg. Aspirin was routinely given unless contraindicated.
All patients in whom either the donor or recipient was cytomegalovirus positive by serologic testing received standard ganciclovir prophylaxis, consisting of valganciclovir (900 mg twice daily) for 1 month initiated as soon as the patient could tolerate oral medications, followed by 900 mg daily until the end of the third post-operative month. Thereafter, all patients remained on valganciclovir 450 mg daily for the remainder of the first year post HT. Patients presenting with signs or symptoms of cytomegalovirus infection underwent testing for confirmation and treatment. Patients were monitored for acute rejection by routine surveillance endomyocardial biopsy and echocardiography to evaluate allograft function at 2 weeks and 1, 2, 3, 4, 6, 9 and 12 months after HT.
TITRATION OF STUDY DRUG AND HYPERTENSION MANAGEMENT
Ramipril or placebo was initiated at 2.5 mg per day 1 week after baseline coronary angiography. Two weeks later, if the serum creatinine was stable and the patient had not developed any adverse sequelae, the dose was increased to 5 mg per day and serum creatinine was checked again 2 weeks later. If the creatinine was stable and the patient had not developed any adverse sequelae, the dose was further increased to 10 mg per day in the adult patients. The dose was titrated to a maximum of 20 mg per day in adult patients if a serum creatinine 2 weeks later was stable and there were no adverse sequelae. If a subject developed a side effect felt secondary to study drug, the dose was first decreased and then discontinued if the side effect persisted.
If a subject developed hypertension, diltiazem was increased. If a patient remained hypertensive despite a maximal dose of diltiazem, hydrochlorothiazide was started at 12.5 mg daily and increased to 25 mg as necessary. If a patient continued to be hypertensive despite a maximal dose of hydrochlorothiazide, then clonidine 0.1 mg twice daily was added and titrated upwards as necessary. A beta-blocker or dihydropyridine calcium antagonist was added if the blood pressure was not controlled with the above medications. Patients had routine blood pressure evaluation on a weekly basis during the first month, on a monthly basis during the first 4 months, and then at months 6, 9, and 12 after HT. The blood pressure goal was <130/80 mmHg in all patients. Patients were not allowed to take an angiotensin receptor blocker or direct renin inhibitor during the study period.
The invasive coronary artery assessments are described in the order in which they were performed in each patient.
CORONARY ENDOTHELIAL FUNCTION ASSESSMENT
Endothelial function assessment was performed in the adult patients enrolled at Stanford University and the Palo Alto Veterans Affairs Health Care System. Using a 6 French guiding catheter, baseline coronary angiography of the LAD without panning the table was performed in a cranial projection. Intracoronary acetylcholine (20 mcg) was administered over 30 seconds. An angiogram of the LAD was recorded. A second bolus of intracoronary acetylcholine (50 mcg) was administered over 30 seconds. Another angiogram of the LAD was recorded. Intracoronary nitroglycerin (100 to 200 mcg) was administered and a final angiogram of the LAD was recorded. All coronary angiograms were analyzed by an independent core laboratory (Cardiovascular Core Analysis Laboratory, Stanford, California) blinded to the patient’s clinical and randomization information. Quantitative and qualitative analyses of the LAD at baseline and after injection were done with an automatic edge detection system (QAngio XA 7.3®; MEDIS, Leiden, Netherlands). Endothelial dysfunction was defined as ≥20% decrease in the LAD diameter after acetylcholine infusion compared with baseline.
CORONARY PHYSIOLOGY ASSESSMENT
Coronary physiology assessment was performed in the adult patients enrolled from Stanford University and the Palo Alto Veterans Affairs Health Care System. After endothelial function assessment and administration of intracoronary nitroglycerin, intravenous heparin (60 units/kg) was administered. A coronary pressure wire (Certus, St. Jude Medical) was calibrated outside of the body and advanced so that the sensor was positioned at the tip of the guide catheter and pressures were equalized. The wire was then advanced to the distal two-thirds of the LAD. Care was taken to position the wire in the same location at baseline and at the 1-year follow-up. Three intracoronary injections of approximately 3 ml of room temperature saline were performed using a 3 cc syringe connected to a stopcock at the back end of the manifold. The resting mean transit time was calculated automatically by the Radi Analyzer (St. Jude Medical) as previously described (6). The 3 resting transit times were automatically averaged and displayed as a resting mean transit time. Intravenous adenosine (140 mcg/kg/min) was then administered. Once maximal hyperemia was achieved, 3 injections of approximately 3 ml of room temperature saline were performed and the hyperemic mean transit time was calculated as described above. Coronary flow reserve (CFR) was calculated as the resting mean transit time divided by the hyperemic mean transit time; the index of microcirculatory resistance (IMR) was calculated as the hyperemic mean transit time multiplied by the hyperemic distal coronary pressure; and fractional flow reserve (FFR) was calculated as the mean distal pressure divided by the mean proximal pressure during maximal hyperemia (7).
INTRAVASCULAR ULTRASOUND
In all patients, a commercially available 40 MHz IVUS catheter (Atlantis SR Pro 2® or OptiCross®; Boston scientific, Natick, Massachusetts) was advanced over the coronary guidewire to the mid to distal LAD. A recording was performed using an automated motorized pullback (0.5 mm/s) of the IVUS catheter. IVUS imaging was analyzed offline by an independent core laboratory (Cardiovascular Core Analysis Laboratory, Stanford, California) blinded to the patient’s clinical and randomization information, using commercially available 3-dimensional reconstruction software (echoPlaque 4.0®; INDEC Medical Systems, Santa Clara, California). Lumen and vessel contours were traced with 1 mm axial intervals for the first 50 mm from the ostium of the LAD. For volumetric analyses, lumen and vessel volumes were calculated using Simpson’s rule and divided by the axial analyzed length (mm3) to adjust for any differences of analyzed longitudinal lengths among the cases. Plaque volume was defined as vessel volume – lumen volume; percent plaque volume (%) was defined as 100 x plaque volume/vessel volume. Maximal intimal thickness (MIT) was also obtained from the interpolated data set as a 2-dimensional IVUS index.
QUANTIFICATION OF ENDOTHELIAL PROGENITOR CELL NUMBER
Whole blood was collected in heparinized collection tubes. Most samples were processed the same day. Other samples were shipped overnight to Stanford University from Cedars Sinai, Los Angeles at ambient temperature and then processed immediately. Peripheral blood mononuclear cells were separated via centrifugation over Ficoll-Paque Plus (GE Healthcare) following the manufacturer’s directions and preserved in 10% DMSO/90% fetal calf serum (FCS) in liquid nitrogen vapor. To perform flow cytometry, samples were thawed and rested for 2 hours in complete media (RPMI 1640 with10%FCS) at 37°C and 5% CO2 prior to surface staining with a cocktail of fluorochrome-conjugated monoclonal antibodies reactive with human CD3, CD14, CD16, CD19, CD20, CD56, CD31, CD34, and CD45 (BD Biosciences) (Online Table 1). Samples were then stained with LiveDead Blue (ThermoFisher), fixed with 2% paraformaaldehyde in phosphate-buffered saline (Electron Microscopy Sciences) and stored at 4°C until acquisition. Data was acquired on LSR II instrument (BD Biosciences) running FACSDIVA software in the Shared FACS Facility obtained. Post-acquisition analysis was done using FlowJo (Treestar). PBMCs, initially gated on light scatter properties and viability staining, were tested for surface expression of CD45 and multiple cell lineage markers (CD3, CD14, CD16, CD19, CD20, CD56). CD31 and CD34 surface expression was then further assessed on those events that were CD45(−) and lineage(−) (Supplemental Figure 1). Events that were CD34(++) and CD31(+) were recorded as EPCs and reported as a percent of total live cells, which were transformed to log10 values (8,9).
STATISTICAL ANALYSIS
Categorical variables are presented as counts and percentages. Pearson’s chi-square test or Fisher’s exact test was used for comparisons of categorical variables, as appropriate. Normality of the continuous variables was confirmed with the Shapiro-Wilk test. Depending on the result of the Levene test for homoscedasticity, variables with normal distribution were compared with Student t-test or Welch t-test. If the normality test failed, variables were compared with Mann-Whitney U test. For paired comparisons, variables with normal distribution were compared with paired t-test. If the normality test failed, variables were compared with Wilcoxon signed-rank test. A p value of <0.05 was considered statistically significant. Assuming a plaque volume at 1 year of 170 mm3 in the control arm and a plaque volume of 133 mm3 in the ramipril treated patients with a standard deviation of 60 mm3, a sample size of 42 was required in each arm to have 80% power to detect a significant difference. All analyses were performed using SPSS® version 21.
RESULTS
Ninety-six patients, including 7 pediatric patients (12 to 19 years old) were enrolled into the study and randomized to ramipril or matching placebo. As outlined in Figure 1, 39 patients in the ramipril arm and 38 patients in the placebo arm completed both baseline and 1-year IVUS examinations. Baseline characteristics of the 2 groups were well matched and are described in Table 1. After 1 year, there were no significant adverse effects related to treatment with ramipril or to the matching placebo. Of this group, 1 patient stopped ramipril because of low blood pressure. The mean dosage of ramipril was 13.8 ± 7.7 mg. As outlined in Table 2, at 1 year, the systolic and diastolic blood pressures were similar in both groups. There was a significantly lower rate of calcium channel blocker use in the ramipril-treated patients as compared to the placebo group (40 vs. 64%, p =0.026). This was primarily because a significantly lower proportion of patients who received amlodipine in the ramipril compared to the placebo group (22.2% vs. 45.5%, respectively; p =0.02), as well as a significantly lower dose of amlodipine in the ramipril versus the placebo group (1.3 ± 2.9 vs. 5.9 ± 18.1 mg, respectively; p =0.012). The blood urea nitrogen, creatinine and potassium levels were all significantly higher in the ramipril group, but no patient had to stop ramipril because of renal dysfunction or hyperkalemia. There was no significant difference between the ramipril and placebo groups with regard to the change in creatinine (0.14 ± 0.31 vs. 0.10 ± 0.29 respectively; p =0.56) or potassium (0.0 ± 0.6 vs. −0.2 ± 0.5 respectively; p =0.12). At 1 year, there was no difference in acute cellular rejection (Grade ≥2R diagnosed by right ventricular biopsy) between the ramipril and placebo groups (14.9 vs. 10.2%, p =0.49).
Figure 1. Enrollment flowchart.
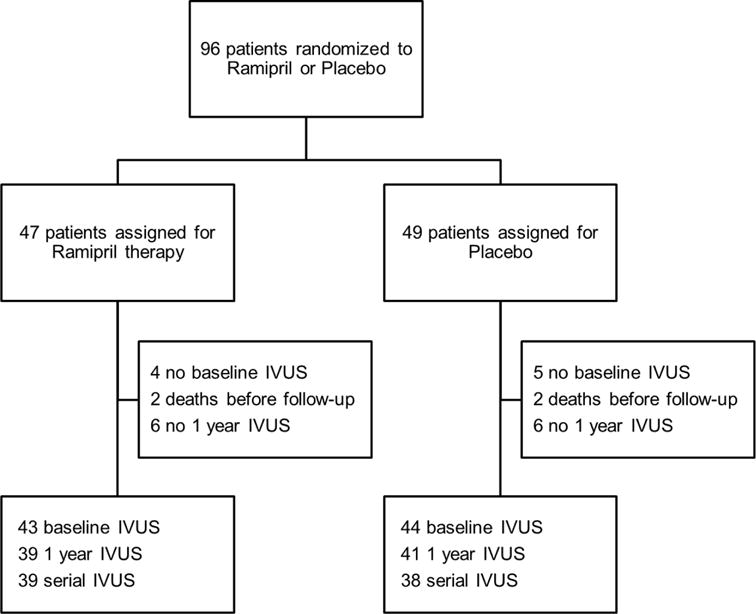
A total of 96 patients were randomized to ramipril (n = 47) or placebo (n = 49). Comparisons of baseline IVUS data were performed between 43 patients in the ramipril group and 44 patients in the placebo group where IVUS images were analyzable. Comparisons of 1 year IVUS data were performed between 39 patients in the ramipril group and 41 patients in the placebo group where IVUS images were available (survived at 1 year) and analyzable. In patients both baseline and 1 year IVUS were analyzed, we performed paired comparisons within the group (39 serial IVUS cases in the ramipril group and 38 serial IVUS cases in the placebo group). IVUS = Intravascular Ultrasound.
Table 1.
Baseline Recipient and Donor Clinical Characteristics
| Variable | Ramipril n = 47 |
Placebo n = 49 |
p value |
|---|---|---|---|
| Recipient characteristics: | |||
| Age, years | 55 ± 15 | 52 ± 17 | 0.57 |
| Male, n (%) | 35 (74.5) | 32 (65.3) | 0.33 |
| Body mass index, kg/m2 | 25.4 ± 4.2 | 25.4 ± 6.0 | 0.57 |
| Comorbidities: | |||
| CMV IgG-positive, n (%) | 33 (70.2) | 34 (70.8) | 0.95 |
| Diabetes, n (%) | 13 (27.7) | 14 (28.6) | 0.92 |
| Hypertension, n (%) | 27 (57.4) | 28 (57.1) | 0.98 |
| Systolic blood pressure (mmHg) | 134 ± 16 | 132 ± 12 | 0.68 |
| Diastolic blood pressure (mmHg) | 82 ± 12 | 84 ± 12 | 0.40 |
| * Hypercholesterolemia, n (%) | 24 (51.1) | 16 (32.7) | 0.07 |
| Ischemic cardiomyopathy, n (%) | 10 (21.3) | 5 (10.2) | 0.14 |
| Laboratory Data: | |||
| Creatinine (mg/dL) | 1.14 ± 0.35 | 1.03 ± 0.36 | 0.16 |
| BUN (mg/dL) | 29.0 ± 8.9 | 30.7 ± 11.8 | 0.81 |
| Sodium (mmol/L) | 139 ± 3 | 138 ± 3 | 0.29 |
| Potassium (mmol/L) | 4.3 ± 0.4 | 4.2 ± 0.5 | 0.61 |
| Medications: | |||
| Calcium channel blocker, n (%) | 26 (55.3) | 27 (55.1) | 0.98 |
| β-blocker, n (%) | 1 (2.1) | 3 (6.1) | 0.32 |
| Diuretics, n (%) | 32 (68.1) | 28 (57.1) | 0.27 |
| Statin, n (%) | 43 (91.5) | 46 (93.9) | 0.48 |
| Induction therapy | 0.35 | ||
| None | 14 (14.7) | 8 (8.4) | |
| γ-antithymocyte globulin, n (%) | 26 (27.4) | 32 (33.7) | |
| Daclizumab, n (%) | 5 (5.3) | 6 (6.3) | |
| Baciliximab, n (%) | 1 (1.1) | 3 (3.2) | |
| Tacrolimus, n (%) | 42 (89.4) | 43 (87.8) | 0.81 |
| Tacrolimus level, ng/mL | 10.8 ± 3.1 | 12.1 ± 3.9 | 0.12 |
| Cyclosporine, n (%) | 4 (8.5) | 5 (10.2) | 0.53 |
| Cyclosporine level, ng/mL | 365.6 ± 57.9 | 401.3 ± 69.6 | 0.39 |
| Mycophenolate mofetil, n (%) | 47 (100) | 49 (100) | NA |
| Corticosteroid dose, mg | 21.1 ± 9.3 | 25.6 ± 21.8 | 0.67 |
|
| |||
| Donor characteristics: | |||
| Age, years | 33 ± 14 | 33 ± 12 | 0.90 |
| Male, n (%) | 28 (59.6) | 32 (66.7) | 0.47 |
| Sex mismatch, n (%) | 9 (19.1) | 11 (22.9) | 0.65 |
| Blood type mismatch, n (%) | 6 (12.8) | 6 (12.5) | 0.97 |
| CMV IgG mismatch, n (%) | 18 (38.3) | 15 (31.3) | 0.47 |
| Cold ischemic time, minutes | 219 ± 39 | 208 ± 50 | 0.24 |
Values are mean and standard deviation or n (%).
Hypercholesterolemia was defined as patient either taking statin or diagnosed as hypercholesterolemia by a treating physician before heart transplantation. BUN = blood urea nitrogen; CMV = cytomegalovirus; IgG = immunoglobulin G.
Table 2.
One-Year Recipient Clinical Characteristics
| Variable | Ramipril n = 45 |
Placebo n = 47 |
p value |
|---|---|---|---|
| Comorbidities: | |||
| Diabetes, n (%) | 16 (34.0) | 18 (36.7) | 0.24 |
| Hypertension, n (%) | 32 (68.1) | 36 (73.5) | 0.64 |
| Systolic blood pressure, mmHg | 125 ± 16 | 127 ± 15 | 0.45 |
| Diastolic blood pressure, mmHg | 78 ± 12 | 80 ± 11 | 0.31 |
| Laboratory Data: | |||
| Creatinine, mg/dL | 1.29 ± 0.33 | 1.11 ± 0.30 | 0.01 |
| BUN, mg/dL | 24.5 ± 7.1 | 20.9 ± 7.5 | 0.01 |
| Sodium, mmol/L | 139 ± 3 | 140 ± 2 | 0.21 |
| Potassium, mmol/L | 4.3 ± 0.4 | 4.0 ± 0.3 | 0.003 |
|
| |||
| Medications: | |||
| Calcium channel blocker, n (%) | 18 (40) | 28 (64) | 0.026 |
| β-blocker, n (%) | 3 (6.4) | 2 (4.1) | 0.76 |
| Diuretics, n (%) | 12 (25.5) | 10 (20.4) | 0.74 |
| Statin, n (%) | 41 (87.2) | 39 (79.6) | 0.45 |
| Tacrolimus, n (%) | 40 (85.1) | 39 (79.6) | 0.74 |
| Tacrolimus level, ng/mL | 8.1 ± 3.6 | 8.0 ± 3.2 | 0.86 |
| Cyclosporine, n (%) | 4 (8.5) | 3 (6.1) | 0.79 |
| Cyclosporine level, ng/mL | 155.8 ± 68.0 | 154.3 ± 109.1 | 0.98 |
| Mycophenolate mofetil, n (%) | 36 (76.6) | 37 (75.5) | 0.86 |
| Steroid dose, mg | 2.7 ± 3.6 | 3.4 ± 7.0 | 0.74 |
Values are mean and standard deviation or n (%). BUN = blood urea nitrogen.
Serial IVUS examination demonstrated significant increases in maximum intimal thickness and plaque volume and significant decreases in lumen and vessel volume in both cohorts of patients during the first year (Online Table 2). At baseline there were no significant differences in IVUS parameters between the 2 groups; at 1 year there were also no significant differences (Table 3). Plaque volume at 1 year was similar between the patients treated with ramipril versus placebo (162.1 ± 70.5 vs. 177.3 ± 94.3 mm3 respectively; p =0.73) (Online Figure 2). The mean change in plaque volume also was similar between the ramipril and placebo groups (26.7 ± 37.5 vs. 29.7 ± 56.9 mm3 respectively; p =0.68). Ramipril did not have a differential effect based on whether the MIT at baseline was greater or less than 0.5 mm. The above results were similar when the 7 pediatric patients were excluded from the analyses.
Table 3.
Baseline and 1-year IVUS indices.
| Variable | Ramipril | Placebo | p value |
|---|---|---|---|
| Baseline | (n = 43) | (n = 44) | |
| Maximal Intimal Thickness (mm) | 0.74 ± 0.44 | 0.72 ± 0.45 | 0.60 |
| Plaque volume (mm3) | 135.4 ± 58.2 | 147.5 ± 70.4 | 0.42 |
| Plaque Volume (mm3/mm)* | 2.8 ± 1.2 | 3.2 ± 1.6 | 0.35 |
| % Plaque Volume | 19.9 ± 7.8 | 20.3 ± 9.2 | 0.95 |
| Lumen volume (mm3) | 558.7 ± 164.5 | 596.8 ± 183.0 | 031 |
| Lumen Volume (mm3/mm)* | 11.5 ± 3.0 | 12.5 ± 3.2 | 0.15 |
| Vessel volume (mm3) | 694.0 ± 185.8 | 744.3 ± 210.7 | 0.29 |
| Vessel Volume (mm3/mm)* | 14.3 ± 3.3 | 15.6 ± 3.7 | 0.14 |
|
| |||
| One year | (n = 39) | (n = 41) | |
| Maximal Intimal Thickness (mm) | 0.89 ± 0.49 | 0.91 ± 0.52 | 0.90 |
| Plaque volume (mm3) | 162.1 ± 70.5 | 177.3 ± 94.3 | 0.73 |
| Plaque Volume (mm3/mm)* | 3.4 ± 1.7 | 3.7 ± 1.9 | 0.55 |
| % Plaque Volume | 25.2 ± 12.0 | 24.3 ± 9.9 | 0.87 |
| Lumen volume (mm3) | 503.3 ± 158.4 | 542.4 ± 176.3 | 0.46 |
| Lumen Volume (mm3/mm)* | 10.2 ± 3.1 | 11.2 ± 3.4 | 0.21 |
| Vessel volume (mm3) | 665.4 ± 162.3 | 719.7 ± 218.2 | 0.21 |
| Vessel Volume (mm3/mm)* | 13.6 ± 3.2 | 14.8 ± 4.2 | 0.22 |
Values are mean and standard deviation.
Plaque, lumen, and vessel volumes were standardized for analyzed longitudinal length (mm3/mm). IVUS = intravascular ultrasound. Includes patients without serial IVUS assessment.
Serial coronary physiology analyses showed that FFR did not change significantly, while CFR increased and IMR tended to decrease for the entire cohort during the first year. Compared to baseline, IMR decreased significantly at 1 year in the ramipril group (21.4 ± 14.7 to 14.4 ± 6.3; p =0.001), but did not change significantly in the placebo arm (17.4 ± 8.4 to 21.5 ± 20.0; p =0.72). CFR increased significantly in the ramipril group (3.8 ± 1.7 to 4.8 ± 1.5; p =0.017) but did not change in the placebo arm (4.1 ± 1.8 to 4.1 ± 2.2; p =0.60). Finally, FFR decreased significantly in the ramipril group (0.90 ± 0.06 to 0.88 ± 0.04; p =0.007) but did not change in the placebo arm (0.89 ± 0.04 to 0.90 ± 0.04; p =0.39) (Figure 2; Table 4). There was no difference in endothelial function at baseline or at 1 year between the 2 groups; nor was there any difference in change of endothelial function from baseline to 1 year in the 2 groups. The log10 percentage of circulating EPCs was similar at baseline between the 2 groups. In the ramipril group this value did not change significantly at 1 year, whereas in the control group there was a significant decline in circulating EPCs at 1 year compared to the baseline value (Figure 3; Table 4). At 1 year, there was a trend towards lower circulating EPCs in the control group compared to the ramipril group (−1.19 ± 0.41 vs. −1.12 ± 0.32 respectively, p =0.17).
Figure 2. Percent change in FFR, CFR and IMR from baseline to 1 year.
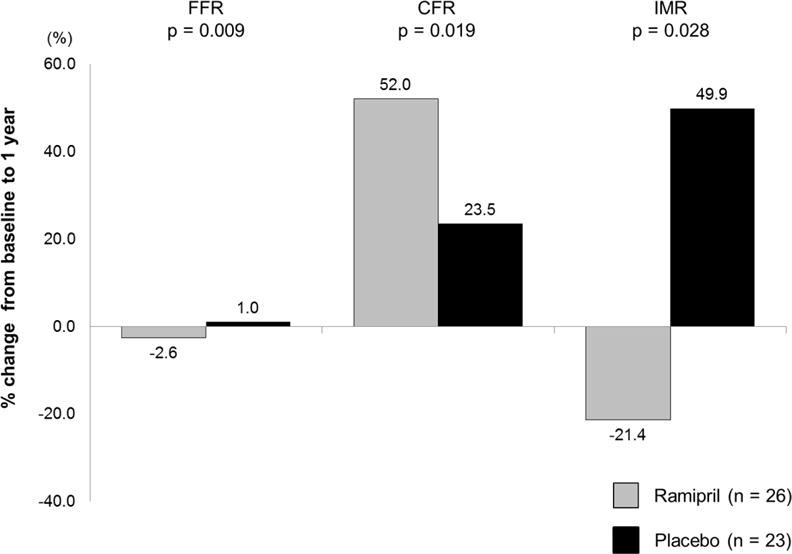
In patients assigned to the ramipril therapy, improvement of microvascular function as assessed by CFR and IMR were observed. CFR = Coronary Flow Reserve; FFR = Fractional Flow Reserve; IMR = Index of Microcirculatory Resistance.
Table 4.
Serial Coronary Physiology, Endothelial Function, and Endothelial Progenitor Cell Data
| Variable | Baseline | One year | p value |
|---|---|---|---|
| Ramipril (n = 26) | |||
| FFR | 0.90 ± 0.06 | 0.88 ± 0.04 | 0.007 |
| CFR | 3.8 ± 1.7 | 4.8 ± 1.5 | 0.017 |
| IMR | 21.4 ± 14.7 | 14.4 ± 6.3 | 0.001 |
| Placebo (n = 23) | |||
| FFR | 0.89 ± 0.04 | 0.90 ± 0.04 | 0.39 |
| CFR | 4.1 ± 1.8 | 4.1 ± 2.2 | 0.60 |
| IMR* | 17.4 ± 8.4 | 21.5 ± 20.0 | 0.72 |
|
| |||
| Ramipril (n = 25) | |||
| Endothelial dysfunction, % | 6.7 ± 20.5 | 9.4 ± 25.6 | 0.63 |
| Placebo (n = 21) | |||
| Endothelial dysfunction, % | 9.3 ± 21.1 | 5.6 ± 27.8 | 0.39 |
|
| |||
| Ramipril (n = 34) | |||
| Log10 % EPC | −1.15 ± 0.55 | −1.12 ± 0.32 | 0.66 |
| Placebo (n = 38) | |||
| Log10 % EPC | −1.05 ± 0.45 | −1.19 ± 0.41 | 0.035 |
Values are mean and standard deviation.
Serial IMR assessment was possible in 22 patients. Numbers are smaller because only Stanford patients underwent physiology and endothelial function assessment. FFR = fractional flow reserve, CFR = coronary flow reserve, IMR = index of microcirculatory resistance, EPC = Endothelial Progenitor Cell.
Figure 3. Log10 of the percentage of circulating EPCs at baseline and at 1 year after transplant for the placebo group or the ramipril-treated group.
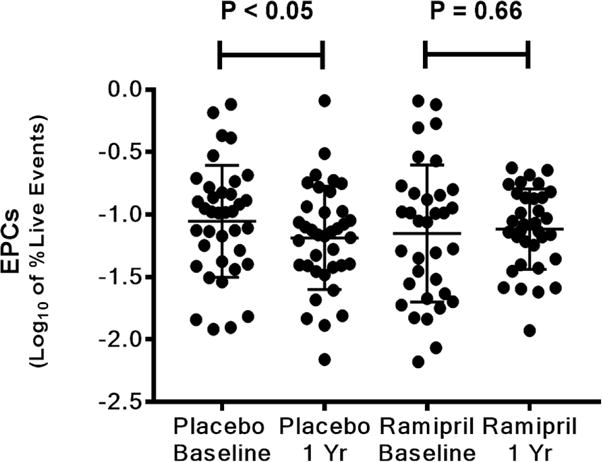
Percentage of circulating EPCs significantly decreased in the placebo group from baseline to 1 year whereas it was similar in the ramipril group. EPC = Endothelial Progenitor Cell.
DISCUSSION
Our main findings regarding the use of the ACEI ramipril early after HT are that 1) the use of ramipril appears safe and effective at lowering blood pressure; 2) the use of ramipril did not significantly affect plaque progression based on IVUS examination; and 3) the use of ramipril improved microvascular function based on IMR and CFR assessment during the first year after HT (Central Illustration).
Central Illustration. Effects of ACE Inhibition Early After Heart Transplantation.

Key positive and negative effects of the ACEI ramipril when administered early after heart transplantation.
Ramipril was well tolerated by transplant patients, even when started within the first few weeks after transplantation. There were no cases of acute renal failure or hyperkalemia requiring discontinuation of the ACEI. Ramipril was also effective at lowering blood pressure, as the ramipril-treated patients required significantly less antihypertensive medication compared to the placebo group. These findings have clinical relevance because of previous concern regarding the tolerability and effectiveness of ACEIs after HT (10). They suggest that ACEIs can be considered a reasonable antihypertensive agent in this population and may have ancillary beneficial effects on the cardiovascular system and renal system with longer-term use, as demonstrated in non-transplant populations.
The renin angiotensin system has been implicated in the development of native coronary atherosclerosis, as well as CAV (11). ACEIs inhibit the renin angiotensin system and decrease angiotensin II, reduce oxidative stress, inhibit smooth muscle cell proliferation, and improve fibrinolysis, all of which likely contribute to their antiatherogenic properties. Previous single center and retrospective studies suggested that ACEIs reduce coronary plaque development after cardiac transplantation (3–5). For example, a small, nonrandomized study comparing the degree of CAV at 1 year after HT based on IVUS measurement of intimal thickness showed that patients receiving an ACEI had less CAV compared to similar controls (3). In a retrospective comparison of transplant recipients treated with ACE I, calcium channel blocker or both, an ACEI alone appeared to be better than a calcium channel blocker with respect to development of CAV, and the combination of ACEI and calcium channel blocker appeared to be best (4). In another retrospective evaluation, 22 transplant recipients with plaque progression based on serial IVUS evaluation were less likely to have been treated with an ACEI compared to 17 patients without plaque regression (5). However, these studies were not prospective, randomized or blinded, and therefore may have been subject to bias.
In this prospective trial, a significant increase in plaque volume, % plaque volume and maximal intimal thickness occurred from baseline to 1 year in both arms, with no apparent protective effect conferred by ramipril. There was concomitant loss of lumen due to a combination of plaque progression and negative vessel remodeling, which occurred to a similar degree in both arms.
Interestingly, although there was no effect on the epicardial anatomy based on IVUS examination in this study, treatment with ramipril did significantly improve microvascular function. IMR (an index specific for the coronary microvasculature) and CFR (an index that interrogates the entire coronary circulation, both the microcirculation and the epicardial vessel) improved significantly in the ramipril-treated patients from baseline to 1 year, while there was no significant change in the placebo-treated patients (Figure 2). FFR (an index that independently assesses the functional significance of epicardial CAV) decreased significantly from baseline to 1 year in the ramipril treated patients, while it did not change in the placebo group. Because plaque progression was similar in the 2 treatment arms, the numerically small but statistically significant decrease in FFR in the ramipril group is likely a reflection of improved microvascular function in this group at 1 year, which allowed greater hyperemic flow, a larger pressure gradient, and a lower FFR, despite the similar plaque volume.
The improved microvascular function at 1 year in the ramipril treated patients is clinically relevant because we demonstrated in a prior study that transplant recipients with low IMR (<20) measured at 1 year, and those with a decrease or no change in IMR from baseline to 1 year after transplantation, had significantly lower rates of long-term mortality or the need for retransplantation (12). In addition, the IMR measurement at 1 year was an independent predictor of death or retransplantation, while IVUS parameters were not, suggesting that microvascular function may be a more important indicator of long-term outcome as compared to epicardial plaque. Moreover, in another study IMR was found to predict development of CAV and graft dysfunction (13). In non-transplant recipients IMR has also been found to be useful in identifying the cause of chest pain in patients with non-obstructive epicardial coronary disease and in predicting poor recovery of left ventricular function and mortality when measured after primary percutaneous coronary intervention in patients with ST segment elevation myocardial infarction (14,15).
Despite this positive effect on microvascular function, ramipril did not appear to improve endothelial function, as the percentage of patients with endothelial dysfunction was similar between the 2 groups. These findings contradict a previous report which found that short-term intracoronary infusion of the ACEI quinaprilat to HT recipients resulted in improved endothelial function based on improved epicardial artery diameter and flow (16). The discordant finding may be explained by the delivery method, the duration of exposure, and/or the ACEI used in the 2 studies.
The role of circulating EPCs in the development of CAV remains unclear. In a clinical study evaluating this topic, Simper et al. found significantly decreased numbers of circulating EPCs in transplant recipients with angiographic evidence of CAV compared to matched recipients without evidence of CAV (17). In the non-transplant population, ACEI therapy may increase the number and the function of circulating EPCs. For example, after 4 weeks of ramipril therapy in patients with stable coronary artery disease, the ACEI-treated patients demonstrated a greater than 2-fold increase in circulating EPC number, with improved proliferative and migratory capacity (18). In the present study, we observed a significant decline in the level of EPCs at 1 year after transplant in the placebo group, whereas the levels at baseline and at 1 year were similar in the ramipril group. This raises the possibility that ramipril treatment may be beneficial for microvascular function following cardiac allograft transplantation, at least in part, by stabilizing circulating EPC number and function.
LIMITATIONS
Limitations of this study include the sample size; however, our study was powered to detect a 20% difference in plaque volume at 1 year and we were able to detect significant differences in microvascular function. Another limitation of this study is the relatively short duration of therapy with ramipril, although other therapies such as calcium channel blockade and statin administration showed benefit during the first year of treatment (19,20). It is unlikely that the dose of ramipril was too low, particularly given the adequate blood pressure control, the changes in potassium and creatinine seen, and the beneficial effects on the microvasculature suggesting a physiologic effect. Additionally, the dosage achieved was similar to previous trials showing a benefit of ramipril (21). Why ramipril improved microvascular function without decreasing epicardial plaque remains unclear. It may be that the positive effects of ACEIs on oxidative stress, endothelial function, smooth muscle cell proliferation and fibrinolysis selectively benefit the microvasculature. Based on this study, we cannot conclude whether this is a class effect associated with all ACEIs, or unique to ramipril.
CONCLUSIONS
Ramipril appears to be a safe and effective antihypertensive early after HT. It does not significantly affect plaque progression during the first year, but it does stabilize circulating endothelial progenitor cell levels and improve microvascular function, which has been associated with improved long-term survival after HT. Further study of the longer term clinical effects of ACEIs in this population are warranted.
Supplementary Material
CONDENSED ABSTRACT.
In this prospective, multicenter, randomized placebo controlled trial we tested the safety and efficacy of the angiotensin converting enzyme inhibitor, ramipril in 96 patients early after heart transplantation (HT). We found that ramipril was safe and effective, but did not slow coronary artery plaque progression during the first year after HT. However, ramipril did stabilize circulating endothelial progenitor cell number and did lead to significant improvement in microvascular function, which has been associated with improved long-term survival after HT.
PERSPECTIVES.
Competency in Medical Knowledge
The angiotensin converting enzyme inhibitor, ramipril, is a safe and effective antihypertensive agent that stabilizes circulating endothelial progenitor cell levels and improves coronary microvascular function early after heart transplantation, but does not prevent thickening of the epicardial coronary artery intima.
Translational Outlook
Further research is needed to understand the interactions between endothelial progenitor cell levels and microvascular function after cardiac transplantation.
Acknowledgments
A NIH S10 Shared instrument Grant (S10RR027431-01) is gratefully acknowledged for the purchase of the LSRII flow cytometer used in this study.
Sources of Funding: 5 R01 HL093475-02 (WFF)
Abbreviations
- ACEI
Angiotensin Converting Enzyme Inhibitor
- CAV
Cardiac Allograft Vasculopathy
- CFR
Coronary Flow Reserve
- EPC
Endothelial Progenitor Cell
- FFR
Fractional Flow Reserve
- IMR
Index of Microcirculatory Resistance
- IVUS
Intravascular Ultrasound
- LAD
Left Anterior Descending
- HT
Heart Transplantation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: WFF receives research support from St. Jude Medical Center (now Abbott Vascular); there are no other disclosures.
Clinical Trials Registration: Clinicaltrials.gov identifier: NCT01078363
References
- 1.Lund LH, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-second official adult heart transplantation report–2015; focus theme: early graft failure. J Heart Lung Transplant. 2015;34:1244–54. doi: 10.1016/j.healun.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Dagenais GR, Pogue J, Fox K, Simoons ML, Yusuf S. Angiotensin-converting-enzyme inhibitors in stable vascular disease without left ventricular systolic dysfunction or heart failure: a combined analysis of three trials. Lancet. 2006;368:581–8. doi: 10.1016/S0140-6736(06)69201-5. [DOI] [PubMed] [Google Scholar]
- 3.Mehra MR, Ventura HO, Smart FW, Collins TJ, Ramee SR, Stapleton DD. An intravascular ultrasound study of the influence of angiotensin-converting enzyme inhibitors and calcium entry blockers on the development of cardiac allograft vasculopathy. Am J Cardiol. 1995;75:853–4. doi: 10.1016/s0002-9149(99)80432-9. [DOI] [PubMed] [Google Scholar]
- 4.Erinc K, Yamani MH, Starling RC, et al. The effect of combined angiotensin-converting enzyme inhibition and calcium antagonism on allograft coronary vasculopathy validated by intravascular ultrasound. J Heart Lung Transplant. 2005;24:1033–8. doi: 10.1016/j.healun.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Bae JH, Rihal CS, Edwards BS, et al. Association of angiotensin-converting enzyme inhibitors and serum lipids with plaque regression in cardiac allograft vasculopathy. Transplantation. 2006;82:1108–11. doi: 10.1097/01.tp.0000230378.61437.a5. [DOI] [PubMed] [Google Scholar]
- 6.De Bruyne B, Pijls NH, Smith L, Wievegg M, Heyndrickx GR. Coronary thermodilution to assess flow reserve: experimental validation. Circulation. 2001;104:2003–6. doi: 10.1161/hc4201.099223. [DOI] [PubMed] [Google Scholar]
- 7.Ng MK, Yeung AC, Fearon WF. Invasive assessment of the coronary microcirculation: superior reproducibility and less hemodynamic dependence of index of microcirculatory resistance compared with coronary flow reserve. Circulation. 2006;113:2054–61. doi: 10.1161/CIRCULATIONAHA.105.603522. [DOI] [PubMed] [Google Scholar]
- 8.Shim Y, Nam MH, Hyuk SW, Yoon SY, Song JM. Concurrent hypermulticolor monitoring of CD31, CD34, CD45 and CD146 endothelial progenitor cell markers for acute myocardial infarction. Anal Chim Acta. 2015;853:501–7. doi: 10.1016/j.aca.2014.10.036. [DOI] [PubMed] [Google Scholar]
- 9.Lachmann R, Lanuti P, Miscia S. OMIP-011: Characterization of circulating endothelial cells (CECs) in peripheral blood. Cytometry A. 2012;81:549–51. doi: 10.1002/cyto.a.22071. [DOI] [PubMed] [Google Scholar]
- 10.Brozena SC, Johnson MR, Ventura H, et al. Effectiveness and safety of diltiazem or lisinopril in treatment of hypertension after heart transplantation. Results of a prospective, randomized multicenter trail. J Am Coll Cardiol. 1996;27:1707–12. doi: 10.1016/0735-1097(96)00057-5. [DOI] [PubMed] [Google Scholar]
- 11.Yousufuddin M, Yamani MH. The renin-angiotensin hypothesis for the pathogenesis of cardiac allograft vasculopathy. Int J Cardiol. 2004;95:123–7. doi: 10.1016/j.ijcard.2003.05.027. [DOI] [PubMed] [Google Scholar]
- 12.Yang HM, Khush K, Luikart H, et al. Invasive assessment of coronary physiology predicts late mortality after heart transplantation. Circulation. 2016;133:1945–50. doi: 10.1161/CIRCULATIONAHA.115.018741. [DOI] [PubMed] [Google Scholar]
- 13.Haddad F, Khazanie P, Deuse T, et al. Clinical and functional correlates of early microvascular dysfunction following heart transplantation. Circ Heart Fail. 2012;5:759–68. doi: 10.1161/CIRCHEARTFAILURE.111.962787. [DOI] [PubMed] [Google Scholar]
- 14.Lee BK, Lim HS, Fearon WF, et al. Invasive evaluation of patients with angina in the absence of obstructive coronary artery disease. Circulation. 2015;131:1054–60. doi: 10.1161/CIRCULATIONAHA.114.012636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fearon WF, Low AF, Yong AS, et al. Prognostic value of the index of microcirculatory resistance measured after primary percutaneous coronary intervention. Circulation. 2013;127:2436–41. doi: 10.1161/CIRCULATIONAHA.112.000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steinhauff S, Pehlivanli S, Bakovic-Alt R, et al. Beneficial effects of quinaprilat on coronary vasomotor function, endothelial oxidative stress, and endothelin activation after human heart transplantation. Transplantation. 2004;77:1859–65. doi: 10.1097/01.tp.0000131148.78203.b7. [DOI] [PubMed] [Google Scholar]
- 17.Simper D, Wang S, Deb A, et al. Endothelial progenitor cells are decreased in blood of cardiac allograft patients with vasculopathy and endothelial cells of noncardiac origin are enriched in transplant atherosclerosis. Circulation. 2003;108:143–9. doi: 10.1161/01.CIR.0000081703.34526.5D. [DOI] [PubMed] [Google Scholar]
- 18.Min TQ, Zhu CJ, Xiang WX, Hui ZJ, Peng SY. Improvement in endothelial progenitor cells from peripheral blood by ramipril therapy in patients with stable coronary artery disease. Cardiovasc Drugs Ther. 2004;18:203–9. doi: 10.1023/B:CARD.0000033641.33503.bd. [DOI] [PubMed] [Google Scholar]
- 19.Schroeder JS, Gao SZ, Alderman EL, et al. A preliminary study of diltiazem in the prevention of coronary artery disease in heart-transplant recipients. N Engl J Med. 1993;328:164–70. doi: 10.1056/NEJM199301213280303. [DOI] [PubMed] [Google Scholar]
- 20.Kobashigawa JA, Katznelson S, Laks H, et al. Effect of pravastatin on outcomes after cardiac transplantation. N Engl J Med. 1995;333:621–7. doi: 10.1056/NEJM199509073331003. [DOI] [PubMed] [Google Scholar]
- 21.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med. 2000;342:145–53. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


