Abstract
Early life adversity (ELA) increases the risk of depression during adolescence that may result from a decline in parvalbumin (PVB) secondary to increased neuroinflammation. In this study, we investigated depressive-like behavior following exposure to two different types of stressors that are relevant for their developmental period: 1) chronic ELA (maternal separation; MS) and 2) an acute emotional stressor during adolescence (witnessing their peers receive multiple shocks; WIT), and their interaction. We also determined whether reducing inflammation by cyclooxygenase-2 (COX-2) inhibition would prevent the onset of depressive-like behavior. Female Sprague-Dawley rat pups underwent MS for four-hours/day or received typical care (CON) between postnatal days (P) 2 and P20. A COX-2 inhibitor (COX-2I) or vehicle was administered every other day between P30 and P38. Subjects were tested for learned helplessness to assess depressive-like behavior at P40 (adolescence). MS females demonstrated increased escape latency and decreased PVB in the prefrontal cortex (PFC) and dorsal raphe that were attenuated by COX-2I intervention. Helplessness was also associated with an increase in D2 receptors in the accumbens. In contrast, WIT elevated escape latency in CON, but reduced latency in MS females. Furthermore, COX-2I intervention decreased escape latency in both CON and MS after WIT. WIT reduced PVB levels in the basolateral amygdala and increased PFC levels to CON levels. Our data suggest that decreased PVB in the PFC is important for the expression of depressive-like behavior and suggest that COX-2I intervention may provide a novel prevention for depression.
Keywords: maternal separation, cyclodeoxygenase-2, depression, female, parvalbumin, prefrontal cortex
1. Introduction
Exposure to adverse experiences early in development may contribute to the expression or exacerbation of a variety of psychiatric disorders including schizophrenia, anxiety, and depression [1, 2]. Symptoms of depression following exposure to early life adversity (ELA) often appear during adolescence, but are delayed in expression, averaging 9.2 years after the abuse was initiated [2]. Sex differences in the prevalence of depression also emerge during adolescence, with a 2:1 ratio of females to males by age 15 [3, 4].
The increase in social awareness that occurs in teens due to continued development of motivation, affect, and decision-making brain regions [5-7] plays a role in heightened sensitivity to social stress during adolescence [8-10]. Exposure to early adversity may further exacerbate risk for depression by increasing sensitivity to subsequent stressors [10, 11]. Greater negative affect and rumination demonstrated by female adolescents may render them even more susceptible to an acute emotional stressor than male adolescents [12]. Even vicariously experiencing an adverse event can be as detrimental to mental health as actually experiencing the event directly [13, 14]. Preclinical studies to date have only focused on adolescent and adult males witnessing social defeat that precipitates increased helplessness in the forced swim test [15, 16]. Here, the effects of an emotional stressor (i.e. witnessing peer distress) on depressive-like behavior in females were investigated.
Increasing evidence suggests that reduced activity in the GABA neurotransmitter system is associated with the pathophysiology of depression, and has been reported in teens with depression and suicide victims [17-20]. In the current study we used an ethologically relevant rodent model of exposure to early adversity, maternal separation (MS) during the neonatal period [21], to study alterations in GABAergic expression in several stress-related brain regions. MS is associated with changes in GABA in the prefrontal cortex (PFC [22-24]) and the amygdala [25]. Rats with a history of MS show changes in PFC-mediated behaviors including learned helplessness (LH), working memory deficits, depressive-like behavior, and reduced social interaction [24, 26-28]. Prior studies show reduced GABAergic calcium-binding protein parvalbumin (PVB) expression in MS adolescent males [24] and females [26] and the basolateral amygdale in MS (BLA; [22, 23]). However, studies have focused primarily on PVB in the PFC, and whether changes in PVB are apparent in other regions in MS subjects is not well known.
While the PFC has been a primary target for investigating the effects of GABA following MS, other brain regions that can influence affective behavior are likely to contribute. Clinical imaging studies show functional differences in PFC, amygdala, and ventral striatum (nucleus accumbens; NAc) connectivity following ELA [29]. The serotonergic dorsal raphe nucleus (DR) plays a critical role in depressive-like behavior [30] and has been implicated in the learned helplessness model [31]. Reciprocal PFC inputs into the DR have shown that increased output from the PFC synapse on GABAergic interneurons to modulate depressive-like behavior [31]. Whether MS influences these interneurons to effect helplessness has not been determined. Similarly, increased excitatory input to D2 receptor-expressing GABAergic medium spiny neurons in the NAc reduces motivation and increases anhedonia [32]; other studies show that D2/D3 receptors directly correlate with elevated motivation in individuals with attention deficit hyperactivity disorder [33]. In the current study, changes in PVB and/or D2 receptors were investigated in the PFC, NAc, amygdala, and the DR of MS animals or controls.
Finally, the delayed onset of depressive symptoms following exposure to early adversity [2] provides a window of opportunity to intervene before symptoms emerge. One possible intervention may be inhibition of the pro-inflammatory molecule cyclooxygenase-2 (COX-2) that is elevated following ELA [24]. Inflammatory responses have been associated with depression in adult human studies [34]. Furthermore, anti-inflammatory agents have efficacy in treating depression in humans [35] and in animals [36]. Our previous work suggests that a cyclooxygenase inhibitor (COX-2I) improves working memory in rats with a history of MS [24]. Whether COX-2I treatment could prophylactically reduce the onset of depressive-like symptoms and/or differentially affect these other target regions is not yet known.
In this study, we examined the effects of MS in females on motivational deficits in the learned helplessness (LH) paradigm and whether these deficits could be prevented by COX-2I. Following MS, PVB levels were examined in the PFC, the BLA, and the DR; dopamine D2 receptor levels were investigated within the NAc. We also investigated whether an acute stress exposure of witnessing their peers receive shocks (WIT) further exacerbated the effects of MS.
2. Material and methods
2.1. Subjects
Pregnant female multiparous Sprague-Dawley rats (250–275 g) were obtained from Charles River Laboratories (Wilmington, MA) on day 16 of gestation. The day of birth was designated as postnatal day 0 (P0). One day after birth, litters were culled to 10 pups (5 males and 5 females), and litters were randomly assigned to either a maternal separation group (MS Group) or animal facility reared control group (CON Group). Pups in the MS Group were isolated for 4 h per day between P2 and P20, and kept at thermoneutral temperature. This procedure is similar to those used previously by this laboratory [24, 37] and others [38]. Pups in the CON Group were not disturbed after day 2, except for routine weekly changes in cage bedding during which all pups were weighed. Rats were housed with food and water available ad libitum in constant temperature and humidity conditions on a 12-h light/dark cycle (light period 07:00-19:00). Rats were weaned on P21, and group-housed in same-sex caging until behavioral testing.
The experimental timeline is outlined in Figure 1A. These experiments were conducted in accordance with the 2011 Guide for the Care and Use of Laboratory Animals (National Research Council, Eighth Edition), and were approved by the Institutional Animal Care and Use Committee at McLean Hospital.
Figure 1.
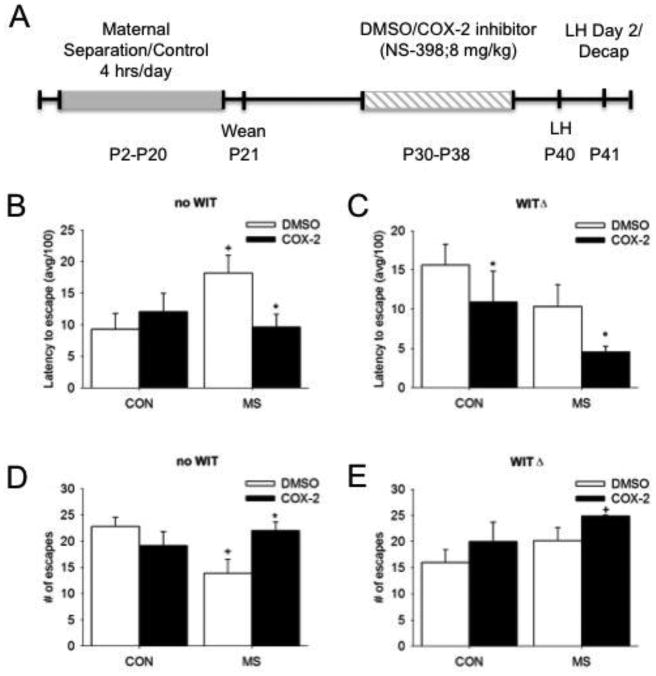
A) Illustration of experimental timeline. The effects of MS and COX-2 intervention on depressive behavior on the latency to escape in B) no WIT and C) WIT females and on the number of escapes in D) no WIT and E) WIT females. Means ± SE presented. ΔP<0.05 compared to non-WIT females, *P<0.05 compared to DMSO within similar MS condition, and +P<0.05 compared to control females within similar Treatment group.
2.2. COX-2 Treatments
The MS or CON subjects (n = 7-8/ Group and Treatment) were treated with either the COX-2I NS-398 (8 mg/kg, diluted in dimethyl sulfoxide [DMSO]) or vehicle (1 ml/kg DMSO) from P30 to P38 once/day every second day and given by i.p. injection. This dose was based on previous studies showing that between 5 and 10 mg/kg NS-398 has neuroprotective effects from excitotoxic and neuroinflammatory damage [24, 39]. Since DMSO is a mild irritant, subjects were dosed every second day to avoid unnecessary stress and discomfort of the animals.
2.3. Depressive-like behavior assessment
2.3.1. Testing paradigm
Females were tested in one of the three conditions used in the triadic model of learned helplessness (LH) between P40 and P42 or in an active avoidance paradigm. The LH paradigm used in this study consisted of the following conditions: 1) an escapable shock (ES) subject and 2) a no shock or “naïve to aversive stimuli” group (NS), where these animals were exposed to, but did not directly experience shock on the first day. Each LH condition assesses different aspects of depressive-like behavior via separate neuronal circuitry [28, 36]. Our previous studies found that females exposed to MS exhibited increased escape latency in the NS group only [26], an LH condition associated with motivational circuitry [26, 40, 41]. Therefore, the current study focused on the effects of MS in females in the NS group.
In the current study, NS rats were gently restrained in the testing apparatus in a separate room (n=7-8/treatment group). This initial group is considered the no witness group (‘no WIT’). Because the rat does not experience any shock this first day, nor witness any in its peers, testing on Day 2 is an active avoidance paradigm. On Day 2, females were placed into a dimly lit shuttle box for 30 trials. Subjects were able to terminate a 1 mA foot-shock by shuttling to the other side for trials 1-5, or by shuttling to the other side and back again for trials 6-30. This response was cued by a tone that preceded the shock by two seconds. The shock remained on for 30 seconds, or until terminated by the appropriate behavioral response. The number of escapes and the mean latency to escape the shock was measured for trials 6-30.
2.3.2. Acute emotional peer distress exposure (witness; WIT)
To measure whether WIT had effects on behavioral and treatment outcomes in rats with different social histories, a separate group of animals were mildly restrained in the testing apparatus on Day 1 of LH where they witnessed (WIT; n=7-8/treatment group) ∼2 hours of an ES rat undergoing 100 trials of an escapable tail shock in a wheel-turn box where paddling the wheel would terminate the shock. The shocks for the ES female were unsignaled, and delivered on a variable time 45 second schedule. Shock intensity escalated from 1.0 to 1.6 mA over the 100 trials to reduce habituation during training. In both the no WIT and the WIT groups, subjects first encounter the aversive stimulus of the shock itself on Day 2 of the LH paradigm [40]. Subjects are tested as described above.
2.4. Western Blots
Ninety minutes following the onset of Day 2 of LH in both no WIT and WIT conditions, animals were decapitated and tissue was regionally dissected in the PFC, NAc, BLA, and DR, frozen on dry ice, and stored at -80 °C until processed. Tissue was then homogenized in 1% sodium dodecylsulfate solution (SDS) containing a protease inhibitor cocktail (Pierce, Rockford, IL). Protein concentration was determined by the Bradford method (BioRad Laboratories, Hercules, CA; [42]) and 40 μg of protein was mixed in 6 × SDS, centrifuged, and boiled for 3 min prior to separation by 15% SDS-PAGE. Following electrophoresis, proteins were transferred to a nitrocellulose membrane (BioRad Laboratories). The membranes were blocked with Odyssey blocking buffer (LI-COR Biosciences, Lincoln, NE) in phosphate-buffered saline for 60 minutes at RT and incubated with primary polyclonal antibodies to PVB neurons (14-17kDa; 1:10,000; Thermo Scientific, Rockford, IL) and D2 receptors (52kDa; 1:400; Santa Cruz Biotechnology, Dallas, TX) in Odyssey blocking buffer (LI-COR Biosciences) in PBS containing 0.1-0.2% Tween (PBS-T) overnight 4 °C. The membranes were rinsed four times for five minutes at RT in PBS-T. After the rinsing procedure, the membranes were incubated for one hour at room temperature in IRDye 800-conjugated affinity purified anti-rabbit (LI-COR Biosciences) or anti-rat (LI-COR Biosciences) 1:20,000 in Odyssey blocking buffer in 0.1% PBS-T. Control for protein loading was achieved by using primary antibodies to mouse β-tubulin (55 kDa; 1:10,000, Covance Laboratories, Dedham, MA) and secondary antibodies to anti-mouse β-tubulin for IRDye 700-conjugated affinity purified anti-IgG (H&L; LI-COR) in PBS-T. Proteins were detected using the Odyssey infrared imaging system (excitation/emission filters at 780 nm/820 nm range; LI-COR Biosciences). For all Western analyses, because each of the eight conditions were included in each run, all data were normalized to its CON DMSO no WIT females as a control.
2.5. Statistical Analyses
Behavioral and Western data were analyzed by a mixed ANOVA (SPSS v 20): a 2 × 2 × 2 design with Treatment (Veh/COX), MS (CON/MS), and WIT (WIT/no WIT) as between-subjects measures. Planned comparisons were conducted across Treatment and MS groups; post-hoc analyses across WIT groups were corrected with Bonferroni's. Correlational analyses (Pearson's r) were used to examine the relationship between biochemical markers and behavior, and comparisons across different correlations were conducted using Fisher's Z transformation. Significance was set at P<0.05.
2. Results
3.1 Depressive-like behavior
In a 2 (Treatment [Veh/COX]) × 2 (MS [CON/MS]) × 2 (WIT [WIT/no WIT]) ANOVA, we found an MS × WIT interaction (F1, 50=5.41, P=0.02) and a main effect of Treatment (F1, 50=5.89, P=0.02) for latency to escape. An MS × Treatment interaction (F1, 24=4.38, P=0.047; Figure 1B) was observed within the no WIT group for the latency to escape. In no WIT females, vehicle females within the MS group took longer to escape the shock than females in the CON Veh group. COX-2I treatment significantly decreased the latency to escape in MS, but not CON females, suggesting that this treatment is effective only in animals with a stress history. In WIT females, our analyses revealed overall Treatment (F1, 26=5.93, P=0.02) and MS main effects (F1, 26=4.61, P=0.04) on the latency to escape (Figure 1C). Surprisingly, MS reduced escape latency in females exposed to an acute vicarious stressor. In addition, COX-2I significantly decreased latency to escape in both MS and CON females.
A 3 way interaction between MS × Treatment × WIT was observed for the number of escapes (F1, 50=4.49, P=0.04). The number of escapes in females in the no WIT group was differentially afected by MS and Treatment (MS × Treatment interaction: F1, 24=5.68, P=0.03; Figure 1D). A decreased number of escapes were observed in MS no WIT females that were reversed by COX-2I treatment. In the WIT group, a main effect of Treatment was observed on the number of escapes (F1, 26=7.52, P=0.01; Figure 1E), where COX-2I increased the number of escapes overall.
3.2. Western blots
Neither main effects nor interactions were observed between MS, Treatment, and WIT on D2 receptor expression in the NAc (F1, 54=0.01, P=0.95; Figure 2A,B). However, when individual differences were analyzed with Pearson's correlational analysis, increased D2 receptors in the NAc were positively associated with increased escape latency in MS no WIT females (r=0.854, P=0.01) that was lost when the subjects were treated with COX-2 inhibition (Figure 2C; r=0.37, P >0.35). Fisher's Z transformation shows that these two correlations are significantly different from each other (Z=1.75, P<0.05).
Figure 2.
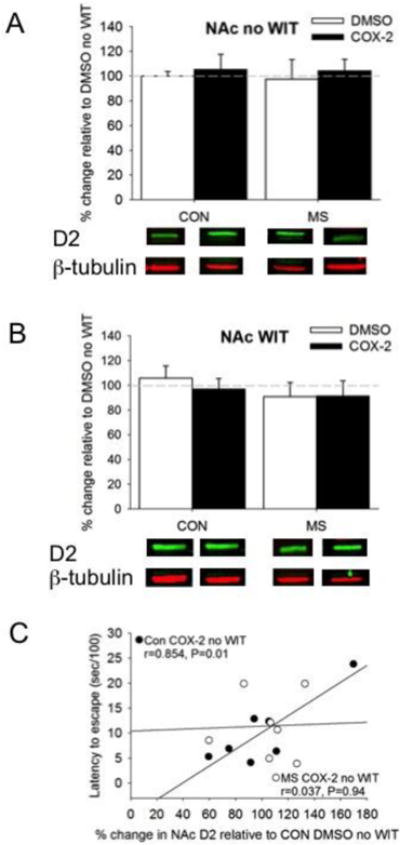
The effects of MS and COX-2 intervention on D2 receptor expression in the NAc of both A) no WIT and B) WIT females. C) Increased NAc D2 receptors in CON COX-2 no WIT (filled circles) females positively correlated with increased latency to escape. Means ± SE presented. Dashed line represents normalization of data to CON no WIT females. Filled circles=Con COX-2I no WIT females. Open circles=MS COX-2I no WIT females.
Within the PFC, a three-way ANOVA between MS, Treatment, and WIT was not observed, but revealed a two-way interaction between MS and Treatment on PVB expression (F1, 47=4.66, P=0.04; Figures 3A,B). The two WIT groups were analyzed separately. First, a MS × Treatment interaction was observed in no WIT females in the PFC (F1, 20=17.11, P=0.001; Figure 3A), where MS reduced, and COX-2I intervention increased, PVB expression. No differences in PVB expression in the PFC following WIT were observed regardless of prior adversity and treatment (Figure 3B). However, a significant correlation between latency to escape and decreased PVB expression in the PFC of the WIT condition was observed in the CON DMSO WIT females (r=-0.829, P=0.02) that was not evident in the CON COX-2I WIT females (P>0.3) suggesting that some degree of stress is needed for COX-2I intervention to be effective (Figure 3C). Fisher's Z transformation suggested that these two correlations are significantly different from each other (Z=-2.79, P<0.01).
Figure 3.
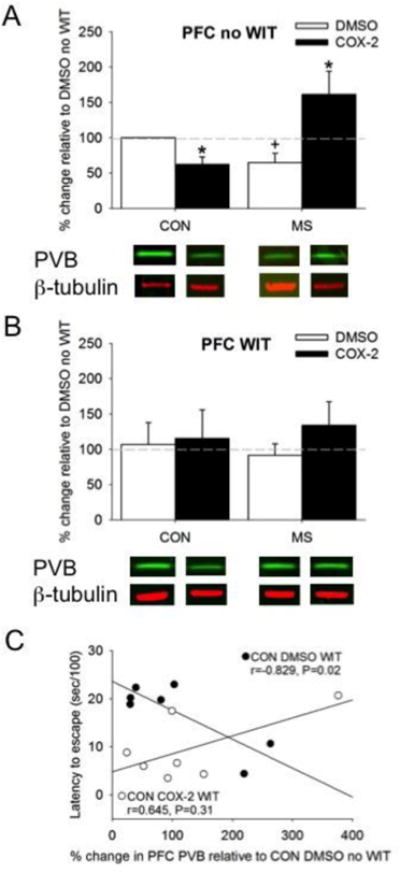
The effects of MS and COX-2 intervention on PVB expression in the PFC of both A) no WIT and B) WIT females. C) Decreased PVB in the PFC of CON DMSO WIT (filled circles) females negatively correlated with increased latency to escape. Means ± SE presented. Dashed line represents normalization of data to CON no WIT females. *P<0.05 compared to DMSO within similar MS condition, and +P<0.05 compared to control females within similar Treatment group. Filled circles=Con DMSO WIT females. Open circles=CON COX-2 WIT females.
In the BLA, only main effects of MS (F1, 38=6.11, P=0.02) and Treatment (F1, 38=5.65, P=0.02) were detected on PVB expression, without a significant three-way interaction. MS decreased PVB expression in the BLA, but more so when females witnessed a peer getting shocked relative to CON (Figure 4A, B). Correlations at the individual level failed to demonstrate a significant relationship between normalized levels of PVB and behavior in non-treated animals (Table 1). Overall, treatment with the COX-2I did not alter PVB expression in the BLA, but comparison of the individual correlations between MS COX-2I WIT and MS DMSO WIT (Fisher's Z=2.22, P<0.05; Figure 4C).
Figure 4.
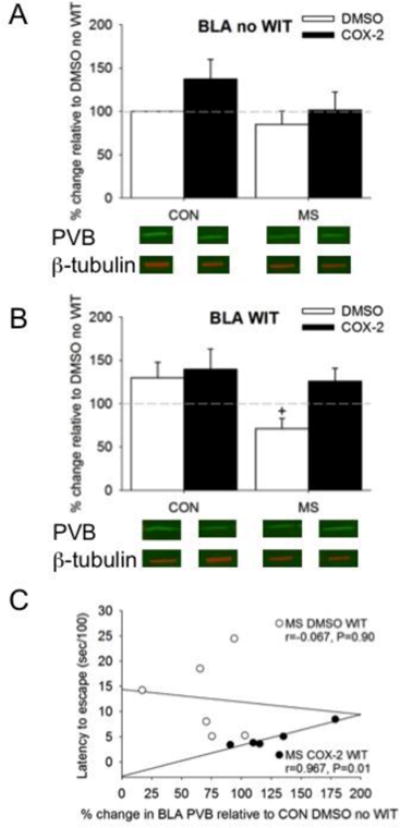
The effects of MS and COX-2 intervention on PVB expression in the BLA of both A) no WIT and B) WIT females. Means ± SE presented. Dashed line represents normalization of data to CON no WIT females. +P<0.05 compared to control females within similar Treatment group.
Table 1. Correlations (Pearson's r) between behavior and PV: effect of social stress.
| Measure | MS Cond | SS Cond | Region | ||
|---|---|---|---|---|---|
| Amygdala | PFC | DR | |||
| # of escapes | CON | no SS | -- | -- | -- |
| SS | -0.21 | 0.33 | 0.36 | ||
|
| |||||
| MS | no SS | 0.11 | 0.62 | -0.14 | |
| SS | 0.17 | 0.05 | -0.4 | ||
|
| |||||
| latency (sec) | CON | no SS | -- | -- | -- |
| SS | 0.04 | -0.83* | 0.16 | ||
|
| |||||
| MS | no SS | -0.22 | -0.68 # | 0.1 | |
| SS | 0.07 | -0.58 | 0.47 | ||
P<0.05
P<0.01
P=0.1 corrected with Bootstrap Analysis
Finally, changes in PVB within the DR were observed. A three-way interaction between MS, Treatment, and WIT on PVB expression was significant (F1, 46=11.91, P=0.001; Figure 5A,B). Analyses were further conducted within WIT groups. An MS × Treatment interaction was observed in no WIT females (F1, 22=14.69, P=0.001; Figure 5A). MS reduced, and COX-2I intervention normalized, PVB expression in the DR of no WIT females. Interestingly, COX-2I intervention in CON no WIT females decreased DR PVB expression. Overall, PVB expression was reduced in the DR of WIT females (Figure 5B). Individual levels of normalized PVB levels did not significantly correlate with behavior (Table 1), and Fisher's Z transformation revealed that the relationship between latency and PVB in the MS WIT animals treated with DMSO or COX-2I were not significantly different (Fisher's Z=1.1, P>0.05; Figure 5C)
Figure 5.
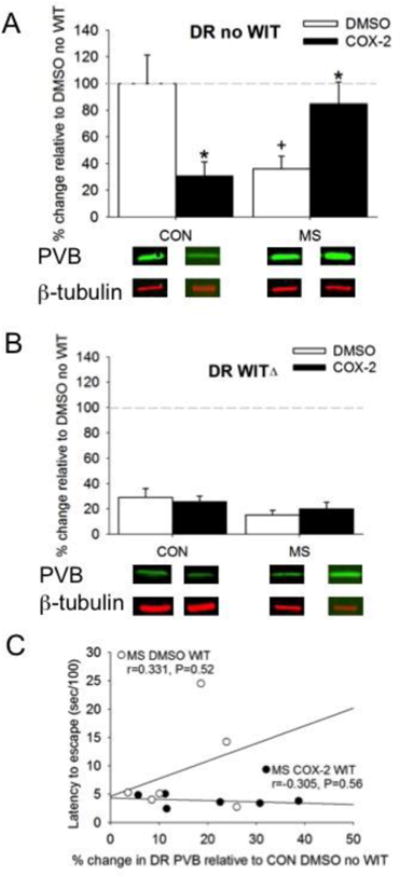
The effects of MS and COX-2 intervention on PVB expression in the DR of both A) no WIT and B) WIT femalesMeans ± SE presented. Dashed line represents normalization of data to CON no WIT females. ΔP<0.05 compared to non-WIT females, *P<0.05 compared to DMSO within similar MS condition, and +P<0.05 compared to control females within similar Treatment group.
4. Discussion
Adolescent females may be particularly sensitive to social stress. Exposure to MS or WIT increased depressive-like behavior in adolescent females. Surprisingly, the effect of WIT did not increase depressive-like behavior relative to the no WIT MS group. Rather, prior exposure to MS reduced the impact of WIT on depressive-like behavior. The no WIT MS group experienced significantly longer latencies to escape and fewer escapes than the WIT MS group, suggesting that this latter group learned something during their Day 1 shock exposure. However, the MS group was still more impaired than the CON group. For these reasons, we will still refer to this paradigm as learned helplessness. The no WIT group experienced an active avoidance response, as they have no prior opportunity to learn about the shock on Day 1 as the WIT group did.
The observation that MS reduced the impact of WIT on depressive behavior deserves further discussion. Exposure to stressful experiences early in life can prepare an individual for a malevolent world [43]. The ability to adapt is protective against later depression. By learning behavioral control over stress or developing coping strategies, the animal is better able to adapt to a subsequent challenge later on [44]. This is the first demonstration that exposure to MS may actually confer some adaptive benefit to the organism. Previous studies have trained animals to control the stress (e.g., such as that found in the ES condition) and these effects are mediated by prefrontal cortex [45]. Changes in PL PVB may contribute to whether animals demonstrate depressive-like behavior or resilience, as suggested by the correlation in this region or modulates serotonin [46].
Preventative intervention with a COX-2I, NSD-398, effectively reduced depressive behavior in both no WIT and WIT conditions. In no WIT females, PVB levels in both the PFC and the DR changed in parallel, but PFC PVB levels mirrored changes in depressive behavior more consistently. Specifically, reduced PVB was associated with increased depressive effects, and like the behavior, COX-2I prevented PVB loss in the PFC. PVB changes in response to WIT were observed in the MS group in the BLA and the DR overall. Finally, no significant effects in D2 receptors in the NAc were observed in any condition, although correlations between NAc D2 receptors and helplessness were observed in the CON no WIT subjects following their first exposure to stress. These data may suggest that NAc D2 receptors change rapidly to stress or are already apparent in susceptible animals [32], whereas PVB changes require minimally ∼24 hours to change in response to stress. Only NAc D2 was measured given its putative role in anhedonia [32].
The results of this study replicate and extend our earlier work [24, 26] by demonstrating MS increases depressive behavior and decreases PVB, but both this decrease in PVB and in depression is preventable with a COX-2I. These effects are most likely mediated by the PFC, where PVB changes paralleled behavioral changes. Changes in PVB in the DR also play a role in this behavioral effect and its reversal in no WIT MS females. In a study by Amat and colleagues [31], reduced GABA in the DR was found in adult males who demonstrated helplessness in the controllable condition of the triadic model. In our studies in MS females, we find helplessness in the motivational condition that was used here, but not the controllable, escapable condition [26, 47]. Levels of PVB were significantly reduced in the DR of MS females and reversed following COX-2I. Surprisingly, low levels of PVB were observed in the WIT group and in females with COX-2I reduced DR PVB in CON subjects in the no WIT group. These data suggest that either a transient stressor such as WIT or COX-2I has detrimental effects if no inflammation is present.
MS animals have increased levels of inflammatory markers in the plasma (IL-6; [48]) and COX-2 in the PFC [24]. Gao et al., has shown that the inflammatory marker IL-6 is present on PVB cells, which may mark them for demise as inflammation rises or stress increases [49]. Other inflammatory markers (IL-1β, IL-4) correlate with working memory performance in MS animals [50]. Alternatively, COX-2 is also involved in glutamate excitoxicity [51]. COX-2I may work by decreasing the NR2A NMDA receptors that are elevated in MS males [48]. We have previously reversed synaptic loss in the frontal cortex of adolescent, socially-stressed rats by treatment with MK-801, an NMDA antagonist [52].
The results of these animal studies of cortical PVB expression and depressive behavior are consistent with clinical studies with magnetic resonance spectroscopy measures of GABA in teenagers or post-mortem analyses [19, 20]. Consistent with the clinical data, we found that MS in no WIT females decreased PVB expression in the PFC and DR, but not the BLA. Moreover, a strong negative association between decreased PVB in the PFC and increased depressive behavior in CON WIT females administered DMSO was observed. In the BLA, both MS and WIT were needed to reduce PVB expression. Other studies found increased PVB expression following MS with cell counting, not Western immunoblot [22].
While recent studies show that witnessing a traumatic event has long lasting effects on behavior in male adult and adolescent rats [15, 53], our study establishes an acute depressive effect in adolescent females may be buffered by prior MS exposure. Exposure to either chronic (MS) or acute (WIT) stress produces behavioral changes that have regional biochemical changes, both of which are preventable by COX-2I.
The current study builds upon our previous observations that adolescent females are more susceptible to motivational deficits that are relevant to depression, but have less controllability issues than adolescent males [8, 26]. We found that MS increased escape latency and decreased the number of escapes in response to shock, which is consistent with deficits in incentive-motivational processes that lead to errors in goal-directed behavior [40, 54]. Earlier studies have shown that MS leads to anhedonic behavior in response to intracranial self-stimulation in adult males rats [55]. Other measures of social stress during later developmental time points, including the social instability paradigm and isolation-rearing of female animals, are associated with anhedonia or alterations in motivational responding [8, 56]. Here, the current manipulation of WIT further expands the ‘stressor arsenal’ of manipulations that produce distress in females. Experiencing a traumatic event secondhand can still have negative effects on mental health [13]. Very few preclinical studies examine this type of emotional stress [15, 16], and fewer in females. We found that witnessing a peer get shocked increased the latency to escape a shock in CON females. Males that witnessed social defeat in a peer have increased sensitivity to stressors, decreased sensitivity to sucrose [15], and increases in anxiety and depressive behavior during adulthood [16]. Our data suggest that experiencing an aversive stimulus, even if its experienced vicariously, is sufficient to increase depressive behaviors similar to females that underwent MS. Future studies will determine whether the effect endures into adulthood, as earlier studies suggest that not all stress exposure effects endure once the females are returned to typical, non-stressful conditions [52].
The observation that a window of opportunity to intervene exists in the MS subjects where preventative intervention of COX-2I reduced depressive behavior is highly clinical relevant. Importantly, COX-2I reduced depressive behavior in animals that had experienced a stressful event, long or short, but did not produce depressive behaviors in individuals where depression (or stress) did not occur. Even though PVB was significantly reduced in the PFC and DR in CON subjects, there was no effect on depressive behavior suggesting that PVB changes are not the sole influence on helplessness. Increased pro-inflammatory activity links MS with the onset of depressive-like behavior [57]. COX-2 is a key mediator of many of the central effects of psychologically relevant stressors and a target of non-steroidal anti-inflammatory agents [58, 59]. We show that treatment with a COX-2I during the juvenile period prevents the expression of depressive-like behavior in female adolescents exposed to an acute, immediate stressor or long-term exposure to three weeks of MS. Our previous studies have found that a COX-2I normalizes PVB expression in the prelimbic PFC and improves working memory deficits due to MS [24]. Therefore, the use of a COX-2I in juvenile females may provide a novel intervention for vulnerable individuals that have a history of early life adversity.
Increased NAc D2 receptors in CON COX-2I no WIT animals led to elevated depressive-like behavior. Recent studies demonstrate that NAc afferent inputs become dysfunctional after stressful stimuli resulting in altered cellular and molecular mechanisms in NAc that mediate depression-like outcomes [60]. Enhancement of D2 receptor synaptic activity in medium spiny neurons of the NAc promotes susceptibility to depressive behavior [32]. Our correlational data suggest that increased D2 receptors in the NAc may be a potential underlying susceptibility mechanism for increased depressive-like behavior. Whether COX-2I helped to unmask this effect is possible, since the relationship between NAc D2 receptors in CON Veh no WIT subjects (R=-0.180) was not significant. We have shown the reverse relationship, where increased PFC D1 receptors are associated with both hedonia and a decrease in D2 receptors in the NAc [61]; reduction of PFC D1 produces helplessness [62]. MS animals have reduced D1 receptors on PFC projections to the NAc [63].
Stressor controllability is regulated by PFC projections to the DR [41]. In turn, serotonergic activation of the DR projecting to the PFC is required for the production of LH [31]. Normal development of this circuit is needed to regulate responses to stress and process information about the controllability of stressors [31]. During the course of normal human development, connections between the PFC and amgydala are immature early in life and become adult-like in late adolescence [29]. Exposure to early life adversity accelerates the development of PFC-amygdala connections [29]. This abnormal rapid development in PFC-amygdala circuitry following early life adversity can lead to an early emergence of adult-like fear learning, amygdala function, and structural maturation [64-66]. Exposure to early adversity also leads to lower NAc reactivity in depressed adolescents [67].
Increased inflammation may underlie the reduced PVB expression in the PFC and DR. For instance, increased COX-2 expression in the PFC of male rats has been observed following MS [24]. In addition, treatment with interleukin-10 (an anti-inflammatory molecule) prevents PVB loss associated with MS in the PFC [48]. In our study, administration with a COX-2 inhibitor increased PVB expression of MS females in both regions. Surprisingly, decreased PVB expression was observed in CON no WIT females in response to COX-2 treatment. In the BLA, MS reduced PVB expression in WIT females but there was no effect of COX-2 treatment.
5. Conclusions
These data suggest that exposure to stress reduces GABA expression possibly due to increased neuroinflammation that may increase risk for the emergence of motivational deficits associated with adolescent depression. These two stress paradigms may also help identify novel targets for prevention of depression in vulnerable individuals during a sensitive period of development.
Acknowledgments
Funding: The project described was supported by a 2013 NARSAD Young Investigator Award from the Brain and Behavior Research Foundation (JLL), a R01 DA-015403 (SLA) from NIDA, a R01 DA-026485 (SLA) from NIDA and the Simches Family (SLA).
Abbreviations
- BLA
basolateral amygdala
- CON
control; animal facility reared control group
- COX-2
cyclodeoxygenase-2
- COX-2I
cyclodeoxygenase-2 inhibitor
- DMSO
dimethyl sulfoxide
- D2
dopamine type 2 receptor
- D3
dopamine type 3 receptor
- DR
dorsal raphe nucleus
- ELA
early life adversity
- ES
escapable shock
- GABA
γ-Aminobutyric acid
- IL-1β
interleukin-1 beta
- IL-4
interleukin-4
- IL-6
interleukin-6
- LH
learned helplessness
- MS
maternal separation
- NAc
nucleus accumbens
- NS
naïve to aversive stimuli
- NMDA
N-methyl-D-aspartate
- NR2A
NMDA subunit NR2A
- PVB
paralbumin
- PBS
phosphate buffered saline
- PBS-T
PBS containing 0.1-0.2% Tween
- PFC
prefrontal cortex
- plPFC
prelimbic prefrontal cortex
- SDS
sodium dodecylsulfate
- WIT
witnessing their peers receive multiple shocks
Footnotes
Disclosure: There are no competing financial interests in relation to the work described.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Penza KM, Heim C, Nemeroff CB. Neurobiological effects of childhood abuse: implications for the pathophysiology of depression and anxiety. Arch Women Ment Health. 2003;6(1):15–22. doi: 10.1007/s00737-002-0159-x. [DOI] [PubMed] [Google Scholar]
- 2.Teicher MH, Samson JA, Polcari A, Andersen SL. Length of time between onset of childhood sexual abuse and emergence of depression in a young adult sample: a retrospective clinical report. The Journal of Clinical Psychiatry. 2009;70(5):684–91. doi: 10.4088/jcp.08m04235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.SAMHSA. 2013-2014 NSDUH State Estimates of Substance Use and Mental Disorders. 2014 http://www.samhsa.gov/data/population-data-nsduh/reports?tab=38.
- 4.Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34(1):13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- 5.Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 2003;27(1-2):3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- 6.Vanderschuren LJ, Niesink RJ, Van Ree JM. The neurobiology of social play behavior in rats. Neurosci Biobehav Rev. 1997;21(3):309–26. doi: 10.1016/s0149-7634(96)00020-6. [DOI] [PubMed] [Google Scholar]
- 7.Casey BJ, Jones RM. Neurobiology of the adolescent brain and behavior: implications for substance use disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49(12):1189–201. doi: 10.1016/j.jaac.2010.08.017. quiz 1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leussis MP, Andersen SL. Is adolescence a sensitive period for depression? Behavioral and neuroanatomical findings from a social stress model. Synapse. 2008;62(1):22–30. doi: 10.1002/syn.20462. [DOI] [PubMed] [Google Scholar]
- 9.Becker JB, Monteggia LM, Perrot-Sinal TS, Romeo RD, Taylor JR, Yehuda R, Bale TL. Stress and disease: is being female a predisposing factor? J Neurosci. 2007;27(44):11851–5. doi: 10.1523/JNEUROSCI.3565-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romeo R. Adoelscence and the ontogeny of the hormonal stress response. Neuroscience and Biobehavioral Reviews. 2016 doi: 10.1016/j.neubiorev.2016.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weber K, Miller GA, Schupp HT, Borgelt J, Awiszus B, Popov T, Elbert T, Rockstroh B. Early life stress and psychiatric disorder modulate cortical responses to affective stimuli. Psychophysiology. 2009;46(6):1234–43. doi: 10.1111/j.1469-8986.2009.00871.x. [DOI] [PubMed] [Google Scholar]
- 12.Ordaz S, Luna B. Sex differences in physiological reactivity to acute psychosocial stress in adolescence. Psychoneuroendocrinology. 2012;37(8):1135–57. doi: 10.1016/j.psyneuen.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beck CT. Secondary traumatic stress in nurses: a systematic review. Archives of Psychiatric Nursing. 2011;25(1):1–10. doi: 10.1016/j.apnu.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Cougle JR, Resnick H, Kilpatrick DG. Does prior exposure to interpersonal violence increase risk of PTSD following subsequent exposure? Behaviour Research and Therapy. 2009;47(12):1012–7. doi: 10.1016/j.brat.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Warren BL, Vialou VF, Iniguez SD, Alcantara LF, Wright KN, Feng J, Kennedy PJ, Laplant Q, Shen L, Nestler EJ, Bolanos-Guzman CA. Neurobiological sequelae of witnessing stressful events in adult mice. Biol Psychiatry. 2013;73(1):7–14. doi: 10.1016/j.biopsych.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patki G, Solanki N, Salim S. Witnessing traumatic events causes severe behavioral impairments in rats. Int J Neuropsychopharmacol. 2014;17(12):2017–29. doi: 10.1017/S1461145714000923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sequeira A, Mamdani F, Ernst C, Vawter MP, Bunney WE, Lebel V, Rehal S, Klempan T, Gratton A, Benkelfat C, Rouleau GA, Mechawar N, Turecki G. Global brain gene expression analysis links glutamatergic and GABAergic alterations to suicide and major depression. PloS One. 2009;4(8):e6585. doi: 10.1371/journal.pone.0006585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanacora G, Mason GF, Rothman DL, Behar KL, Hyder F, Petroff OA, Berman RM, Charney DS, Krystal JH. Reduced cortical gamma-aminobutyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 1999;56(11):1043–7. doi: 10.1001/archpsyc.56.11.1043. [DOI] [PubMed] [Google Scholar]
- 19.Gabbay V, Mao X, Klein RG, Ely BA, Babb JS, Panzer AM, Alonso CM, Shungu DC. Anterior cingulate cortex gamma-aminobutyric acid in depressed adolescents: relationship to anhedonia. Arch Gen Psychiatry. 2012;69(2):139–49. doi: 10.1001/archgenpsychiatry.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klempan TA, Sequeira A, Canetti L, Lalovic A, Ernst C, ffrench-Mullen J, Turecki G. Altered expression of genes involved in ATP biosynthesis and GABAergic neurotransmission in the ventral prefrontal cortex of suicides with and without major depression. Mol Psychiatry. 2009;14(2):175–89. doi: 10.1038/sj.mp.4002110. [DOI] [PubMed] [Google Scholar]
- 21.Lehmann J, Feldon J. Long-term biobehavioral effects of maternal separation in the rat: consistent or confusing? Reviews in the Neurosciences. 2000;11(4):383–408. doi: 10.1515/revneuro.2000.11.4.383. [DOI] [PubMed] [Google Scholar]
- 22.Seidel K, Helmeke C, Poeggel G, Braun K. Repeated neonatal separation stress alters the composition of neurochemically characterized interneuron subpopulations in the rodent dentate gyrus and basolateral amygdala. Developmental Neurobiology. 2008;68(9):1137–52. doi: 10.1002/dneu.20651. [DOI] [PubMed] [Google Scholar]
- 23.Stevenson CW, Meredith JP, Spicer CH, Mason R, Marsden CA. Early life programming of innate fear and fear learning in adult female rats. Behavioural Brain Research. 2009;198(1):51–7. doi: 10.1016/j.bbr.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 24.Brenhouse HC, Andersen SL. Nonsteroidal anti-inflammatory treatment prevents delayed effects of early life stress in rats. Biol Psychiatry. 2011;70(5):434–40. doi: 10.1016/j.biopsych.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(9):5335–40. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leussis MP, Freund N, Brenhouse HC, Thompson BS, Andersen SL. Depressive-like behavior in adolescents after maternal separation: sex differences, controllability, and GABA. Developmental Neuroscience. 2012;34(2-3):210–7. doi: 10.1159/000339162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brenhouse HC, Schwarz JM. Immunoadolescence: Neuroimmune development and adolescent behavior. Neurosci Biobehav Rev. 2016;70:288–299. doi: 10.1016/j.neubiorev.2016.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koe AS, Ashokan A, Mitra R. Short environmental enrichment in adulthood reverses anxiety and basolateral amygdala hypertrophy induced by maternal separation. Translational Psychiatry. 2016;6:e729. doi: 10.1038/tp.2015.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, Hare TA, Bookheimer SY, Tottenham N. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(39):15638–43. doi: 10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gardner KL, Hale MW, Oldfield S, Lightman SL, Plotsky PM, Lowry CA. Adverse experience during early life and adulthood interact to elevate tph2 mRNA expression in serotonergic neurons within the dorsal raphe nucleus. Neuroscience. 2009;163(4):991–1001. doi: 10.1016/j.neuroscience.2009.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci. 2005;8(3):365–71. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- 32.Francis TC, Chandra R, Friend DM, Finkel E, Dayrit G, Miranda J, Brooks JM, Iniguez SD, O'Donnell P, Kravitz A, Lobo MK. Nucleus accumbens medium spiny neuron subtypes mediate depression-related outcomes to social defeat stress. Biol Psychiatry. 2015;77(3):212–22. doi: 10.1016/j.biopsych.2014.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Volkow ND, Wang GJ, Newcorn JH, Kollins SH, Wigal TL, Telang F, Fowler JS, Goldstein RZ, Klein N, Logan J, Wong C, Swanson JM. Motivation deficit in ADHD is associated with dysfunction of the dopamine reward pathway. Mol Psychiatry. 2011;16(11):1147–54. doi: 10.1038/mp.2010.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lindqvist D, Janelidze S, Hagell P, Erhardt S, Samuelsson M, Minthon L, Hansson O, Bjorkqvist M, Traskman-Bendz L, Brundin L. Interleukin-6 is elevated in the cerebrospinal fluid of suicide attempters and related to symptom severity. Biol Psychiatry. 2009;66(3):287–92. doi: 10.1016/j.biopsych.2009.01.030. [DOI] [PubMed] [Google Scholar]
- 35.Muller N. COX-2 inhibitors as antidepressants and antipsychotics: clinical evidence. Curr Opin Investig Drugs. 2010;11(1):31–42. [PubMed] [Google Scholar]
- 36.Hennessy MB, Stafford NP, Yusko-Osborne B, Schiml PA, Xanthos ED, Deak T. Naproxen attenuates sensitization of depressive-like behavior and fever during maternal separation. Physiol Behav. 2015;139:34–40. doi: 10.1016/j.physbeh.2014.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andersen SL, Teicher MH. Delayed effects of early stress on hippocampal development. Neuropsychopharmacology. 2004;29(11):1988–93. doi: 10.1038/sj.npp.1300528. [DOI] [PubMed] [Google Scholar]
- 38.Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Research Molecular Brain Research. 1993;18(3):195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- 39.Madrigal JL, Moro MA, Lizasoain I, Lorenzo P, Leza JC. Stress-induced increase in extracellular sucrose space in rats is mediated by nitric oxide. Brain Res. 2002;938(1-2):87–91. doi: 10.1016/s0006-8993(02)02467-8. [DOI] [PubMed] [Google Scholar]
- 40.Pryce CR, Azzinnari D, Spinelli S, Seifritz E, Tegethoff M, Meinlschmidt G. Helplessness: a systematic translational review of theory and evidence for its relevance to understanding and treating depression. Pharmacology & therapeutics. 2011;132(3):242–67. doi: 10.1016/j.pharmthera.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 41.Maier SF, Watkins LR. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci Biobehav Rev. 2005;29(4-5):829–41. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 42.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical biochemistry. 1976;72:248–54. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 43.Teicher MH. Scars that won't heal: the neurobiology of child abuse. Scientific American. 2002;286(3):68–75. doi: 10.1038/scientificamerican0302-68. [DOI] [PubMed] [Google Scholar]
- 44.Lucas M, Ilin Y, Anunu R, Kehat O, Xu L, Desmedt A, Richter-Levin G. Long-term effects of controllability or the lack of it on coping abilities and stress resilience in the rat. Stress. 2014;17(5):423–30. doi: 10.3109/10253890.2014.930430. [DOI] [PubMed] [Google Scholar]
- 45.Amat J, Paul E, Watkins LR, Maier SF. Activation of the ventral medial prefrontal cortex during an uncontrollable stressor reproduces both the immediate and long-term protective effects of behavioral control. Neuroscience. 2008;154(4):1178–86. doi: 10.1016/j.neuroscience.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amat J, Paul E, Zarza C, Watkins LR, Maier SF. Previous experience with behavioral control over stress blocks the behavioral and dorsal raphe nucleus activating effects of later uncontrollable stress: role of the ventral medial prefrontal cortex. J Neurosci. 2006;26(51):13264–72. doi: 10.1523/JNEUROSCI.3630-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Freund N, Thompson BS, Norman KJ, Einhorn P, Andersen SL. Developmental emergence of an obsessive-compulsive phenotype and binge behavior in rats. Psychopharmacology (Berl) 2015;232(17):3173–81. doi: 10.1007/s00213-015-3967-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wieck A, Andersen SL, Brenhouse HC. Evidence for a neuroinflammatory mechanism in delayed effects of early life adversity in rats: relationship to cortical NMDA receptor expression. Brain, Behavior, and Immunity. 2013;28:218–26. doi: 10.1016/j.bbi.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 49.Gao R, Ji MH, Gao DP, Yang RH, Zhang SG, Yang JJ, Shen JC. Neuroinflammation-Induced Downregulation of Hippocampacal Neuregulin 1-ErbB4 Signaling in the Parvalbumin Interneurons Might Contribute to Cognitive Impairment in a Mouse Model of Sepsis-Associated Encephalopathy. Inflammation. 2017;40(2):387–400. doi: 10.1007/s10753-016-0484-2. [DOI] [PubMed] [Google Scholar]
- 50.Grassi-Oliveira R, Honeycutt JA, Holland FH, Ganguly P, Brenhouse HC. Cognitive impairment effects of early life stress in adolescents can be predicted with early biomarkers: Impacts of sex, experience, and cytokines. Psychoneuroendocrinology. 2016;71:19–30. doi: 10.1016/j.psyneuen.2016.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Araki E, Forster C, Dubinsky JM, Ross ME, Iadecola C. Cyclooxygenase-2 inhibitor ns-398 protects neuronal cultures from lipopolysaccharide-induced neurotoxicity. Stroke; a Journal of Cerebral Circulation. 2001;32(10):2370–5. doi: 10.1161/hs1001.096057. [DOI] [PubMed] [Google Scholar]
- 52.Leussis MP, Lawson K, Stone K, Andersen SL. The enduring effects of an adolescent social stressor on synaptic density, part II: Poststress reversal of synaptic loss in the cortex by adinazolam and MK-801. Synapse. 2008;62(3):185–92. doi: 10.1002/syn.20483. [DOI] [PubMed] [Google Scholar]
- 53.Warren BL, Sial OK, Alcantara LF, Greenwood MA, Brewer JS, Rozofsky JP, Parise EM, Bolanos-Guzman CA. Altered gene expression and spine density in nucleus accumbens of adolescent and adult male mice exposed to emotional and physical stress. Developmental Neuroscience. 2014;36(3-4):250–60. doi: 10.1159/000362875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Balleine BW, Dickinson A. Goal-directed instrumental action: contingency and incentive learning and their cortical substrates. Neuropharmacology. 1998;37(4-5):407–19. doi: 10.1016/s0028-3908(98)00033-1. [DOI] [PubMed] [Google Scholar]
- 55.Der-Avakian A, Markou A. The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci. 2012;35(1):68–77. doi: 10.1016/j.tins.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Herzog CJ, Czeh B, Corbach S, Wuttke W, Schulte-Herbruggen O, Hellweg R, Flugge G, Fuchs E. Chronic social instability stress in female rats: a potential animal model for female depression. Neuroscience. 2009;159(3):982–92. doi: 10.1016/j.neuroscience.2009.01.059. [DOI] [PubMed] [Google Scholar]
- 57.Miura H, Ozaki N, Shirokawa T, Isobe K. Changes in brain tryptophan metabolism elicited by ageing, social environment, and psychological stress in mice. Stress. 2008;11(2):160–9. doi: 10.1080/10253890701685908. [DOI] [PubMed] [Google Scholar]
- 58.Madrigal JL, Garcia-Bueno B, Moro MA, Lizasoain I, Lorenzo P, Leza JC. Relationship between cyclooxygenase-2 and nitric oxide synthase-2 in rat cortex after stress. Eur J Neurosci. 2003;18(6):1701–5. doi: 10.1046/j.1460-9568.2003.02888.x. [DOI] [PubMed] [Google Scholar]
- 59.Dhir A, Padi SS, Naidu PS, Kulkarni SK. Protective effect of naproxen (non-selective COX-inhibitor) or rofecoxib (selective COX-2 inhibitor) on immobilization stress-induced behavioral and biochemical alterations in mice. Eur J Pharmacol. 2006;535(1-3):192–8. doi: 10.1016/j.ejphar.2006.01.064. [DOI] [PubMed] [Google Scholar]
- 60.Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59(12):1151–9. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 61.Sonntag KC, Brenhouse HC, Freund N, Thompson BS, Puhl M, Andersen SL. Viral over-expression of D1 dopamine receptors in the prefrontal cortex increase high-risk behaviors in adults: Comparison with adolescents. Psychopharmacology (Berl) 2014;231(8):1615–26. doi: 10.1007/s00213-013-3399-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Freund N, Thompson BS, Sonntag K, Meda S, Andersen SL. When the party is over: depressive-like states in rats following termination of cortical D1 receptor overexpression. Psychopharmacology (Berl) 2016;233(7):1191–201. doi: 10.1007/s00213-015-4200-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brenhouse HC, Lukkes JL, Andersen SL. Early life adversity alters the developmental profiles of addiction-related prefrontal circuitry. Brain Sciences. 2012;2(1) doi: 10.3390/brainsci3010143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Callaghan BL, Richardson R. Maternal separation results in early emergence of adult-like fear and extinction learning in infant rats. Behavioral Neuroscience. 2011;125(1):20–8. doi: 10.1037/a0022008. [DOI] [PubMed] [Google Scholar]
- 65.Moriceau S, Roth TL, Okotoghaide T, Sullivan RM. Corticosterone controls the developmental emergence of fear and amygdala function to predator odors in infant rat pups. International Journal of Developmental Neuroscience : the Official Journal of the International Society for Developmental Neuroscience. 2004;22(5-6):415–22. doi: 10.1016/j.ijdevneu.2004.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ono M, Kikusui T, Sasaki N, Ichikawa M, Mori Y, Murakami-Murofushi K. Early weaning induces anxiety and precocious myelination in the anterior part of the basolateral amygdala of male Balb/c mice. Neuroscience. 2008;156(4):1103–10. doi: 10.1016/j.neuroscience.2008.07.078. [DOI] [PubMed] [Google Scholar]
- 67.Goff B, Gee DG, Telzer EH, Humphreys KL, Gabard-Durnam L, Flannery J, Tottenham N. Reduced nucleus accumbens reactivity and adolescent depression following early-life stress. Neuroscience. 2013;249:129–38. doi: 10.1016/j.neuroscience.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]


