Abstract
Children with cerebral palsy (CP) expend more energy to walk compared to typically-developing peers. One of the most prevalent gait patterns among children with CP, crouch gait, is often singled out as especially exhausting. The dynamics of crouch gait increase external flexion moments and the demand on extensor muscles. This elevated demand is thought to dramatically increase energy expenditure. However, the impact of crouch severity on energy expenditure has not been investigated among children with CP. We evaluated oxygen consumption and gait kinematics for 573 children with bilateral CP. The average net nondimensional oxygen consumption during gait of the children with CP (0.18 ± 0.06) was 2.9 times that of speed-matched typically-developing peers (0.10 ± 0.03). Crouch severity was only modestly related to oxygen consumption, with measures of knee flexion angle during gait explaining only 5–20% of the variability in oxygen consumption. While knee moment and muscle activity were moderately to strongly correlated with crouch severity (r2 = 0.13 – 0.73), these variables were only weakly correlated with oxygen consumption (r2 = 0.02 – 0.04). Thus, although the dynamics of crouch gait increased muscle demand, these effects did not directly result in elevated energy expenditure. In clinical gait analysis, assumptions about an individual’s energy expenditure should not be based upon kinematics or kinetics alone. Identifying patient-specific factors that contribute to increased energy expenditure may provide new pathways to improve gait for children with CP.
Keywords: cerebral palsy, crouch gait, oxygen consumption, electromyography, co-contraction, energy expenditure
Introduction
Children with cerebral palsy (CP) expend more energy during walking compared to typically-developing children (Campbell and Ball, 1978; Rose et al., 1990). Crouch gait, characterized by excessive hip and knee flexion, is one of the most common gait patterns among children with CP (Wren et al., 2005), and is often singled out as especially exhausting (Sutherland and Davids, 1993; Waters and Mulroy, 1999). In a crouched posture, the ground reaction force moves away from the hip and knee joint centers, increasing the required internal extensor joint moments (Arnold et al., 2005). Further, the ability of key hip and knee extensor muscles to accelerate these joints into extension decreases with crouch severity (Hicks et al., 2008). During crouch gait, sustained activity of the quadriceps throughout stance also acts to decelerate the body, like driving with your parking brake on (Steele et al., 2013). Together, these factors suggest greater and more persistent muscle force would be needed to support and propel the body during crouch gait, thereby requiring greater energy expenditure compared to more upright gait patterns (Hsu et al., 1993; Perry et al., 1975; Steele et al., 2013).
Although these biomechanical and dynamic factors suggest energy expenditure increases with crouch severity, oxygen consumption during crouch gait in children with CP has not been previously reported. In unimpaired adults, prior research has demonstrated that the maximum knee flexion angle during stance strongly correlates (r = 0.75) with energy expenditure during walking (Winter, 1983). Increasing knee flexion during gait among unimpaired adults using a unilateral knee brace has also been shown to increase energy expenditure: 13%, 19%, and 38% with 15°, 30°, and 45° of imposed knee flexion, respectively (Waters and Mulroy, 1999). In typically-developing children, however, wearing bilateral knee braces to restrict knee motion led to inconsistent changes in oxygen consumption, with only six of ten children showing a progressive increase in oxygen consumption with increasing knee flexion (Duffy et al., 1997). In children with CP, energy expenditure during walking has been shown to improve after surgery to correct crouch gait (Nene et al., 1993), but it remains unknown if these improvements are due to a reduction in crouch severity or other changes from surgery.
Factors other than gait dynamics may also impact energy expenditure during walking. Prior research has demonstrated that differences in neuromuscular control can impact energy expenditure. During walking, muscle co-contraction and electromyography (EMG) signal magnitudes have been associated with energy expenditure in CP (Damiano et al., 2000; Unnithan et al., 1996). Spasticity, or other inappropriate muscle activity, may also contribute to increased energy expenditure during walking (Hemingway et al., 2001; Johnston et al., 2004; Olney et al., 1987). During other activities, such as cycling, prior research has shown that coordination of muscle activity, rather than intensity of muscle activity, is related to peak power output; with improper timing contributing to higher energy expenditure (Wakeling et al., 2010). Thus, the magnitude of muscle force and the coordination among muscle groups may influence energy expenditure and performance.
The aim of this study was to quantify oxygen consumption during crouch gait among children with CP. We hypothesized that there would be a strong correlation between crouch severity and oxygen consumption, due to the confluence of biomechanical factors that increase joint moments and muscle activity during crouch gait. We used previously collected clinical motion analysis data to examine the relationship between knee flexion angles, knee moments, and electromyography (EMG) with oxygen consumption during gait. Understanding how crouch severity influences energy expenditure can help guide treatment goals and inform rehabilitation.
Methods
Approval for this research was obtained from the Institutional Review Boards at both the University of Washington and the University of Minnesota.
We analyzed the relationship between oxygen consumption during gait and crouch severity for children with bilateral CP who had previously undergone clinical motion analysis at Gillette Children’s Specialty Healthcare (St. Paul, MN) between February 1994 and March 2014. We included all individuals with a primary diagnosis of bilateral CP who were under the age of 21 and whose clinical analysis included oxygen consumption during a 6-minute walk test and instrumented gait analysis with kinematics and EMG data. These analyses are part of the standard of care at Gillette when patients visit the laboratory, and thus the sampling method results in a representative sample of individuals with CP who have undergone gait analysis at this hospital. We excluded individuals with knee hyperextension (defined as a minimum knee flexion angle more than one standard deviation below the average of typically-developing controls). For each child, one randomly selected limb was included in the analysis.
Instrumented gait analysis was performed with a 12-camera system (Vicon Motion Systems, Lake Forest, CA) and all kinematic data was processed using either the Vicon Clinical Manager or Vicon Plug-in-Gait Model. Knee flexion angles were extracted at 2% increments over the gait cycle and averaged over a minimum of three gait cycles for each individual. To evaluate crouch gait, we used three common metrics previously used to define crouch severity: minimum knee flexion angle during gait, average stance phase (0–60% of gait cycle) knee flexion angle, and knee flexion angle at initial contact. For each child, these three variables were extracted from the average kinematics recorded during instrumented gait analysis at self-selected speed.
Energy expenditure during gait was evaluated from net nondimensional oxygen consumption during a 6-minute self-selected speed walking trial that followed a 3–10 minute rest period (Schwartz et al., 2006). The duration of the resting period depended on the date of visit. Visits prior to 2006 used a 3-minute rest period that analyzed the last minute of the rest period and the most level three minutes of the six-minute walking test. The more recent visits used a 10-minute rest period and averaged all breaths occurring during three minute steady state intervals during both the rest period and six-minute walking test, as described in Schwartz, 2007. These changes were implemented following a review of protocol changes to improve the reliability of energy expenditure measures (see Schwartz, 2007).
Volume flow and oxygen concentration of inspired and expired gases were measured with a breath-by-breath oximeter (CPXD, Medical Graphics Corporation, St. Paul, MN). Net oxygen consumption was calculated as the difference between oxygen consumption at rest and during steady-state walking, and nondimensionalized as:
where and represent the rate of oxygen consumption (J/s) during gait and rest, m is the child’s mass, g is acceleration due to gravity and Lleg is the child’s average leg length. Note that oxygen consumption was selected for this analysis versus oxygen cost because of the large, non-linear impact of walking speed on oxygen cost. For individuals with very slow walking speeds, oxygen cost approaches infinity due to the small fixed amount of energy needed to stand compared to resting which is not removed in calculating net oxygen cost. This effect makes walking speed a significant confounding factor in analyses that use oxygen cost.
We also compared oxygen consumption for each individual to control data from a database of typically-developing children walking at a similar speed (Koop et al., 1989). Briefly, this analysis uses a third-order polynomial that was fit to oxygen consumption data from typically-developing controls who walked at three speeds (slow, self-selected, and fast):
where v is nondimensional walking speed, calculated as . Using this low-order polynomial, we can calculate the average oxygen consumption of typically-developing controls at a given nondimensional walking speed and then calculate a patient’s oxygen consumption as a multiple of this speed matched oxygen consumption.
A crouch posture increases the internal knee extensor moment and knee extensor muscle activity (Hsu et al., 1993; Steele et al., 2012), which have been hypothesized to increase energy expenditure during crouch gait. To evaluate the relationship between the internal knee extensor moment and oxygen consumption, we calculated the knee extensor angular impulse, defined as the integral of the sagittal plane knee extensor moment. To focus on quadriceps demand, only the positive area (i.e., extensor moment resisted by quadriceps) was included in the analysis to calculate the knee extensor angular impulse. Similar to knee kinematics, the sagittal knee moment was averaged over all trials at 2% increments of the gait cycle. The average sagittal knee moment normalized by body mass and average step time were then used to calculate the knee extensor angular impulse. Note that kinetics are collected as standard of care; however, some individuals did not have kinetics collected due to an inability to capture clean force plate strikes because of assistive device use or extremely short step length.
The integral of rectus femoris electromyography (EMG, Motion Laboratory Systems, Baton Rouge, LA) data was used to evaluate persistence of knee extensor muscle activity. At this clinical center, EMG data from the rectus femoris was collected as part of standard clinical care. However, EMG data was not available from the other primary knee extensors, the vasti. The EMG data was sampled at 1080 Hz, band-pass filtered between 20 and 400 Hz, rectified, and then low-pass filtered at 10 Hz. Since maximum voluntary contractions are not taken as part of standard clinical care, the EMG data was normalized to the peak value across all walking trials for each child. Although this normalization limits comparison of the magnitude of rectus femoris muscle activity with crouch severity, the integrated area of rectus femoris activity provides a measure of sustained muscle activity.
As co-contraction has previously been shown to be related to energy expenditure in children with CP (Damiano et al., 2000; Unnithan et al., 1996), we also evaluated the co-contraction index of the rectus femoris (RF) and biceps femoris long head (BFLH). The co-contraction index was calculated as:
where area of RF and area of BFLH represent the integral of the EMG data over one gait cycle and common area is the overlapping integrated area of the two muscles (Winter, 1990).
Linear regression with a robust fit to reduce the impact of outliers was used to examine the dependence of oxygen consumption during gait on knee flexion angle, knee extensor angular impulse, rectus femoris activity, and co-contraction (Matlab, Mathworks, Inc. Natick, MA). Subgroups of children who used walking aids, including walkers or crutches, were also identified to determine whether correlations differed among these groups compared to children who did not use walking aids.
Results
We analyzed oxygen consumption and gait characteristics for 573 children with bilateral CP who met the inclusion criteria for this study (average ± one standard deviation, age 10.3 ± 3.9 years, height 1.32 ± 0.19 m, mass 33.6 ± 15.0 kg, M:F 316:257). The average oxygen consumption was 0.18 ± 0.06 for this group with CP (Figure 1) compared to 0.10 ± 0.03 among the typically-developing children (N = 77, age 10.7 ± 4.2 years, height 1.41 ± 0.22 m, mass 39.0 ± 17.6 kg). The children with CP walked slower than the typically-developing children with a walking speed of 0.83 ± 0.30 versus 1.09 ± 0.13 m/s. Comparing oxygen consumption to typically-developing controls with a similar walking speed, the average oxygen consumption for the children with CP was 2.9 times that of speed-matched controls (xSMC). Only 11% of the children had an oxygen consumption during walking within 1.5 xSMC and only 29% had an oxygen consumption within 2 xSMC. We excluded 55 children who had knee hyperextension during gait (age 8.6 ± 2.8 years, height 1.23 ± 0.14 m, mass 27.0 ± 12.5 kg, M:F 26:29). These subjects also demonstrated increased oxygen consumption during walking compared to typically-developing peers, 0.20 ± 0.08.
Figure 1.
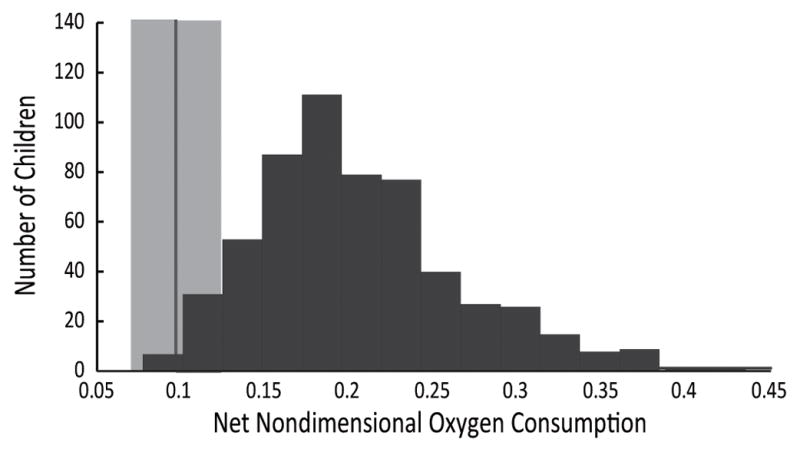
Histogram of the distribution of net nondimensional oxygen (O2) consumption during gait of children with bilateral cerebral palsy and no knee hyperextension (N = 573) compared to the average ± one standard deviation of typically-developing children walking at free speed (light gray band, N = 77).
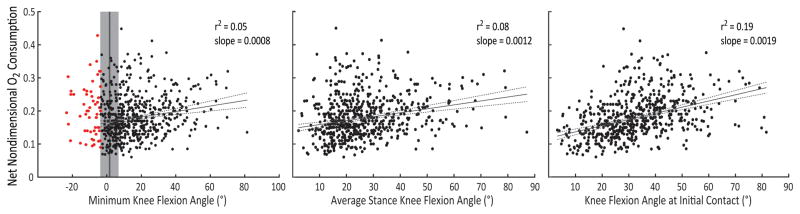
Measures of knee flexion angle during gait in children with CP were only weakly to moderately correlated with oxygen consumption (Figure 2). Knee flexion angle at initial contact explained the greatest variance in oxygen consumption during walking, 19%. Minimum knee flexion angle during and average knee flexion angle during stance explained only 5% and 8% of the variance in oxygen consumption, respectively.
Figure 2.
Comparison of minimum knee flexion angle, average knee flexion angle during stance, and knee flexion angle at initial contact with net nondimensional oxygen (O2) consumption. Children with knee hyperextension (red dots, > 1 SD below minimum knee flexion angle of typically-developing children, gray area) were excluded from analyses.
Oxygen consumption did increase with crouch severity, with an average increase in oxygen consumption of 8%, 15%, and 23% with a minimum knee flexion angle of 15°, 30°, and 45°, respectively. There was a large degree of variability in oxygen consumptions between individuals with similar crouch severity. The average [5–95 percentile] oxygen consumption was 0.18 [0.10–0.31] during mild crouch gait (15–30° minimum knee flexion angle, N = 164), 0.20 [0.12–0.28] during moderate crouch gait (30–50° minimum knee flexion angle, N = 83), and 0.22 [0.14–0.31] during severe crouch gait (> 50° minimum knee flexion angle, N = 24).
Children who used a walker (N = 34) or crutches (N = 15) also had higher oxygen consumption than children who did not walk with aids, with an oxygen consumption of 0.21 ± 0.06 for both the walker and crutches groups. The correlations of oxygen consumption with knee flexion angle were similar for children who used a walker (minimum knee flexion angle r2 = 0.05 and slope = 0.001, knee flexion angle at initial contact r2 = 0.16 and slope = 0.002), but these correlations were greatly reduced among the children who used crutches (r2 < 0.01 and slope < 0.001).
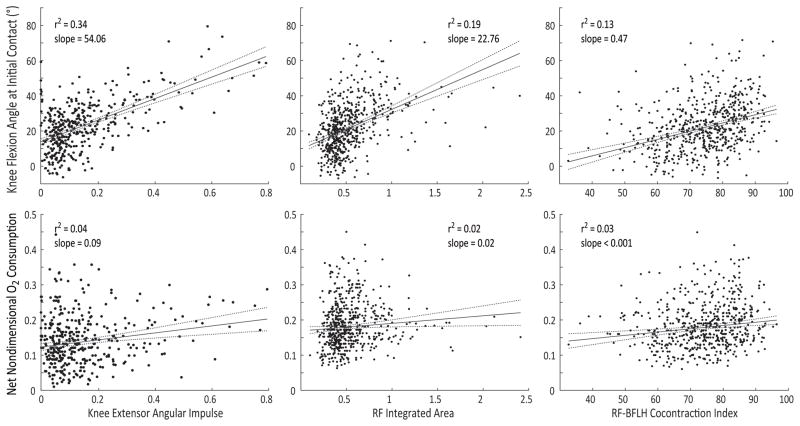
The internal knee extensor moment and EMG activity during gait were moderately to strongly correlated with crouch severity; however, these variables were only weakly correlated with oxygen consumption (Figure 3). Note that not all children with CP had kinetics collected as part of their gait analysis (N = 389). For this group, the knee extensor moment angular impulse explained 72% and 34% of the variance in minimum knee flexion angle and knee flexion angle at initial contact, respectively, but only 4% of the variance in oxygen consumption. Similarly, integrated rectus femoris EMG activity explained 27% of the variance in minimum knee flexion angle and 19% of the variance in knee flexion angle at initial contact, but only 2% of the variance in oxygen consumption. The co-contraction index of the rectus femoris and biceps femoris longhead explained 11% of the variance in minimum knee flexion angle, 13% of the variance in knee flexion angle at initial contact, and 3% of the variance in oxygen consumption.
Figure 3.
Comparison of knee flexion angle at initial contact (top row) and net nondimensional oxygen (O2) consumption (bottom row) with the internal knee extensor moment angular impulse, rectus femoris (RF) integrated area, and the rectus femoris and biceps femoris long head (RF-BFLH) co-contraction index. Regressions include children with bilateral CP without knee hyperextension. Note that only a subset of the children with CP (N = 389) had kinetic data collected during their gait analysis to evaluate the knee moment.
Discussion
Increasing crouch severity led to only modest increases in oxygen consumption during walking among individuals with bilateral CP. Crouch severity explained only 5–19% of the variance in oxygen consumption during walking in these children. Our original hypothesis was that there would be a strong correlation between crouch severity and oxygen consumption due to the dynamics of this gait pattern. However, the results of this study suggest that increased knee flexion during gait is not a dominant factor in determining energy expenditure during crouch gait. This study highlights the variability in energy expenditure across this population, and the complex array of factors that may influence an individual’s oxygen consumption. This study demonstrates that two individuals with CP walking with similar crouch severity can have vastly different energy expenditure.
Although biomechanical factors, such as internal knee extensor moment and rectus femoris EMG activity, were strongly correlated with crouch severity, these variables were only weakly correlated with oxygen consumption. These results support the conclusions drawn by Duffy and colleagues (1997), who found that a flexed-knee gait did not cause consistent increases in energy expenditure during walking in typically-developing children (Duffy et al., 1997). These researchers theorized that in CP a flexed-knee gait pattern does not inherently require greater energy expenditure than other pathologic gait patterns. A wide array of other factors, such as cardiovascular health, selective motor control, or altered muscle properties (e.g., strength or fiber composition), likely contribute to the variability in energy expenditure among individuals with CP.
While crouch gait is often defined by minimum or average knee flexion angle during stance, it is also interesting to note that knee flexion at initial contact had a larger correlation with oxygen consumption during gait. While we expected that sustained knee flexion and activity of the quadriceps during stance would be most relevant, these results suggest that shorter step lengths and increased cadence resulting from increased knee flexion at initial contact may play an important role in determining oxygen consumption during gait. Poor knee extension at initial contact may also be indicative of other factors, such as increased spasticity or poor selective motor control, which may influence an individual’s oxygen consumption. Crouch severity was also less associated with knee flexion during gait among the individuals who used crutches in this study. These results point to the additional demands placed on the arms, rather than lower-extremity dynamics, when using crutches. However, there was only a small group of children with assistive devices included in this study, which highlights the need for further research on the impacts of assistive device use on oxygen consumption in CP (Konop et al., 2009; Park et al., 2001).
Prior research on the impact of muscle activity and co-contraction on energy expenditure during gait have reported mixed results. Unnithan et al. (1996) found that co-contraction of the quadriceps and hamstrings explained 51.4% of the variance in gross oxygen consumption among children with CP walking on a treadmill at 3 km/hr (N = 9, 7 bilateral, 1 with crutches). However, Damiano et al. (2000) found the opposite relationship, with greater co-contraction between the quadriceps and hamstrings related to a lower Energy Expenditure Index (r = −0.71, N = 5). In this study, we found that integrated rectus femoris EMG area and co-contraction of the rectus femoris and biceps femoris long head only explained 2–3% of the variance in oxygen consumption during gait. Both variables did have a positive slope, indicating individuals with greater rectus activity or co-contraction had slightly greater oxygen consumption.
A limitation of this study was that we only had EMG data from the rectus femoris and not the vasti. However, prior research has demonstrated increased vasti demand during in crouch (Hsu et al., 1993; Steele et al., 2013), which would imply a stronger correlation between vasti activity and knee kinematics and kinetics than demonstrated for the rectus femoris in this study. Exclusion of the vasti from this study does not impact our conclusions that crouch severity is a poor predictor of oxygen consumption during gait. However, understanding how other muscles and differences in neuromuscular control influence patient-specific differences in energy expenditure during walking represents an important area for future research to guide new interventions and treatment planning.
The large variability in oxygen consumption among children with CP also has important implications for treatment and clinical decision making. Physical fatigue is prevalent among individuals with CP (Jahnsen et al., 2003), and can hinder activities of daily living. Prior research has demonstrated that orthopaedic surgery and selective dorsal rhizotomy can modestly improve energy expenditure during gait (Nene et al., 1993; Schwartz et al., 2004; Thomas et al., 2004). However, changes in oxygen consumption after treatment are variable, with some patients experiencing no change, or even an increase in oxygen consumption. Treatments that improve gait kinematics or spasticity may not have a significant impact on oxygen consumption if other factors, such as impaired neuromuscular control, remain unchanged. This research highlights the need for treatments that can reduce oxygen consumption to improve participation and quality of life for individuals with CP.
Crouch gait clearly requires greater energy expenditure compared to unimpaired gait. However, this work demonstrates the challenges in predicting energy consumption in CP and that some of our common assumptions about factors that contribute to increased energy expenditure in CP may be incorrect. Although there was a modest increase in oxygen consumption with increasing knee flexion, the relationship was poor compared to that expected from the dynamics of crouch gait. Assumptions about energy expenditure during gait cannot be based on kinematics or kinetics alone for individuals with CP. Rather, quantitative measurements of an individual’s oxygen consumption should be included in gait analyses to inform treatment planning. Future research needs to identify additional factors that may contribute to energy expenditure during gait, evaluate how energy expenditure relates to measures of quality of life, and guide new strategies for improving oxygen consumption and community participation for individuals with CP.
Acknowledgments
The authors would like to thank the staff at the James R. Gage Center for Gait and Motion Analysis at Gillette Children’s Specialty Healthcare for data collection and feedback. Research reported in this publication was supported by the National Institutes of Health under awards R01NS091056 and R01EB021935.
Footnotes
Conflicts of Interest:
The authors have no conflicts of interest to disclose related to this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnold AS, Anderson FC, Pandy MG, Delp SL. Muscular contributions to hip and knee extension during the single limb stance phase of normal gait: a framework for investigating the causes of crouch gait. Journal of Biomechanics. 2005;38:2181–2189. doi: 10.1016/j.jbiomech.2004.09.036. [DOI] [PubMed] [Google Scholar]
- Campbell J, Ball J. Energetics of walking in cerebral palsy. Orthopedic Clinics of North America. 1978;9:374–377. [PubMed] [Google Scholar]
- Damiano DL, Martellotta TL, Sullivan DJ, Granata KP, Abel MF. Muscle force production and functional performance in spastic cerebral palsy: relationship of cocontraction. Archives of physical medicine and rehabilitation. 2000;81:895–900. doi: 10.1053/apmr.2000.5579. [DOI] [PubMed] [Google Scholar]
- Duffy CM, Hill AE, Graham HK. The influence of flexed-knee gait on the energy cost of walking in children. Developmental Medicine and Child Neurology. 1997;39:234–238. doi: 10.1111/j.1469-8749.1997.tb07417.x. [DOI] [PubMed] [Google Scholar]
- Hemingway C, McGrogan J, Freeman JM. Energy requirements of spasticity. Developmental Medicine & Child Neurology. 2001;43:277–278. doi: 10.1017/s0012162201000524. [DOI] [PubMed] [Google Scholar]
- Hicks JL, Schwartz MH, Arnold AS, Delp SL. Crouched postures reduce the capacity of muscles to extend the hip and knee during the single-limb stance phase of gait. Journal of Biomechanics. 2008;41:960–967. doi: 10.1016/j.jbiomech.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu AT, Perry J, Gronley JK, Hislop HJ. Quadriceps force and myoelectric activity during flexed knee stance. Clinical Orthopaedics and Related Research. 1993:254–262. [PubMed] [Google Scholar]
- Jahnsen R, Villien L, Stanghelle JK, Holm I. Fatigue in adults with cerebral palsy in Norway compared with the general population. Developmental Medicine & Child Neurology. 2003;45:296–303. doi: 10.1017/s0012162203000562. [DOI] [PubMed] [Google Scholar]
- Johnston TE, Moore SE, Quinn LT, Smith BT. Energy cost of walking in children with cerebral palsy: relation to the Gross Motor Function Classification System. Developmental Medicine & Child Neurology. 2004;46:34–38. doi: 10.1017/s0012162204000064. [DOI] [PubMed] [Google Scholar]
- Konop KA, Strifling K, Wang M, Cao K, Eastwood D, Jackson S, Ackman J, Altiok H, Schwab J, Harris GF. Upper extremity kinetics and energy expenditure during walker-assisted gait in children with cerebral palsy. Acta Orthop Traumatol Turc. 2009;43:156–164. doi: 10.3944/AOTT.2009.156. [DOI] [PubMed] [Google Scholar]
- Koop S, Stout J, Starr R, Drinken B. Oxygen consumption during walking in children with cerebral palsy. Developmental Medicine & Child Neurology. 1989;31:6. [Google Scholar]
- Nene AV, Evans GA, Patrick JH. Simultaneous multiple operations for spastic diplegia. Outcome and functional assessment of walking in 18 patients. Journal of Bone & Joint Surgery, British Volume. 1993;75:488–494. doi: 10.1302/0301-620X.75B3.8496229. [DOI] [PubMed] [Google Scholar]
- Olney SJ, Costigan PA, Hedden DM. Mechanical energy patterns in gait of cerebral palsied children with hemiplegia. Physical Therapy. 1987;67:1348–1354. doi: 10.1093/ptj/67.9.1348. [DOI] [PubMed] [Google Scholar]
- Park ES, Park CI, Kim JY. Comparison of anterior and posterior walkers with respect to gait parameters and energy expenditure of children with spastic diplegic cerebral palsy. Yonsei medical journal. 2001;42:180–184. doi: 10.3349/ymj.2001.42.2.180. [DOI] [PubMed] [Google Scholar]
- Perry J, Antonelli D, Ford W. Analysis of knee-joint forces during flexed-knee stance. Journal of Bone & Joint Surgery, American Volume. 1975;57:961–967. [PubMed] [Google Scholar]
- Rose J, Gamble JG, Burgos A, Medeiros J, Haskell WL. Energy expenditure index of walking for normal children and for children with cerebral palsy. Developmental Medicine & Child Neurology. 1990;32:333–340. doi: 10.1111/j.1469-8749.1990.tb16945.x. [DOI] [PubMed] [Google Scholar]
- Schwartz MH. Protocol changes can improve the reliability of net oxygen cost data. Gait & Posture. 2007;26:494–500. doi: 10.1016/j.gaitpost.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Schwartz MH, Koop SE, Bourke JL, Baker R. A nondimensional normalization scheme for oxygen utilization data. Gait & Posture. 2006;24:14–22. doi: 10.1016/j.gaitpost.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Schwartz MH, Viehweger E, Stout J, Novacheck TF, Gage JR. Comprehensive treatment of ambulatory children with cerebral palsy: an outcome assessment. Journal of Pediatric Orthopaedics. 2004;24:45–53. [PubMed] [Google Scholar]
- Steele KM, Demers MS, Schwartz MH, Delp SL. Compressive tibiofemoral force during crouch gait. Gait & Posture. 2012;35:556–560. doi: 10.1016/j.gaitpost.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele KM, Seth A, Hicks JL, Schwartz MH, Delp SL. Muscle contributions to vertical and fore-aft accelerations are altered in subjects with crouch gait. Gait & Posture. 2013;38:86–91. doi: 10.1016/j.gaitpost.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland DH, Davids JR. Common gait abnormalities of the knee in cerebral palsy. Clinical Orthopaedics and Related Research. 1993:139–147. [PubMed] [Google Scholar]
- Thomas SS, Buckon CE, Piatt JH, Aiona MD, Sussman MD. A 2-year follow-up of outcomes following orthopedic surgery or selective dorsal rhizotomy in children with spastic diplegia. Journal of Pediatric Orthopaedics B. 2004;13:358–366. doi: 10.1097/01202412-200411000-00002. [DOI] [PubMed] [Google Scholar]
- Unnithan VB, Dowling JJ, Frost G, Bar-Or O. Role of cocontraction in the O2 cost of walking in children with cerebral palsy. Medicine & Science in Sports & Exercise. 1996;28:1498–1504. doi: 10.1097/00005768-199612000-00009. [DOI] [PubMed] [Google Scholar]
- Wakeling J, Blake O, Chan H. Muscle coordination is key to the power output and mechanical efficiency of limb movements. The Journal of experimental biology. 2010;213:487–492. doi: 10.1242/jeb.036236. [DOI] [PubMed] [Google Scholar]
- Waters RL, Mulroy S. The energy expenditure of normal and pathologic gait. Gait & Posture. 1999;9:207–231. doi: 10.1016/s0966-6362(99)00009-0. [DOI] [PubMed] [Google Scholar]
- Winter D. Biomechanics and motor control of human movement. 4. Wiley; 1990. [Google Scholar]
- Winter DA. Knee flexion during stance as a determinant of inefficient walking. Physical Therpay. 1983;63:331–333. doi: 10.1093/ptj/63.3.331. [DOI] [PubMed] [Google Scholar]
- Wren TA, Rethlefsen S, Kay RM. Prevalence of specific gait abnormalities in children with cerebral palsy: influence of cerebral palsy subtype, age, and previous surgery. Journal of Pediatric Orthopaedics. 2005;25:79–83. doi: 10.1097/00004694-200501000-00018. [DOI] [PubMed] [Google Scholar]