Abstract
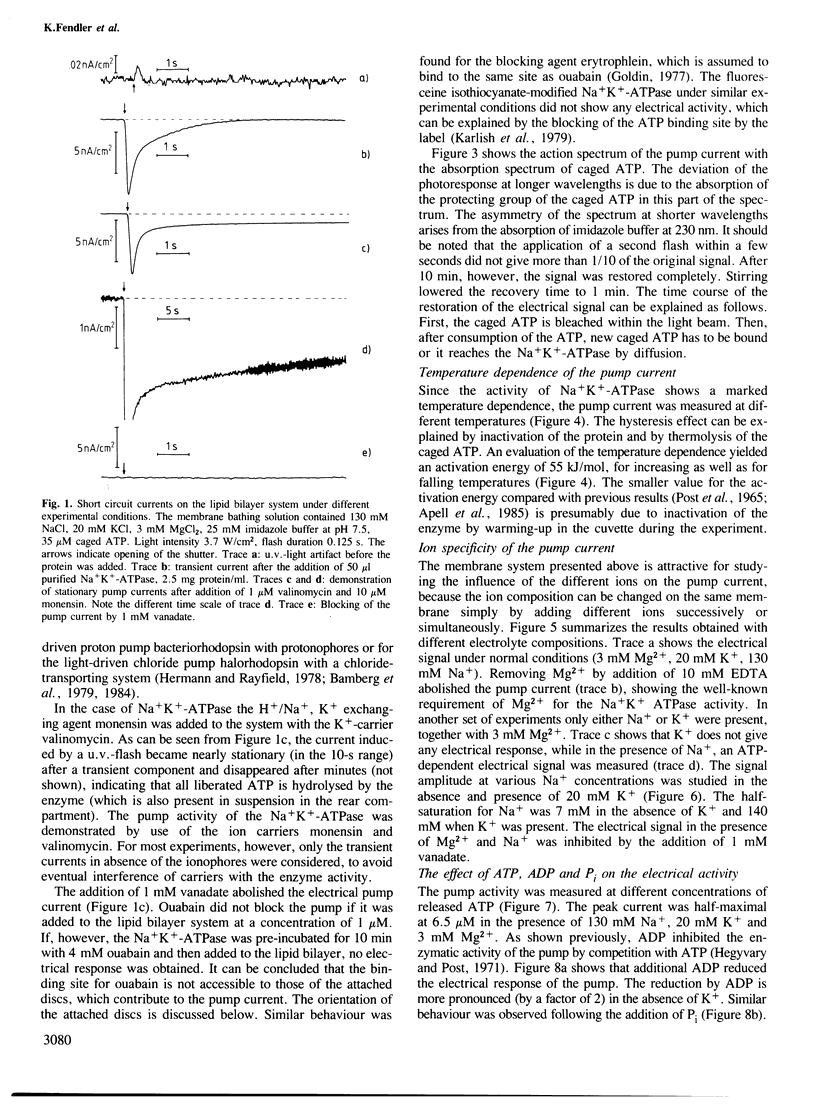
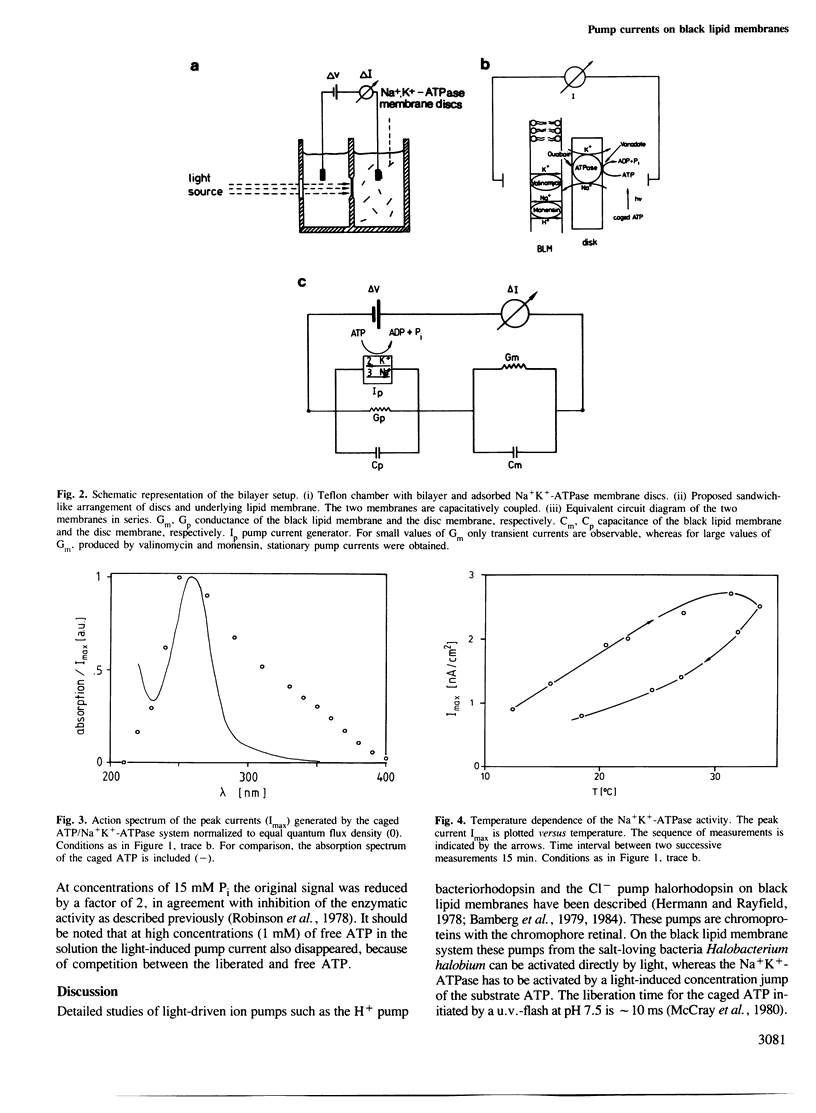
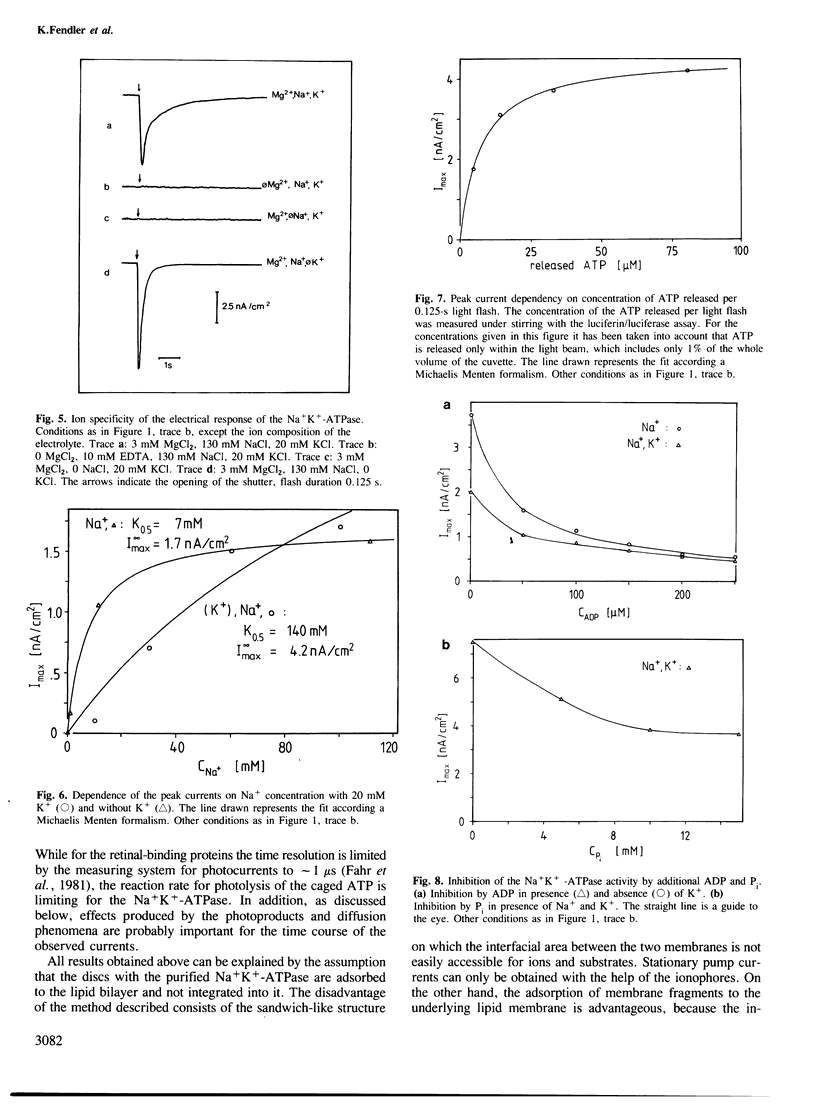
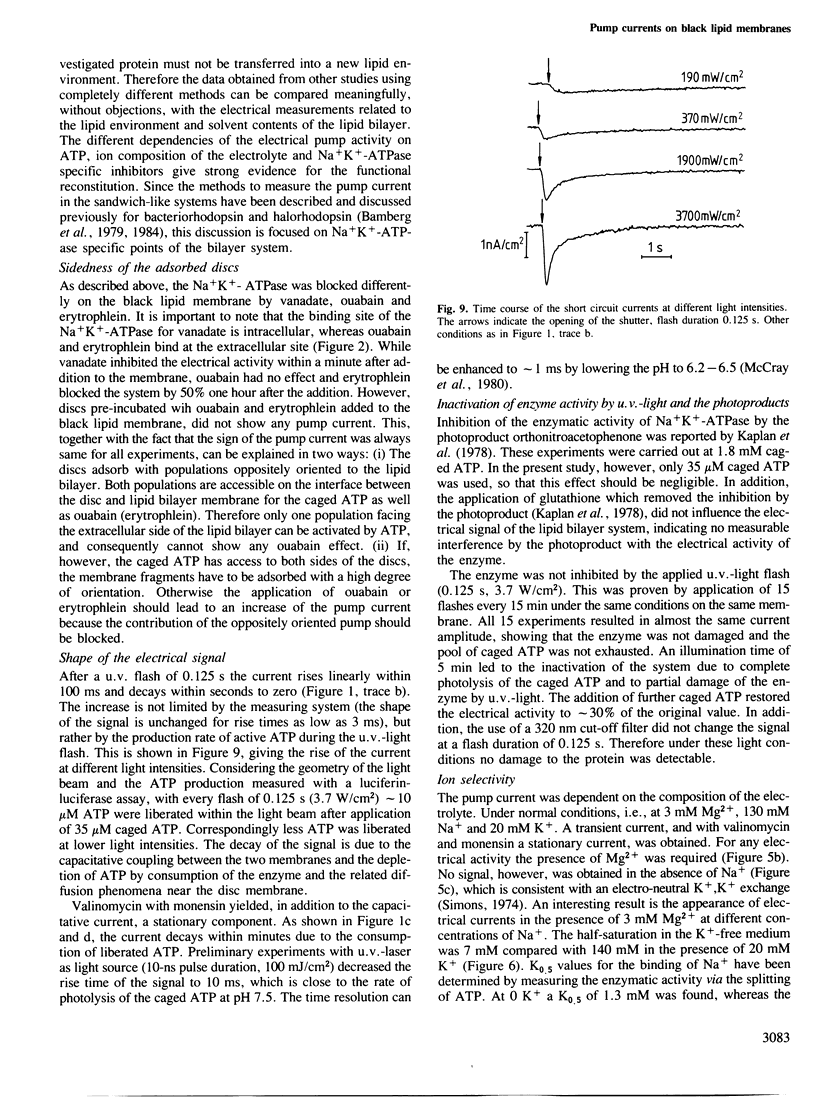
The transport activity of purified Na+K+-ATPase was investigated by measuring the electrical pump current induced on black lipid membranes. Discs containing purified Na+K+-ATPase from pig kidney were attached to planar lipid bilayers in a sandwich-like structure. After the addition of only microM concentrations of an inactive photolabile ATP derivative [P3-1-(2-nitro)phenylethyladenosine 5'-triphosphate, caged ATP] ATP was released after illumination with u.v.-light, which led to a transient current in the system. The transient photoresponse indicates that the discs and the underlying membrane are capacitatively coupled. Stationary pump currents were obtained after the addition of the H+, Na+ exchanging agent monensin together with valinomycin to the membrane system, which increased the permeability of the black lipid membrane for the pumped ions. In the absence of ADP and Pi the half saturation for the maximal photoeffect was obtained at 6.5 microM released ATP. The addition of ADP decreased the pump activity. Pump activity was obtained only in the presence of Mg2+ together with Na+ and Na+ and K+. No pump current was obtained in the presence of Mg2+ together with K+. The electrical response was blocked completely by the Na+K+-ATPase-specific inhibitors vanadate and ouabain. No pump currents were observed with a chemically modified protein, which was labelled on the ATP binding site with fluoresceine isothiocyanate. The method described offers the possibility of investigating by direct electrical measurements ion transport of Na+K+-ATPase with a large variety of different parameters.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apell H. J., Marcus M. M., Anner B. M., Oetliker H., Läuger P. Optical study of active ion transport in lipid vesicles containing reconstituted Na,K-ATPase. J Membr Biol. 1985;85(1):49–63. doi: 10.1007/BF01872005. [DOI] [PubMed] [Google Scholar]
- Brotherus J. R., Jacobsen L., Jørgensen P. L. Soluble and enzymatically stable (Na+ + K+)-ATPase from mammalian kidney consisting predominantly of protomer alpha beta-units. Preparation, assay and reconstitution of active Na+, K+ transport. Biochim Biophys Acta. 1983 Jun 10;731(2):290–303. doi: 10.1016/0005-2736(83)90021-4. [DOI] [PubMed] [Google Scholar]
- Cornelius F., Skou J. C. Reconstitution of (Na+ + K+)-ATPase into phospholipid vesicles with full recovery of its specific activity. Biochim Biophys Acta. 1984 May 30;772(3):357–373. doi: 10.1016/0005-2736(84)90153-6. [DOI] [PubMed] [Google Scholar]
- Dancsházy Z., Karvaly B. Incorporation of bacteriorhodopsin into a bilayer lipid membrane; a photoelectric-spectroscopic study. FEBS Lett. 1976 Dec 15;72(1):136–138. doi: 10.1016/0014-5793(76)80829-0. [DOI] [PubMed] [Google Scholar]
- Forbush B., 3rd Na+ movement in a single turnover of the Na pump. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5310–5314. doi: 10.1073/pnas.81.17.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadsby D. C., Kimura J., Noma A. Voltage dependence of Na/K pump current in isolated heart cells. Nature. 1985 May 2;315(6014):63–65. doi: 10.1038/315063a0. [DOI] [PubMed] [Google Scholar]
- Garay R. P., Garrahan P. J. The interaction of sodium and potassium with the sodium pump in red cells. J Physiol. 1973 Jun;231(2):297–325. doi: 10.1113/jphysiol.1973.sp010234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrahan P. J., Glynn I. M. Facftors affecting the relative magnitudes of the sodium:potassium and sodium:sodium exchanges catalysed by the sodium pump. J Physiol. 1967 Sep;192(1):189–216. doi: 10.1113/jphysiol.1967.sp008296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn I. M., Karlish S. J. ATP hydrolysis associated with an uncoupled sodium flux through the sodium pump: evidence for allosteric effects of intracellular ATP and extracellular sodium. J Physiol. 1976 Apr;256(2):465–496. doi: 10.1113/jphysiol.1976.sp011333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn I. M., Karlish S. J. The sodium pump. Annu Rev Physiol. 1975;37:13–55. doi: 10.1146/annurev.ph.37.030175.000305. [DOI] [PubMed] [Google Scholar]
- Goldin S. M. Active transport of sodium and potassium ions by the sodium and potassium ion-activated adenosine triphosphatase from renal medulla. Reconstitution of the purified enzyme into a well defined in vitro transport system. J Biol Chem. 1977 Aug 25;252(16):5630–5642. [PubMed] [Google Scholar]
- Goldin S. M., Tong S. W. Reconstitution of active transport catalyzed by the purified sodium and potassium ion-stimulated adenosine triphosphatase from canine renal medulla. J Biol Chem. 1974 Sep 25;249(18):5907–5915. [PubMed] [Google Scholar]
- Hegyvary C., Jorgensen P. L. Conformational changes of renal sodium plus potassium ion-transport adenosine triphosphatase labeled with fluorescein. J Biol Chem. 1981 Jun 25;256(12):6296–6303. [PubMed] [Google Scholar]
- Hegyvary C., Post R. L. Binding of adenosine triphosphate to sodium and potassium ion-stimulated adenosine triphosphatase. J Biol Chem. 1971 Sep 10;246(17):5234–5240. [PubMed] [Google Scholar]
- Herrmann T. R., Rayfield G. W. The electrical response to light of bacteriorhodopsin in planar membranes. Biophys J. 1978 Feb;21(2):111–125. doi: 10.1016/S0006-3495(78)85512-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman J. F., Kaplan J. H., Callahan T. J. The Na:K pump in red cells is electrogenic. Fed Proc. 1979 Oct;38(11):2440–2441. [PubMed] [Google Scholar]
- Jensen J., Norby J. G. On the specificity of the ATP-binding site of (Na+ + K+)-activated ATPase from brain microsomes. Biochim Biophys Acta. 1971 Apr 13;233(2):395–403. doi: 10.1016/0005-2736(71)90336-1. [DOI] [PubMed] [Google Scholar]
- Jorgensen P. L. Purification and characterization of (Na+ plus K+ )-ATPase. 3. Purification from the outer medulla of mammalian kidney after selective removal of membrane components by sodium dodecylsulphate. Biochim Biophys Acta. 1974 Jul 12;356(1):36–52. doi: 10.1016/0005-2736(74)90292-2. [DOI] [PubMed] [Google Scholar]
- Jørgensen P. L. Mechanism of the Na+, K+ pump. Protein structure and conformations of the pure (Na+ +K+)-ATPase. Biochim Biophys Acta. 1982 Aug 11;694(1):27–68. doi: 10.1016/0304-4157(82)90013-2. [DOI] [PubMed] [Google Scholar]
- Kaplan J. H., Forbush B., 3rd, Hoffman J. F. Rapid photolytic release of adenosine 5'-triphosphate from a protected analogue: utilization by the Na:K pump of human red blood cell ghosts. Biochemistry. 1978 May 16;17(10):1929–1935. doi: 10.1021/bi00603a020. [DOI] [PubMed] [Google Scholar]
- Karlish S. J., Beaugé L. A., Glynn I. M. Vanadate inhibits (Na+ + K+)ATPase by blocking a conformational change of the unphosphorylated form. Nature. 1979 Nov 15;282(5736):333–335. doi: 10.1038/282333a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Last T. A., Gantzer M. L., Tyler C. D. Ion-gated channel induced in planar bilayers by incorporation of (Na+,K+)-ATPase. J Biol Chem. 1983 Feb 25;258(4):2399–2404. [PubMed] [Google Scholar]
- Lederer W. J., Nelson M. T. Sodium pump stoicheiometry determined by simultaneous measurements of sodium efflux and membrane current in barnacle. J Physiol. 1984 Mar;348:665–677. doi: 10.1113/jphysiol.1984.sp015132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCray J. A., Herbette L., Kihara T., Trentham D. R. A new approach to time-resolved studies of ATP-requiring biological systems; laser flash photolysis of caged ATP. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7237–7241. doi: 10.1073/pnas.77.12.7237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POST R. L., SEN A. K., ROSENTHAL A. S. A PHOSPHORYLATED INTERMEDIATE IN ADENOSINE TRIPHOSPHATE-DEPENDENT SODIUM AND POTASSIUM TRANSPORT ACROSS KIDNEY MEMBRANES. J Biol Chem. 1965 Mar;240:1437–1445. [PubMed] [Google Scholar]
- Reinhardt R., Lindemann B., Anner B. M. Leakage-channel conductance of single (Na+ + K+)-ATPase molecules incorporated into planar bilayers by fusion of liposomes. Biochim Biophys Acta. 1984 Jul 11;774(1):147–150. doi: 10.1016/0005-2736(84)90285-2. [DOI] [PubMed] [Google Scholar]
- Robinson J. D., Flashner M. S., Marin G. K. Inhibition of the (Na+ + K+)-dependent ATPase by inorganic phosphate. Biochim Biophys Acta. 1978 Jun 2;509(3):419–428. doi: 10.1016/0005-2736(78)90236-5. [DOI] [PubMed] [Google Scholar]
- Robinson J. D. Na+ sites of the (Na+ + K+)-dependent ATPase. Biochim Biophys Acta. 1977 Jun 10;482(2):427–437. doi: 10.1016/0005-2744(77)90257-1. [DOI] [PubMed] [Google Scholar]
- Schuurmans Stekhoven F., Bonting S. L. Transport adenosine triphosphatases: properties and functions. Physiol Rev. 1981 Jan;61(1):1–76. doi: 10.1152/physrev.1981.61.1.1. [DOI] [PubMed] [Google Scholar]
- Simons T. J. Potassium: potassium exchange catalysed by the sodium pump in human red cells. J Physiol. 1974 Feb;237(1):123–155. doi: 10.1113/jphysiol.1974.sp010474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEHRLI W. E., VERHEYDEN D. L., MOFFATT J. G. DISMUTATION REACTIONS OF NUCLEOSIDE POLYPHOSPHATES. II. SPECIFIC CHEMICAL SYNTHESES OF ALPHA-, BETA-, AND GAMMA-P32-NUCLEOSIDE 5'-TRIPHOSPHATES. J Am Chem Soc. 1965 May 20;87:2265–2277. doi: 10.1021/ja01088a028. [DOI] [PubMed] [Google Scholar]



