Abstract
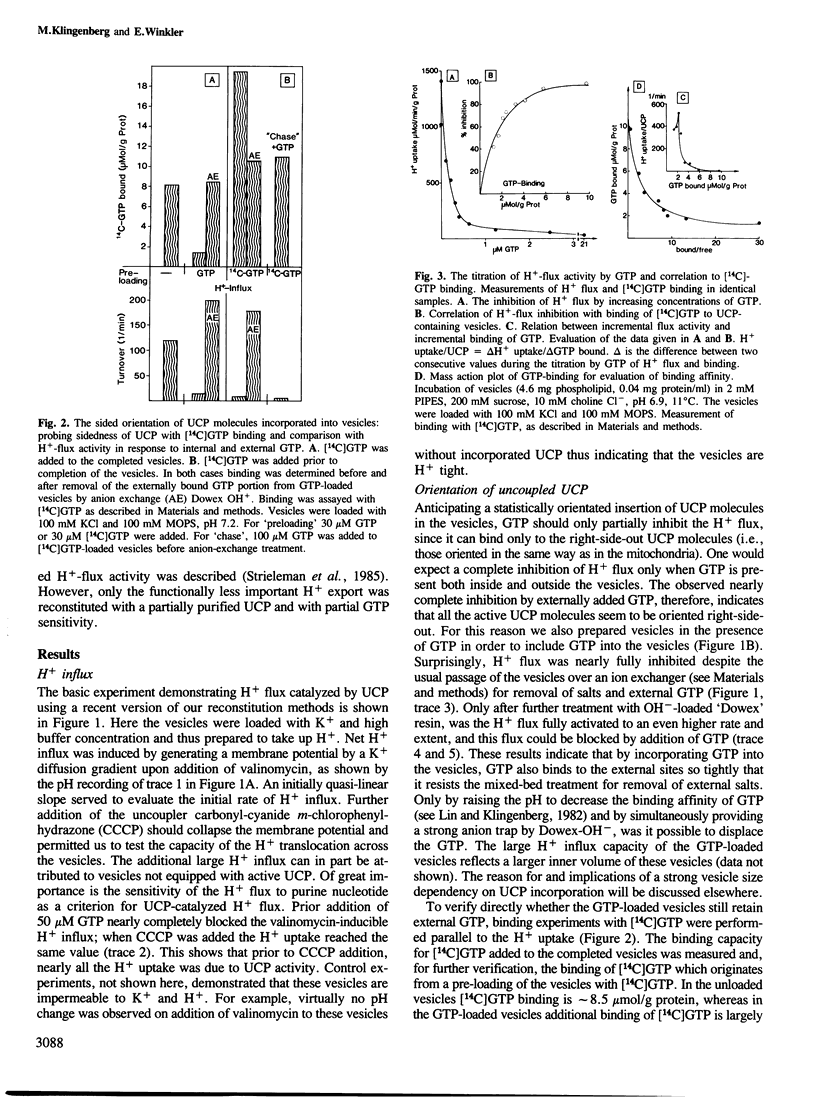
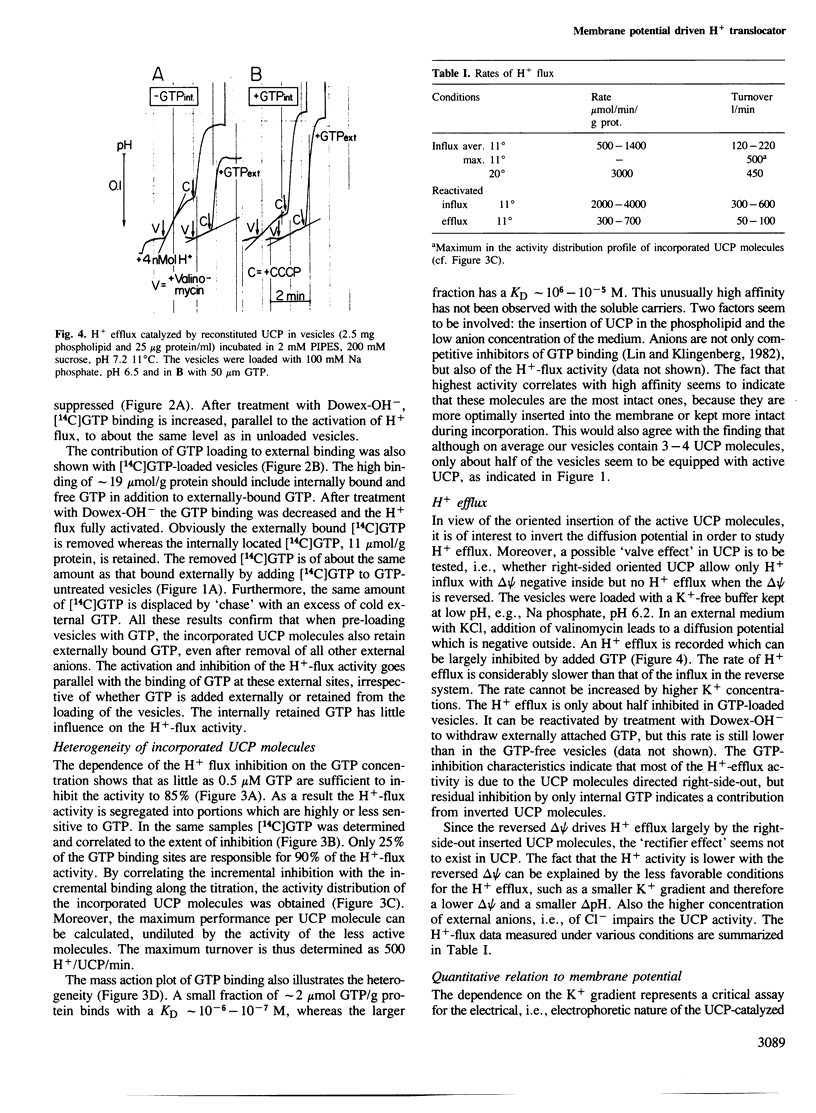
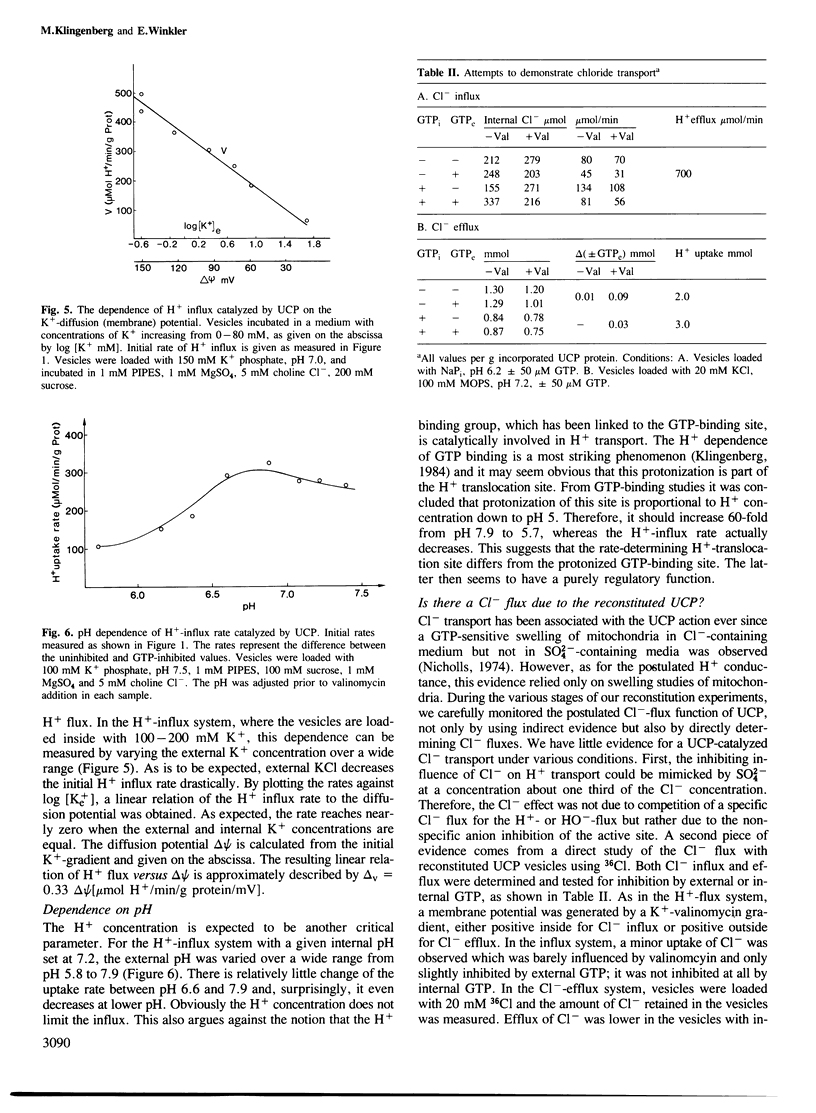
The isolated uncoupling protein (UCP) from brown fat adipose tissue mitochondria has been reconstituted into artificial phospholipid vesicles. Because of the high lability of H+ transport, several new steps have been introduced in the reconstitution; the detergent octyl-POE, the addition of phospholipids to mitochondria prior to solubilization and purification, the vesicle formation by rapid removal of detergent with polystyrene beads and of external salts by a mixed ion exchange. In the K+-loaded proteoliposomes, H+ influx can be induced by a diffusion potential on addition of valinomycin. H+ influx is inhibited to more than 90% by GTP addition, in the assay for UCP activity. By reversing delta psi with external K+, H+ efflux is measured, however, at a four times lower rate. In vesicles loaded with internal GTP, H+ influx is fully inhibited but can be activated by Dowex-OH treatment to an even higher rate than that found in the GTP-free vesicles. Binding studies with GTP show that most of the active UCP are oriented with the binding site outside as in mitochondria, and that in GTP-loaded vesicles GTP is also bound at the outside. The rate of H+ transport is linearly dependent on the membrane potential. Despite the ordered orientation, there is no 'valve' mechanism, since there is H+ efflux with a reversed potential. pH dependency is only small between pH 6.5 and 7.5, indicating that the H+-translocating site differs from the highly pH-dependent nucleotide-binding site. The turnover number of reconstituted UCP is commensurate with mitochondrial function and indicates a carrier instead of a channel-type H+ transport.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF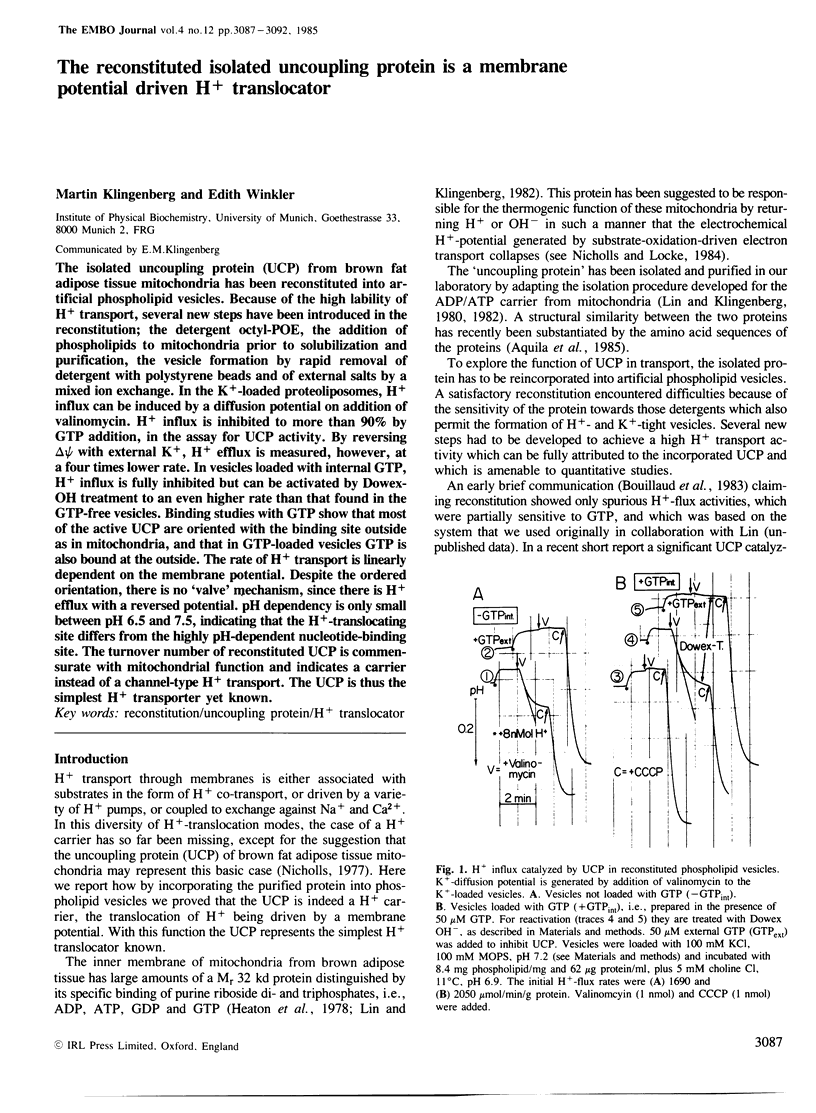


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bouillaud F., Ricquier D., Gulik-Krzywicki T., Gary-Bobo C. M. The possible proton translocating activity of the mitochondrial uncoupling protein of brown adipose tissue. Reconstitution studies in liposomes. FEBS Lett. 1983 Dec 12;164(2):272–276. doi: 10.1016/0014-5793(83)80300-7. [DOI] [PubMed] [Google Scholar]
- Chappell J. B. Systems used for the transport of substrates into mitochondria. Br Med Bull. 1968 May;24(2):150–157. doi: 10.1093/oxfordjournals.bmb.a070618. [DOI] [PubMed] [Google Scholar]
- Guérin Bernard, Guérin Martine, Klingenberg Martin. Differential inhibition of phosphate efflux and influx and a possible discrimination between an inner and outer location of the phosphate carrier in mitochondria. FEBS Lett. 1970 Oct 16;10(4):265–268. doi: 10.1016/0014-5793(70)80644-5. [DOI] [PubMed] [Google Scholar]
- Heaton G. M., Wagenvoord R. J., Kemp A., Jr, Nicholls D. G. Brown-adipose-tissue mitochondria: photoaffinity labelling of the regulatory site of energy dissipation. Eur J Biochem. 1978 Jan 16;82(2):515–521. doi: 10.1111/j.1432-1033.1978.tb12045.x. [DOI] [PubMed] [Google Scholar]
- Klingenberg M. Characteristics of the uncoupling protein from brown-fat mitochondria. Biochem Soc Trans. 1984 Jun;12(3):390–393. doi: 10.1042/bst0120390. [DOI] [PubMed] [Google Scholar]
- Klingenberg M., Durand R., Guérin B. Analysis of the reactivity of SH-reagents with the mitochondrial phosphate carrier. Eur J Biochem. 1974 Feb 15;42(1):135–150. doi: 10.1111/j.1432-1033.1974.tb03323.x. [DOI] [PubMed] [Google Scholar]
- Klingenberg M., Palmieri F., Quagliariello E. Quantitative correlation between the distribution of anions and the pH difference across the mitochondrial membrane. Eur J Biochem. 1970 Dec;17(2):230–238. doi: 10.1111/j.1432-1033.1970.tb01158.x. [DOI] [PubMed] [Google Scholar]
- Klingenberg M. The ADP-ATP translocation in mitochondria, a membrane potential controlled transport. J Membr Biol. 1980 Sep 30;56(2):97–105. doi: 10.1007/BF01875961. [DOI] [PubMed] [Google Scholar]
- Kopecký J., Guerrieri F., Jezek P., Drahota Z., Houstek J. Molecular mechanism of uncoupling in brown adipose tissue mitochondria. The non-identity of proton and chloride conducting pathways. FEBS Lett. 1984 May 7;170(1):186–190. doi: 10.1016/0014-5793(84)81396-4. [DOI] [PubMed] [Google Scholar]
- Krämer R., Klingenberg M. Electrophoretic control of reconstituted adenine nucleotide translocation. Biochemistry. 1982 Mar 2;21(5):1082–1089. doi: 10.1021/bi00534a040. [DOI] [PubMed] [Google Scholar]
- Krämer R., Klingenberg M. Reconstitution of adenine nucleotide transport from beef heart mitochondria. Biochemistry. 1979 Sep 18;18(19):4209–4215. doi: 10.1021/bi00586a027. [DOI] [PubMed] [Google Scholar]
- Lin C. S., Klingenberg M. Characteristics of the isolated purine nucleotide binding protein from brown fat mitochondria. Biochemistry. 1982 Jun 8;21(12):2950–2956. doi: 10.1021/bi00541a023. [DOI] [PubMed] [Google Scholar]
- Lin C. S., Klingenberg M. Isolation of the uncoupling protein from brown adipose tissue mitochondria. FEBS Lett. 1980 May 5;113(2):299–303. doi: 10.1016/0014-5793(80)80613-2. [DOI] [PubMed] [Google Scholar]
- Nicholls D. G. Hamster brown-adipose-tissue mitochondria. Purine nucleotide control of the ion conductance of the inner membrane, the nature of the nucleotide binding site. Eur J Biochem. 1976 Feb 16;62(2):223–228. doi: 10.1111/j.1432-1033.1976.tb10151.x. [DOI] [PubMed] [Google Scholar]
- Nicholls D. G. Hamster brown-adipose-tissue mitochondria. The chloride permeability of the inner membrane under respiring conditions, the influence of purine nucleotides. Eur J Biochem. 1974 Dec 2;49(3):585–593. doi: 10.1111/j.1432-1033.1974.tb03862.x. [DOI] [PubMed] [Google Scholar]
- Nicholls D. G., Lindberg O. Brown-adipose-tissue mitochondria. The influence of albumin and nucleotides on passive ion permeabilities. Eur J Biochem. 1973 Sep 3;37(3):523–530. doi: 10.1111/j.1432-1033.1973.tb03014.x. [DOI] [PubMed] [Google Scholar]
- Nicholls D. G., Locke R. M. Thermogenic mechanisms in brown fat. Physiol Rev. 1984 Jan;64(1):1–64. doi: 10.1152/physrev.1984.64.1.1. [DOI] [PubMed] [Google Scholar]
- Nicholls D. G. The effective proton conductance of the inner membrane of mitochondria from brown adipose tissue. Dependency on proton electrochemical potential gradient. Eur J Biochem. 1977 Jul 15;77(2):349–356. doi: 10.1111/j.1432-1033.1977.tb11674.x. [DOI] [PubMed] [Google Scholar]
- Rial E., Nicholls D. G. The regulation of the proton conductance of brown fat mitochondria. Identification of functional and non-functional nucleotide-binding sites. FEBS Lett. 1983 Sep 19;161(2):284–288. doi: 10.1016/0014-5793(83)81026-6. [DOI] [PubMed] [Google Scholar]
- Rial E., Poustie A., Nicholls D. G. Brown-adipose-tissue mitochondria: the regulation of the 32000-Mr uncoupling protein by fatty acids and purine nucleotides. Eur J Biochem. 1983 Dec 1;137(1-2):197–203. doi: 10.1111/j.1432-1033.1983.tb07815.x. [DOI] [PubMed] [Google Scholar]
- Strieleman P. J., Schalinske K. L., Shrago E. Partial purification and functional reconstitution of GDP sensitive brown adipose tissue mitochondria uncoupling protein using octyl glucoside. Biochem Biophys Res Commun. 1985 Mar 15;127(2):509–516. doi: 10.1016/s0006-291x(85)80189-3. [DOI] [PubMed] [Google Scholar]



